Collagen Based Materials in Cosmetic Applications: A Review
Abstract
1. Introduction
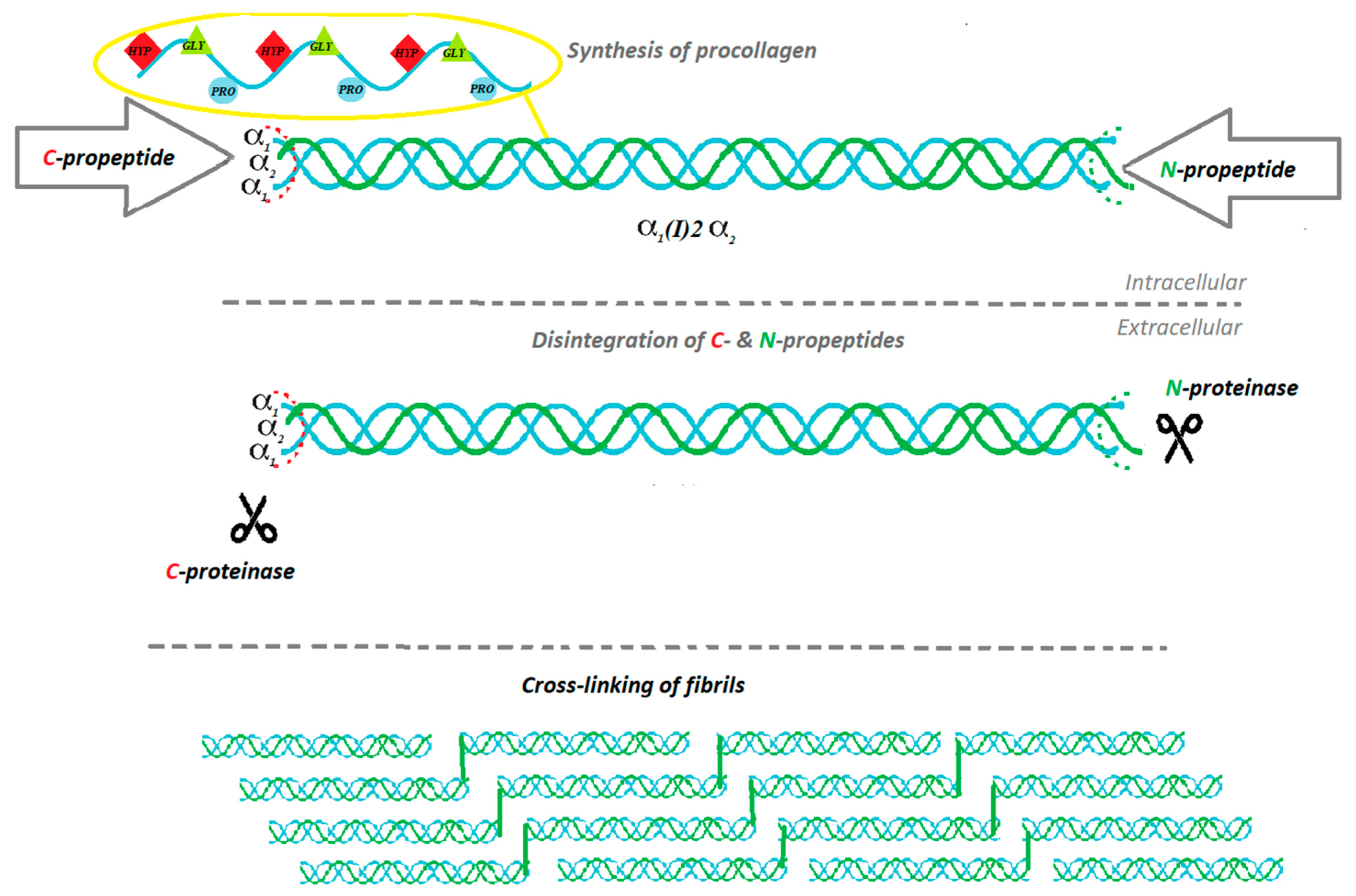
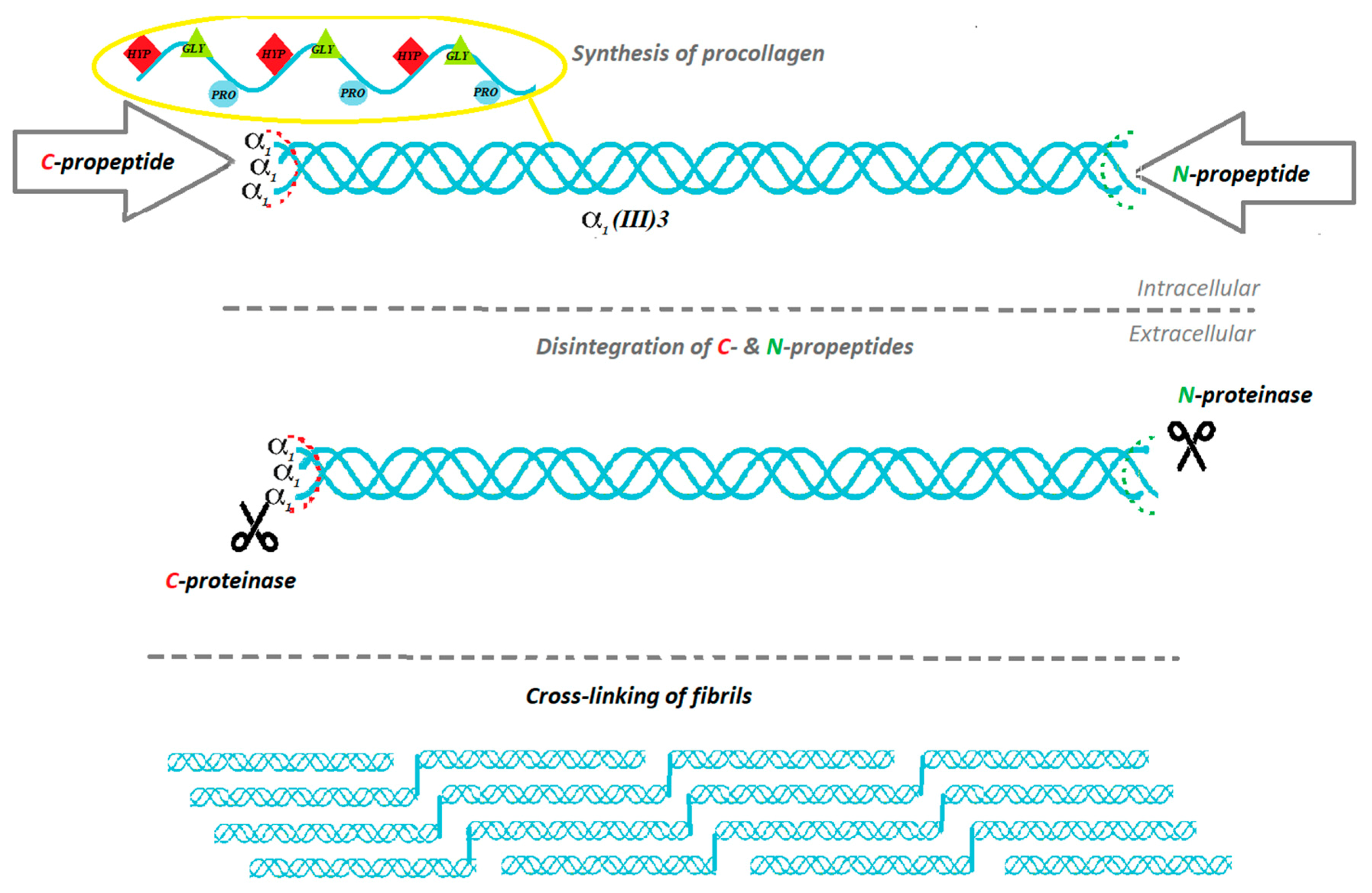
2. The Biosynthesis of Collagen
3. Sources of Collagen for Cosmetic Use
4. Collagen Extraction Methods for Cosmetic Use
5. Types of Collagen in Cosmetics
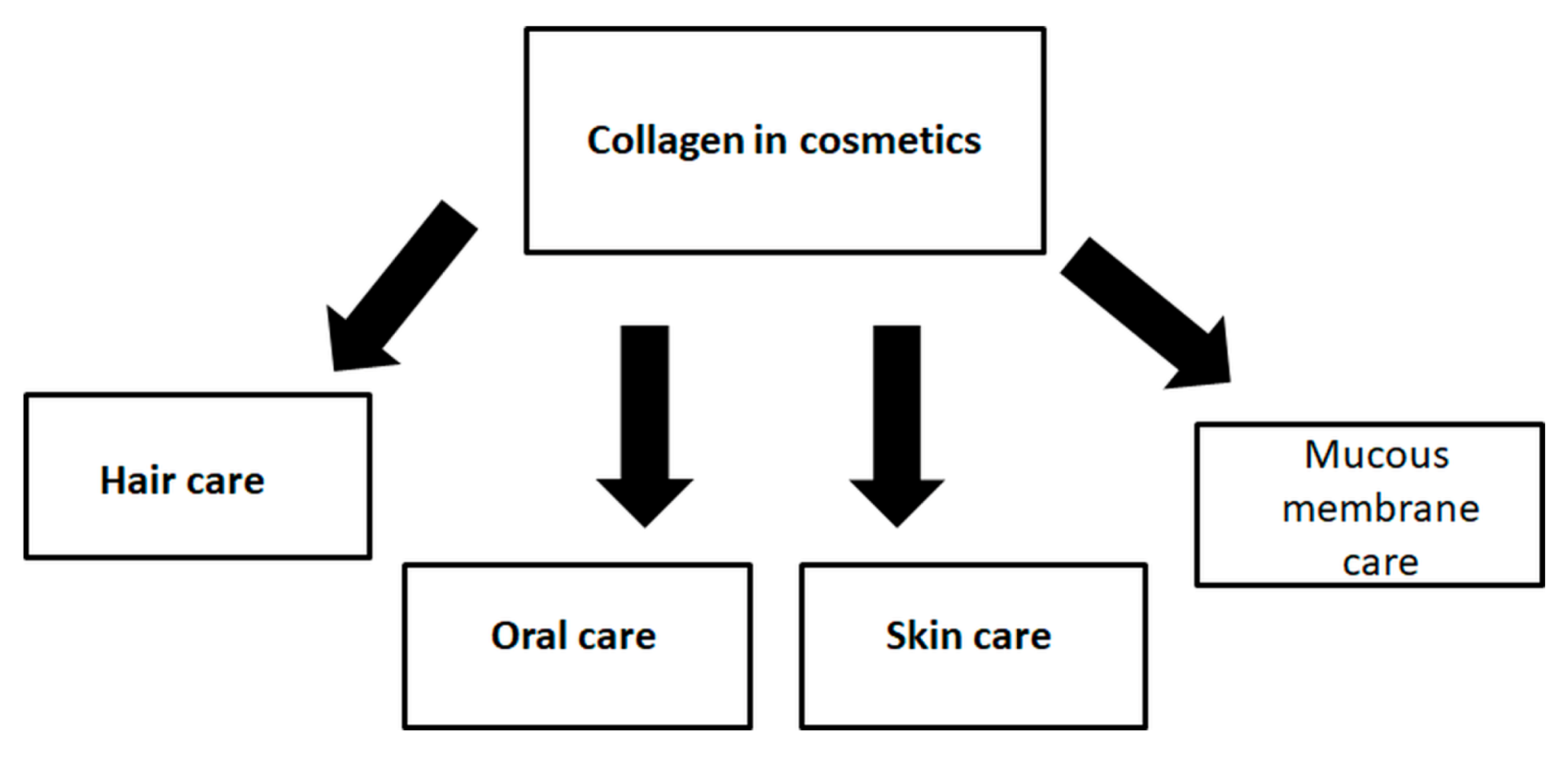
6. Collagen Role in Cosmetics
7. Hydrolyzed Collagen for Cosmetic Purposes
8. Collagen Modification by Cross-Linking
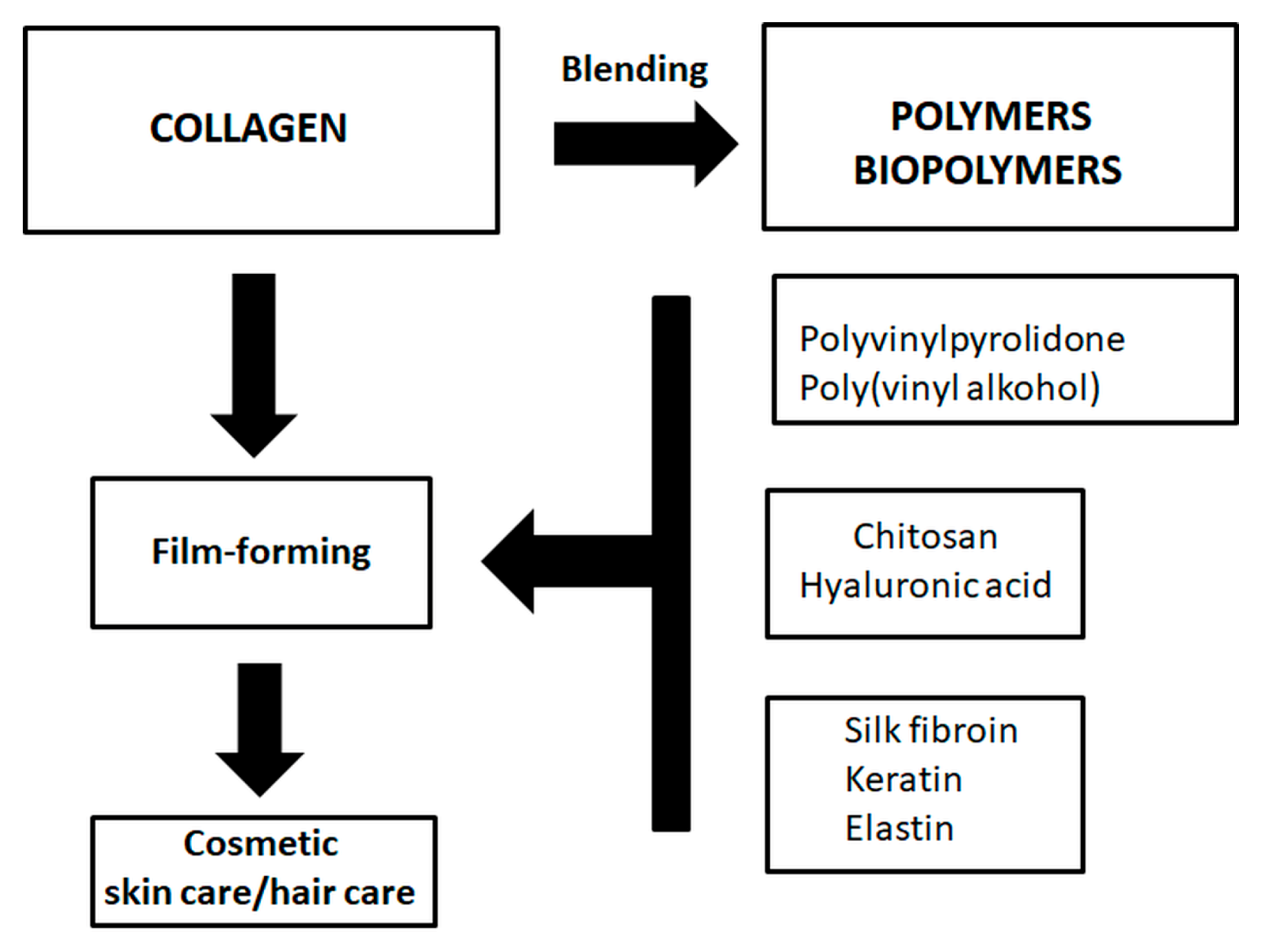
9. Collagen Blends for Cosmetic Applications
10. The Comparison of the Existing Knowledge in the Field of Collagen Application in Cosmetics
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodríguez, M.; Rodriguez, I.A.; Barrosso, L.G.R.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2017, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Leon-Lopez, A.; Morales-Penaloza, A.; Martinez-Juarez, V.; Vergas-Torres, A.; Zeugolis, D.; Aguirre-Alvarez, G. Hydrolyzed collagen—Sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harbor Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, P.; Dandge, P.B. Isolation, characterization and valorizable applications of fish scale collagen in food and agriculture industries. Biocatal. Agric. Biotechnol. 2016, 7, 234–240. [Google Scholar] [CrossRef]
- Bailey, A.J.; Paul, R.G. Collagen is not so simple protein. J. Soc. Leather Technol. Chem. 1998, 82, 104–108. [Google Scholar]
- Prockop, D.J.; Berg, R.A.; Kivirikko, K.I.; Uitto, J. Intracellular Steps in the Biosynthesis of Collagen; Springer Science and Business Media LLC: Berlin, Germany, 1976; pp. 163–273. [Google Scholar]
- Sorushanova, A.; Delgado, L.; Zhuning, W.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamiliy: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, M.; Bruckner, P.; Steinmann, B. Delayed triple helix formation of mutant collagen from patient with osteogenesis imperfecta. J. Mol. Boil. 1994, 236, 940–949. [Google Scholar] [CrossRef]
- Koide, T.; Nagata, K. Collagen biosynthesis. Top. Curr. Chem. 2005, 247, 85–114. [Google Scholar]
- Engel, J.; Prockop, D. The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper. Annu. Rev. Biophys. Biophys. Chem. 1991, 20, 137–152. [Google Scholar] [CrossRef]
- Bailey, A.J.; Paul, R.G.; Knott, L. Mechanisms of mutaration and ageing of collagen. Mech. Ageing Dev. 1998, 106, 1–56. [Google Scholar] [CrossRef]
- Privalov, P.; Tiktopulo, E.; Tischenko, V. Stability and mobility of the collagen structure. J. Mol. Boil. 1979, 127, 203–216. [Google Scholar] [CrossRef]
- Sricholpech, M.; Perdivara, I.; Yokoyama, M.; Nagaoka, H.; Terajima, M.; Tomer, K.; Yamauchi, M. Lysyl hydroxylase 3-mediated glycosylation in type I collagen molecular loci and biological significance. J. Biol. Chem. 2012, 287, 22998–23009. [Google Scholar] [CrossRef] [PubMed]
- Tasab, M.; Batten, M.R.; Bulleid, N.J. Hsp47: A molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J. 2000, 19, 2204–2211. [Google Scholar] [CrossRef]
- Bottomley, M.; Batten, M.; Lumb, R.; Bulleid, N. Quality control in the endoplasmatic reticulum: PDI mediates the ER retention of unassembled procollagen C-propeptides. Curr. Biol. 2001, 11, 1114–1118. [Google Scholar] [CrossRef]
- Kessler, E.; Takahara, K.; Biniaminov, L.; Brusel, M.; Greenspan, D.S.; Morell, V. Bone morphogenetic protein-1: The type i procollagen C-proteinase. Science 1996, 271, 360–362. [Google Scholar] [CrossRef]
- Mao, Y.; Schwarzbauer, J.E. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Boil. 2005, 24, 389–399. [Google Scholar] [CrossRef]
- Kadler, K.E.; Hill, A.; Canty-Laird, E.G. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Boil. 2008, 20, 495–501. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Brøndsted, H.; Carlsen, F. A cortical cytoskeleton in expanded epithelium cells of sponge gemmules. Exp. Cell Res. 1951, 2, 90–96. [Google Scholar] [CrossRef]
- Gross, J.; Sokal, Z.; Rougvie, M. Structural and chemical studies on the connective tissue of marine sponges. J. Histochem. Cytochem. 1956, 4, 227–246. [Google Scholar] [CrossRef]
- Junqua, S.; Robert, L.; Garrone, R.; De Ceccatty, M.P.; Vacelet, J. Biochemical and morphological studies on collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [PubMed]
- Pallela, R.; Bojja, S.; Janapala, V.R. Biochemical and biophysical characterization of collagens of marine sponge, Ircinia fusca (Porifera: Demospongiae: Irciniidae). Int. J. Boil. Macromol. 2011, 49, 85–92. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Nagai, T.; Worawattanamateekul, W.; Suzuki, N.; Nakamura, T.; Ito, T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhophilema asamushi). Food Chem. 2000, 70, 205–208. [Google Scholar]
- Nagai, T.; Ogawa, T.; Nakamura, T.; Nakamura, H. Collagen of edicle jellyfish exumbrella. J. Sci. Food Agric. 1999, 79, 855–858. [Google Scholar]
- Calejo, M.; Morais, Z.; Fernandes, A. Isolation and biochemical characterization of a novel collagen from Catostylus tagi. J. Biomater. Sci. Polym. Ed. 2009, 20, 2073–2087. [Google Scholar]
- Kimura, S.; Miura, S.; Park, Y.-H. Collagen as the major edible component of jellyfish (Stomolophus nomural). J. Food Sci. 1983, 48, 1758–1760. [Google Scholar] [CrossRef]
- Miura, S.; Kimura, S. Jellyfish mesoglea collagen. Characterization of molecules as aplha 1 alpha 2 heterotrimers. J. Biol. Chem. 1985, 260, 15352–15356. [Google Scholar]
- Hsieh, Y. Use of Jellyfish Collagen (Type II) in the Treatment of Rheumatoid Arthritis. U.S. Patent 6,894,029, 17 May 2005. [Google Scholar]
- Nagai, T.; Yamashita, E.; Taniguchi, K.; Suzuki, N. Isolation and characterization of collagen from the outer skin waste material of cuttlefish (Sepia lycidas). Food Chem. 2001, 72, 425–429. [Google Scholar]
- Nagai, T.; Nagamori, K.; Yamashita, E.; Suzuki, N. Collagen of octopus Callistoctopus arakawai arm. Int. J. Food Sci. Technol. 2002, 37, 285–289. [Google Scholar] [CrossRef]
- Nagai, T. Collagen from diamondback squid (Thysanoteuthis rhombus) outer skin. Zeitschrift für Naturforschung C 2004, 59, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Senaratne, L.; Park, P.-J.; Kim, S.-K. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresour. Technol. 2006, 97, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, W.; Li, G. Isolation and characterization of collages from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar]
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes). Food Chem. 2002, 78, 173–177. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Khan, S.B.; Qian, Z.-J.; Ryu, B.; Kim, S.-K. Isolation and biochemical characterization of collagens from seaweed pipefish, Syngnathus schlegeli. Biotechnol. Bioprocess. Eng. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Miller, A.T.; Karmas, E.; Lu, M.F. Age-related changes in the collagen of bovine corium: Studies on extractability, solubility and molecular size distribution. J. Food Sci. 1983, 48, 681–685. [Google Scholar] [CrossRef]
- Rodziewicz-Motowidło, S.; Śladewska, A.; Mulkiewicz, E.; Kołodziejczyk, A.; Aleksandrowicz, A.; Miszkiewicz, J.; Stepnowski, P. Isolation and characterization of a thermally stable collagen preparation from the outer skin of the silver carp Hypophthalmichthys molitrix. Aquaculture 2008, 285, 130–134. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the potential of collagen from codfish skin as a biomaterial for biomedical applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, M.; Qiao, Y.; Tian, Y.; Liu, J.; Qin, S.; Wu, W. Extraction and characterization of type I collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process. Biochem. 2018, 74, 156–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Li, G.; Shi, B.; Miao, Y.; Wu, X. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem. 2007, 103, 906–912. [Google Scholar] [CrossRef]
- Fielding, A.M. Preparation of neutral salt soluble collagen. In The Methodology of Connective Tissue Research, 1st ed.; Hall, D.A., Ed.; Joynson-Bruvvers: Oxford, UK, 1976; pp. 9–12. [Google Scholar]
- Trelstad, R. Immunochemistry of the Extracellular Matrix, 1st ed.; CRC Press: Boca Raton, FL, USA, 1982. [Google Scholar]
- Piez, K.; Reddi, K. Extracellular Matrix Biochemistry, 1st ed.; Elsevier: New York, NY, USA, 1984. [Google Scholar]
- Hema, G.; Shyni, K.; Mathew, S.; Anandan, R.; Ninan, G. A simple method for isolation of fish skin collagen- biochemical characterization of skin collagen extracted from Albacore Tuna (Thunnus Alalunga), Dog Shark (Scoliodon Sorrakowah), and Rohu (Labeo Rohita). Ann. Biol. Res. 2013, 4, 271–278. [Google Scholar]
- Ervin, H.; Epstein, J.; Munderloh, N. Human skin collagen. J. Biol. Chem. 1978, 253, 1336–1337. [Google Scholar]
- Song, W.-K.; Liu, D.; Sun, L.-L.; Li, B.-F.; Hou, H. Physicochemical and biocompatibility properties of type i collagen from the skin of Nile Tilapia (Oreochromis Niloticus) for biomedical applications. Mar. Drugs 2019, 17, 137. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, R.; Tian, Y.; Konno, K. Characterisation of acid-soluble collagen from skin of silver carp (Hypophthalmichthys molitrix). Food Chem. 2009, 116, 318–322. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, R.; Ye, C.; Konno, K. Isolation and characterisation of collagens from scale of silver carp (Hypophthalmichthys molitrix). J. Food Biochem. 2010, 34, 6. [Google Scholar]
- Aidos, I.; Lie, Ø.; Espe, M. Collagen content in farmed Atlantic salmon (Salmo salar L.). J. Agric. Food Chem. 1999, 47, 1440–1444. [Google Scholar] [CrossRef]
- Andersen, C.M.; Wold, J.P. Fluorescence of muscle and connective tissue from cod and salmon. J. Agric. Food Chem. 2003, 51, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zheng, H.; Shi, Z.; Li, D.; Xiang, Y. Isolation and characterization of acid-soluble collagen and pepsin-soluble collagen from the skin of hybrid sturgeon. J. Wuhan Univ. Technol. Sci. Ed. 2019, 34, 950–959. [Google Scholar] [CrossRef]
- Helfrich, Y.; Sachs, D.; Voorhese, J. Overview of skin aging and photoaging. Dermatol. Nurs. 2008, 20, 177–183. [Google Scholar] [PubMed]
- Chung, J.-H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.-E.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Secchi, G. Role of protein in cosmetics. Clin. Dermatol. 2008, 26, 321–325. [Google Scholar] [CrossRef]
- Peng, Y.; Glattauer, V.; Werkmeister, J.; Ramshaw, J. Evaluatio for collagen products for cosmetic application. J. Cosmet. Sci. 2004, 55, 327–341. [Google Scholar]
- Nagelschmidt, M.; Struck, H. Collagen as cosmetic compound? Arch. Dermatol. Res. 1974, 250, 237–243. [Google Scholar]
- Li, G.; Fukunaga, S.; Takenouchi, K.; Nakamura, F. Comprative study of the physiological properties of collagen, gelatin and collagen hydrolysate as cosmetic materials. Int. J. Cosm. Sci. 2005, 27, 101–106. [Google Scholar]
- Pachence, J.; Berg, R.; Silver, F. Collagen: Its place in the medical device industry. Med. Device Diagn. Ind. 1987, 9, 49–55. [Google Scholar]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Boil. Macromol. 2015, 77, 369–374. [Google Scholar] [CrossRef]
- Newman, J. Review of soft tissue augmentation in the face. Clin. Cosmet. Investig. Dermatol. 2009, 2, 141–150. [Google Scholar] [CrossRef]
- Requena, L.; Requena, C.; Christensen, L.; Zimmermann, U.S.; Kutzner, H.; Cerroni, L. Adverse reactions to injectable soft tissue fillers. J. Am. Acad. Dermatol. 2011, 64, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Buck, D.W.; Alam, M.; Kim, J.Y. Injectable fillers for facial rejuvenation: A review. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 11–18. [Google Scholar] [CrossRef] [PubMed]
- DeVore, D.; Kelman, C.; Fagien, S.; Casson, P. Autologen: Autologous, injectable, dermal collagen. In Principles and Practice of Ophthalmic Plastic and Reconstructive Surgery; Bosniak, S.W.B., Ed.; Saunders: Philadelphia, PA, USA, 1996; pp. 670–675. [Google Scholar]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2016, 28, 4–9. [Google Scholar] [CrossRef]
- Araujo, J.; Sica, P.; Costa, C.; Márquez, M. Enzymatic hydrolysis of fish waste as an alternative to produce high value-added products. Waste Biomass-Valoriz. 2020, 1–9, in press. [Google Scholar] [CrossRef]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Alvarado, R.J.; Aguirre-Álvarez, G. Collagen hydrolysates for skin protection: Oral administration and topical formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef]
- Campos, P.M.B.G.M.; Melo, M.O.; César, F.C.S. Topical application and oral supplementation of peptides in the improvement of skin viscoelasticity and density. J. Cosmet. Dermatol. 2019, 18, 1693–1699. [Google Scholar] [CrossRef]
- Mejía-Calvo, I.; López-Juárez, L.E.; Vázquez-Leyva, S.; López-Morales, C.A.; Montoya-Escutia, D.; Rivera, P.G.M.; Herbert-Pucheta, J.E.; Zepeda-Vallejo, L.G.; Velasco-Velázquez, M.A.; Pavón, L.; et al. Quality attributes of partially hydrolyzed collagen in a liquid formulation used for skin care. J. Cosmet. Dermatol. 2020, in press. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed collagen—Sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Lupu, M.-A.; Pircalabioru, G.G.; Chifiriuc, M.-C.; Albulescu, R.; Tanase, C. Beneficial effects of food supplements based on hydrolyzed collagen for skin care (Review). Exp. Ther. Med. 2019, 20, 12–17. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Battino, M.; Benjakul, S. Effect of stabilizing agents on characteristics, antioxidant activities and stability of liposome loaded with hydrolyzed collagen from defatted Asian sea bass skin. Food Chem. 2020, 328, 127127. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, A.; Jameela, S. Glutaraldehyde as a fixative in bioprosthetic and drug delivery matrices. Biomaterials 1996, 17, 471–484. [Google Scholar] [CrossRef]
- Zeugolis, D.; Paul, G.; Attenburrow, G. Cross-linking of extruded collagen fibers—A biomimetic three-dimensional scaffold for tissue engineering applications. Mater. Res. A 2009, 89, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. Part. A 2008, 87, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.K.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef]
- Gaudio, C.; Baiguera, S.; Boieri, M. Induction of angiogenesis using VEGF realising genipin-crosslinked electrospun gelatin mats. Biomaterials 2013, 34, 7754–7765. [Google Scholar] [CrossRef]
- Kwon, Y.-S.; Lim, E.-S.; Kim, H.-M.; Hwang, Y.-C.; Lee, K.-W.; Min, K.-S. Genipin, a cross-linking agent, promotes odontogenic differentiation of human dental pulp cells. J. Endod. 2015, 41, 501–507. [Google Scholar] [CrossRef]
- Kozlowska, J.; Stachowiak, N.; Prus, W. Stability studies of collagen-based microspheres with Calendula officinalis flower extract. Polym. Degrad. Stab. 2019, 163, 214–219. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen-gelatin hydrogel using EDC/NHS for corneal tissue engeneering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef]
- Pfeifer, V.F.; Sohns, V.E.; Conway, H.F.; Lancaster, E.B.; Dabic, S.; Griffin, E.L. Two stage process for dialdehyde starch using electrolytic regeneration of periodic acid. Ind. Eng. Chem. 1960, 52, 201–206. [Google Scholar] [CrossRef]
- Mu, C.; Liu, F.; Cheng, Q.; Li, H.; Wu, B.; Zhang, G.; Lin, W. Collagen cryogel cross-linked by dialdehyde starch. Macromol. Mater. Eng. 2010, 295, 100–107. [Google Scholar] [CrossRef]
- Langmaier, F.; Mládek, M.; Mokrejš, P.; Kolomazník, K. Biodegradable packing materials based on waste collagen hydrolysate cured with dialdehyde starch. J. Therm. Anal. Calorim. 2008, 93, 547–552. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-chemical characterization and biological tests of collagen/silk fibroin/chitosan scaffolds cross-linked by dialdehyde starch. Polymers 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Michalska-Sionkowska, M.; Walczak, M. Preparation and characterization of collagen/hyaluronic acid/chitosan film crosslinked with dialdehyde starch. Int. J. Boil. Macromol. 2020, 149, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Leedy, M.; Martin, H.; Norowski, P.; Jennings, J.; Haggard, W.; Bumgardner, J. Use of chitosan as a bioactive implant coating for bone-implant applications. In Chitosan for Biomaterials II (Advances in Polymer Science); Jayakumar, R., Prabaharan, M., Muzzarelli, R., Eds.; Springer: Berlin, Germany, 2011; Volume 5, pp. 129–165. [Google Scholar]
- Yan, J.; Li, X.; Liu, L.; Wang, F.; Zhu, T.W.; Zhang, Q. Potential use of collagen-chitosan-hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Artif. Cells Blood Substit. Biotechnol. 2006, 34, 27–39. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, H.; Long, X.; Wei, L.; Li, J.; Wu, Y.; Lin, Z. Use of synovium-derived stromal cells and chitosan/collagen type I scaffolds for cartilage tissue engineering. Biomed. Mater. 2010, 5, 055005. [Google Scholar] [CrossRef] [PubMed]
- Khor, E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials 1997, 18, 95–105. [Google Scholar] [CrossRef]
- Trackman, P.C. Diverse biological functions of extracellular collagen processing enzymes. J. Cell. Biochem. 2005, 96, 927–937. [Google Scholar] [CrossRef]
- Crescenzi, V.; Francescangeli, A.; Taglienti, A. New gelatin-based hydrogels via enzymatic networking. Biomacromolecules 2002, 3, 1384–1391. [Google Scholar] [CrossRef]
- Chen, T.; Embree, H.D.; Brown, E.M.; Taylor, M.M.; Payne, G.F. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003, 24, 2831–2841. [Google Scholar] [CrossRef]
- Garcia, Y.; Collighan, R.; Griffin, M.; Pandit, A. Assessment of cell viability in a three-dimensional enzymatically cross-linked collagen scaffold. J. Mater. Sci. Mater. Electron. 2007, 18, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Toki, S.; Ishii, Y.; Shirai, K. Effect of Transglutaminase on reconstruction and physicochemical properties of collagen gel from shark type I collagen. Biomacromolecules 2001, 2, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Folk, J.E. Transglutaminases. Annu. Rev. Biochem. 1980, 49, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Garcia, Y.; Hemantkumar, N.; Collighan, R.; Griffin, M.; Cabello, J.C.R.; Pandit, A. In vitro characterization of a collagen scaffold enzymatically cross-linked with a tailored elastin-like polymer. Tissue Eng. Part A 2009, 15, 887–899. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Boil. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Sionkowska, A. Interaction of collagen and poly(vinyl pyrrolidone) in blends. Eur. Polym. J. 2003, 39, 2135–2140. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skopinska-Wisniewska, J.; Wiśniewski, M. Collagen–synthetic polymer interactions in solution and in thin films. J. Mol. Liq. 2009, 145, 135–138. [Google Scholar] [CrossRef]
- Taravel, M.N.; Domard, A. Collagen and its interaction with chitosan. II. Influence of the physicochemical characteristics of collagen. Biomaterials 1995, 16, 865–871. [Google Scholar] [CrossRef]
- Sionkowska, A. Molecular interactions in collagen and chitosan blends. Biomaterials 2004, 25, 795–801. [Google Scholar] [CrossRef]
- Ye, Y.; Dan, W.; Zeng, R.; Lin, H.; Dan, N.; Guan, L.; Mi, Z. Miscibility studies on the blends of collagen/chitosan by dilute solution viscometry. Eur. Polym. J. 2007, 43, 2066–2071. [Google Scholar] [CrossRef]
- Machado, A.A.S.; Martins, V.D.C.A.; Plepis, A.M.D.G. Thermal and rheological behavior of collagen. Chitosan blends. J. Therm. Anal. Calorim. 2002, 67, 491–498. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewski, M.; Skopinska, J.; Poggi, G.; Marsano, E.; Maxwell, C.; Wess, T. Thermal and mechanical properties of UV irradiated collagen/chitosan thin films. Polym. Degrad. Stab. 2006, 91, 3026–3032. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K. Modification of collagen and chitosan mixtures by the addition of tannic acid. J. Mol. Liq. 2014, 199, 318–323. [Google Scholar] [CrossRef]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual crosslinked collagen/chitosan film for potential biomedical applications. Polymers 2019, 11, 2094. [Google Scholar] [CrossRef]
- Bax, D.V.; Smalley, H.E.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Cellular response to collagen-elastin composite materials. Acta Biomater. 2019, 86, 158–170. [Google Scholar] [CrossRef]
- Niculescu, M.-D.; Epure, D.-G.; Lasoń-Rydel, M.; Gaidau, C.; Gidea, M.; Enascuta, C. Biocomposites based on collagen and keratin with properties for agriculture and industrie applications. EuroBiotech J. 2019, 3, 160–166. [Google Scholar] [CrossRef]
- Ghaeli, I.; De Moraes, M.A.; Beppu, M.M.; Lewandowska, K.; Sionkowska, A.; Ferreira-Da-Silva, F.; Ferraz, M.P.; Monteiro, F. Phase behaviour and miscibility studies of collagen/silk fibroin macromolecular system in dilute solutions and solid state. Molecules 2017, 22, 1368. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, C.; Ma, X.; Chen, C.; Zhu, H. Study on the preparation of collagen-modified silk fibroin films and their properties. Biomed. Mater. 2006, 1, 242–246. [Google Scholar] [CrossRef]
- Sionkowska, A.; Grabska-Zielińska, S.; Lewandowska, K.; Andrzejczyk, A. Polymer films based on silk fibroin and collagen—The physico-chemical properties. Mol. Cryst. Liq. Cryst. 2016, 640, 13–20. [Google Scholar] [CrossRef]
- Lin, X.-L.; Gao, L.; Li, R.; Cheng, W.; Zhang, C.-Q.; Zhang, X.-Z. Mechanical property and biocompatibility of silk fibroin-collagen type II composite membrane. Mater. Sci. Eng. C 2019, 105, 110018. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Jakus, A.E.; Shah, R.N. In situ forming collagen–hyaluronic acid membrane structures: Mechanism of self-assembly and applications in regenerative medicine. Acta Biomater. 2013, 9, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Ikoma, T.; Tanaka, J. An improved method to prepare hyaluronic acid and type II collagen composite matrices. J. Biomed. Mater. Res. 2002, 61, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-N.; Lee, H.-J.; Lee, K.-H.; Suh, H. Biological characterization of EDC-crosslinked collagen-hyaluronic acid matrix in dermal tissue restoration. Biomaterials 2003, 24, 1631–1641. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Grabska-Zielińska, S.; Kaczmarek, B.; Michalska, M. The miscibility of collagen/hyaluronic acid/chitosan blends investigated in dilute solutions and solids. J. Mol. Liq. 2016, 220, 726–730. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Grabska-Zielińska, S.; Kaczmarek, B. Surface and thermal properties of collagen/hyaluronic acid blends containing chitosan. Int. J. Boil. Macromol. 2016, 92, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kaczmarek, B.; Michalska, M.; Lewandowska, K.; Grabska-Zielińska, S. Preparation and characterization of collagen/chitosan/hyaluronic acid thin films for application in hair care cosmetics. Pure Appl. Chem. 2017, 89, 1829–1839. [Google Scholar] [CrossRef]
- Oliveira, V.D.M.; De Assis, C.R.D.; Costa, B.D.A.M.D.; Neri, R.C.D.A.; Monte, F.T.D.D.; Freitas, H.M.S.D.C.V.D.; França, R.C.P.; Dos Santos, J.F.; Bezerra, R.D.S.; Porto, A.L.F. Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2020, 1224, 129023. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the marine sponges Axinella cannabina and Suberites carnosus: Isolation and morphological, biochemical, and biophysical characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef]
- Berillis, P. Marine collagen: Extraction and applications. In Research Trends in Biochemistry, Molecular Biology and Microbiology; SM Group: Dover, DE, USA, 2015; pp. 1–13. [Google Scholar]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Dıíaz, M.; Ulmo, N.; Lizarbe, M.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: a comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Stoichevska, V.; Vashi, A.; Howell, L.; Fehr, F.; Dumsday, G.J.; Werkmeister, J.A.; Ramshaw, J.A. Non-animal collagens as new options for cosmetic formulation. Int. J. Cosmet. Sci. 2015, 37, 636–641. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. https://doi.org/10.3390/ma13194217
Sionkowska A, Adamiak K, Musiał K, Gadomska M. Collagen Based Materials in Cosmetic Applications: A Review. Materials. 2020; 13(19):4217. https://doi.org/10.3390/ma13194217
Chicago/Turabian StyleSionkowska, Alina, Katarzyna Adamiak, Katarzyna Musiał, and Magdalena Gadomska. 2020. "Collagen Based Materials in Cosmetic Applications: A Review" Materials 13, no. 19: 4217. https://doi.org/10.3390/ma13194217
APA StyleSionkowska, A., Adamiak, K., Musiał, K., & Gadomska, M. (2020). Collagen Based Materials in Cosmetic Applications: A Review. Materials, 13(19), 4217. https://doi.org/10.3390/ma13194217






