Energy Conversion Capacity of Barium Zirconate Titanate
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sukwisute, P.; Muensit, N.; Soontaranon, S.; Rugmai, S. Micropower energy harvesting using poly (vinylidene fluoride hexafluoropropylene). Appl. Phys. Lett. 2013, 103, 063905. [Google Scholar] [CrossRef]
- Rakbamrung, P.; Lallart, M.; Guyomar, D.; Muensit, N.; Thanachayanont, C.; Lucat, C.; Guiffard, B.; Petit, L.; Sukwisut, P. Performance comparison of PZT and PMN-PT piezoceramics for vibration energy harvesting using standard or nonlinear approach. Sens. Actuators A Phys. 2010, 163, 493–500. [Google Scholar] [CrossRef]
- Lü, C.; Zhang, Y.; Zhang, H.; Zhang, Z.; Shen, M.; Chen, Y. Generalized optimization method for energy conversion and storage efficiency of nanoscale flexible piezoelectric energy harvesters. Energy Convers. Manag. 2019, 182, 34–40. [Google Scholar]
- Acosta, M.; Novak, N.; Rojas, V.; Patel, S.; Vaish, R.; Koruza, J.; Rossetti, G.A.; Rödel, J. BaTiO3-based piezoelectrics: Fundamentals, current status, and perspectives. Appl. Phys. Rev. 2017, 4, 041305. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, Q.; Zhai, J.; Xu, Z. DC field effect on dielectric property of Ba (ZrxTi1−x) O3 ceramics. Ceram. Int. 2013, 39, S9–S13. [Google Scholar] [CrossRef]
- Kuang, S.J.; Tang, X.G.; Li, L.Y.; Jiang, Y.P.; Liu, Q.X. Influence of Zr dopant on the dielectric properties and Curie temperatures of Ba(ZrxTi1−x)O3 (0 ≤ x ≤ 0.12) ceramics. Scr. Mater. 2009, 61, 68–71. [Google Scholar] [CrossRef]
- Yang, L.; Kong, X.; Li, F.; Hao, H.; Cheng, Z.; Liu, H.; Li, J.-F.; Zhang, S. Perovskite lead-free dielectrics for energy storage applications. Prog. Mater. Sci. 2019, 102, 72–108. [Google Scholar] [CrossRef]
- Julphunthong, P.; Chootin, S.; Bongkarn, T. Phase formation and electrical properties of Ba (ZrxTi1−x) O3 ceramics synthesized through a novel combustion technique. Ceram. Int. 2013, 39, S415–S419. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Huang, Y.H.; Song, K.X.; Wu, Y.J. Enhanced dielectric strength and energy storage density in BaTi0.7Zr0.3 O3 ceramics via spark plasma sintering. J. Mater. Sci. 2019, 54, 4511–4517. [Google Scholar] [CrossRef]
- Puli, V.S.; Pradhan, D.K.; Riggs, B.C.; Chrisey, D.B.; Katiyar, R.S. Investigations on structure, ferroelectric, piezoelectric and energy storage properties of barium calcium titanate (BCT) ceramics. J. Alloys Compd. 2014, 584, 369–373. [Google Scholar] [CrossRef]
- Dhakar, L.; Liu, H.; Tay, F.E.H.; Lee, C. A new energy harvester design for high power output at low frequencies. Sens. Actuators A Phys. 2013, 199, 344–352. [Google Scholar] [CrossRef]
- Selvan, K.V.; Muhammad, M.S. Micro-scale energy harvesting devices: Review of methodological performances in the last decade. Renew. Sust. Energ. Rev. 2016, 54, 1035–1047. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, S.; Li, D.; Jin, L.; Wu, Q.; Liu, F. Frequency up-converted piezoelectric energy harvester for ultralow-frequency and ultrawide-frequency-range operation. Appl. Phys. Lett. 2018, 112, 163902. [Google Scholar] [CrossRef]
- Jiwei, Z.; Xi, Y.; Liangying, Z.; Bo, S.; Chen, H. Orientation control and dielectric properties of sol–gel deposited Ba(Ti, Zr)O3 thin films. J. Cryst. Growth 2004, 262, 341–347. [Google Scholar] [CrossRef]
- Binhayeeniyi, N.; Sukvisut, P.; Thanachayanont, C.; Muensit, S. Physical and electromechanical properties of barium zirconium titanate synthesized at low-sintering temperature. Mater. Lett. 2010, 64, 305–308. [Google Scholar] [CrossRef]
- Thanachayanont, C.; Yordsri, V.; Kijamnajsuk, S.; Binhayeeniyi, N.; Muensit, N. Microstructural investigation of sol–gel BZT powders. Mater. Lett. 2012, 82, 205–207. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, K.; Guo, X.; Sun, W.; Jiang, H.; Han, X.; Tao, X.; Cheng, Z.; Zhao, H.; Kimura, H.; et al. Crystallization, phase evolution and ferroelectric properties of sol-gel-synthesized Ba(Ti0.8Zr0.2)O3–x(Ba0.7Ca0.3)TiO3 thin films. J. Mater. Chem. C 2013, 1, 522–530. [Google Scholar] [CrossRef]
- Buscaglia, M.T.; Buscaglia, V.; Alessio, R. Coating of BaCO3 crystals with TiO2: Versatile approach to the synthesis of BaTiO3 tetragonal nanoparticles. Chem. Mater. 2007, 19, 711–718. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Tsubouchi, N.; Sugawara, K. Synthesis of BaTiO3 nanoparticles from TiO2-coated BaCO3 particles derived using a wet-chemical method. J. Asian Ceram. Soc. 2014, 2, 68–76. [Google Scholar] [CrossRef]
- Maiwa, H. Electromechanical properties of Ba (Zr0.2Ti0.8) O3 ceramics prepared by spark plasma sintering. Ceram. Int. 2012, 38, S219–S223. [Google Scholar] [CrossRef]
- Xu, Q.; Zhan, D.; Huang, D.P.; Liu, H.X.; Chen, W.; Zhang, F. Dielectric inspection of BaZr0.2Ti0.8O3 ceramics under bias electric field: A survey of polar nano-regions. Mater. Res. Bull. 2012, 47, 1674–1679. [Google Scholar] [CrossRef]
- Hemeda, O.M.; Salem, B.I.; Abdelfatah, H.; Abdelsatar, G.; Shihab, M. Dielectric and ferroelectric properties of barium zirconate titanate ceramics prepared by ceramic method. Phys. B 2019, 574, 411680. [Google Scholar] [CrossRef]
- Tang, X.G.; Wang, J.; Wang, X.X.; Chan, H.L.W. Effects of grain size on the dielectric properties and tunabilities of sol-gel derived Ba (Zr0.2Ti0.8) O3 ceramics. Solid State Commun. 2004, 131, 163–168. [Google Scholar] [CrossRef]
- Xue, D.; Gao, J.; Zhou, Y.; Ding, X.; Sun, J.; Lookman, T.; Ren, X. Phase transitions and phase diagram of Ba(Zr0.2Ti 0.8)O3−x(Ba0.7Ca0.3)TiO3 Pb-free system by anelastic measurement. J. Appl. Phys. 2015, 117, 124107. [Google Scholar] [CrossRef]
- Trainer, M. Ferroelectrics and the Curie–Weiss law. Eur. J. Phys. 2000, 21, 459–464. [Google Scholar] [CrossRef]
- Sun, Z.; Li, L.; Zheng, H.; Yu, S.; Xu, D. Effects of sintering temperature on the microstructure and dielectric properties of BaZr0.2Ti0.8O3 ceramics. Ceram. Int. 2015, 41, 12158–12163. [Google Scholar] [CrossRef]
- Uchino, K.; Nomura, S. Critical exponents of the dielectric constants in diffused-phase-transition crystals. Ferroelectr. Lett. Sect. 1982, 44, 55–61. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Alismail, L.; Alshoaibi, A.; Aljaafari, A.; Kotb, H.M.; Hassanien, R. Dielectric behavior of spark plasma sintered BaTi0.7Zr0.3O3 relaxor ferroelectrics. Results Phys. 2019, 15, 102799. [Google Scholar] [CrossRef]



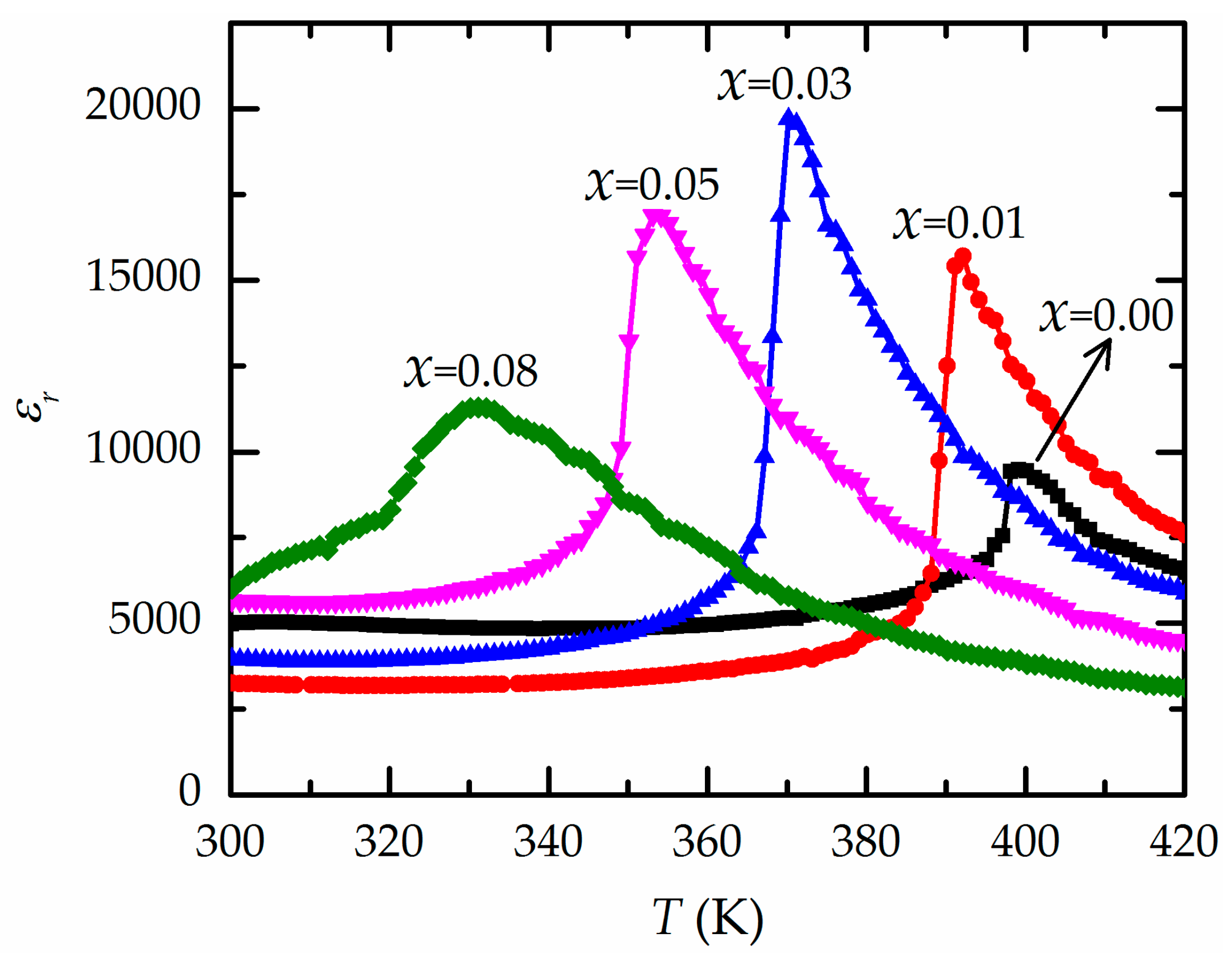
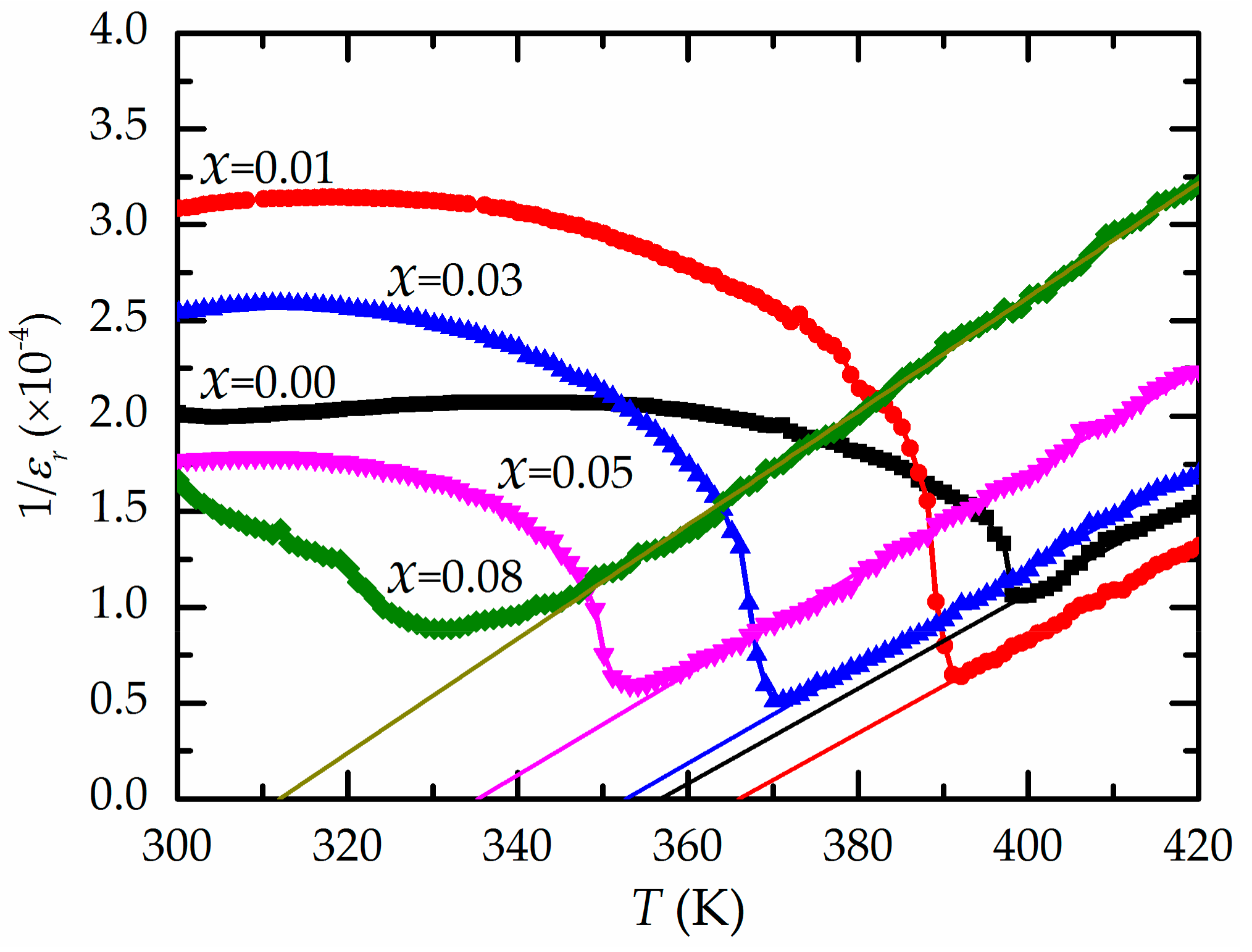
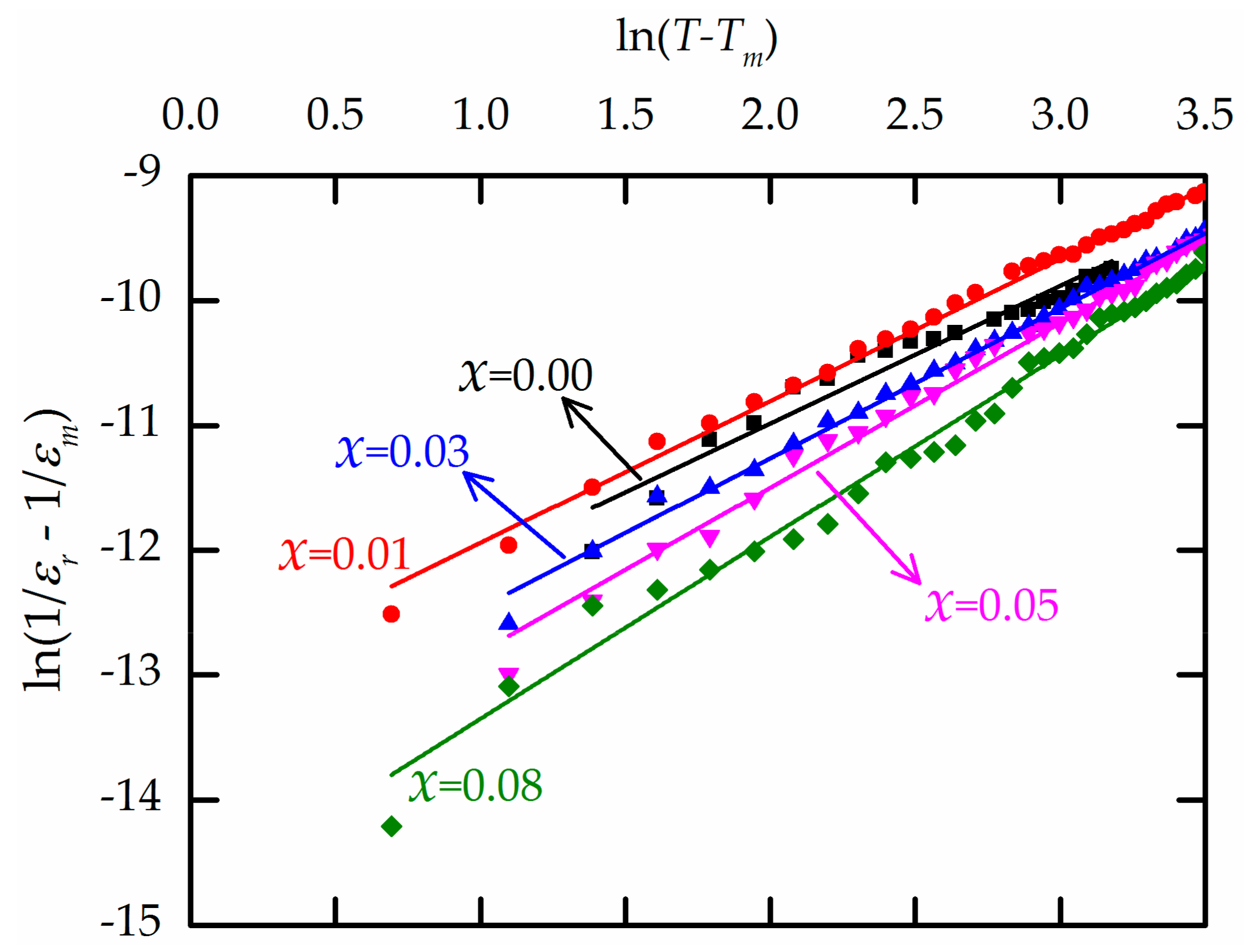
| Ba(ZrxTi1−x)O3 | Relative Density (%) | εr at Tm (1 kHz) | tanδ at Tm (1 kHz) | T0 (K) | C (×105 K) | Tcw (K) | Tm (K) | ΔTm (K) | γ |
|---|---|---|---|---|---|---|---|---|---|
| x = 0.00 | 93.26 | 9,496 | 0.0072 | 357 | 4.04 | 400 | 399 | 1 | 1.01 |
| x = 0.01 | 93.66 | 15,702 | 0.0207 | 366 | 4.08 | 395 | 392 | 3 | 1.05 |
| x = 0.03 | 93.76 | 19,698 | 0.0314 | 353 | 3.92 | 378 | 370 | 8 | 1.21 |
| x = 0.05 | 93.49 | 16,891 | 0.0382 | 335 | 3.79 | 368 | 353 | 14 | 1.26 |
| x = 0.08 | 93.32 | 11,294 | 0.0392 | 312 | 3.36 | 355 | 331 | 24 | 1.38 |
| Ba(ZrxTi1−x)O3 | V (±0.05 V) | R (MΩ) | Pac (nW) |
|---|---|---|---|
| x = 0.00 | 0.24 | 132 | 0.044 |
| x = 0.01 | 0.26 | 90 | 0.075 |
| x = 0.03 | 0.86 | 30 | 2.47 |
| x = 0.05 | 0.68 | 50 | 0.92 |
| x = 0.08 | 0.28 | 90 | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binhayeeniyi, N.; Sukwisute, P.; Nawae, S.; Muensit, N. Energy Conversion Capacity of Barium Zirconate Titanate. Materials 2020, 13, 315. https://doi.org/10.3390/ma13020315
Binhayeeniyi N, Sukwisute P, Nawae S, Muensit N. Energy Conversion Capacity of Barium Zirconate Titanate. Materials. 2020; 13(2):315. https://doi.org/10.3390/ma13020315
Chicago/Turabian StyleBinhayeeniyi, Nawal, Pisan Sukwisute, Safitree Nawae, and Nantakan Muensit. 2020. "Energy Conversion Capacity of Barium Zirconate Titanate" Materials 13, no. 2: 315. https://doi.org/10.3390/ma13020315
APA StyleBinhayeeniyi, N., Sukwisute, P., Nawae, S., & Muensit, N. (2020). Energy Conversion Capacity of Barium Zirconate Titanate. Materials, 13(2), 315. https://doi.org/10.3390/ma13020315





