Synthesis of Vanadium Carbide by Mechanical Activation Assisted Carbothermic Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
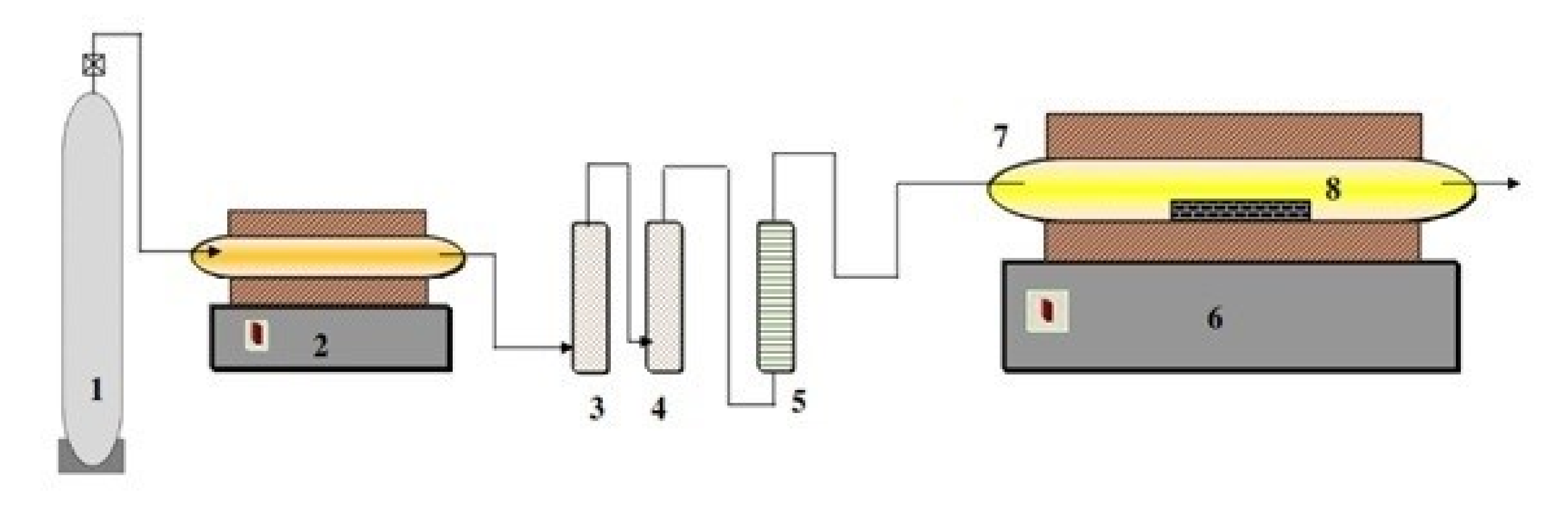
2.2. Procedure
2.3. Characterization
3. Results and Discussion
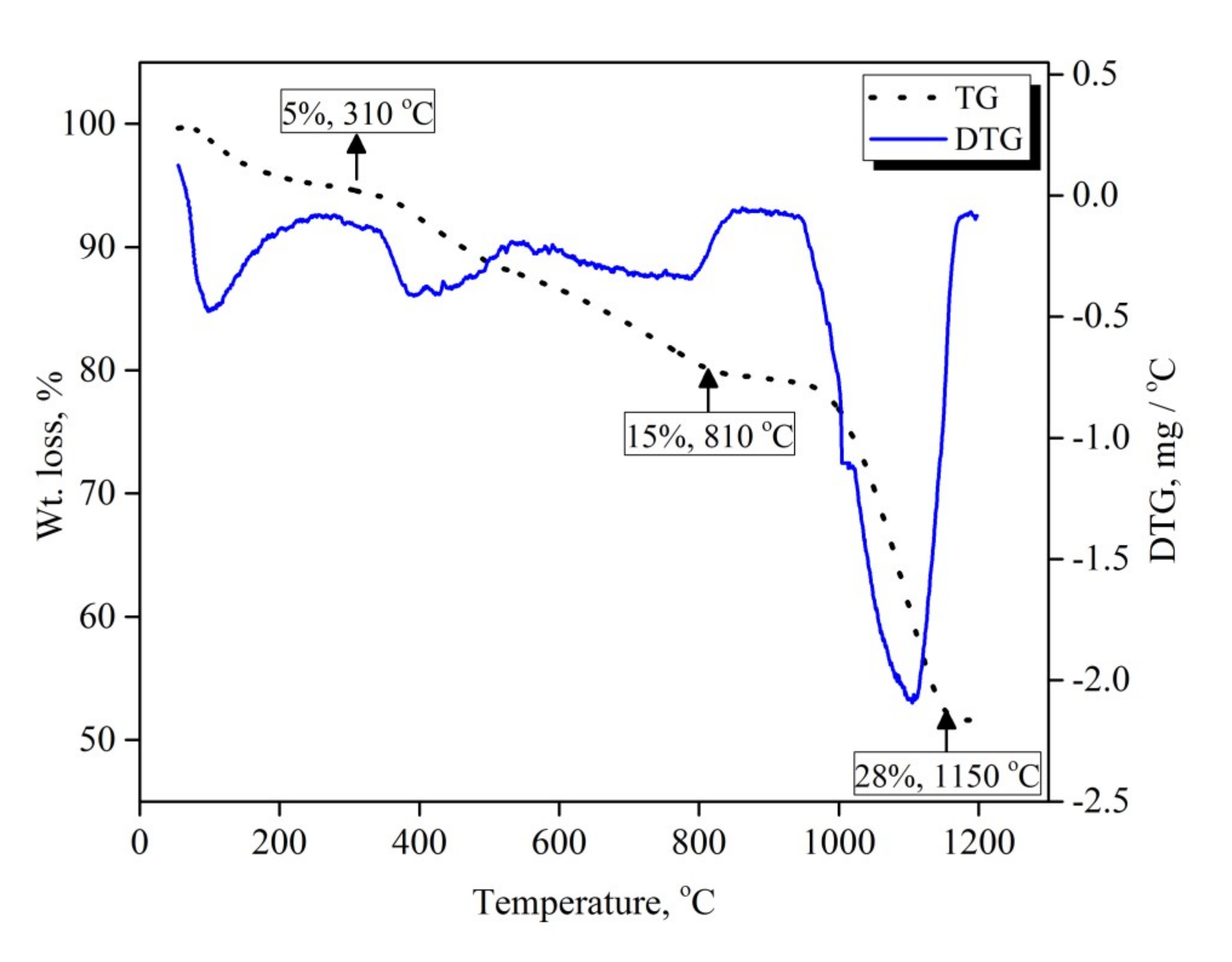
3.1. Thermogravimetric Analysis
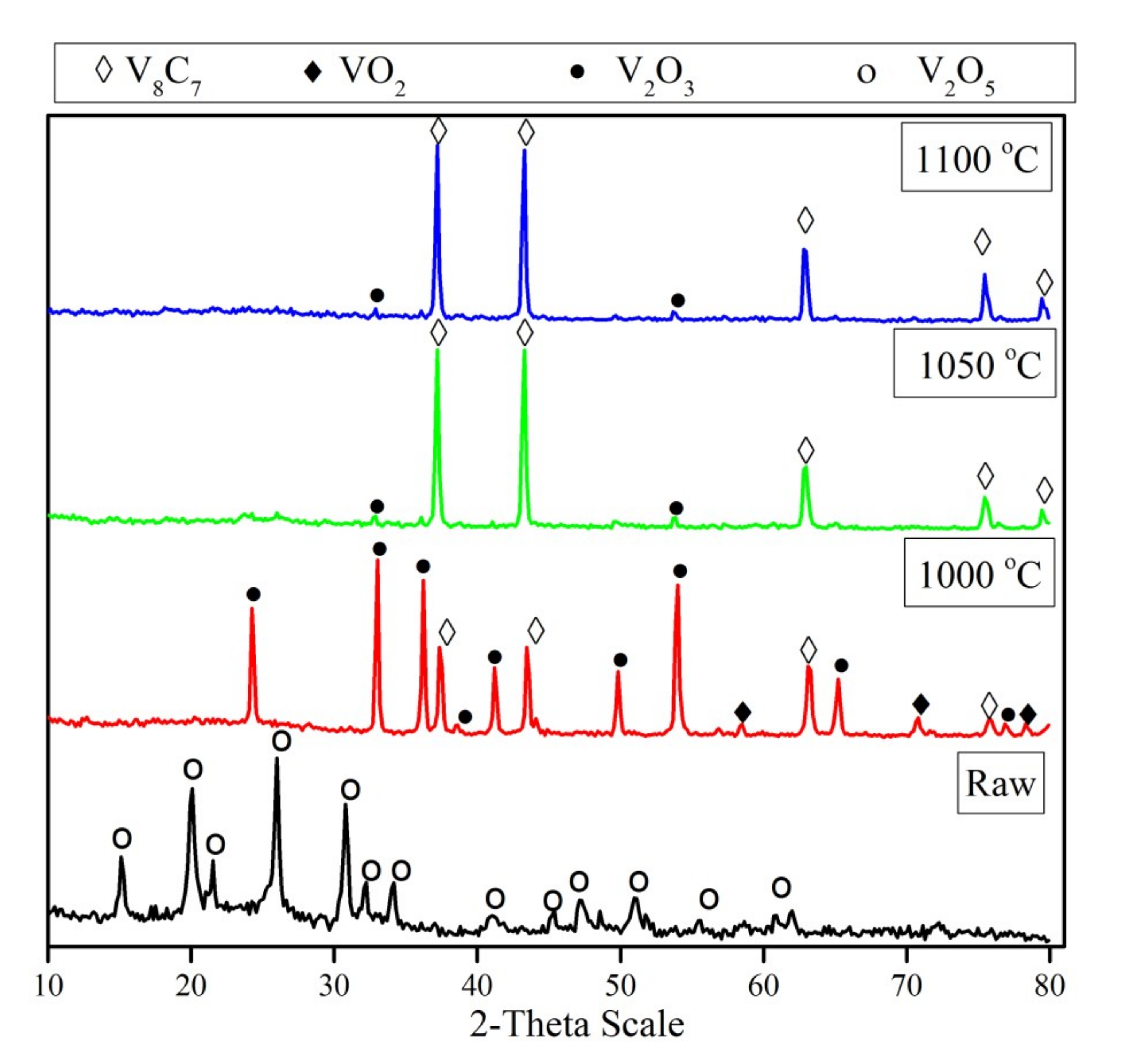
3.2. Effect of Reduction Temperature
3.3. Effect of Reduction Time
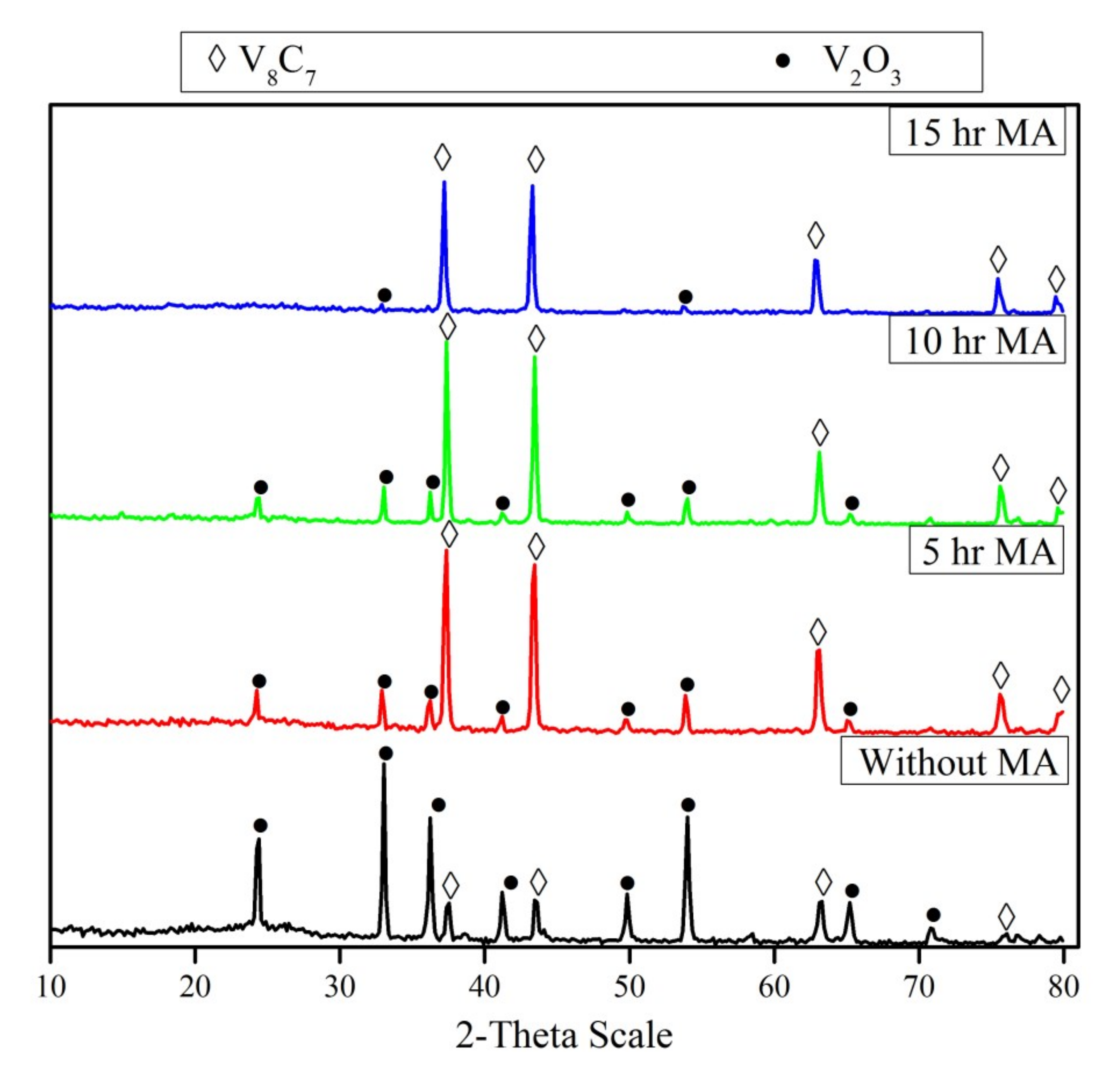
3.4. Effect of Mechanical Activation
3.5. Microstructure Characterization
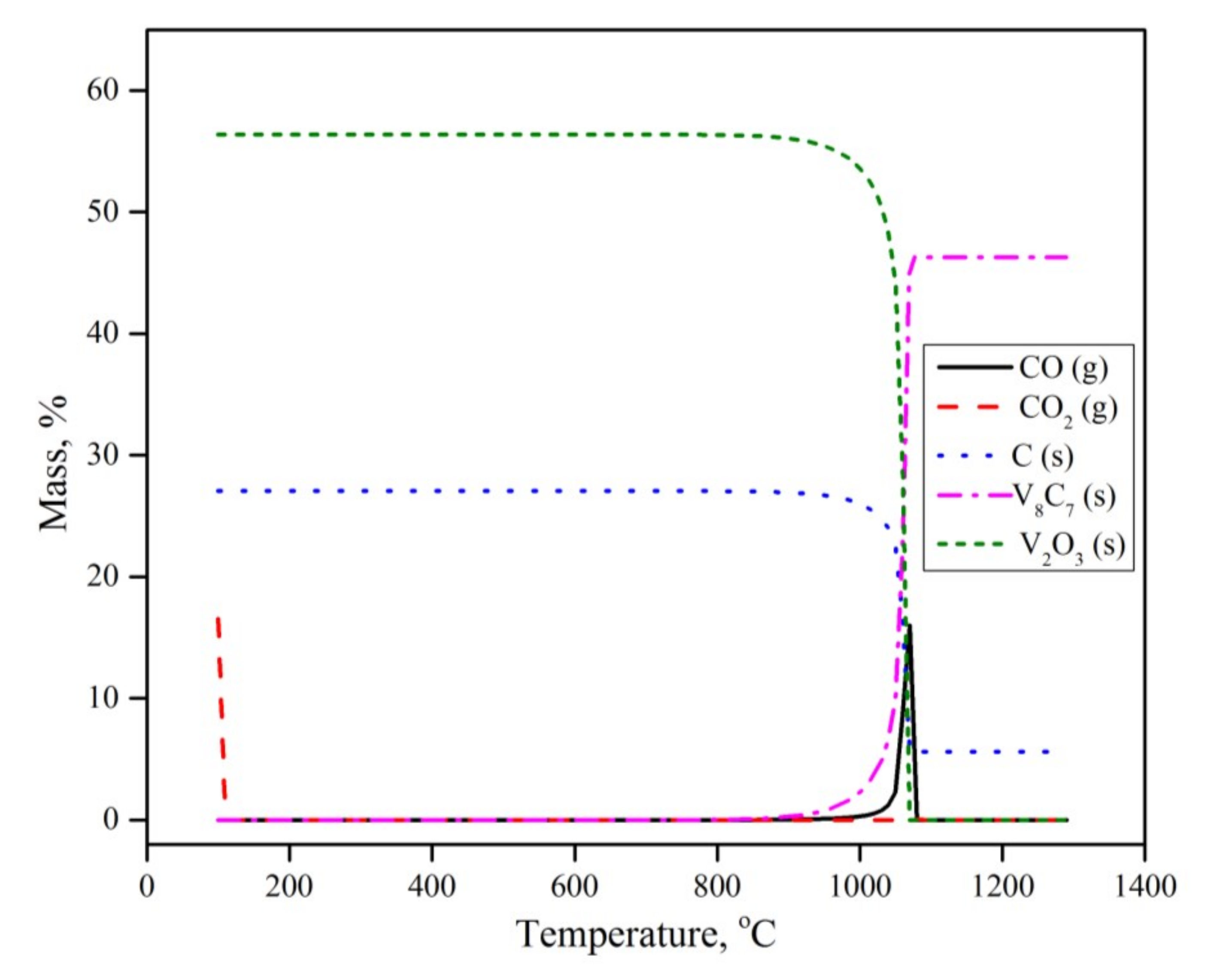
3.6. Thermodynamic Calculation
4. Conclusions
- Under the conventional conditions of the carbothermic reduction of vanadium oxide, a very small portion of the oxide can be converted to carbide, resulting in a very poor yield.
- The mechanical activation-assisted carbothermic reduction of vanadium oxide, on the other hand, gives promising results, with a very high yield of about 95%.
- The effects of reaction parameters like temperature, reduction duration and mechanical activation duration on the formation of vanadium carbide and the final product’s properties were investigated, and the optimum conditions were identified.
- V8C7 nanoparticles with a crystallite size of 88 nm were prepared from a mechanically activated V2O5-C mixture after milling for 15 h and further heating at 1050 °C for 1 h.
- The experimental results at and above 950 °C were successfully explained by equilibrium calculations. At lower temperatures, the reactions were kinetically limited.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hossein-Zadeh, M.; Razavi, M.; Safa, M.; Abdollahi, A.; Mirzaee, O. Synthesis and structural evolution of vanadium carbide in nano scale during mechanical alloying. J. King Saud Univ. Eng. Sci. 2016, 28, 207–212. [Google Scholar] [CrossRef]
- Ma, J.; Wu, M.; Du, Y.; Chen, S.; Ye, J.; Jin, L. Low temperature synthesis of vanadium carbide (VC). Mater. Lett. 2009, 63, 905–907. [Google Scholar] [CrossRef]
- Hossein-Zadeh, M.; Mirzaee, O. Synthesis and characterization of V8C7 nanocrystalline powder by heating milled mixture of V2O5, C and Ca via mechanochemical activation. Adv. Powder Technol. 2014, 25, 978–982. [Google Scholar] [CrossRef]
- Mahajan, M.; Singh, K.; Pandey, O.P. Single step synthesis of nano vanadium carbide—V8C7 phase. Int. J. Refract. Met. Hard Mater. 2013, 36, 106–110. [Google Scholar] [CrossRef]
- Kurlov, A.S.; Gusev, A.I. Preparation and microstructure of VC0.875 nanopowder. Inorg. Mater. 2013, 49, 347–354. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Ye, H.; Ma, J. A simple and novel route to synthesize nano-vanadium carbide using magnesium powders, vanadium pentoxide and different carbon source. Int. J. Refract. Met. Hard Mater. 2011, 29, 528–531. [Google Scholar] [CrossRef]
- Bauer, G.; Güther, V.; Hess, H.; Otto, A.; Roidl, O.; Roller, H.; Sattelberger, S. Vanadium and Vanadium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; American Cancer Society: New York, NY, USA, 2017; pp. 1–22. [Google Scholar]
- Matsushita, J.-I.; Nakayama, T. Oxidation behavior of vanadium carbide powder. J. Adv. Sci. 1998, 10, 97–99. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Murray, M.J. Elastic moduli measurements of some cubic transition metal carbides and alloyed carbides. J. Mater. Sci. 1974, 9, 223–228. [Google Scholar] [CrossRef]
- Kato, C.; Bailey, J.A. Wear Characteristics of a Woodworking Knife with a Vanadium Carbide Coating only on the Clearance Surface (Back Surface). Key Eng. Mater. 1997, 138, 479–520. [Google Scholar] [CrossRef]
- Borisova, A.; Borisov, Y.; Shavlovsky, E.; Mits, I.; Castermans, L.; Jongbloed, R. Vanadium carbide coatings: deposition process and properties. 15th International Plansee Seminar: Powder Metallurgical High Performance Materials, Reutte, Austria, 15–17 May 2001; Wildner, H., Ed.; Volume 2: P/M Hard Materials. p. 896. [Google Scholar]
- Krajewski, A.; D’Alessio, L.; De Maria, G. Physiso-Chemical and Thermophysical Properties of Cubic Binary Carbides. Cryst. Res. Technol. 1998, 33, 341–374. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, J.; Xu, L. Research on wear resistance of high speed steel with high vanadium content. Mater. Sci. Eng. A 2005, 404, 138–145. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Vanadium; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Claridge, J.B.; York, A.P.E.; Brungs, A.J.; Green, M.L.H. Study of the Temperature-Programmed Reaction Synthesis of Early Transition Metal Carbide and Nitride Catalyst Materials from Oxide Precursors. Chem. Mater. 2000, 12, 132–142. [Google Scholar] [CrossRef]
- Kapoor, R.; Oyama, S. Synthesis of Vanadium Carbide by Temperature Programmed Reaction. J. Solid State Chem. 1995, 120, 320–326. [Google Scholar] [CrossRef]
- Hossein-Zadeh, M.; Tebyani, F.; Cherati, J.Y.; Mirzaee, O. Mechanochemical Carboaluminothermic Reduction of V2O5 to Produce VC-Al2O3 Nanocomposite. J. Teknol. 2012, 59. [Google Scholar] [CrossRef]
- Venugopalan, R.; Sathiyamoorthy, D. Investigation through factorial design on novel method of preparing vanadium carbide using carbon during aluminothermic reduction. J. Mater. Process. Technol. 2006, 176, 133–139. [Google Scholar] [CrossRef]
- Li, P.; Lei, M.; Tang, W. Route to transition metal carbide nanoparticles through cyanamide and metal oxides. Mater. Res. Bull. 2008, 43, 3621–3626. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying: For Fabrication of Advanced Engineering Materials; University Press of Mississippi: Jackson, MS, USA, 2013. [Google Scholar]
- Hoseinpur, A.; Khaki, J.V.; Marashi, M.S. Mechanochemical synthesis of tungsten carbide nano particles by using WO3/Zn/C powder mixture. Mater. Res. Bull. 2013, 48, 399–403. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Z. Synthesis of vanadium carbide by mechanical alloying. J. Alloy. Compd. 2005, 392, 183–186. [Google Scholar] [CrossRef]
- Bale, C.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaki, Z.I.; El-Sadek, M.H.; Ali, H.H.; Ahmed, H. Synthesis of Vanadium Carbide by Mechanical Activation Assisted Carbothermic Reduction. Materials 2020, 13, 4408. https://doi.org/10.3390/ma13194408
Zaki ZI, El-Sadek MH, Ali HH, Ahmed H. Synthesis of Vanadium Carbide by Mechanical Activation Assisted Carbothermic Reduction. Materials. 2020; 13(19):4408. https://doi.org/10.3390/ma13194408
Chicago/Turabian StyleZaki, Zaki I., Mohamed H. El-Sadek, Heba H. Ali, and Hesham Ahmed. 2020. "Synthesis of Vanadium Carbide by Mechanical Activation Assisted Carbothermic Reduction" Materials 13, no. 19: 4408. https://doi.org/10.3390/ma13194408
APA StyleZaki, Z. I., El-Sadek, M. H., Ali, H. H., & Ahmed, H. (2020). Synthesis of Vanadium Carbide by Mechanical Activation Assisted Carbothermic Reduction. Materials, 13(19), 4408. https://doi.org/10.3390/ma13194408




