Environmental Impact of the Reclaimed Sand Addition to Molding Sand with Furan and Phenol-Formaldehyde Resin—A Comparison
Abstract
1. Introduction
- Evaporation of solvent, byproduct or chemical constituent occurring during mixing, core/mold making and core/mold storage, prior to pouring
- Thermal decomposition during pouring, cooling and shakeout operations.
2. Materials and Methods
2.1. Materials
- Furan no-bake acid catalyzed (FNB) was composed of 1% urea-furfuryl resin (free furfuryl alcohol 85%; nitrogen <0.9%; free formaldehyde <0.1%); hardener (p-toluenesulfonic acid) and fresh silica sand or reclaimed sand.
- Phenolic esters no-bake (PFNB) was composed of 1.2% phenol-formaldehyde resin (free phenol, 1.0%; free formaldehyde <0.20%); hardener (mix of organic esters) and fresh silica sand or reclaimed sand.
2.2. Methods
2.2.1. Gas Chromatography/Mass Spectrometry (GC/MS)
- BTEX analysis: HP-5MS; 30 m × 0.25 mm × 0.25 μm (film thickness) capillary column
- PAHs analysis: ZB-PAH; 20 m × 0.18 mm × 0.14 μm (film thickness) capillary column
- BTEX analysis (source temperature: 250 °C; operating mode: SIM)
- PAHs analysis (source temperature: 250 °C; operating mode: SCAN)
2.2.2. Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS)
- During the first stage of the research, the composition of gases evolving from molding sands, prepared on fresh sand matrices and poured with cast iron with a temperature of 1350 °C, was tested (samples marked: FNB and PFNB) [11].
- During the second stage, the emission of substances evolving from molding sands, prepared on fresh sand matrices with various fractions of a reclaim and poured with cast iron of a temperature of 1350 °C, was measured (samples marked: FNBXRYFS, where XR is the percent of reclaim fraction and YFS is the percent of fresh sand fraction).
- Molding sand with furan resin: FNB100FS (100% fresh sand), FNB50R50FS (50% reclaimed sand + 50% fresh sand) and FNB100R (100% reclaimed sand).
- Molding sand with phenol-formaldehyde resin: PFNB100FS (100% fresh sand), PFNB50R50FS (50% reclaimed sand + 50% fresh sand) and PFNB100R (100% reclaimed sand).
3. Results and Discussion
3.1. Investigations of the Gases Emitted from Molding Sands Prepared on the Fresh Sand Matrix
3.2. Investigations of the Gases Emitted from Molding Sands Prepared on the Reclaimed Sand Matrices
4. Conclusions
- Molding sands with the PFNB binder release a low amount of substances from the BTEX group (by up to 25%) than molding sands with the FNB binder. In both cases, benzene constituted more than 90%.
- Molding sands with the PFNB binder release nearly 50% less substances from the PAHs group than molding sands with the FNB binder.
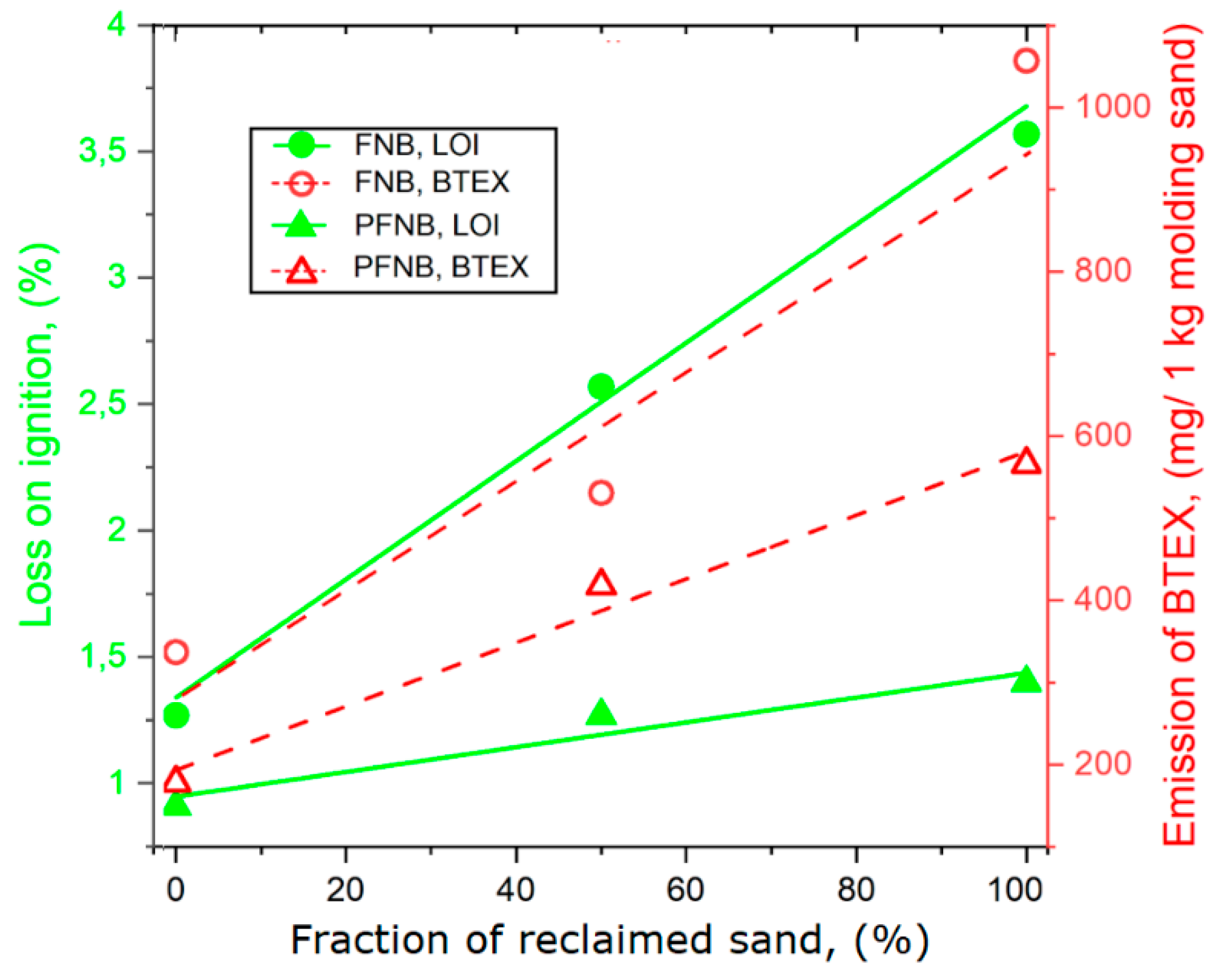
- The fresh sand substitution by a reclaimed sand in the matrix causes a significant increase (up to threefold) of the released gases from both groups. In addition, BTEX emission from the PFNB binder is twice lower than that from the FNB binder. The reclaimed sand addition to the PFNB sand influences the emission of PAHs only to a small degree.
- The LOI parameter can be useful in the case of these molding sands—for assessing amounts and approximated composition (e.g., benzene content) of evolving gases.
Author Contributions
Funding

Conflicts of Interest
References
- La Fay, V.; Schifo, J. Reducing seacoal in practice. Mod. Cast. 2012, 102, 32–34. [Google Scholar]
- Campbell, J. Complete Casting Handbook, 1st ed.; Elsevier: Amsterdam, Netherlands, 2011. [Google Scholar]
- Czerwinski, F.; Mir, M.; Kasprzak, W. Application of cores and binders in metalcasting. Int. J. Cast Met. Res. 2015, 28, 129–139. [Google Scholar] [CrossRef]
- Brown, J. Foceco Ferrous Foundryman’s Handbook, 11th. ed.; Elsevier: Amsterdam, Netherlands, 2000. [Google Scholar]
- Kmita, A.; Benko, A.; Roczniak, A.; Holtzer, M. Evaluation of pyrolysis and combustion products from foundry binders: potential hazards in metal casting. J. Therm. Anal. Calorim. 2020, 140, 2347–2356. [Google Scholar] [CrossRef]
- Allen, G.R.; Archibald, J.J.; Keenan, T. Hazardous air pollutants: A challenge to metal casting industry. Trans. Am. Foundrymen’s Soc. 1991, 99, 585–593. [Google Scholar]
- Dungan, R.S. Polycyclic aromatic hydrocarbons and phenolics and ferrous and non-ferrous waste foundry sands. J. Residuals Sci. Technol. 2006, 3, 203–209. [Google Scholar]
- Baker, D. Environmental responsibility—Foundry binders. Met. Cast. Surf. Finish. 1997, 43, 28–31. [Google Scholar]
- Menapace, C.; Leonardi, M.; Secchi, M.; Bonfanti, A.; Gialanella, S.; Straffelini, G. Thermal behavior of a phenolic resin for brake pad manufacturing. J. Therm. Anal. Calorim. 2019, 137, 759–766. [Google Scholar] [CrossRef]
- Ghosh, D.K. Comparison of molding sand technology between Alphaset (APNB) and Furan (FNB). Arch. Foundry Eng. 2019, 4, 11–20. [Google Scholar]
- Giese, S.R.; Shepard, A. Understanding emission characteristics of a foundry sand binder. In Proceedings of the 71st World Foundry Congress: Advanced Sustainable Foundry, WFC 2014, Bilbao, Spain, 19–21 May 2014; pp. 19–21. [Google Scholar]
- Kmita, A.; Knauer, W.; Holtzer, M.; Hodor, K.; Piwowarski, G.; Roczniak, A.; Górecki, K. The decomposition process and kinetic analysis of commercial binder based on phenol-formaldehyde resin, using in metal casting. Appl. Therm. Eng. 2019, 156, 263–275. [Google Scholar] [CrossRef]
- Zhong, Y.; Jing, X.; Wang, S.; Jia, Q.X. Behavior investigation of phenolic hydroxyl groups during the pyrolysis of cured phenolic resin via molecular dynamics simulation. Polym. Degrad. Stab. 2016, 125, 97–104. [Google Scholar] [CrossRef]
- Stevenson, M. The taxing problem of waste sand disposal. Foundry Trade J. 2006, 170, 580–582. [Google Scholar]
- Echard, J.B.; Regan, R.W.; Voigt, R.C. Environmental impact of foundry residuals: Pennsylvania, beneficial use approach. Trans. Am. Foundrymen’s Soc. 1995, 103, 463–467. [Google Scholar]
- Leidel, D.S.; Novakowski, M.; Pohlman, D.; MacRunnels, Z.D.; Mackay, M.H. External beneficial reuse of spent foundry sand. Trans. Am. Foundrymen’s Soc. 1994, 102, 235–243. [Google Scholar]
- Alves, B.S.Q.; Dungan, R.S.; Carnin, R.L.P.; Galvez, R.; De Carvalho Pinto, C.R.S. Metals in waste foundry sands and an evaluation of their leaching and transport to groundwater. Water Air Soil Pollut. 2014, 225, 1963. [Google Scholar] [CrossRef]
- Fox, J.R.; Adamovits, M.; Henry, C. Strategies for reducing foundry emissions. Trans. Am. Foundrymen’s Soc. 2002, 110, 1299–1309. [Google Scholar]
- Lefebvre, J.; Mamleev, V.; Le Bras, M.; Bourbigot, S. Kinetic analysis of pyrolysis of cross-linked polymers. Polym. Degrad. Stab. 2005, 88, 85–91. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stab. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Ribeiro, M.G.; Filho, W.R.P. Risk assessment of chemicals in foundries: The International Chemical Toolkit pilot-project. J. Hazard. Mater. 2006, 136, 432–437. [Google Scholar] [CrossRef]
- Bouajila, J.; Raffin, G.; Alamercery, S.; Waton, H.; Sanglar, C.; Grenier-Loustalot, M.F. Phenolic resins (IV). Thermal degradation of crosslinked resins in controlled atmospheres. Polym. Polym. Compos. 2003, 11, 345–357. [Google Scholar] [CrossRef]
- Skrzyński, M.; Dańko, R. Influence of the Reclamation Process Intensity in the REGMAS Reclaimer on the Purification Degree of the High-silica Matrix. Arch. Foundry Eng. 2016, 16, 69–72. [Google Scholar] [CrossRef][Green Version]
- Dungan, S.R.; Reeves, J.B. Pyrolysis of foundry sand resins: a determination of organic products by mass spectrometry. J. Environ. Sci. Health 2005, 40, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, H.; Zheng, K.; Li, X.; Liu, G.; Wang, Y. Diminishing hazardous air pollutant emissions from pyrolysis of furan no-bake binders using methanesulfonic acid as the binder catalyst. J. Therm. Anal. Calorim. 2014, 116, 373–381. [Google Scholar] [CrossRef]
- Psimenos, A.C.; Scheitz, W.; Eder, G.E. Konventioeneltle No-bake Systeme mit extreme reduziertem Monomergehalt. Giess. Prax. 2008, 9, 318–320. [Google Scholar]
- Liang, J.J.; Tsay, G.S. Composition and yield of the pernicious and stench gases in furan resin sand model founding process. Sustain. Environ. Res. 2010, 20, 115–125. [Google Scholar]
- Ren, Y.; Li, Y. Substitute materials of furfuryl alcohol in furan resin used for foundry and their technical properties. China Foundry 2009, 6, 339–342. [Google Scholar]
- Holtzer, M.; Dańko, J.; Lewandowski, J.L.; Solarski, W.; Dańko, R.; Grabowska, B.; Bobrowski, A.; Żymankowska-Kumon, S.; Sroczyński, A.; Różycki, A.; et al. Station for Research of the Volume and Harmfulness of Gases Compounds from the Materials Used in Foundry and Metallurgical Processes. Patent no. PL 224705 B1, 31 January 2017. [Google Scholar]
- Chen, Y.; Chen, Z.; Xiao, S.; Liu, H. A novel thermal degradation mechanism of phenol-formaldehyde type resins. Thermochim. Acta 2008, 476, 39–43. [Google Scholar] [CrossRef]
- Roczniak, A. Study of the Influence of Temperature and Type of Atmosphere on Release Chemical Compounds from Alphaset Molding Sands. Ph.D. Thesis, AGH-University of Science and Technology, Kraków, Poland, 2019. [Google Scholar]
- Holtzer, M. Influence of the Addition of the Reclaimed Sand on the Quality of Castings and Harmfulness of the New Generation Core and Moulding Sands; AKAPIT: Krakow, Poland, 2015. [Google Scholar]
- Holtzer, M.; Dańko, R. The Assesment of Harmfulness of Binding Materials Used for a New Generation of Core and Molding Sands; AKAPIT: Krakow, Poland, 2013. [Google Scholar]

| Component | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO |
| Mass (%) | 98.10 | 0.65 | 1.07 | 0.01 | 0.10 | 0.07 |
| Grain Size (mm) | 1.00 | 0.800 | 0.630 | 0.400 | 0.320 | 0.200 | 0.160 | 0.100 | 0.071 | 0.560 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh Silica Sand | |||||||||||
| Fraction (%) | 0.00 | 2.50 | 6.68 | 23.95 | 23.17 | 34.15 | 6.23 | 3.29 | 0.00 | 0.00 | 100 |
| Reclaimed Silica Sand | |||||||||||
| Fraction (%) | 0.02 | 1.15 | 5.13 | 34.04 | 25.88 | 25.86 | 5.51 | 2.16 | 0.05 | 0.00 | 100 |
| Sam-ple | Volume of Gases per 1 kg | Benzene per 1 kg | Toluene per 1 kg | Ethylbenzene per 1 kg | Xylenes per 1 kg | Total per 1 kg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | of molding sand, dm3 | of binder,dm3 | of molding sand, mg | of binder, mg | of molding sand, mg | of binder, mg | of molding sand, mg | of binder, mg | of molding sand, mg | of binder, mg | of molding sand, mg | of binder, mg |
| Molding Sand with Furan Resin (85% of Free Furfuryl Alcohol) (FNB) | ||||||||||||
| FNB | 14.466 | 964 | 602 | 40,158 | 51 | 3384 | 0.77 | 51 | 4 | 260 | 658 ± 66 | 43,835 ± 4385 |
| Molding Sand with Phenol Formaldehyde Resin (PFNB) | ||||||||||||
| PFNB | 17.482 | 1161 | 464 | 30,911 | 26 | 1738 | 0.84 | 56 | 4 | 289 | 495 ± 50 | 32,994 ± 3299 |
| Compound | FNB Sand Molding | PFNB Sand Molding | ||
|---|---|---|---|---|
| Results per 1 kg of Binder, mg | Results per 1 kg of Molding Sand, mg | Results per 1 kg of Binder, mg | Results per 1 kg of Molding Sand, mg | |
| Naphtalene | 93 | 1.39 | 193 | 2.89 |
| Acenaphtylene | 26 | 0.40 | 128 | 1.92 |
| Acenaphtene | 2 | 0.03 | 2 | 0.02 |
| Fluorene | 9 | 0.14 | 15 | 0.23 |
| Phenanthrene | 102 | 1.52 | 33 | 0.50 |
| Anthracene | 59 | 0.88 | 8 | 0.15 |
| Fluoranthene | 176 | 2.63 | 66 | 1.00 |
| Pyrene | 129 | 1.92 | 64 | 0.96 |
| Benz(a)anthracene | 22 | 0.32 | 15 | 0.22 |
| Chrysene | 29 | 0.43 | 19 | 0.28 |
| Benzo(b)fluoranthene | 17 | 0.26 | 9 | 0.13 |
| Benzo(k)fluoranthene | 10 | 0.15 | 5 | 0.08 |
| Benzo(a)pyrene | 16 | 0.24 | 11 | 0.17 |
| Indeno[1,2,3-cd]pyrene | 55 | 0.83 | 34 | 0.51 |
| Dibenz[a,h]anthracene | 24 | 0.36 | 20 | 0.30 |
| Benzo[g,h,i]perylene | 40 | 0.60 | 34 | 0.51 |
| Total PAHs ± 20% | 806 ± 161 | 12.09 ± 2.42 | 658 ± 132 | 9.87 ± 2 |
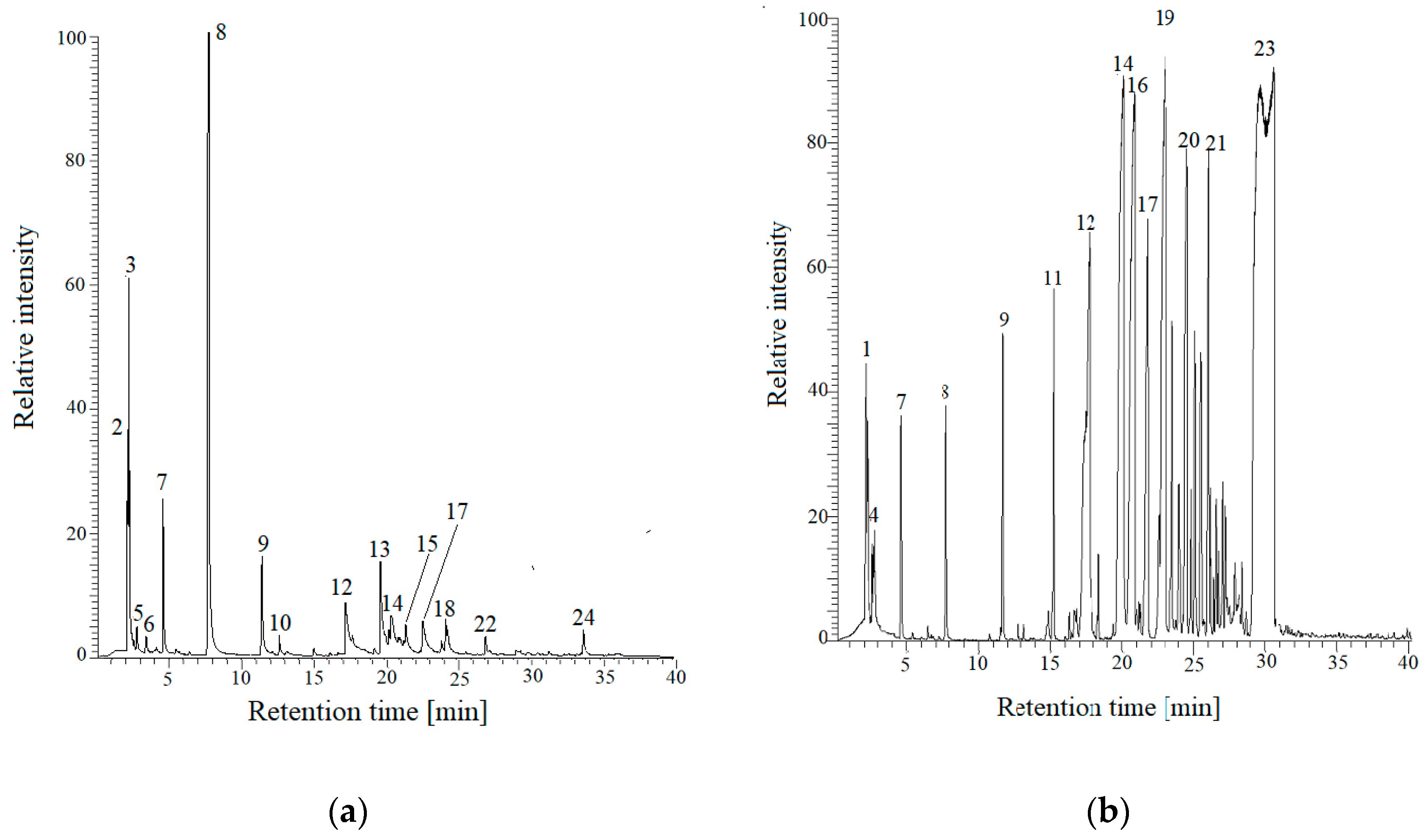
| Peak Number | Retention Time (min) | CAS Registry Number | Compound Name/ Summary Formula | Molecular Weight g/mol | Relative Area *(%) | |
|---|---|---|---|---|---|---|
| FNB Resin | PFNB Resin [31] | |||||
| 1 | 2.12 | 75–31–0 | 2-Propanamine, C3H9N | 59 | - | 0.59 |
| 2 | 2.14 | 140308 | Benzeneethanamine, 3-fluoro-beta,5-dihydroxy-N-methyl-, C9H12FNO2 | 185 | 1.43 | - |
| 3 | 2.20 | 7446–09–5 | Sulfur dioxide-, SO2 | 64 | 10.78 | - |
| 4 | 2.23 | 67–56–1 | Methanol, CH4O | 32 | - | 0.27 |
| 5 | 2.77 | 646–05–9 | 1-Penten-3-yne C5H6 | 66 | 0.87 | - |
| 6 | 3.39 | 930–27–8 | Furan, 3-methyl-, C5H6O | 82 | 1.22 | - |
| 7 | 4.61 | 71–43–2 | Benzene, C6H6 | 78 | 6.38 | 0.75 |
| 8 | 7.77 | 108–88–3 | Toluene, C7H8 | 92 | 48.63 | 0.98 |
| 9 | 11.65 | 108–38–3 | Benzene, 1,3-dimethyl-, C8H10 | 106 | 5.91 | 1.45 |
| 10 | 12.68 | 108–38–3 | m-Xylene, C8H10 | 106 | 1.17 | - |
| 11 | 15.23 | 526–73–8 | Benzene, 1,2,3-trimethyl-, C9H12 | 120 | - | 1.98 |
| 12 | 17.62 | 108–95–2 | Phenol, C6H6O | 94 | 3.24 | 2.95 |
| 13 | 20.47 | 108–40–7 | Benzenethiol, 3-methyl-, C7H8S | 124 | 3.24 | - |
| 14 | 20.65 | 106–44–5 | m-Cresol, C7H8O | 108 | 5.56 | 61.74 |
| 15 | 21.48 | 576–26–1 | Phenol, 2,6-dimethyl-, C8H10O | 122 | 1.69 | - |
| 16 | 21.68 | 95–87–4 | Phenol, 2,5-dimethyl-, C8H10O | 122 | - | 4.28 |
| 17 | 22.64 | 105–67–9 | Phenol, 2,4-dimethyl-, C8H10O | 122 | 3.57 | 14.35 |
| 18 | 24.28 | 28715–26–6 | Benzofuran, 4,7-dimethyl-, C10H10O | 146 | 2.90 | |
| 19 | 24.43 | 2416–94–6 | Phenol, 2,3,6-trimethyl-, C9H12O | 136 | - | 9.44 |
| 20 | 26.19 | 627–93–0 ● | Hexanedioic acid, dimethyl ester, C8H14O4 | 174 | - | - |
| 21 | 26.56 | 2219–78–5 | 2-methyl-4,5-dimethylphenol, C10H14O | 150 | - | 1.22 |
| 22 | 27.02 | 1904–26–4 | 3-Buten-2-one, 3-methyl-4-phenyl-, C11H12O | 160 | 1.85 | - |
| 23 | 29.54 | 102–62–5 ● | Glycerol 1,2-diacetate, C7H12O5 | 176 | - | - |
| 24 | 33.83 | 620–47–3 | 1-benzyl-3-methylbenzene, C14H14 | 182 | 2.78 | - |
| Sample Code | Volume of Gases per 1 kg of Molding Sand, dm3 | Emission of Gases, mg/1 kg Molding Sand | Loss on Ignition LOI % | |||||
|---|---|---|---|---|---|---|---|---|
| Benzene | Toluene | Ethylbenzene | Xylenes | Total BTEX | ||||
| Molding Sand FNB (Free Furfuryl Alcohol 50%) | ||||||||
| FNB100FS | 12.804 | 333 | 3 | 0.6 | 0 | 336.6 | 1.27 | |
| FNB50R50FS | 18.261 | 513 | 18 | 0 | 0 | 531 | 2.57 | |
| FNB100R | 24.369 | 957 | 91 | 1 | 8 | 1057 | 3.57 | |
| Molding Sand PFNB | ||||||||
| PFNB100FS | 10.606 | 175 | 2 | 0.6 | 0 | 177.5 | 0.91 | |
| PFNB50R50FS | 13.199 | 407 | 9.5 | 0.8 | 0.7 | 418 | 1.27 | |
| PFNB100R | 17.568 | 553 | 11 | 1.2 | 0.8 | 566 | 1.40 | |
| Compound | Results per 1 kg of Molding Sand, mg | |||||
|---|---|---|---|---|---|---|
| Molding Sand PFNB | Molding Sand FNB | |||||
| PFNB100FS | PFNB50R50FS | PFNB100R | FNB100FS | FNB50R50F | FNB100R | |
| Naphtalene | 1.66 | 2.18 | 2.02 | 3.12 | 5.12 | 10.18 |
| Acenaphtylene | 0.00 | 0.00 | -- | -- | 0.00 | 0.01 |
| Fluorene | 0.02 | 0.07 | 0.03 | 0.08 | 0.10 | 0.90 |
| Phenanthrene | 0.17 | 0.45 | 0.40 | 0.37 | 0.46 | 0.85 |
| Anthracene | 0.09 | 0.10 | 0.26 | 0.14 | 0.17 | 0.32 |
| Fluoranthene | 0.60 | 0.57 | 0.76 | 0.84 | 1.84 | 0.95 |
| Pyrene | 0.60 | 0.59 | 0.68 | 0.59 | 1.48 | 0.71 |
| Benz(a)anthracene | 0.20 | 0.23 | 0.10 | 0.26 | 0.40 | 0.17 |
| Chrysene | 0.54 | 0.73 | 0.08 | 0.14 | 0.38 | 0.09 |
| Benzo(b)fluoranthene | 0.37 | 0.36 | 0.28 | 0.49 | 0.75 | 0.18 |
| Benzo(k)fluoranthene | 0.14 | 0.15 | 0.08 | 0.19 | 0.25 | 0.07 |
| Benzo(a)pyrene | 0.48 | 0.48 | 0.33 | 0.80 | 1.04 | 0.23 |
| Dibenz[a.h]anthracene | -- | -- | 0.00 | 0.08 | 0.02 | 0.02 |
| Benzo[g.h.i]perylene | 0.36 | 0.48 | 0.30 | 0.36 | 0.05 | 0.13 |
| Indeno[1.2.3 cd]pyrene | 0.05 | 0.49 | 0.36 | 0.48 | 0.83 | 0.16 |
| Total PAHs ± 20% | 5.28 ± 1.06 | 6.88 ± 1.38 | 5.68 ± 1.14 | 7.94 ± 1.59 | 12.89 ± 2.58 | 14.97 ± 2.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtzer, M.; Dańko, R.; Kmita, A.; Drożyński, D.; Kubecki, M.; Skrzyński, M.; Roczniak, A. Environmental Impact of the Reclaimed Sand Addition to Molding Sand with Furan and Phenol-Formaldehyde Resin—A Comparison. Materials 2020, 13, 4395. https://doi.org/10.3390/ma13194395
Holtzer M, Dańko R, Kmita A, Drożyński D, Kubecki M, Skrzyński M, Roczniak A. Environmental Impact of the Reclaimed Sand Addition to Molding Sand with Furan and Phenol-Formaldehyde Resin—A Comparison. Materials. 2020; 13(19):4395. https://doi.org/10.3390/ma13194395
Chicago/Turabian StyleHoltzer, Mariusz, Rafał Dańko, Angelika Kmita, Dariusz Drożyński, Michał Kubecki, Mateusz Skrzyński, and Agnieszka Roczniak. 2020. "Environmental Impact of the Reclaimed Sand Addition to Molding Sand with Furan and Phenol-Formaldehyde Resin—A Comparison" Materials 13, no. 19: 4395. https://doi.org/10.3390/ma13194395
APA StyleHoltzer, M., Dańko, R., Kmita, A., Drożyński, D., Kubecki, M., Skrzyński, M., & Roczniak, A. (2020). Environmental Impact of the Reclaimed Sand Addition to Molding Sand with Furan and Phenol-Formaldehyde Resin—A Comparison. Materials, 13(19), 4395. https://doi.org/10.3390/ma13194395







