The Influence of UV Light on Rheological Properties of Collagen Extracted from Silver Carp Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Collagen Preparation
2.2. Measurement of Rheological Properties
2.3. UV Irradiation of Collagen Solution
3. Results
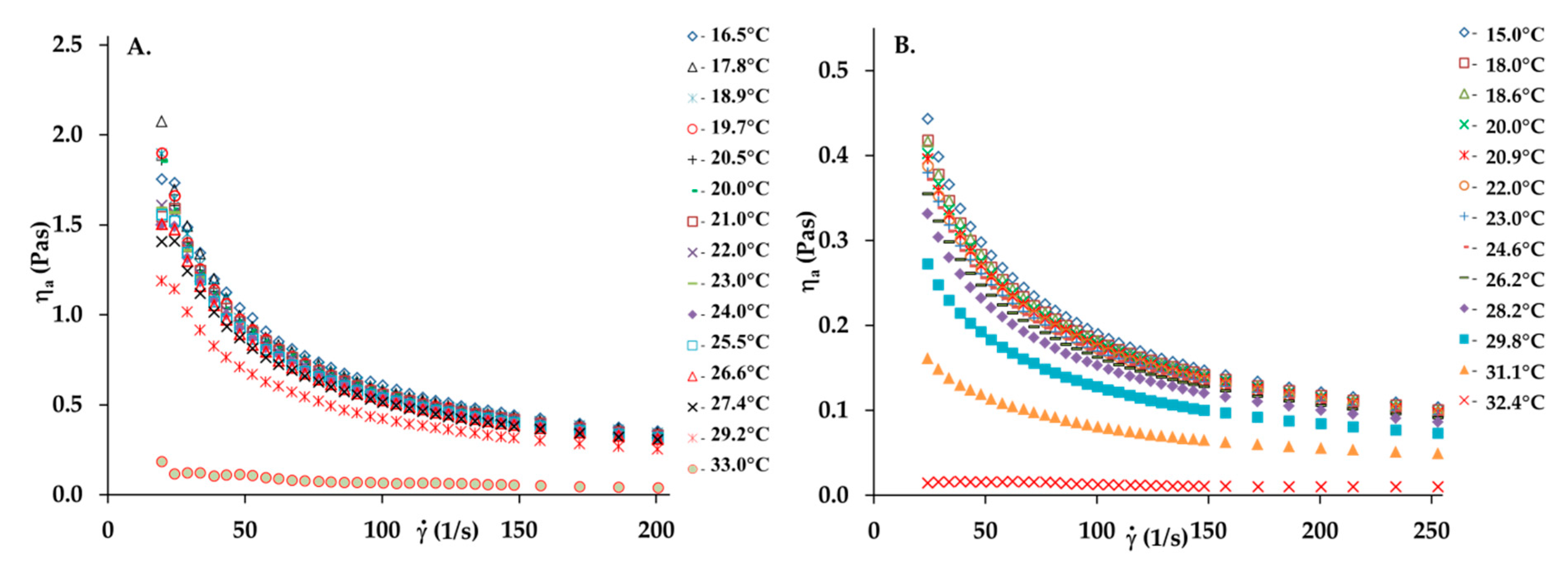
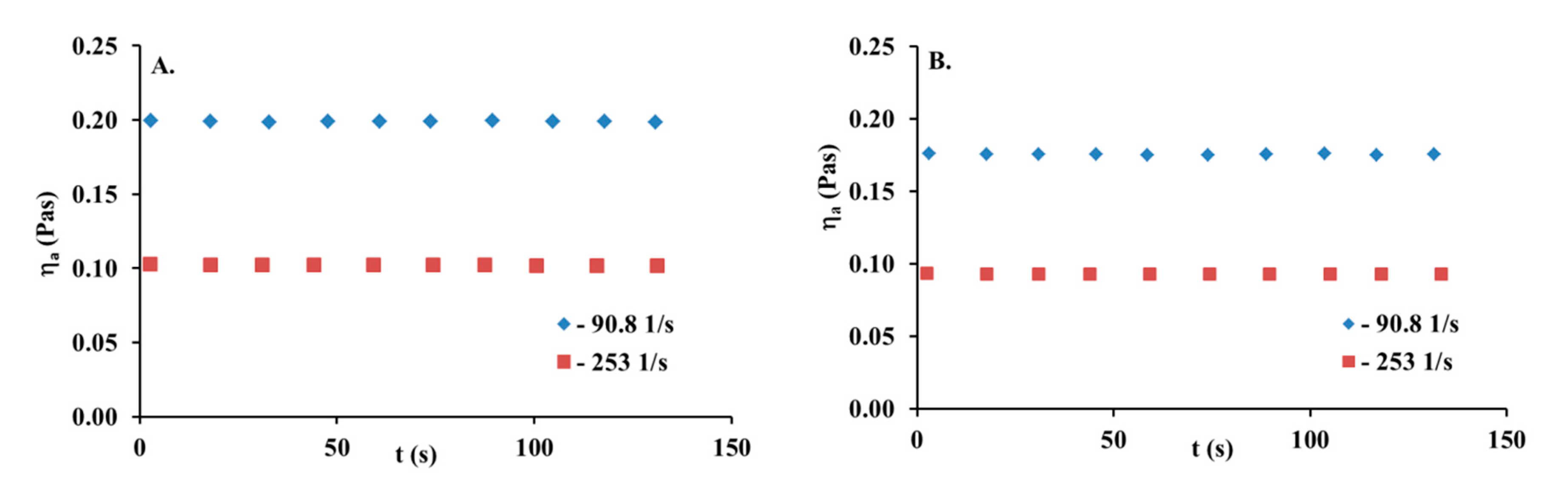
3.1. Rheological Behaviour of Collagen Solutions and Gels
3.2. The Ostwald de Waele and Cross Models
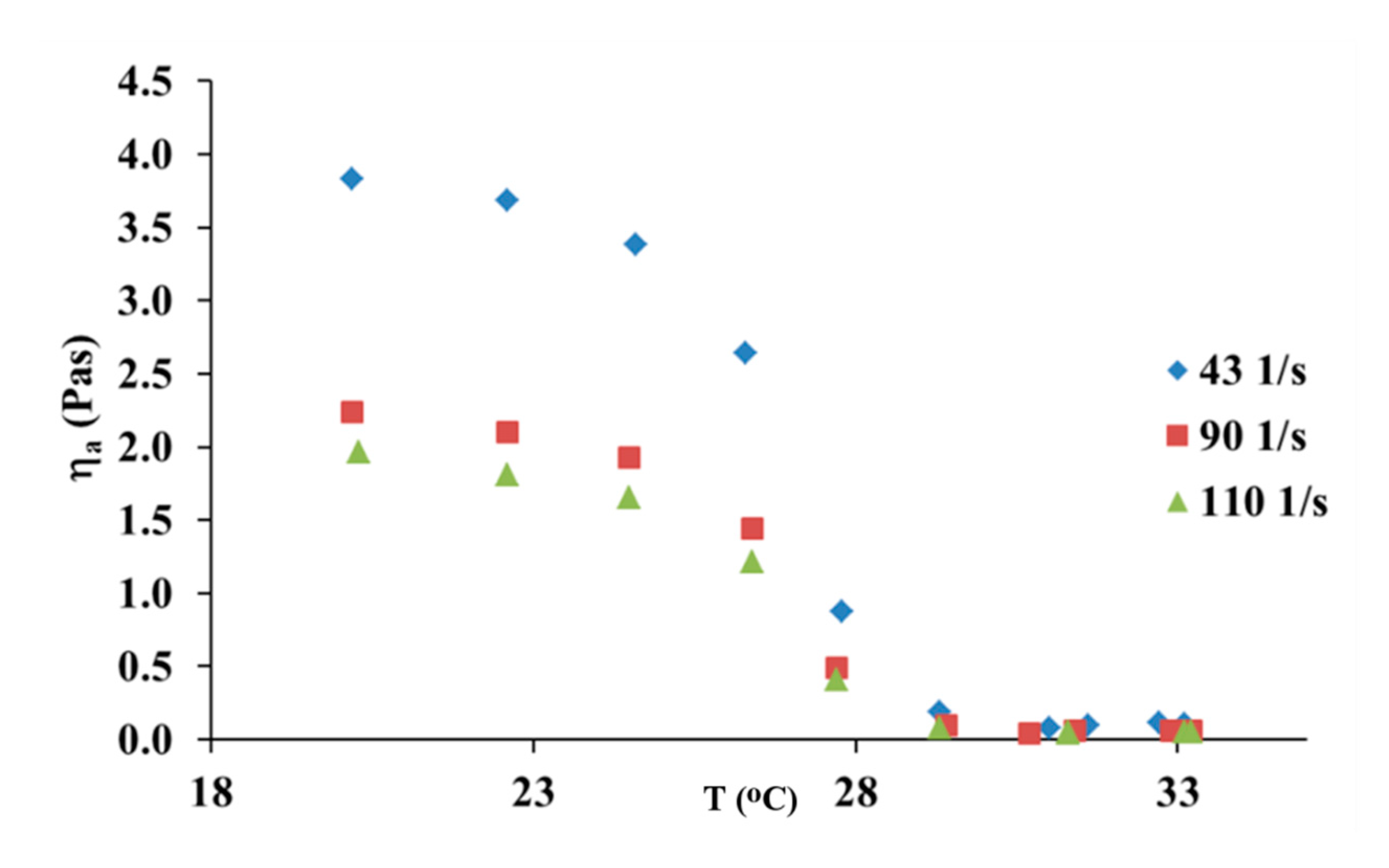
3.3. The Effect of Temperature and the Viscous Flow Activation Energy
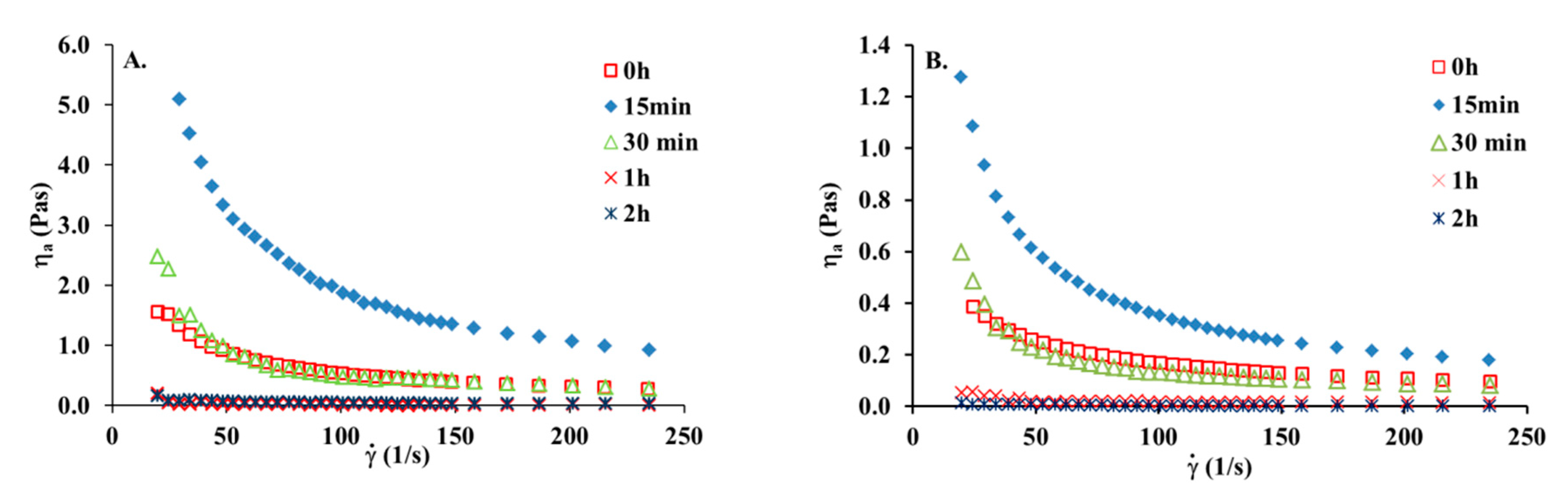
3.4. The Effect of UV Irradiation on the Collagen Solution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miyata, T.; Sohde, T.; Rubin, A.L.; Stenzel, K.H. Effects of ultraviolet irradiation on native and telopeptide-poor collagen. Biochim. Biophys. Acta 1971, 229, 672–680. [Google Scholar] [CrossRef]
- Hyashi, T.; Curran-Patel, S.; Prockop, D.J. Thermal stability of the triple helix of type I procollagen and collagen. Precautions for minimizing ultraviolet damage to proteins during circular dichroism studies. Biochemistry 1979, 18, 4182–4187. [Google Scholar] [CrossRef]
- Fujimori, E. Changes induced by ozone and ultraviolet light in type I collagen. Bovine Achilles tendon collagen versus rat tail tendon collagen. Eur. J. Biochem. 1985, 152, 299–306. [Google Scholar] [CrossRef]
- Kaminska, A.; Sionkowska, A. The effect of UV radiation on the thermal parameters of collagen degradation. Polym. Degrad. Stab. 1996, 51, 15–18. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaminska, A. Thermal helix-coil transition in UV irradiated collagen from rat tail tendon. Int. J. Biol. Macromol. 1999, 24, 337–340. [Google Scholar] [CrossRef]
- Miles, C.A.; Sionkowska, A.; Hulin, S.L.; Sims, T.J.; Avery, N.C.; Bailey, A.J. Identification of an intermediate state in the helix-coil degradation of collagen by ultraviolet light. J. Biol. Chem. 2000, 275, 33014–33020. [Google Scholar] [CrossRef] [PubMed]
- Jariashvili, K.; Madhan, B.; Brodsky, B.; Kuchava, A.; Namicheishvili, L.; Metreveli, N. UV-damage of collagen: Insides from model collagen peptides. Biopolymers 2012, 7, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Wess, T.J. Mechanical properties of UV irradiated rat tail tendon (RTT) collagen. Int. J. Biol. Macromol. 2004, 34, 9–12. [Google Scholar] [CrossRef]
- Vizarova, K.; Bakos, D.; Rehakova, M.; Macho, V. Modification of layered atelocollagen by ultraviolet irradiation and chemical cross-linking: Structure stability and mechanical properties. Biomaterials 1994, 15, 1082–1086. [Google Scholar] [CrossRef]
- Sionkowska, A. Modification of collagen films by ultraviolet irradiation. Polym. Degrad. Stab. 2020, 68, 147–151. [Google Scholar] [CrossRef]
- Sionkowska, A. Flash photolysis and pulse radiolysis studies on collagen Type I in acetic acid solution. J. Photochem. Photobiol. B Biol. 2006, 84, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Bailey, A.J.; Paul, R.G. Collagen—Is not so simple protein. J. Soc. Leather Technol. Chem. 1998, 82, 104–108. [Google Scholar]
- Orgel, J.P.; Miller, A.; Irving, T.C.; Fischetti, R.F.; Hammersley, A.P.; Wess, T.J. The in situ supermolecular structure of type I collagen. Structure 2001, 9, 1061–1069. [Google Scholar] [CrossRef]
- Bella, J. A new method for describing the helical conformation of collagen: Dependence of the triple helical twist on amino acid sequence. J. Struct. Biol. 2010, 170, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Poly. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, R.; Tian, Y.; Konno, K. Characterisation of acid-soluble collagen from skin of silver carp (Hypophthalmichthys molitrix). Food Chem. 2009, 116, 318–322. [Google Scholar] [CrossRef]
- Knapp, V.D.M.; Barocas, V.H.; Moon, A.G.; Tranquillo, R.T. Rheology of reconstituted type I collagen gel in confined compression. J. Rheol. 1997, 415. [Google Scholar] [CrossRef]
- Lai, G.; Li, Y.; Li, G. Effect of concentration and temperature on the rheological behavior of collagen solution. Int. J. Biol. Macromol. 2008, 42, 285–291. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, C.; Deng, F.; Ding, C.; Yang, C.; Wu, H.; Ni, Y.; Huang, L.; Chen, L.; Zhang, M. Fabrication of highly concentrated collagens using cooled urea/HAc as novel binary solvent. J. Mol. Liq. 2019, 291, 1–10. [Google Scholar] [CrossRef]
- Yang, H.; Duan, L.; Li, Q.; Tian, Z.; Li, G. Experimental and modeling investigation on the rheological behavior of collagen solution as a function of acetic acid concentration. J. Mech. Behav. Biomed. Mater. 2018, 77, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, M.T.; Esmail, M.N. Rheological properties of carboxymethyl cellulose. J. Appl. Polym. Sci. 1997, 64, 289–301. [Google Scholar] [CrossRef]
- Lewandowska, K. Comparative studies of rheological properties of polyacrylamide and partially hydrolyzed polyacrylamide solutions. J. Appl. Polym. Sci. 2007, 103, 2235–2241. [Google Scholar] [CrossRef]
- Wang, S.; Le, H.; Guo, J.; Zhao, J.; Tang, H. Intrinsic viscosity and rheological properties of natural and substituted guar gums in seawater. Int. J. Biol. Macromol. 2015, 76, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Duan, L.; Wu, L.; Shen, L.; Li, G. Rheological properties of glutaraldehyde-crosslinked collagen solutions analyzed quantitatively using mechanical models. Mater. Sci. Eng. C 2016, 63, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Martuscelli, E. Melt rheology of a compatible blend: Poly (ethylene oxide)/poly (methyl methacrylate). Die Makromol. Chem. Rapid Commun. 1984, 5, 255–261. [Google Scholar] [CrossRef]
- Razavi, S.M.; Cui, S.W.; Ding, H. Structural and physicochemical characteristics of a novel water-soluble gum from Lallemantia royleana seed. Int. J. Biol. Macromol. 2016, 83, 142–151. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M.G. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef]
- Xing, J.Y.; Bai, B.; Xue, W.B.; Yang, M.Y. Effect of UV on stability of collagen with consideration of hydratation and fibrillogenesis. Food Sci. Biotechnol. 2013, 22, 1–5. [Google Scholar] [CrossRef]
- Mori, H.; Hara, M. UV irradiation of type I collagen gels changed the morphology of the interconnected brain capillary endothelial cells on them. Mater. Sci. Eng. C 2020, 112, 110907. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Pei, Y.; Liu, J.; Wang, K.; Tang, K.Y. Effect of UV irradiation on the properties of goatskin collagen matrices. J. Soc. Leather Technol. Chem. 2017, 101, 66–71. [Google Scholar]

| T (°C) | Ostwald de Waele Model | Cross Model | ||||||
|---|---|---|---|---|---|---|---|---|
| n | k (Pasn) | R2 | η0 (Pas) | η∞ (Pas) | λ (s) | m | R2 | |
| collagen c = 5 mg/mL | ||||||||
| 15 | 0.39 | 3.18 | 0.999 | 0.673 | 0.0443 | 0.0185 | 1.13 | 0.999 |
| 18 | 0.39 | 3.00 | 0.999 | 0.657 | 0.0420 | 0.0225 | 1.09 | 0.999 |
| 20 | 0.39 | 2.88 | 0.999 | 0.582 | 0.0446 | 0.0148 | 1.15 | 0.999 |
| 25 | 0.41 | 2.52 | 0.999 | 0.538 | 0.0410 | 0.0175 | 1.12 | 0.998 |
| 30 | 0.43 | 1.76 | 0.999 | 0.362 | 0.0382 | 0.00980 | 1.21 | 0.998 |
| 31 | 0.48 | 0.87 | 0.999 | 0.184 | 0.0331 | 0.00292 | 1.43 | 0.997 |
| collagen c = 10 mg/mL | ||||||||
| 16 | 0.25 | 19.1 | 0.998 | 3.079 | 0.126 | 0.0225 | 1.18 | 0.998 |
| 18 | 0.26 | 17.5 | 0.998 | 3.272 | 0.286 | 0.761 | 0.38 | 1.00 |
| 20 | 0.27 | 16.5 | 0.999 | 2.742 | 0.263 | 0.0101 | 1.41 | 0.998 |
| 25 | 0.26 | 16.3 | 0.999 | 2.867 | 0.101 | 0.0286 | 1.13 | 0.998 |
| 30 | 0.22 | 7.45 | 0.963 | 0.629 | 0.070 | 7.69 × 10−4 | 1.80 | 0.994 |
| c (mg/mL) | Ea (kJ/mol) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 s−1 | R2 | 43.3 s−1 | R2 | 90.9 s−1 | R2 | 110 s−1 | R2 | |
| 10 | 17.1 | 0.914 | 13.9 | 0.975 | 11.4 | 0.975 | 10.9 | 0.979 |
| 19.5 | 0.657 | 10.3 | 0.943 | 9.3 | 0.944 | 8.7 | 0.940 | |
| T (°C) | Ostwald de Waele Model | Cross Model | ||||||
|---|---|---|---|---|---|---|---|---|
| n | k (Pasn) | R2 | η0 (Pas) | η∞ (Pas) | λ (s) | m | R2 | |
| collagen c = 10 mg/mL | ||||||||
| 20 | 0.27 | 58.5 | 0.998 | 22.04 | 0.0605 | 0.2205 | 0.84 | 1.00 |
| 25 | 0.23 | 61.0 | 0.997 | 17.17 | 0.2083 | 0.1031 | 0.99 | 0.999 |
| 30 | 0.13 | 4.79 | 0.835 | 0.358 | 0.0313 | 1.38 × 10−3 | 1.78 | 0.991 |
| c (mg/mL) | Ea (kJ/mol) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 s−1 | R2 | 43.3 s−1 | R2 | 90.9 s−1 | R2 | 110 s−1 | R2 | |
| 10 | 49.8 | 0764. | 41.5 | 0.801 | 49.1 | 0.836 | 54.2 | 0.859 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sionkowska, A.; Lewandowska, K.; Adamiak, K. The Influence of UV Light on Rheological Properties of Collagen Extracted from Silver Carp Skin. Materials 2020, 13, 4453. https://doi.org/10.3390/ma13194453
Sionkowska A, Lewandowska K, Adamiak K. The Influence of UV Light on Rheological Properties of Collagen Extracted from Silver Carp Skin. Materials. 2020; 13(19):4453. https://doi.org/10.3390/ma13194453
Chicago/Turabian StyleSionkowska, Alina, Katarzyna Lewandowska, and Katarzyna Adamiak. 2020. "The Influence of UV Light on Rheological Properties of Collagen Extracted from Silver Carp Skin" Materials 13, no. 19: 4453. https://doi.org/10.3390/ma13194453
APA StyleSionkowska, A., Lewandowska, K., & Adamiak, K. (2020). The Influence of UV Light on Rheological Properties of Collagen Extracted from Silver Carp Skin. Materials, 13(19), 4453. https://doi.org/10.3390/ma13194453







