Porous Poly(Hexamethylene Biguanide) Hydrochloride Loaded Silk Fibroin Sponges with Antibacterial Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Silk Fibroin (SF) Solution
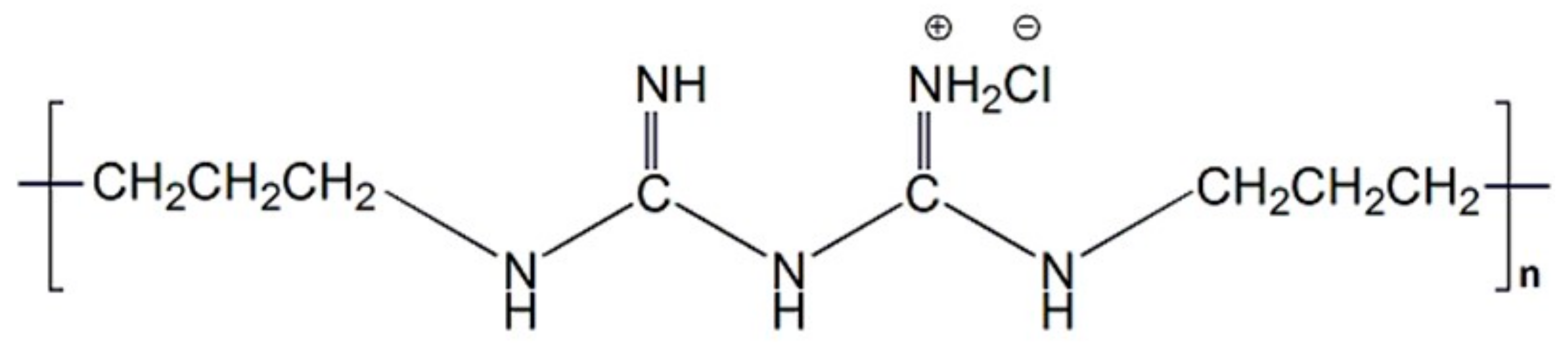
2.2. Preparation of Poly(Hexamethylene Biguanide) Hydrochloride (PHMB)/SF Sponges
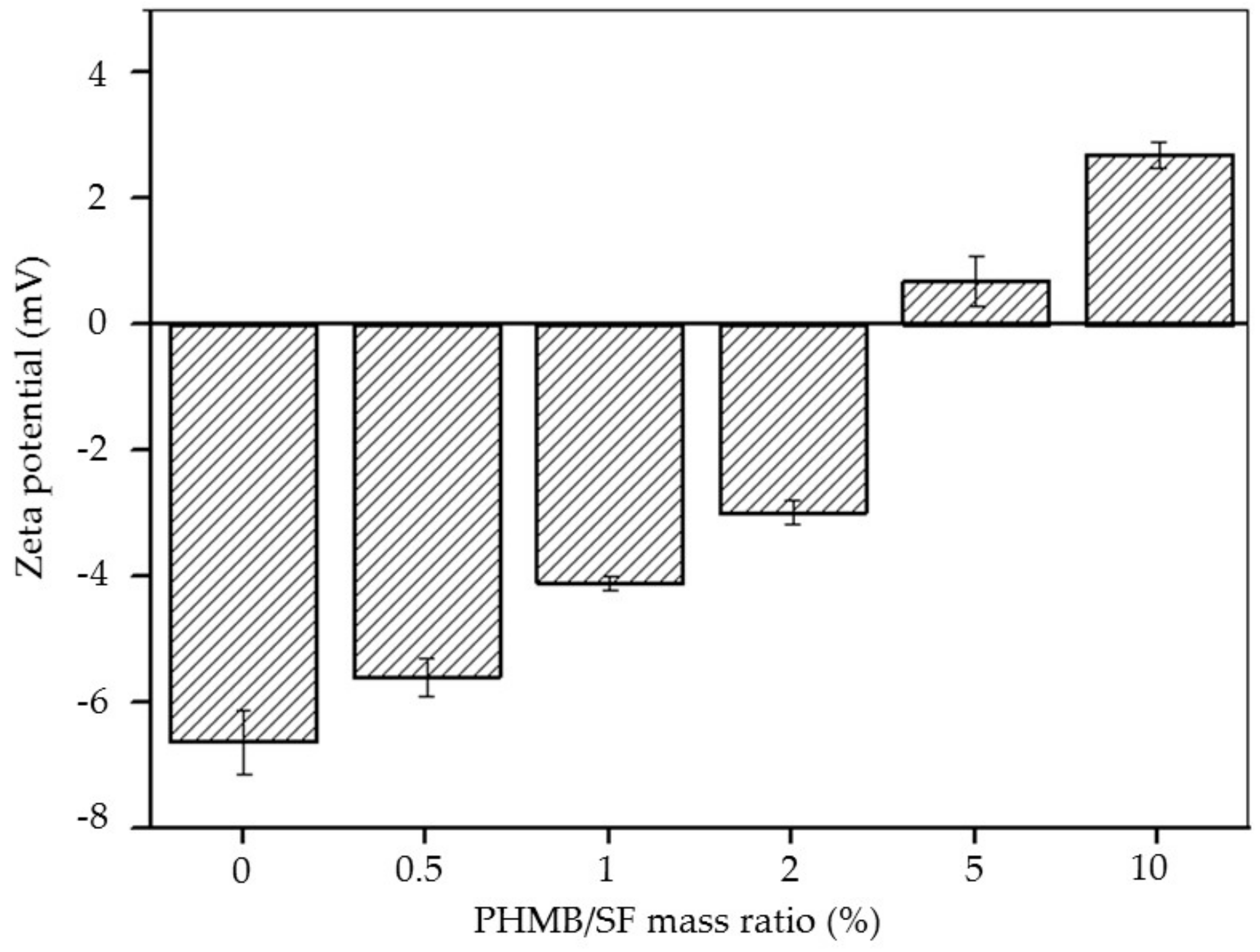
2.3. Zeta Potential of the PHMB/SF Complexes
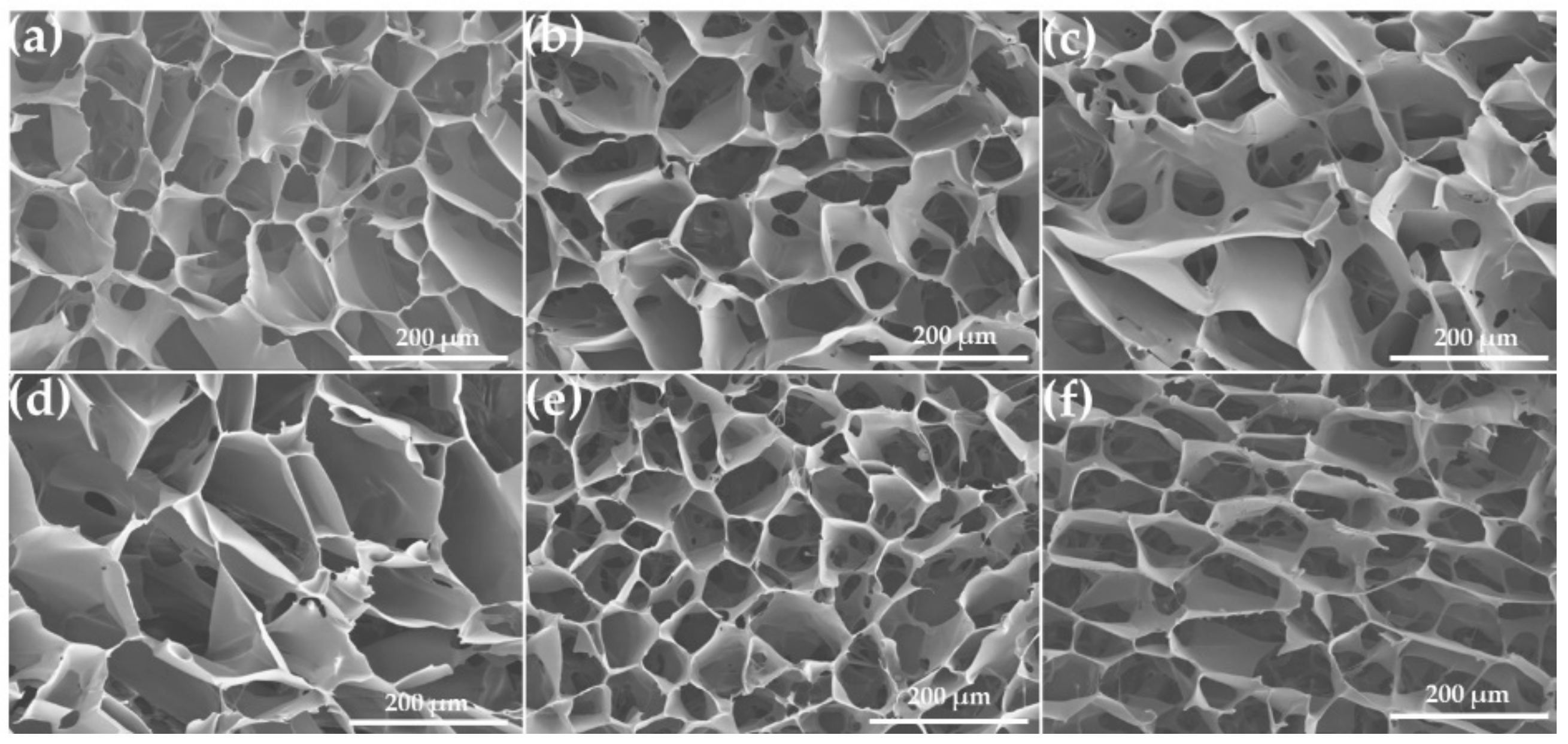
2.4. Scanning Electron Microscopy (SEM)
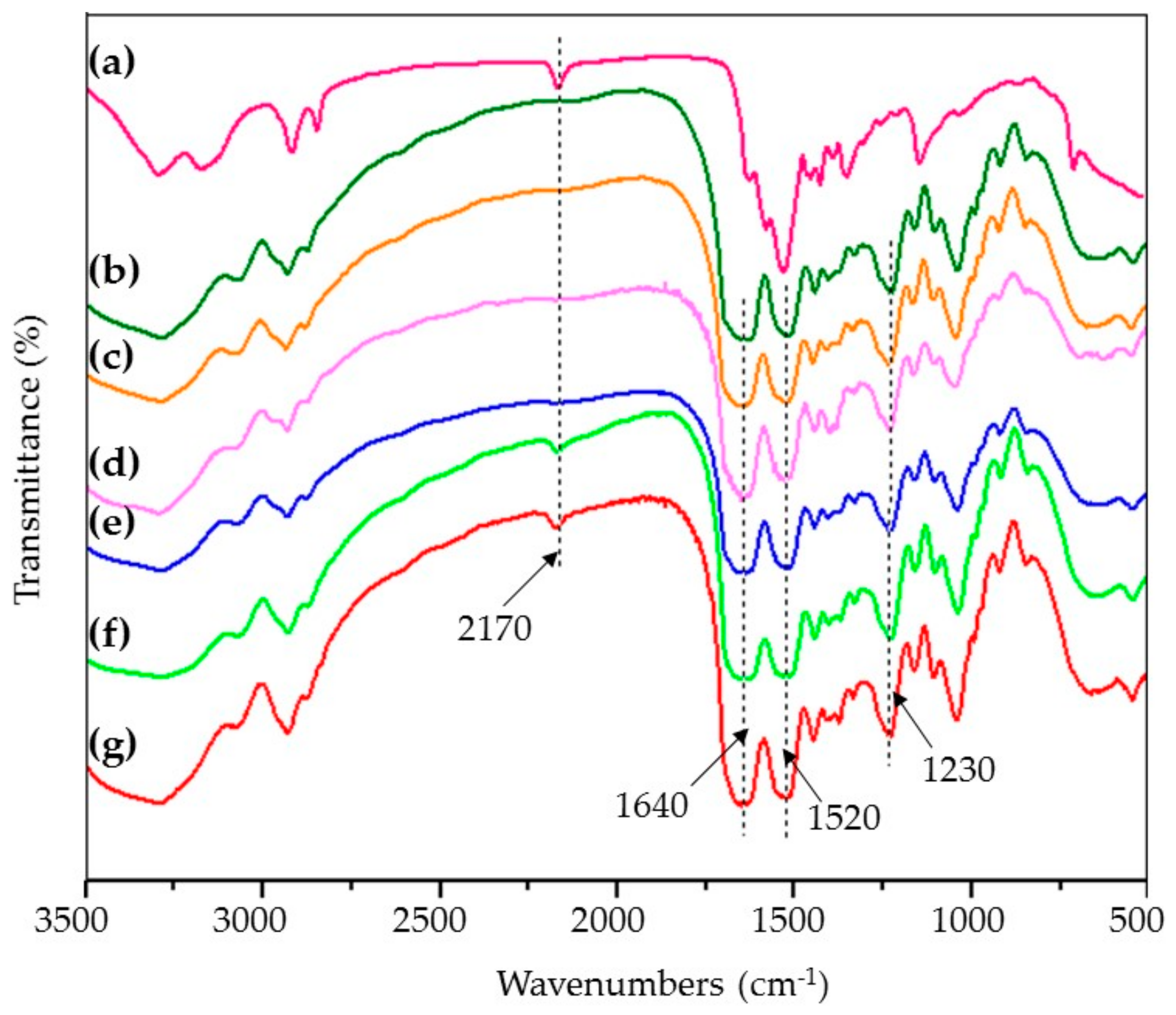
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. PHMB release from the PHMB/SF Sponges
2.7. Antibacterial Activity Test
2.8. Statistical Analysis
3. Results
3.1. Zeta Potential of the PHMB/SF Complexes
3.2. Morphology of PHMB/SF Sponges
3.3. FTIR Spectra
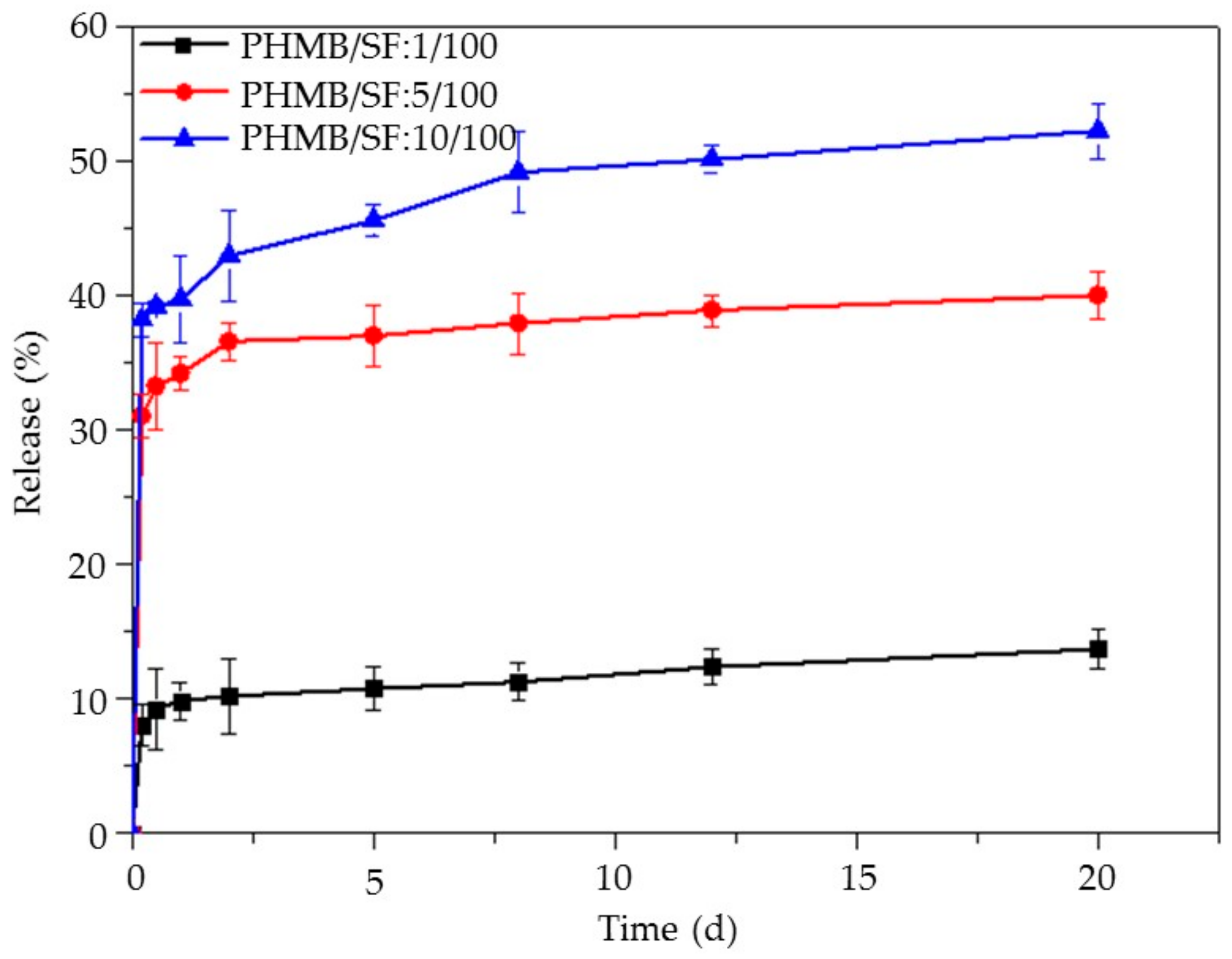
3.4. Release of PHMB from the PHMB/SF Sponges
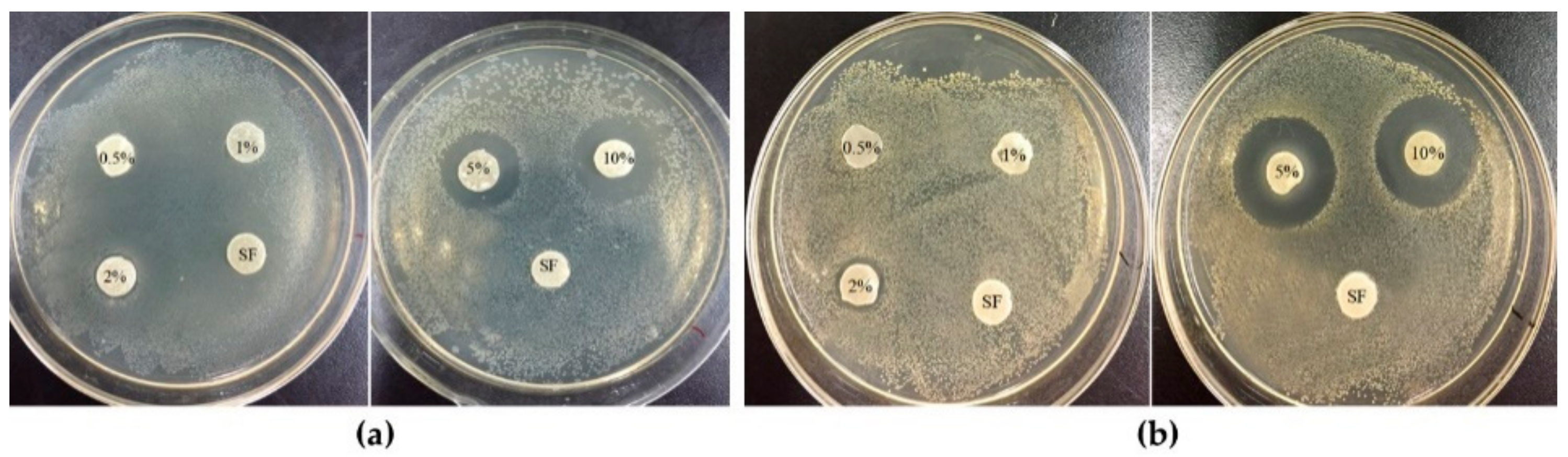
3.5. Antibacterial Activity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Selig, H.F.; Lumenta, D.B.; Giretzlehner, M.; Jeschke, M.G.; Upton, D.; Kamolz, L.P. The properties of an “ideal” burn wound dressing-what do we need in daily clinical practice? results of a worldwide online survey among burn care specialists. Burns 2012, 38, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Bulman, S.; Tronci, G.; Goswami, P.; Carr, C.; Russell, S. Antibacterial properties of nonwoven wound dressings coated with manuka honey or methylglyoxal. Materials 2017, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Di Franco, C.; Panico, A.; Scamarcio, G.; Sannino, A.; Pollini, M. In vitro assessment of the antibacterial potential of silver nano-coatings on cotton gauzes for prevention of wound infections. Materials 2016, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Fracchia, L.; Marchetti, A.; Rinaldi, M.; Bosetti, M. Injectable scaffolds enriched with silver to inhibit bacterial invasion in tissue regeneration. Materials 2019, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Shefa, A.A.; Amirian, J.; Kang, H.J.; Bae, S.H.; Jung, H.I.; Choi, H.J.; Lee, S.Y.; Lee, B.T. In vitro and in vivo evaluation of effectiveness of a novel TEMPO-oxidized cellulose nanofiber-silk fibroin scaffold in wound healing. Carbohydr. Polym. 2017, 177, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A promising wound dressing material with excellent cytocompatibility and proangiogenesis action for wound healing: Strontium loaded silk fibroin/sodium alginate (SF/SA) blend films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef]
- Kang, Y.; Jung, J.Y.; Cho, D.; Kwon, O.H.; Cheon, J.; Park, W. Antimicrobial silver chloride nanoparticles stabilized with chitosan oligomer for the healing of burns. Materials 2016, 9, 215. [Google Scholar] [CrossRef]
- Deveci, S.S.; Basal, G. Preparation of PCM microcapsules by complex coacervation of silk fibroin and chitosan. Colloid. Polym. Sci. 2009, 287, 1455–1467. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.; Li, M. Silk fibroin based porous materials. Materials 2009, 2, 2276–2295. [Google Scholar] [CrossRef]
- Ju, H.W.; Lee, O.J.; Lee, J.M.; Moon, B.M.; Park, H.J.; Park, Y.R.; Lee, M.C.; Kim, S.H.; Chao, J.R.; Ki, C.S.; et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X.; Shi, T.; Lu, L. Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor κB subtype-regulated CCCTC binding factor (CTCF) activation. J. Biol. Chem. 2013, 288, 24363–24371. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Chen, Y.; Yang, Z.; You, B.; Ruan, Y.C.; Peng, Y. Epidermal CFTR suppresses MAPK/NF-κB to promote cutaneous wound healing. Cell. Physiol. Biochem. 2016, 39, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.; Wang, W.; Zhang, M.; Li, M. Bombyx mori silk fibroin scaffolds with Antheraea pernyi silk fibroin micro/nano fibers for promoting EA. hy926 cell proliferation. Materials 2017, 10, 1153. [Google Scholar] [CrossRef]
- Martínez-Mora, C.; Mrowiec, A.; García-Vizcaíno, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolás, F.J. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, Q.; Wang, J.; Liu, Y.; Lu, S.; Li, M.; Kaplan, D.L. Silk fibroin/chondroitin sulfate/hyaluronic acid ternary scaffolds for dermal tissue reconstruction. Acta Biomater. 2013, 9, 6771–6782. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, J.; You, R.; Qu, J.; Li, M. Functionalized silk fibroin dressing with topical bioactive insulin release for accelerated chronic wound healing. Mater. Sci. Eng. C 2017, 72, 394–404. [Google Scholar] [CrossRef]
- Li, X.; You, R.; Luo, Z.; Chen, G.; Li, M. Silk fibroin scaffolds with a micro-/nano-fibrous architecture for dermal regeneration. J. Mater. Chem. B 2016, 4, 2903–2912. [Google Scholar] [CrossRef]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Roth, C.; Beule, A.G.; Kramer, A.; Hosemann, W.; Kohlmann, T.; Scharf, C. Response analysis of stimulating efficacy of polihexanide in an in vitro wound model with respiratory ciliary epithelial cells. Skin Pharmacol. Phys. 2010, 23, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Roth, B.; Müller, G.; Rudolph, P.; Klöcker, N. Influence of the antiseptic agents polyhexanide and octenidine on FL cells and on healing of experimental superficial aseptic wounds in piglets. Skin Pharmacol. Phys. 2004, 17, 141–146. [Google Scholar] [CrossRef] [PubMed]
- De Paula, G.F.; Netto, G.I.; Mattoso, L.H.C. Physical and chemical characterization of poly (hexamethylene biguanide) hydrochloride. Polymers 2011, 3, 928–941. [Google Scholar] [CrossRef]
- Roth, B.; Brill, F.H.H. Polihexanide for wound treatment–how it began. Skin Pharmacol. Phys. 2010, 23, 4–6. [Google Scholar] [CrossRef]
- Mulder, G.D.; Cavorsi, J.P.; Lee, D.K. Polyhexamethylene Biguanide (PHMB): An addendum to current topical antimicrobials. Wounds 2007, 19, 173–182. [Google Scholar]
- Magina, S.; Santos, M.; Ferra, J.; Cruz, P.; Portugal, I.; Evtuguin, D. High pressure laminates with antimicrobial properties. Materials 2016, 9, 100. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Li, M.; Ye, D.; Zhang, Q.; You, R.; Xu, W. Facile preparation of biocompatible silk fibroin/cellulose nanocomposite films with high mechanical performance. ACS Sustain. Chem. Eng. 2017, 5, 6227–6236. [Google Scholar] [CrossRef]
- Bueno, C.Z.; Moraes, A.M. Influence of the incorporation of the antimicrobial agent polyhexamethylene biguanide on the properties of dense and porous chitosan-alginate membranes. Mater. Sci. Eng. C 2018, 93, 671–678. [Google Scholar] [CrossRef]
- Schillinger, U.; Lücke, F.K. Antibacterial activity of lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Yan, S.; Yang, Y.; Zhao, H.; Li, M.; Kaplan, D.L. Preparation of uniaxial multichannel silk fibroin scaffolds for guiding primary neurons. Acta Biomater. 2012, 8, 2628–2638. [Google Scholar] [CrossRef]
- Urciuolo, F.; Garziano, A.; Imparato, G.; Panzetta, V.; Fusco, S.; Casale, C.; Netti, P.A. Biophysical properties of dermal building-blocks affect extra cellular matrix assembly in 3D endogenous macrotissue. Biofabrication 2016, 8, 015010–015022. [Google Scholar] [CrossRef] [PubMed]
- Britz, J.; Meyer, W.H.; Wegner, G. Poly (alkylene biguanides) as proton conductors for high-temperature PEMFCs. Adv. Mater. 2010, 22, E72–E76. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, G.; Singaravelu, S.; Raja, M.D.; Nagiah, N.; Padmapriya, P.; Ruban, K.; Kaveri, K.; Natarajan, T.S.; Sivagnanam, U.T.; Perumal, P.T. Fabrication and characterization of a collagen coated electrospun poly (3-hydroxybutyric acid)–gelatin nanofibrous scaffold as a soft bio-mimetic material for skin tissue engineering applications. Rsc Adv. 2016, 6, 7914–7922. [Google Scholar] [CrossRef]
- Xu, W.; Song, Q.; Xu, J.F.; Serpe, M.J.; Zhang, X. Supramolecular hydrogels fabricated from supramonomers: A novel wound dressing material. ACS Appl. Mater. Interfaces 2017, 9, 11368–11372. [Google Scholar] [CrossRef]
- Ignacio, C.; Barcellos, L.; Ferreira, M.D.; Moura, S.A.L.D.; Soares, I.A.; Oréfice, R.L. In vivo tests of a novel wound dressing based on biomaterials with tissue adhesion controlled through external stimuli. J. Mater. Sci. 2011, 22, 1357–1364. [Google Scholar] [CrossRef]
- Xie, R.J.; Zhang, M. Effect of glycerol on structure and properties of silk fibroin/pearl powder blend films. Adv. Mater. Res. 2013, 796, 126–131. [Google Scholar] [CrossRef]
- Qu, J.; Wang, L.; Niu, L.; Lin, J.; Huang, Q.; Jiang, X.; Li, M. Porous silk fibroin microspheres sustainably releasing bioactive basic fibroblast growth factor. Materials 2018, 11, 1280. [Google Scholar] [CrossRef]
- Dilamian, M.; Montazer, M.; Masoumi, J. Antimicrobial electrospun membranes of chitosan/poly (ethylene oxide) incorporating poly (hexamethylene biguanide) hydrochloride. Carbohyd. Polym. 2013, 94, 364–371. [Google Scholar] [CrossRef]
- Llorens, E.; Calderón, S.; Del Valle, L.J.; Puiggalí, J. Polybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties. Mater. Sci. Eng. C 2015, 50, 74–84. [Google Scholar] [CrossRef]
- Müller, G.; Koburger, T.; Kramer, A. Interaction of polyhexamethylene biguanide hydrochloride (PHMB) with phosphatidylcholine containing o/w emulsion and consequences for microbicidal efficacy and cytotoxicity. Chem. Biol. Interact. 2013, 201, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Song, D.W.; Kim, S.H.; Kim, H.H.; Lee, K.H.; Ki, C.S.; Park, Y.H. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, A.; Zhang, M.; Luo, H.; Niu, L.; Feng, Y.; Li, M. Porous Poly(Hexamethylene Biguanide) Hydrochloride Loaded Silk Fibroin Sponges with Antibacterial Function. Materials 2020, 13, 285. https://doi.org/10.3390/ma13020285
Liang A, Zhang M, Luo H, Niu L, Feng Y, Li M. Porous Poly(Hexamethylene Biguanide) Hydrochloride Loaded Silk Fibroin Sponges with Antibacterial Function. Materials. 2020; 13(2):285. https://doi.org/10.3390/ma13020285
Chicago/Turabian StyleLiang, Ahui, Min Zhang, Hong Luo, Longxing Niu, Yanfei Feng, and Mingzhong Li. 2020. "Porous Poly(Hexamethylene Biguanide) Hydrochloride Loaded Silk Fibroin Sponges with Antibacterial Function" Materials 13, no. 2: 285. https://doi.org/10.3390/ma13020285
APA StyleLiang, A., Zhang, M., Luo, H., Niu, L., Feng, Y., & Li, M. (2020). Porous Poly(Hexamethylene Biguanide) Hydrochloride Loaded Silk Fibroin Sponges with Antibacterial Function. Materials, 13(2), 285. https://doi.org/10.3390/ma13020285





