Investigation of Microstructure of Al-5Ti-0.62C System and Synthesis Mechanism of TiC
Abstract
1. Introduction
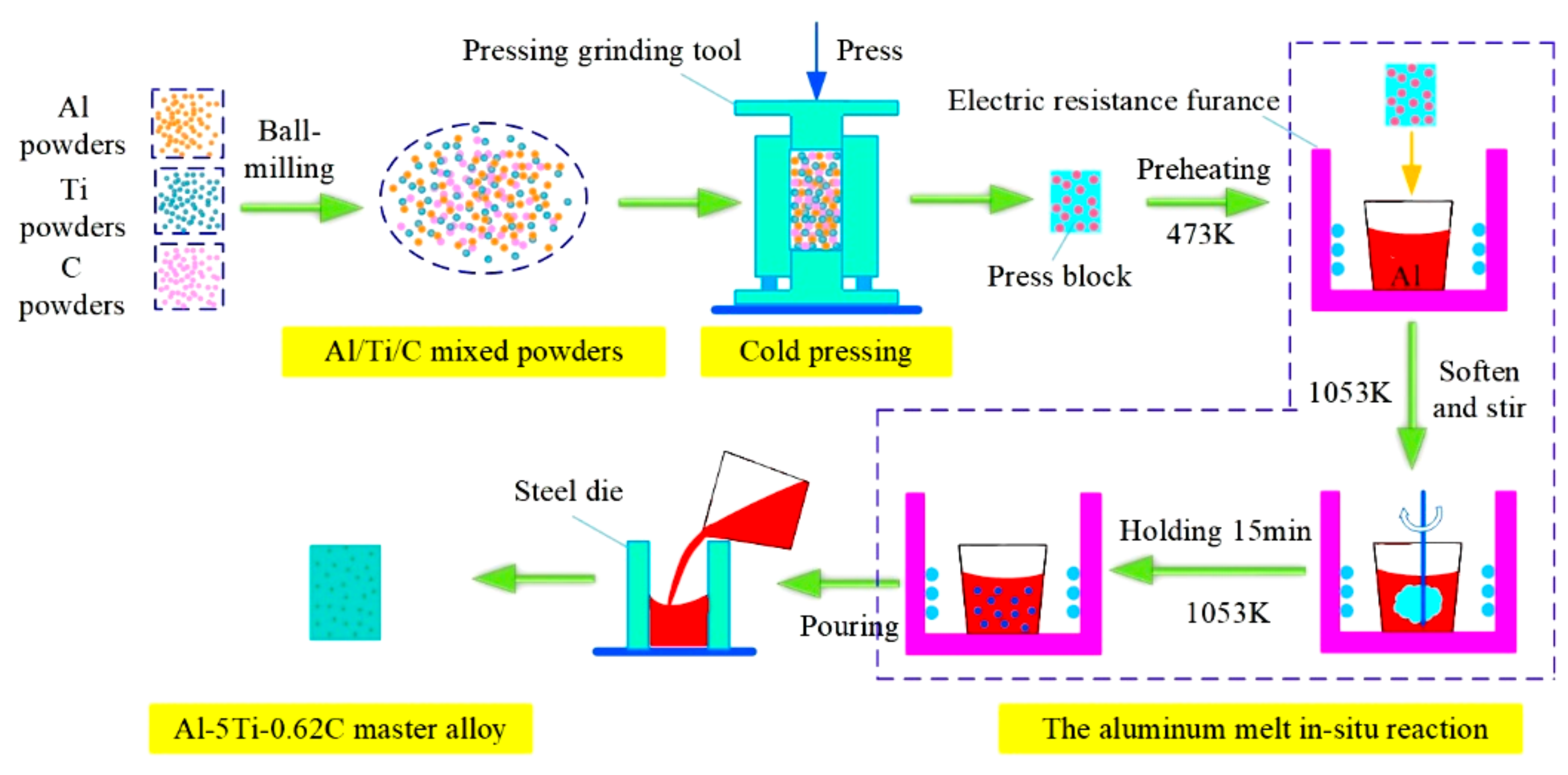
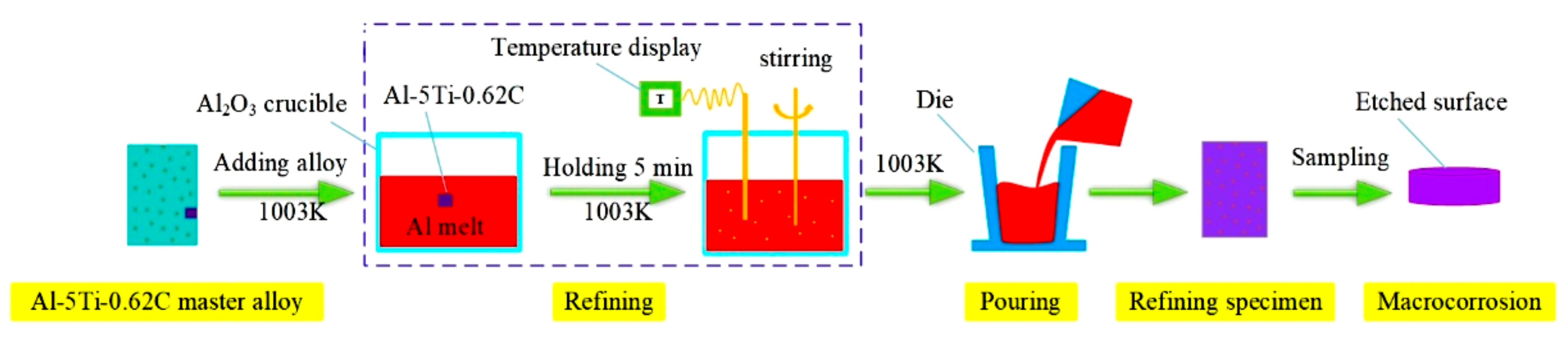
2. Experimental Materials and Methods
3. Results and Discussion
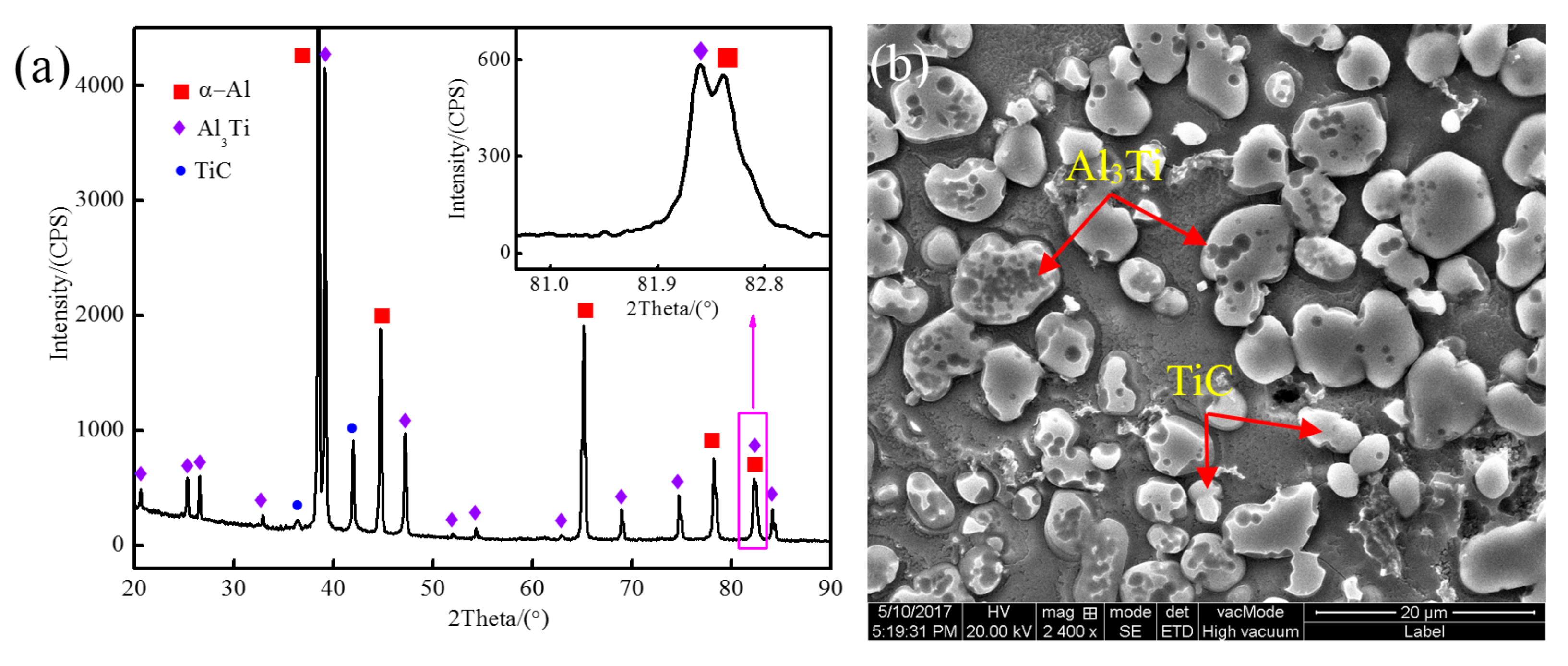
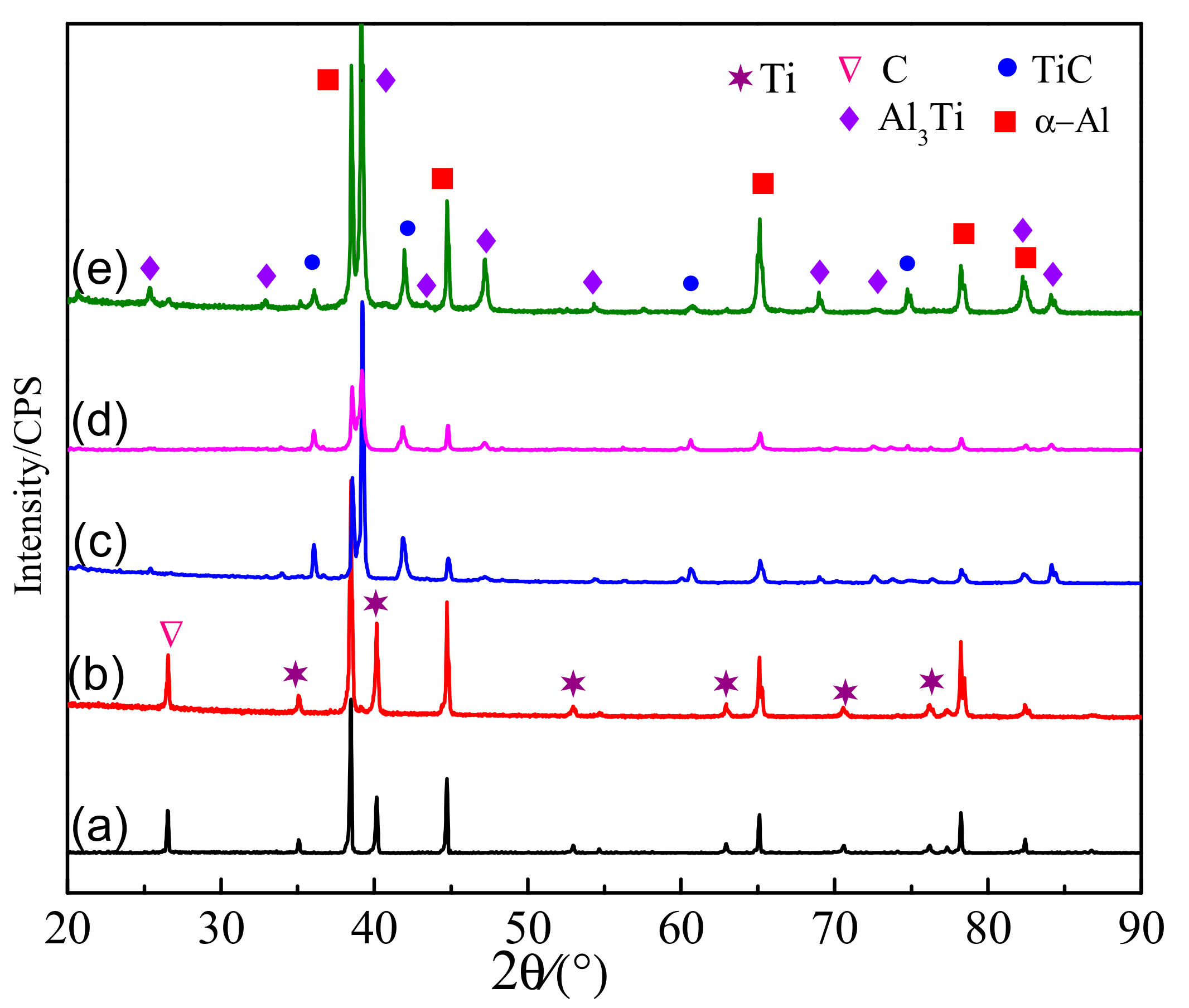
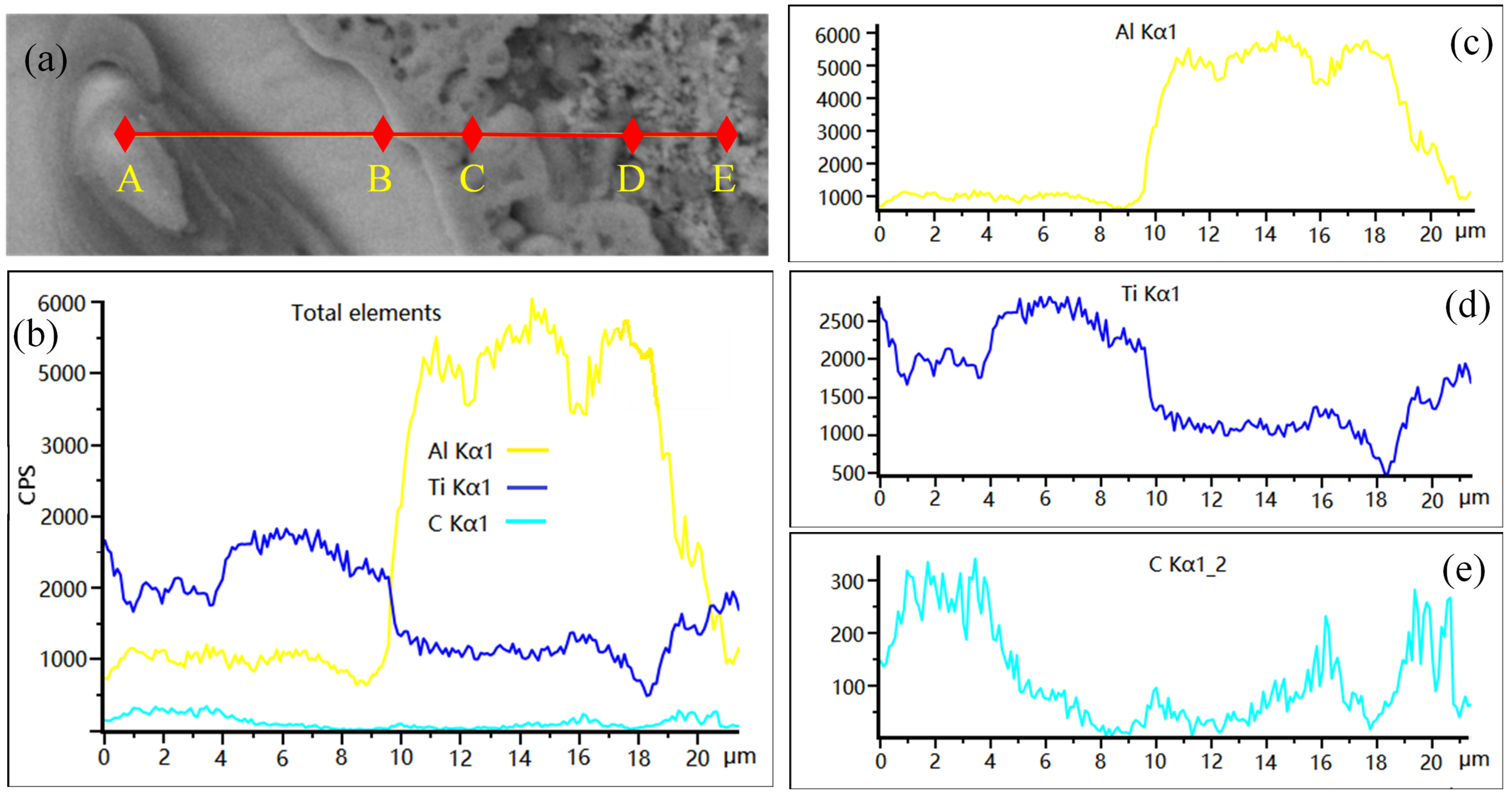
3.1. The Microstructure of Al-5Ti-0.62C Master Alloy
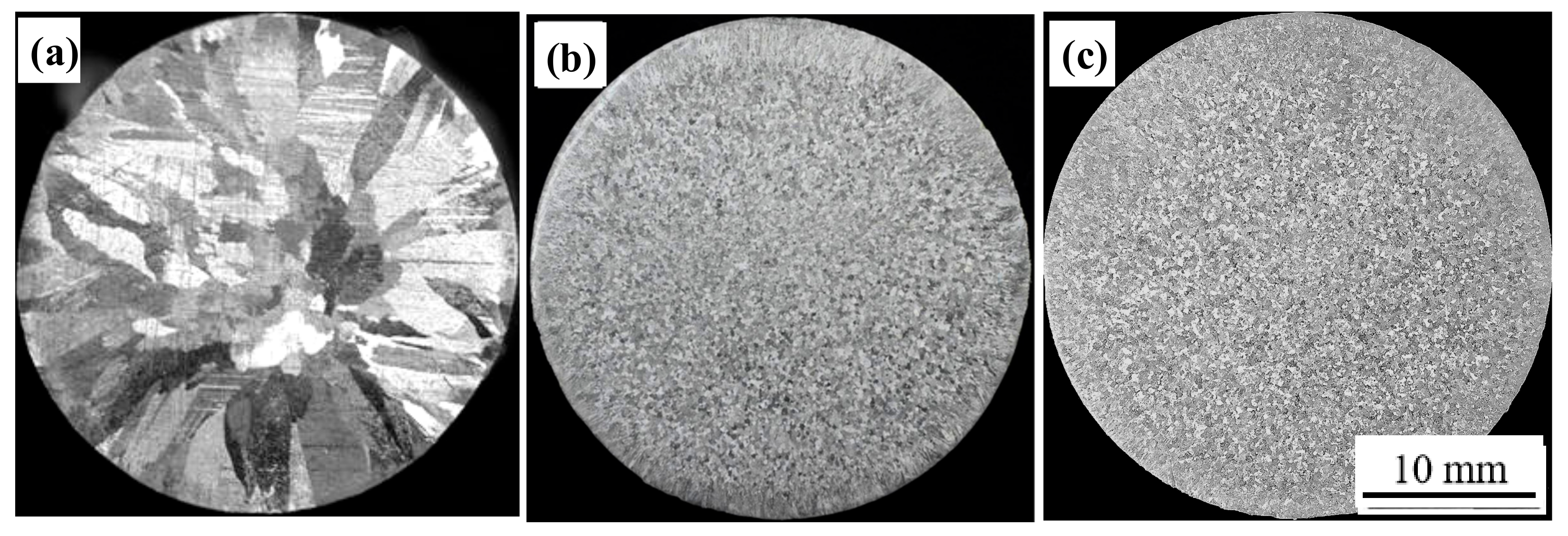
3.2. Refining Effect Evaluation of Al-5Ti-0.62C Master Alloy
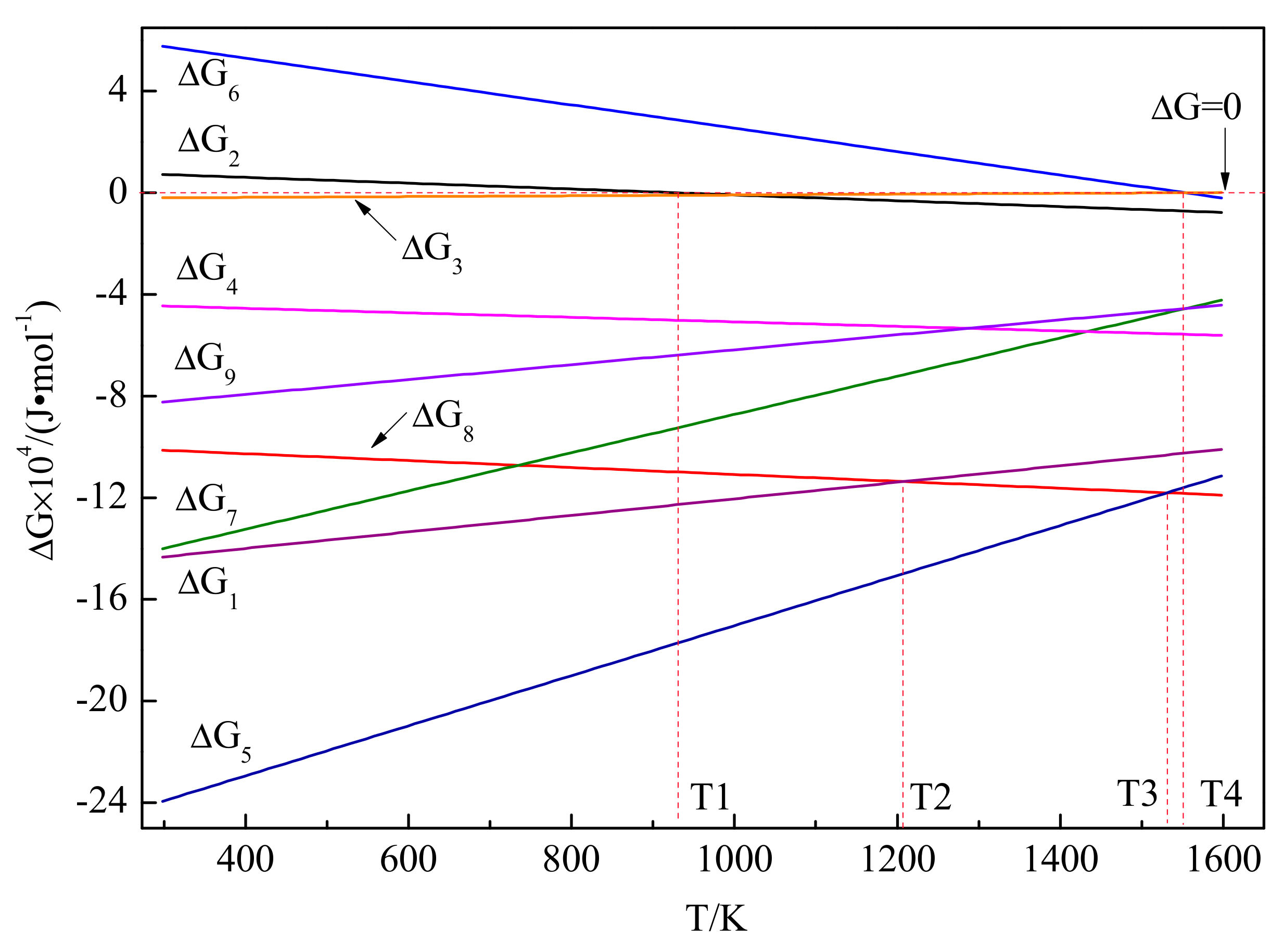
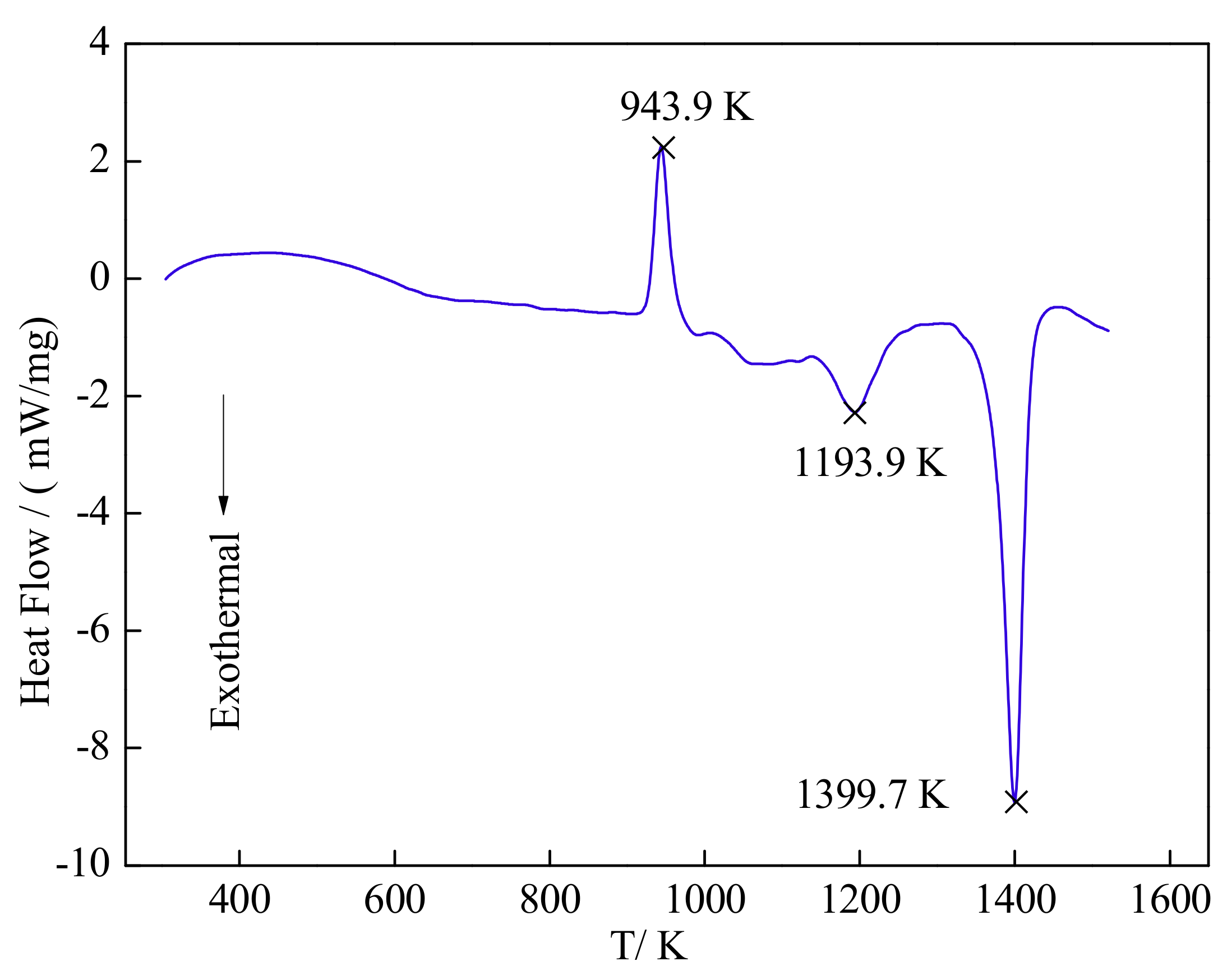
3.3. Phase Transformation and Microstructure Transformation of Al-Ti-C System
4. Conclusions
- (1)
- Al-5Ti-0.62C master alloys have a good refining effect on A99.7. A99.7 is completely refined into the fine equiaxed structure by adding 0.3% Al-5Ti-0.62C master alloy.
- (2)
- When the Al-5Ti-0.62C master alloy is added into the melt, TiC particles mainly distribute in the grain interior and aggregate on the grain boundaries, excess Ti provided by the dissolved Al3Ti will spread around TiC and finally form a Ti-rich zone to promote the nucleation of α-Al.
- (3)
- From the view of thermodynamics and dynamics, TiC is formed by the reaction between solid C and Ti atom provided by the dissolved Al3Ti.
- (4)
- The main reactions in the Al-5Ti-0.62C system are confirmed by DSC, that is, solid Al is transferred into liquid Al, and then liquid Al reacts with solid Ti to form the Al3Ti, and release a lots of heat to promote the formation of TiC which is formed by the Ti atoms and solid C.
Author Contributions
Funding
Conflicts of Interest
References
- Lin, Y.C.; Luo, S.C.; Huang, J.; Yin, L.X.; Jiang, X.Y. Effects of solution treatment on microstructures and micro-hardness of a Sr-modified Al-Si-Mg alloy. Mater. Sci. Eng. A 2018, 725, 530–540. [Google Scholar] [CrossRef]
- Karakoc, H.; Karabulut, S.; Citak, R. Study on mechanical and ballistic performances of boron carbide reinforced Al 6061 aluminum alloy produced by powder metallurgy. Compos. Part B 2018, 148, 68–80. [Google Scholar] [CrossRef]
- Li, Q.L.; Li, B.Q.; Li, J.B.; Zhu, Y.Q.; Xia, T.D. Effect of yttrium addition on the microstructures and mechanical properties of hypereutectic Al-20Si alloy. Mater. Sci. Eng. A 2018, 732, 47–57. [Google Scholar] [CrossRef]
- Liu, G.L.; Si, N.C.; Sun, S.C.; Wu, Q.F. Effects of grain refining and modification on mechanical properties and microstructure of Al-7Si-4Cu cast alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 946–953. [Google Scholar] [CrossRef]
- Li, P.T.; Liu, S.D.; Zhang, L.L.; Liu, X.F. Grain refinement of A356 alloy by Al-Ti-B-C master alloy and its effect on mechanical properties. Mater. Des. 2013, 47, 522–528. [Google Scholar] [CrossRef]
- Yang, H.B.; Gao, T.; Zhang, H.N.; Nie, J.F.; Liu, X.F. Enhanced age-hardening behavior in Al-Cu alloys induced by in-situ synthesized TiC nanoparticles. J. Mater. Sci. Technol. 2019, 35, 374–382. [Google Scholar] [CrossRef]
- Nie, J.F.; Wang, F.; Li, Y.S.; Liu, Y.F.; Liu, X.F.; Zhao, Y.H. Microstructure and mechanical properties of Al-TiB2/TiC in situ composites improved via hot rolling. Trans. Nonferrous Met. Soc. China 2017, 27, 2548–2554. [Google Scholar] [CrossRef]
- Pramod, S.L.; Rao, A.K.P.; Murty, B.S.; Srinivasa, R.B. Microstructure and mechanical properties of as-cast and T6 treated Sc modified A356-5TiB2 in-situ composite. Mater. Sci. Eng. A 2019, 739, 383–394. [Google Scholar] [CrossRef]
- Cibula, A. The mechanism of grain refinement of sand casting in aluminum alloy. J. Inst. Met. 1949, 76, 321–360. [Google Scholar]
- Kori, S.A.; Auradi, V. Influence of reaction temperature for the manufacturing of Al-3Ti and Al-3B master alloys and their grain refining efficiency on a Al-7Si alloy. Adv. Mater. Res. 2007, 29, 111–115. [Google Scholar] [CrossRef]
- Sun, J.Y.; Li, C.; Liu, X.F.; Li, H.J.; Liu, Y.C. Investigation on AlP as the heterogeneous nucleus of Mg2Si in Al-Mg2Si alloys by experimental observation and first-principles calculation. Results Phys. 2018, 8, 146–152. [Google Scholar] [CrossRef]
- Kumar, G.S.V.; Murty, B.S.; Chakraborty, M. Grain refinement response of LM25 alloy towards Al-Ti-C and Al-Ti-B grain refiners. J. Alloys Compd. 2009, 472, 112–120. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, C.M.; Zhou, L.; Hashimoto, T.; Zhou, X.; Ramasse, Q.M.; Fan, Z. Mechanism for Zr poisoning of Al-Ti-B based grain refiner. Acta Mater. 2019, 164, 428–439. [Google Scholar] [CrossRef]
- Jiang, H.X.; Sun, Q.; Zhang, L.L.; Zhao, J.Z. Al-Ti-C master alloy with nano-sized TiC particles dispersed in the matrix prepared by using carbon nanotubes as C source. J. Alloys Compd. 2018, 748, 774–782. [Google Scholar] [CrossRef]
- Ding, H.M.; Liu, X.F.; Yu, L. Influence of zirconium on grain refining efficiency of Al-Ti-C master alloys. J. Mater. Sci. 2007, 42, 9817–9821. [Google Scholar] [CrossRef]
- Yang, H.B.; Qian, Z.; Zhang, H.N.; Nie, J.F.; Liu, X.F. The grain refinement performance of B-doped TiC on Zr-containing Al alloys. J. Alloys Compd. 2018, 731, 774–783. [Google Scholar] [CrossRef]
- Ding, H.M.; Liu, X.F.; Yu, L.; Zhao, G.Q. The influence of the forming processes on the distribution and morphologies of TiC in Al-Ti-C master alloys. Scr. Mater. 2007, 57, 575–578. [Google Scholar] [CrossRef]
- Liu, X.F.; Wang, Z.Q.; Zhang, Z.G.; Bian, X.F. The relationship between microstructures and refining performances of Al-Ti-C master alloys. Mater. Sci. Eng. A 2002, 332, 70–74. [Google Scholar] [CrossRef]
- Yang, H.B.; Gao, T.; Wang, H.C.; Nie, J.F.; Liu, X.F. Influence of C/Ti stoichiometry in TiCx on the grain refinement efficiency of Al-Ti-C master alloy. J. Mater. Sci. Technol. 2017, 33, 616–622. [Google Scholar] [CrossRef]
- Gezer, B.T.; Toptan, F.; Daglilar, S.; Kerti, I. Production of Al-Ti-C grain refiners with the addition of elemental carbon. Mater. Des. 2010, 31, S30–S35. [Google Scholar] [CrossRef]
- Svynaarenko, K.; Zhang, Y.B.; Jie, J.C.; Kutsova, V.; Li, T.J. Microstructure and refinement performance of Al-Ti-C master alloy: Effect of excess Ti on the growth and nucleating ability of TiC particles. Met. Mater. Int. 2017, 5, 994–1001. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, J.M.; Kim, S.H. Al-TiC composites fabricated by a thermally activated reaction process in an Al melt using Al-Ti-C-CuO powder mixtures. Part I: Microstructure evolution and reaction mechanism. Metall. Mater. Trans. A 2017, 45, 5667–5678. [Google Scholar] [CrossRef]
- Li, Y.X.; Hu, J.D.; Wang, H.Y.; Guo, Z.X.; Chumakov, A.N. Thermodynamic and lattice parameter calculation of TiCx produced from Al-Ti-C powders by laser igniting self-propagating high-temperature synthesis. Mater. Sci. Eng. A 2007, 458, 235–239. [Google Scholar] [CrossRef]
- Li, P.J.; Kanndalova, E.G.; Ni, V.I. In situ synthesis of Al-TiC in aluminum melt. Mater. Lett. 2005, 59, 2545–2548. [Google Scholar] [CrossRef]
- Wang, W.Q.; Liu, X.F.; Zhang, J.Y.; Bian, X.F. Reaction mechanism in an Al-TiO2-C system for producing in situ Al/(TiC + Al2O3) composite. J. Mater. Sci. 2004, 39, 667–669. [Google Scholar] [CrossRef]
- Crossly, F.A.; Mondolfo, L.F. Mechanism of grain refinement in aluminum alloys. J. Met. 1951, 12, 1143–1148. [Google Scholar] [CrossRef]
- Wang, E.Z.; Gao, T.; Nie, J.F.; Liu, X.F. Grain refinement limit and mechanical properties of 6063 alloy inoculated by Al-Ti-C(B) master alloys. J. Alloys Compd. 2014, 594, 7–11. [Google Scholar] [CrossRef]
- Birol, Y. Grain refining efficiency of Al-Ti-C alloys. J. Alloys Compd. 2006, 422, 128–131. [Google Scholar] [CrossRef]
- Ju, Z.C.; Fan, N.; Ma, X.C.; Li, J.; Ma, X.J.; Xu, L.Q.; Qian, Y.T. Synthesis of uniform TiC hollow spheres by a Co-reduction route at low temperature. J. Phys. Chem. C 2007, 111, 16202–16206. [Google Scholar] [CrossRef]
- Wu, X.Z.; Sun, T.; Wang, R.; Liu, L.L.; Liu, Q. Energy investigations on the adhesive properties of Al/TiC interfaces: First-principles study. Phys. B Condens. Matter 2014, 449, 269–273. [Google Scholar] [CrossRef]
- Ma, H.T.; Li, J.G.; Zhang, B.Q.; Fang, H.S. Research on crystal nuclei of aluminum alloy refined by Al-Ti-C master alloy. Heat Treat. Met. 1999, 10, 3–5. [Google Scholar]
- Li, Y.L.; Chen, Y.B.; Cao, F.R.; Wen, J.L. Study on nucleation and attenuation mechanism of Al-Ti-C grain refiner. Spec. Cast. Nonferrous Alloys 2005, 25, 712–714. [Google Scholar]
- Kattner, U.R.; Lin, J.C.; Chang, Y.A. Thermodynamic assessment and calculation of the Ti-Al system. Metall. Trans. A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Arpón, R.; Narciso, J.; Louis, E.; García-Cordovilla, C. Interfacial reactions in Al/TiC particulate composites produced by pressure infiltration. Mater. Sci. Technol. 2003, 19, 1225–1230. [Google Scholar] [CrossRef]
- Tronche, A.; Vandyoussefi, M.; Greer, A.L. Instability of TiC particles in aluminum melts inoculated with an Al-Ti-C grain refiner. Mater. Sci. Technol. 2002, 18, 1072–1078. [Google Scholar] [CrossRef]
- Hou, Y.F. Structure Macro Kinetics of Combustion Synthesis Al-Ti-C and Grain Refining Performance Evaluation. Ph.D. Thesis, Lanzhou University of Technology, Lanzhou, China, 2007. [Google Scholar]
- Dorward, R.C. Discussion of comments on the solubility of carbon in molten aluminium. Metall. Trans. A 1990, 21, 255–257. [Google Scholar] [CrossRef]
- Banerji, A.; Reif, W. Metallographic investigation of TiC nucleants in the newly developed Al-Ti-C grain refiner. J. Mater. Sci. 1994, 29, 1958–1965. [Google Scholar] [CrossRef]
- Zhao, H.L.; Zhao, K.X.; Sun, Q.Y. Preparation and solidification process of Al-3Ti-0.2C-5Sr grain refiner. J. Zhengzhou Univ. Eng. Sci. 2015, 36, 14–17. [Google Scholar]
- Kumar, G.S.; Murty, B.S.; Chakroabtry, M. Development of Al-Ti-C grain refiners and study of their grain refining efficiency on Al and Al-7Si alloy. J. Alloys Compd. 2005, 396, 143–150. [Google Scholar] [CrossRef]
- Balog, M.; Florek, R.; Nosko, M.; Simancik, F. Self-Propagating Synthesis of Ti-Al-C Powder Mixtures. Key Eng. Mater. 2012, 520, 347–352. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Hallstedt, B.; Bondar, A.A.; Hecht, U.; Sleptsov, S.V.; Velikanova, T.Y. Thermodynamic description of the Al-C-Ti system. J. Alloys Compd. 2015, 623, 480–496. [Google Scholar] [CrossRef]

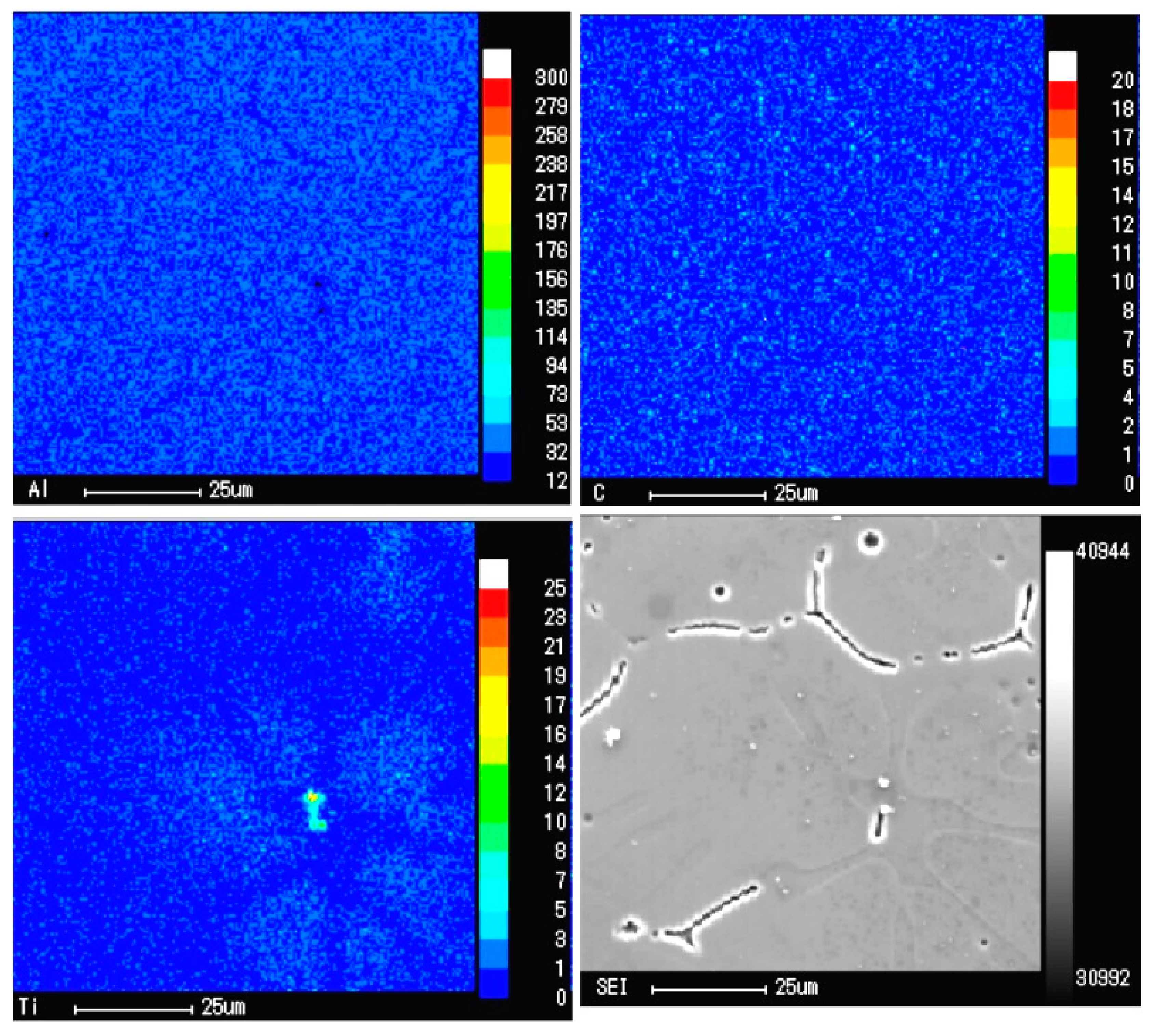




| Point No. | Atomic (Al)/% | Atomic (Ti)/% | Atomic (C)/% |
|---|---|---|---|
| A | 70.6 | 25.7 | 3.6 |
| B | 13.2 | 41.7 | 45.2 |
| C | 10.4 | 40.5 | 49.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Chen, T.; Zhao, X.; Cheng, Y.; Liu, X.; Gou, L. Investigation of Microstructure of Al-5Ti-0.62C System and Synthesis Mechanism of TiC. Materials 2020, 13, 310. https://doi.org/10.3390/ma13020310
Ding W, Chen T, Zhao X, Cheng Y, Liu X, Gou L. Investigation of Microstructure of Al-5Ti-0.62C System and Synthesis Mechanism of TiC. Materials. 2020; 13(2):310. https://doi.org/10.3390/ma13020310
Chicago/Turabian StyleDing, Wanwu, Taili Chen, Xiaoyan Zhao, Yan Cheng, Xiaoxiong Liu, and Lumin Gou. 2020. "Investigation of Microstructure of Al-5Ti-0.62C System and Synthesis Mechanism of TiC" Materials 13, no. 2: 310. https://doi.org/10.3390/ma13020310
APA StyleDing, W., Chen, T., Zhao, X., Cheng, Y., Liu, X., & Gou, L. (2020). Investigation of Microstructure of Al-5Ti-0.62C System and Synthesis Mechanism of TiC. Materials, 13(2), 310. https://doi.org/10.3390/ma13020310





