Collagen-Based Hydrogels Composites from Hide Waste to Produce Smart Fertilizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
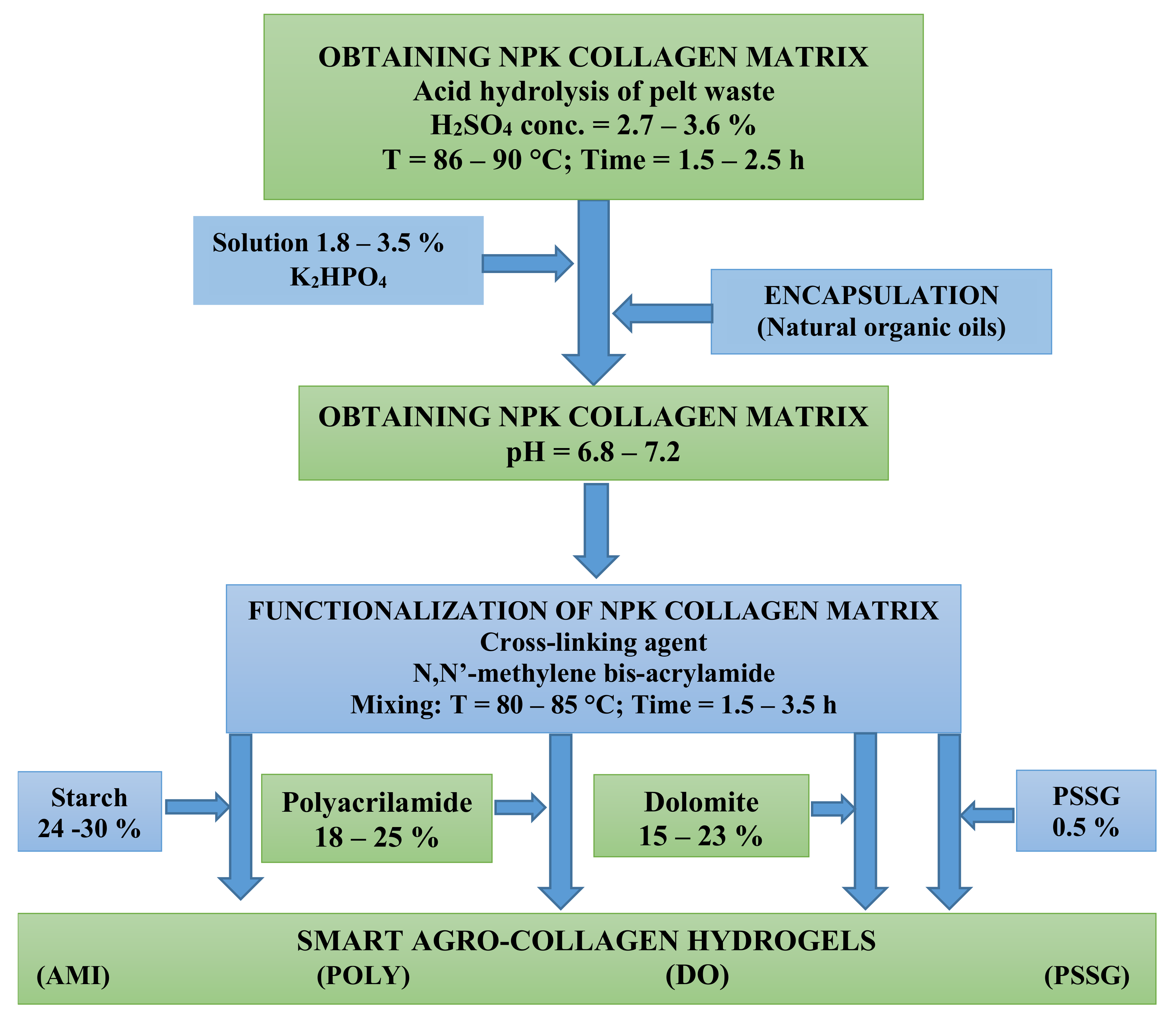
2.2. Preparation of NPK Collagen Hydrolysate and its Functionalization
- obtaining the collagenous matrix by acid hydrolysis of the gelatine hide at 86–90 °C, with 2.7–3.6% H2SO4 for 1.5–3.5 h;
- additivation of collagen hydrolysate with PK nutrients by treating with a K2HPO4 solution of 10–20% concentration;
- preparing in parallel a polymeric solution of starch or polyacrylamide gel by continuous mixing, dezaeration and addition of N,N’-methylene bis-acrilamide as reticulant agent;
- functionalization of NPK collagen hydrolysate by copolymerization with starch, poly-acrilamide, or dolomite suspension;
- obtaining smart collagen-based fertilizers by encapsulation in natural organic oils.
2.3. Characterisation of Compounded Hydrogels
2.4. Determination of Biodegradability
- SR EN ISO 14852/2005 – Determination of the final aerobic biodegradability in aqueous medium of plastic materials, by analyzing the evolved carbon dioxide;
- SR EN ISO 14855-1/2008 - Determination of the final aerobic biodegradability in composting controlled conditions by measuring the quantity of evolved carbon dioxide.
3. Results and Discussion
3.1. Ecological and Mechanistic Approaches on Smart Biofertilizers
- Polymer-analogous transformations, when only the functional groups belonging to amino-acids residues are involved;
- Grafting, characterized by linking at collagen macromolecule of oligomer type structures ( < 10,000 Da);
- Reticulation, devoted to transformation of linear and branching configurations in tridimensional configurations.
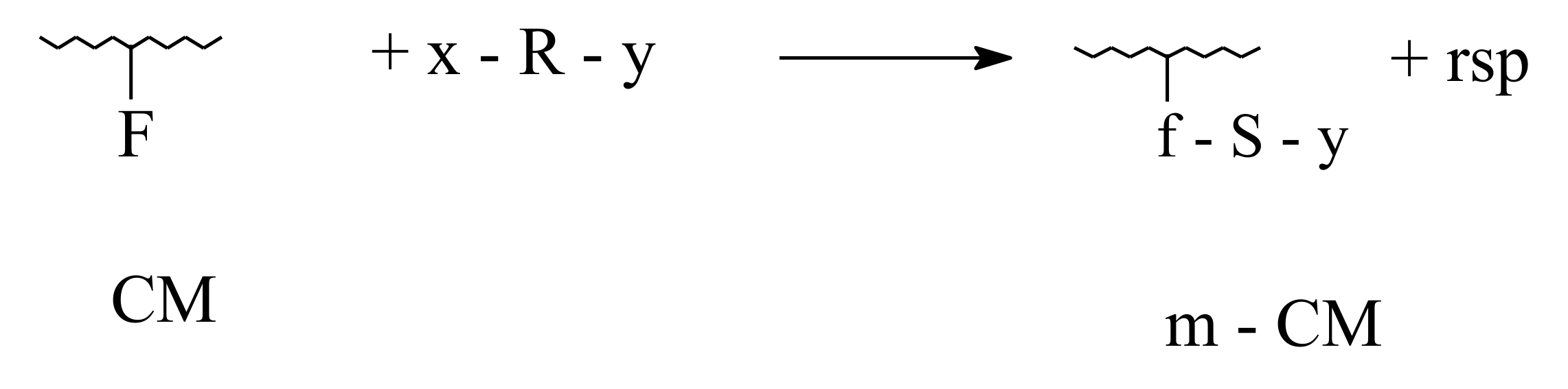
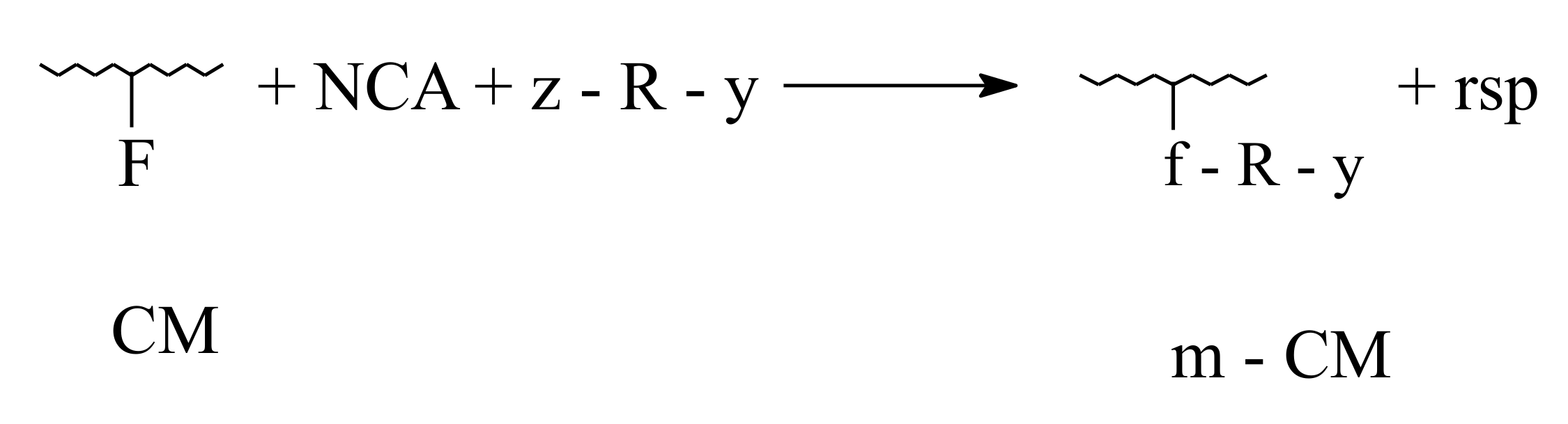
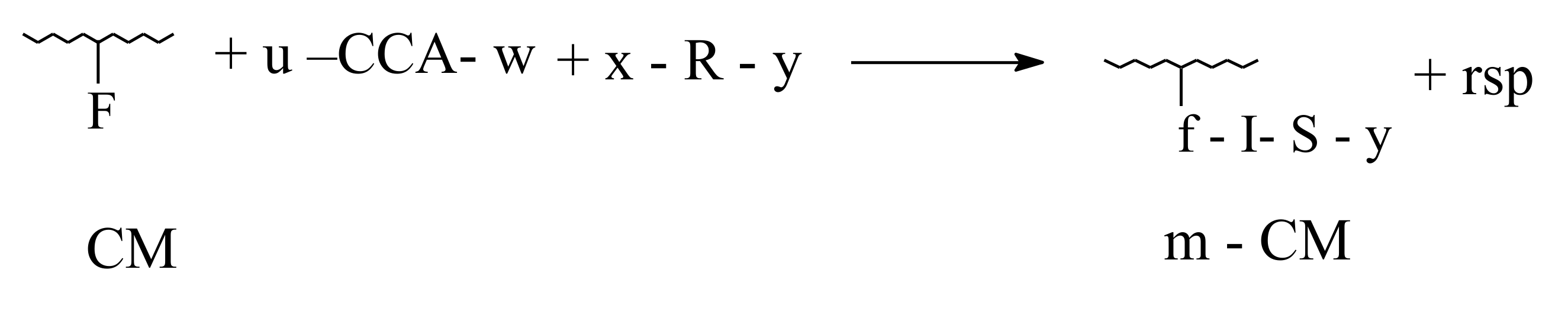
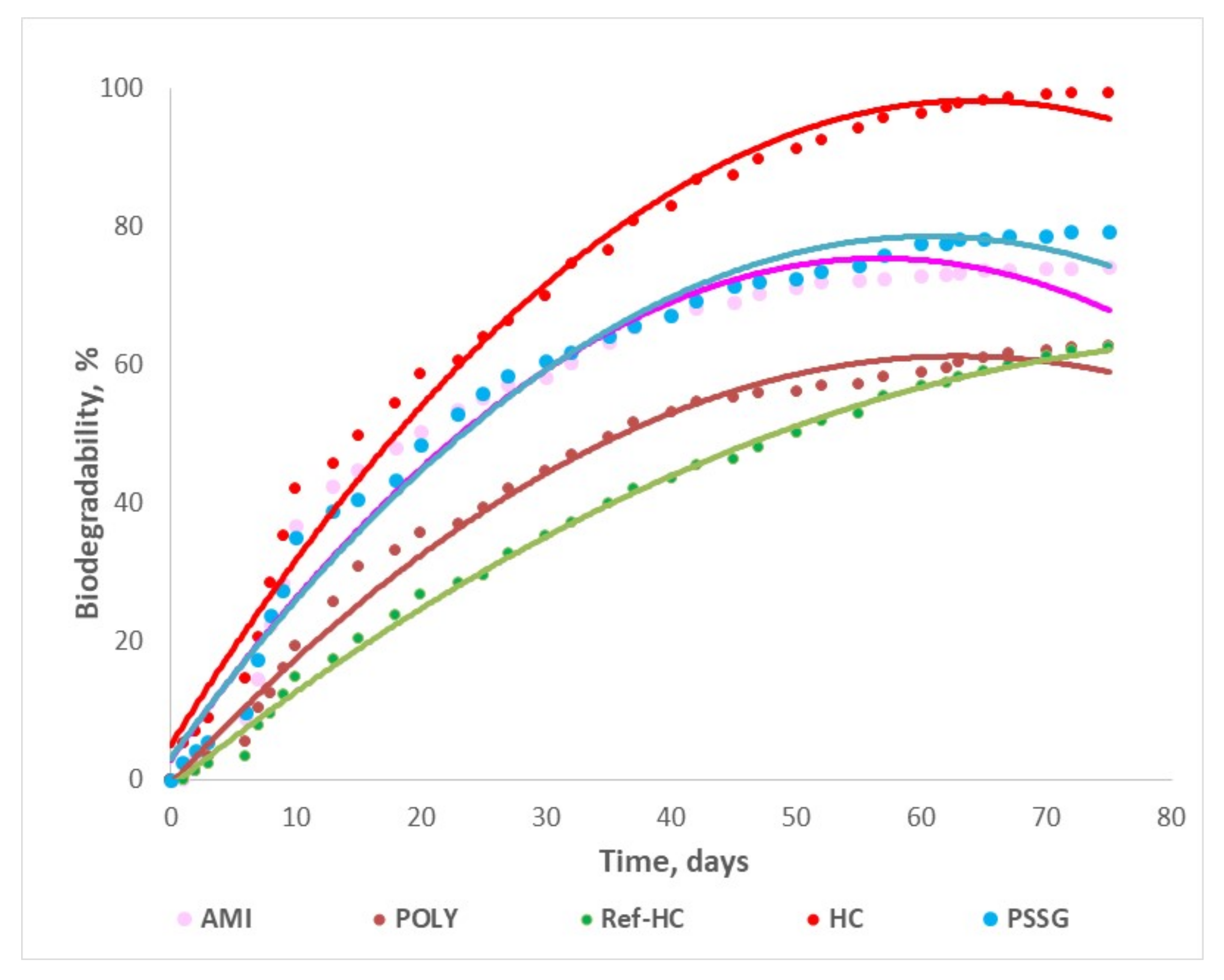
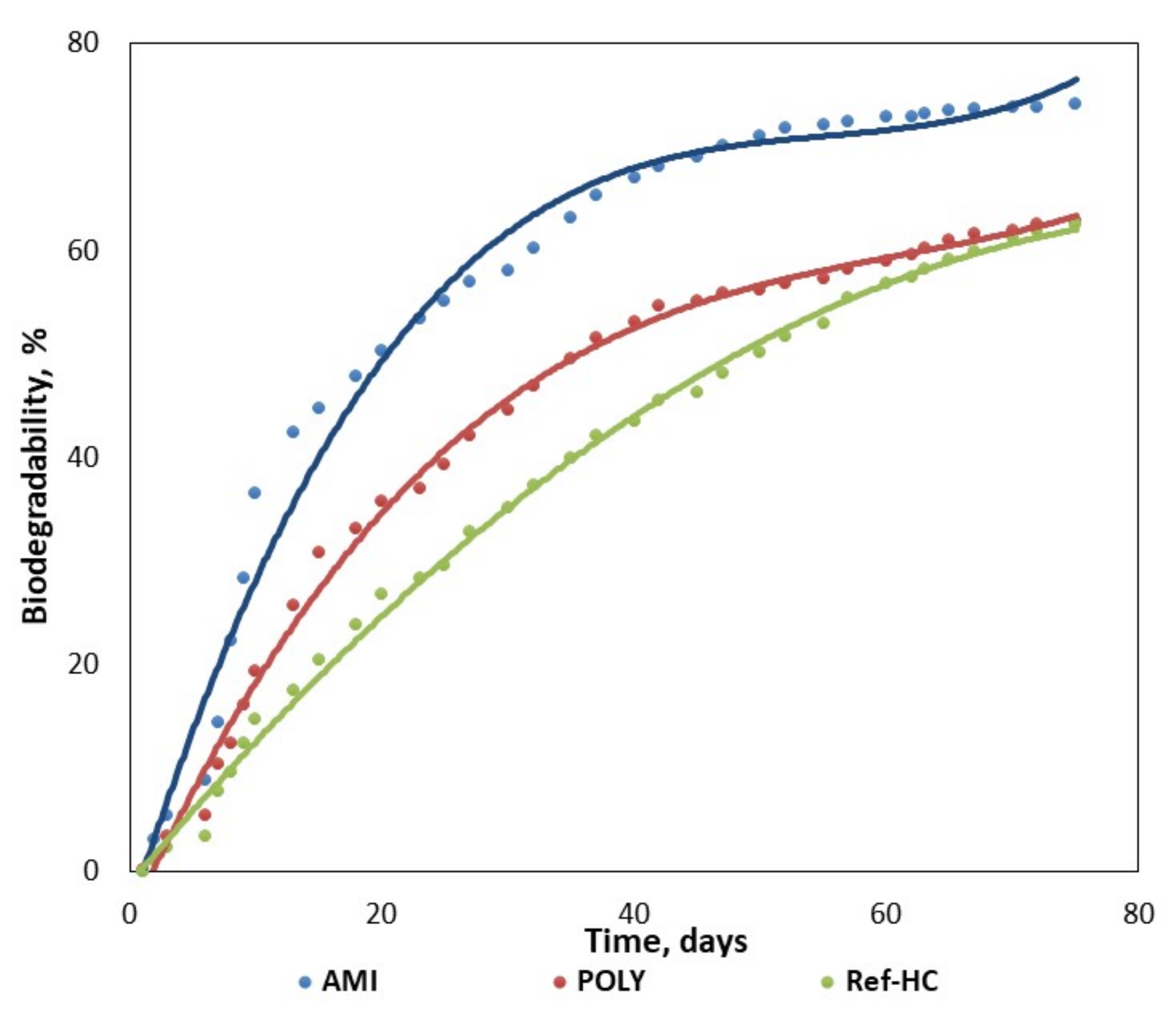
3.2. Biodegradability of Fertilizers in Aqueous or Composting Environments
- The stagnant zone corresponds to the first step, where the biodegradation process is initiated and the biodegradation rate is small (0–6 days);
- The acceleration zone, when biodegradation degree is linearly increasing in time (2–50 days);
- The slowing zone, where even though there is an increasing tendency, the biodegradation rate is smaller (14–56 days);
- The stationary zone, where the biodegradation degree reaches its maximum value and the process rate tends to zero (41–75 days).
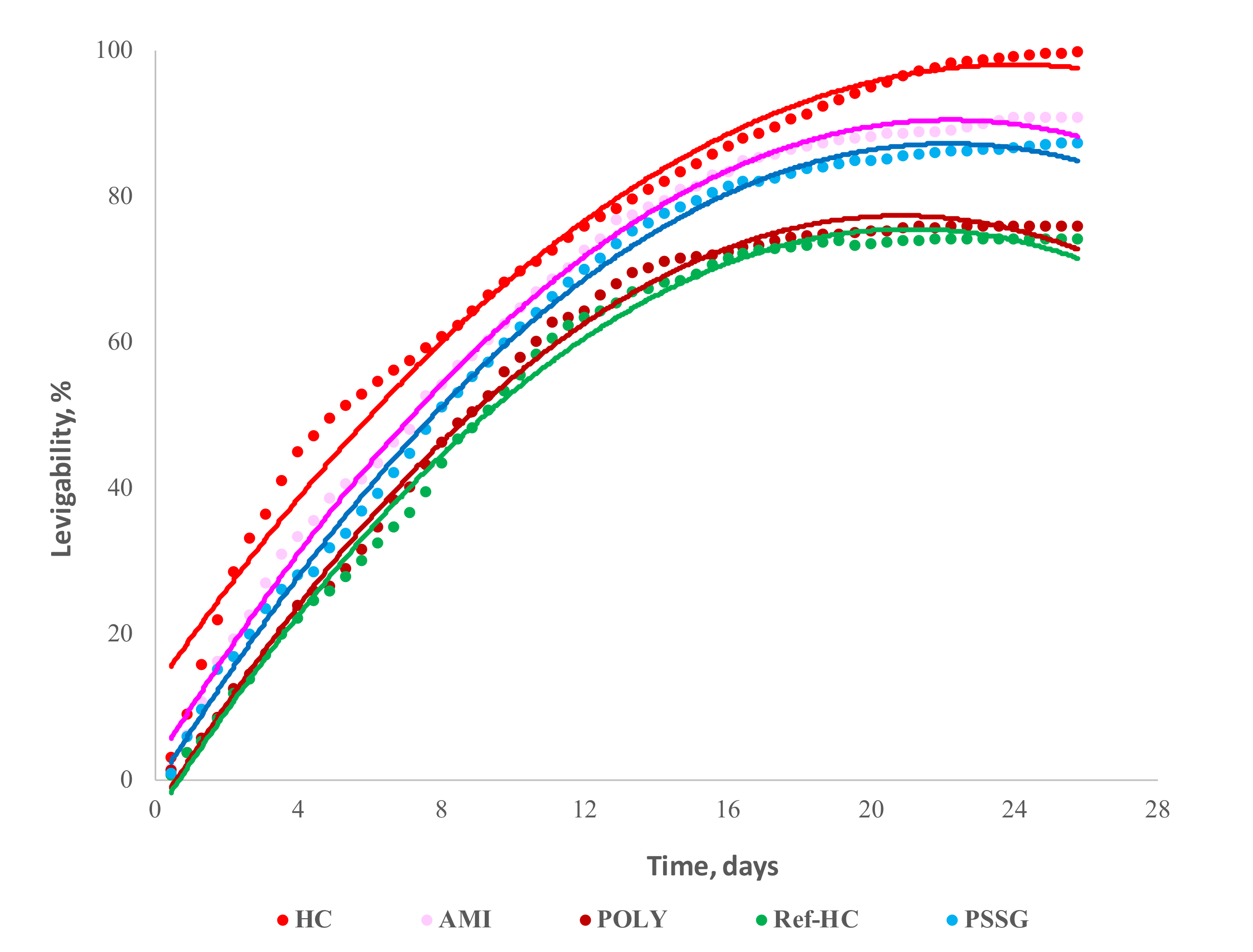
3.3. Leaching the Oxidable Compounds in Laboratory Dynamic Systems
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Coupling agent |
| CM | Collagenic material |
| f-S-y | Novel type of functional group attached to polypeptidic chain |
| F | Functional groups of collagenic material |
| m-CM | Modified collagenic material |
| Rsp | Reaction secondary product |
| R | Support of the reactive functional group |
| S—R | A spacer of the novel functional group |
| X-R | Modification reagents |
| X | Reactive functional group |
| Y | Functional group attached to the reagent |
| Z | Connecting functional group |
References
- Zainescu, G.; Albu, L.; Deselnicu, D.; Constantinescu, R.R.; Vasilescu, A.M.; Nichita, P.; Sirbu, C. A new concept of complex valorization of leather wastes. Mater. Plast. 2014, 51, 90–93. [Google Scholar]
- Bajza, Ž.; Vrček, V. Thermal and enzymatic recovering of proteins from untanned leather waste. Waste Manag. 2001, 21, 79–84. [Google Scholar] [CrossRef]
- Rangel-Serrano, A.; Maldonado, V.M.; Kosters, K. Characterization of waste materials in tanners for better ecological uses. J. Am. Leather Chem. Assoc. 2003, 98, 43–48. [Google Scholar]
- Eurostat. Record recycling rates and use of recycled materials in the EU. Available online: https://ec.europa.eu/eurostat/documents/2995521/9629294/8-04032019-BP-EN.pdf/295c2302-4ed1-45b9-af86-96d1bbb7acb1 (accessed on 30 September 2020).
- Stefan, D.S.; Constantinescu, R.R.; Meghea, A.; Anghel, R.; Stefan, M.; Tudosie, M.S. Obtaining of biofertilisers using pelt skin wastes. Rev. Chim. 2016, 67, 1401–1405. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Spizzirri, U.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in Agriculture: A Review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–95. [Google Scholar] [CrossRef]
- El-Rehim, H.A.A.; Hegazy, E.-S.A.; El-Mohdy, H.L.A. Effect of various environmental conditions on the swelling property of PAAm/PAAcK superabsorbent hydrogel prepared by ionizing radiation. J. Appl. Polym. Sci. 2006, 101, 3955–3962. [Google Scholar] [CrossRef]
- Yazaki, M.; Ito, Y.; Yamada, M.; Goulas, S.; Teramoto, S.; Nakaya, M.-A.; Ohno, S.; Yamaguchi, K. Oral Ingestion of Collagen Hydrolysate Leads to the Transportation of Highly Concentrated Gly-Pro-Hyp and Its Hydrolyzed Form of Pro-Hyp into the Bloodstream and Skin. J. Agric. Food Chem. 2017, 65, 2315–2322. [Google Scholar] [CrossRef]
- Yorgancioglu, A.; Bayramoglu, E.E. Production of cosmetic purpose collagen containing antimicrobial emulsion with certain essential oils. Ind. Crop. Prod. 2013, 44, 378–382. [Google Scholar] [CrossRef]
- Tzoumani, I.; Lainioti, G.C.; Aletras, A.J.; Zainescu, G.; Stefan, S.; Meghea, A.; Kallitsis, J.K. Modification of Collagen Derivatives with Water-Soluble Polymers for the Development of Cross-Linked Hydrogels for Controlled Release. Materials 2019, 12, 4067. [Google Scholar] [CrossRef] [PubMed]
- Deselnicu, D.C.; Militaru, G.; Deselnicu, V. Obtaining of biodegradable plastic materials. Mater. Plast. 2014, 51, 72–74. [Google Scholar]
- Rodrigues, F.T.; Martins, V.D.C.A.; Plepis, A.M.D.G. Porcine skin as a source of biodegradable matrices: alkaline treatment and glutaraldehyde crosslinking. Polímeros 2010, 20, 92–97. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Zainescu, G.; Constantinescu, R.R.; Sirbu, C. Smart Hydrogels with Collagen Structure Made of Pelt Waste. Rev. de Chim. 2017, 68, 393–395. [Google Scholar] [CrossRef]
- Manea-Saghin, A.-M.; Stefan, D.S.; Zainescu, G.; Kallitsis, J.K.; Meghea, A. Release of organic compounds from different types of fertilizers obtained from skin waste. In Proceedings of the 19th International Multidisciplinary Scientific GeoConference (SGEM 2019), Sophia, Bulgaria, 9–11 December 2019; pp. 127–133. [Google Scholar] [CrossRef]
- Zainescu, G.A.; Albu, L.; Constantinescu, R.R. Study of Collagen Hydrogel Biodegradability Over Time. Rev. de Chim. 2018, 69, 101–104. [Google Scholar] [CrossRef]
- Aftab, M.N.; Hameed, A.; Haq, I.; Run-Sheng, C. Biodegradation of Leather Waste by Enzymatic Treatment. Chin. J. Proc. Eng. 2006, 6, 462–465. [Google Scholar]
- Stefan, D.S.; Manea-Saghin, A.-M.; Zainescu, G.; Meghea, A.; Stefan, M. Comparative methods for skin and hide waste capitalization. In Proceedings of the 18th International Multidisciplinary Scientific GeoConference (SGEM 2018), Albena, Bulgaria, 2–8 December 2018; pp. 95–102. [Google Scholar] [CrossRef]
- Lima, D.Q.; Oliveira, L.C.A.; Bastos, A.R.R.; Carvalho, G.S.; Marques, J.G.S.M.; Carvalho, J.G.; De Souza, G.A. Leather Industry Solid Waste as Nitrogen Source for Growth of Common Bean Plants. Appl. Environ. Soil Sci. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Nogueira, F.G.; Prado, N.T.D.; Oliveira, L.C.; Bastos, A.R.; Lopes, J.H.; De Carvalho, J.G. Incorporation of mineral phosphorus and potassium on leather waste (collagen): A new NcollagenPK-fertilizer with slow liberation. J. Hazard. Mater. 2010, 176, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.G.; Castro, I.A.; Bastos, A.R.; Souza, G.A.; De Carvalho, J.G.; Oliveira, L.C. Recycling of solid waste rich in organic nitrogen from leather industry: Mineral nutrition of rice plants. J. Hazard. Mater. 2011, 186, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Pooley, S.A.; Rivas, B.L.; Lillo, F.E.; Pizzaro, G.C. Hydrogels from acrylic acids with N,N-dimethylacrylamide: synthesisi, characterization, and water absorption properties. J. Chil. Chem. Soc. 2010, 55, 19–24. [Google Scholar] [CrossRef]
- Ho, E.; Lowman, A.; Marcolongo, M. Synthesis and Characterization of an Injectable Hydrogel with Tunable Mechanical Properties for Soft Tissue Repair. Biomacromolecules 2006, 7, 3223–3228. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Verheijen, L.A.H.M.; Wiersema, D.; Hulshoff Pol, L.W.; De Wit, J. Management of Waste from Animal Product Processing; International Agriculture Centre: Wageningen, The Netherlands, January 1996. [Google Scholar]
- United Nations Industrial Development Organization. Introduction to treatment of tannery effluents, What every tanner should know about effluent treatment. Available online: https://www.unido.org/resources/publications/creating-shared-prosperity/agribusiness-and-rural-entrepreneurship/introduction-treatment-tannery-effluents (accessed on 30 September 2020).
- Ashmead, H.H. Synergistic plant regulatory compositions. US Patent 4169717, 2 October 1979. [Google Scholar]
- Ashmead, H.H.; Hsu, H.-H. Potassium polyphosphate protein hydrolysate fertilizer. US Patent 4491464, 1 January 1985. [Google Scholar]
- Yanagawa, S.; Kondo, H.; Hara, M. Sulfonic acid group-containing carbonaceous material. U.S. Patent 2010/0048835 A1, 11 November 2009. [Google Scholar]
- Hennink, W.E.; Van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Lee, P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277–288. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]



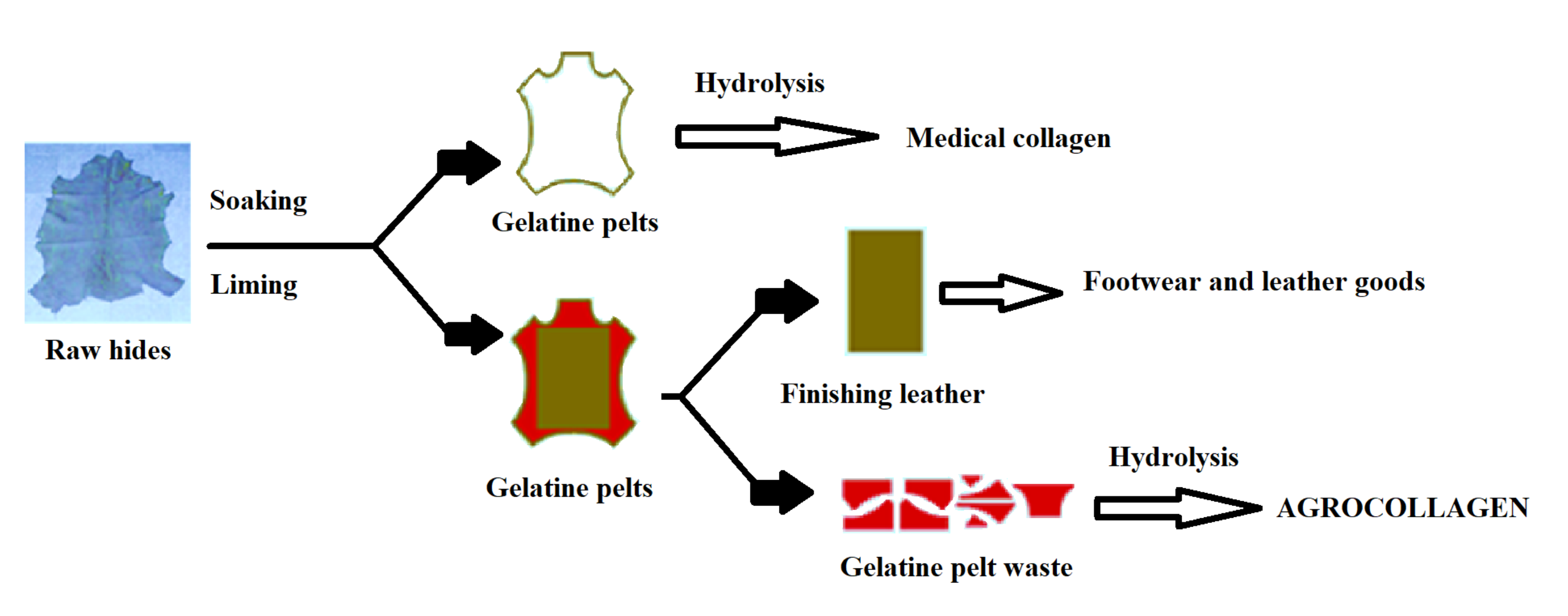
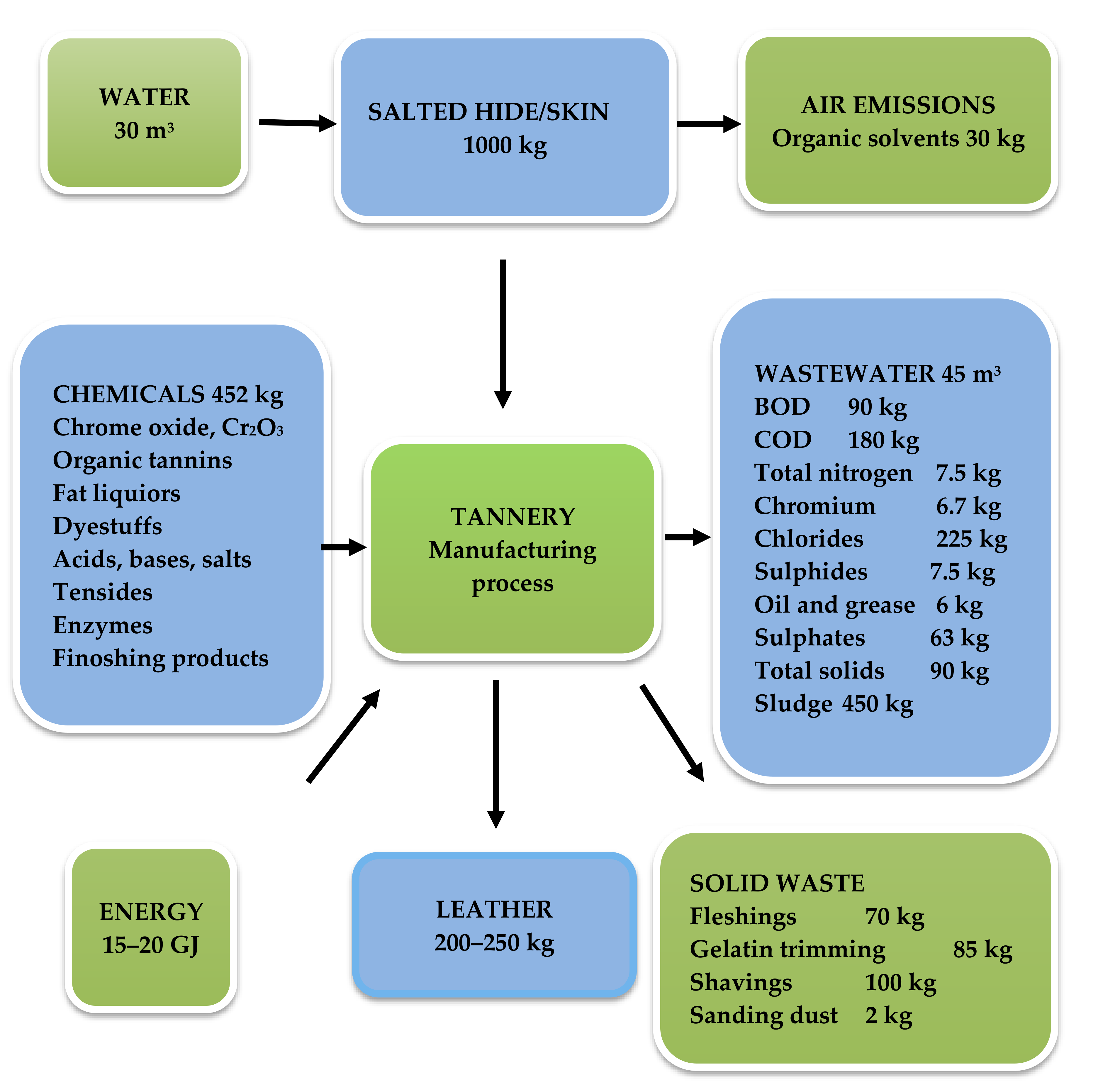
| Component | Hide Fleshing | Trimming Gelatin | Splitting Gelatin | Lime Raw Hide |
|---|---|---|---|---|
| Proteins, % | 10–50 * | 21–84 * | 22–90 * | 23–61 * |
| Water Content, % | 80 | 75 | 75 | 62 |
| Fat Substances, % | 7–35 * | 1–4 * | 0.3–1.2 * | 13.5–36 * |
| Mineral Substances, % | 3–15 * | 3–12 * | 2.2–8.8 * | 1–3.0 * |
| Nr.Crt. | Parameter | Values | Standard Methods |
|---|---|---|---|
| 1 | Volatile mater, % | 68.74 | SR EN ISO 4684-2006 |
| 2 | Fat maters, % | 2.30 | STAS 145/20-1988 |
| 3 | Total, ash, % | 1.18 | SR EN ISO 4047-2002 |
| 4 | Total nitrogen, % | 13.76 | SR ISO 5397-1996 |
| 5 | Dermal substance, % | 77.33 | SR ISO 5397-1996 |
| 6 | Metal oxides, % | 1.01 | ICPI Methods |
| 7 | pH- aqueous extract | 7.34 | SR EN ISO 4045-2008 |
| No | Fertilizer Parameter | UM | HC | Ref-HC | PSSG | POLY | AMI | Method of Analysis |
|---|---|---|---|---|---|---|---|---|
| 1 | Dry Substance | % | 24.18 | 61.22 | 65.8 | 21.59 | 26.38 | SR EN ISO 4684: 2006 |
| 2 | Ash | % | 2.48 | 22.36 | 25.18 | 17.23 | 16.38 | SR EN ISO 4047: 2002 |
| 3 | Total Nitrogen | % | 10.36 | 10.55 | 10.14 | 12.13 | 8.29 | SR ISO 5397: 1996 |
| 4 | Soluble Phosphorus, P2O5 | % | - | 7.67 | 6.75 | 5.79 | 5.54 | SR EN 15959: 2012 |
| 5 | Soluble Potassium, K2O | % | - | 10.62 | 8.21 | 8.40 | 10.07 | SR ISO 5397: 1996 |
| 6 | Total Organic Carbon, TOC | % | 46.2 | 45.2 | 37.56 | 48.1 | 64.32 | SR EN 13137/2005 |
| 7 | pH | units | 6.70 | 7.20 | 6.87 | 6.76 | 6.20 | STAS 8619/3-1990 |
| Fertiliser Type | Biodegradability, %, in Time Polynomial Regresion Ecuation | R² |
|---|---|---|
| HC-W | y = −0.0226x2 + 2.903x + 5.0378 | 0.9872 |
| Ref-HC-W | y = −0.0081x2 + 1.4468x − 0.9437 | 0.9972 |
| Ref-HC-C | y = −0.0082x2 + 1.457x − 1.1262 | 0.997 |
| AMI-W | y = −0.0225x2 + 2.5537x + 2.972 | 0.9672 |
| AMI-C | y = 0.0004x3 − 0.0683x2 + 3.8722x − 4.0301 | 0.9844 |
| POLY-W | y = -0.0156x2 + 1.9655x − 0.5645 | 0.9891 |
| POLY-C | y = 0.0002x3 − 0.0399x2 + 2.6786x − 4.6409 | 0.9956 |
| PSSG-W | y = −0.0205x2 + 2.4853x + 3.1857 | 0.9811 |
| No | Biodegradation Degree, % Days | HC | Ref-HC | AMI | POLY | PSSG | |||
|---|---|---|---|---|---|---|---|---|---|
| W | W | C | W | C | W | C | W | ||
| 1 | 10 | 42 | 35 | 17 | 37 | 33 | 15 | 12 | 20 |
| 2 | 20 | 58 | 48 | 33 | 50 | 48 | 27 | 21 | 35 |
| 3 | 75 | 99 | 74 | 50 | 80 | 64 | 62 | 40 | 63 |
| Fertiliser Type | k | n | R2 |
|---|---|---|---|
| HC | 0.137 | 0.6693 | 0.9159 |
| Ref-HC | 0.048 | 0.9514 | 0.924 |
| POLY | 0.056 | 0.9059 | 0.9212 |
| PSSG | 0.071 | 0.8644 | 0.9097 |
| AMI | 0.088 | 0.7995 | 0.9212 |
| Fertiliser Type | Time Interval, Days | k | n | R2 |
|---|---|---|---|---|
| HC | 0–2.67 | 0.097 | 1.2466 | 0.9838 |
| 2.67–25.78 | 0.242 | 0.4527 | 0.9929 | |
| Ref-HC | 0–9.78 | 0.034 | 1.2524 | 0.9725 |
| 9.78–25.78 | 0.338 | 0.4551 | 0.8232 | |
| POLY | 0–10.22 | 0.041 | 1.1732 | 0.9906 |
| 10.22–25.78 | 0.383 | 0.4309 | 0.8333 | |
| PSSG | 0–5.11 | 0.048 | 1.3215 | 0.9325 |
| 5.11–25.78 | 0.132 | 0.6285 | 0.9373 | |
| AMI | 0–3.56 | 0.06 | 1.3017 | 0.9707 |
| 3.56–25.78 | 0.171 | 0.5497 | 0.9511 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefan, D.S.; Zainescu, G.; Manea-Saghin, A.-M.; Triantaphyllidou, I.-E.; Tzoumani, I.; Tatoulis, T.I.; Syriopoulos, G.T.; Meghea, A. Collagen-Based Hydrogels Composites from Hide Waste to Produce Smart Fertilizers. Materials 2020, 13, 4396. https://doi.org/10.3390/ma13194396
Stefan DS, Zainescu G, Manea-Saghin A-M, Triantaphyllidou I-E, Tzoumani I, Tatoulis TI, Syriopoulos GT, Meghea A. Collagen-Based Hydrogels Composites from Hide Waste to Produce Smart Fertilizers. Materials. 2020; 13(19):4396. https://doi.org/10.3390/ma13194396
Chicago/Turabian StyleStefan, Daniela Simina, Gabriel Zainescu, Ana-Maria Manea-Saghin, Irene-Eva Triantaphyllidou, Ioanna Tzoumani, Triantafyllos I. Tatoulis, George T. Syriopoulos, and Aurelia Meghea. 2020. "Collagen-Based Hydrogels Composites from Hide Waste to Produce Smart Fertilizers" Materials 13, no. 19: 4396. https://doi.org/10.3390/ma13194396
APA StyleStefan, D. S., Zainescu, G., Manea-Saghin, A.-M., Triantaphyllidou, I.-E., Tzoumani, I., Tatoulis, T. I., Syriopoulos, G. T., & Meghea, A. (2020). Collagen-Based Hydrogels Composites from Hide Waste to Produce Smart Fertilizers. Materials, 13(19), 4396. https://doi.org/10.3390/ma13194396







