Corrosion Resistance of Inconel 625 CMT-Cladded Layers after Long-Term Exposure to Biomass and Waste Ashes in High-Temperature Conversion Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. X-ray Fluorescence (XRF) and Chemical Analysis
2.2.2. Long-Term Exposure Tests
2.2.3. Analyses of the Samples after the Exposure Tests
3. Results and Discussion
3.1. Analyses of the Ashes Prior to the Exposure Tests
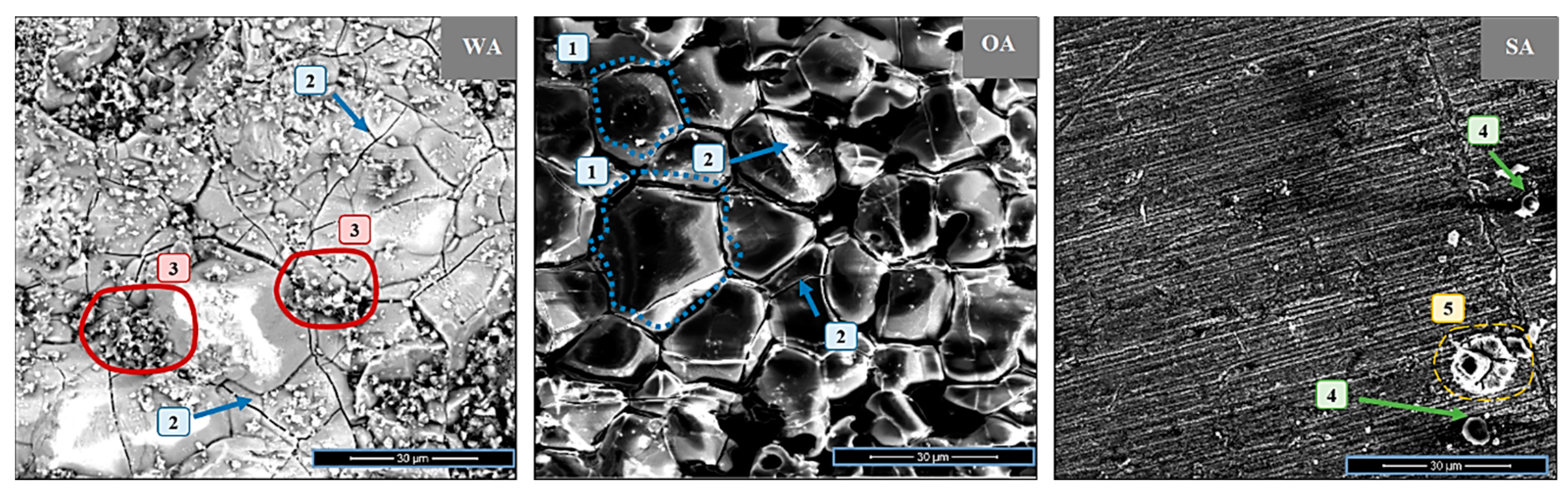
3.2. SEM Analysis of Inconel Surface after Exposure Tests
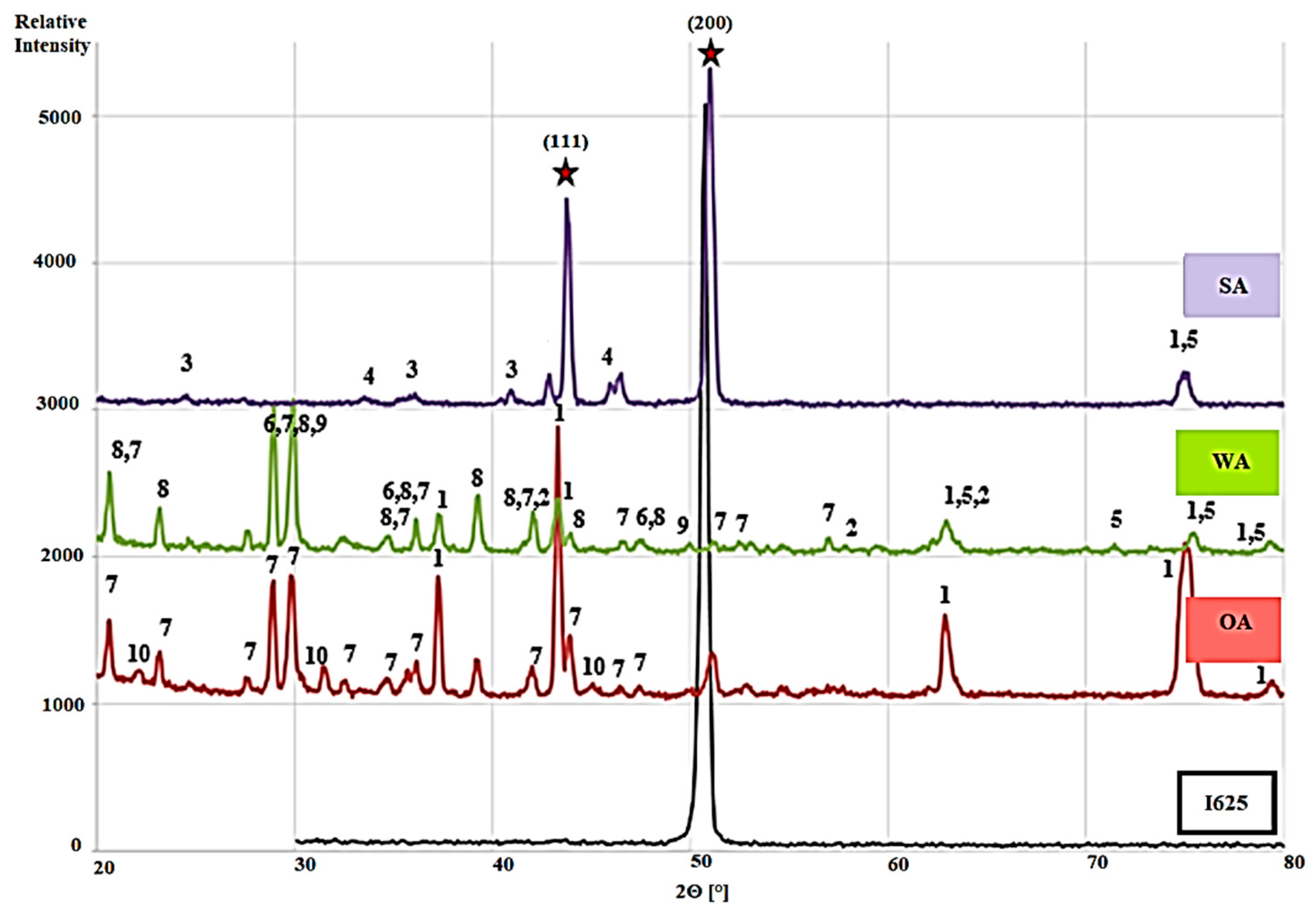
3.3. Mineral Phase Analysis after Exposure Tests
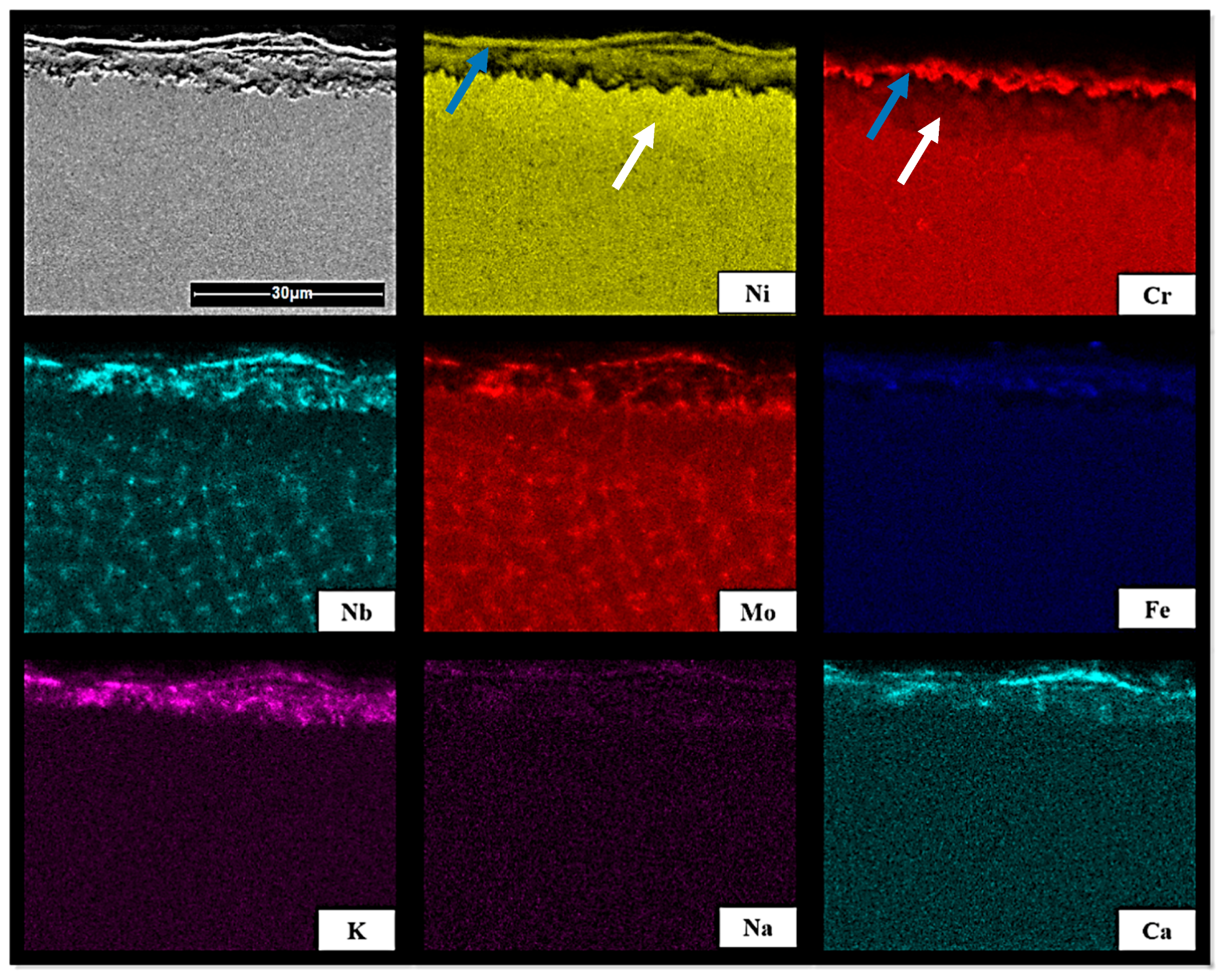
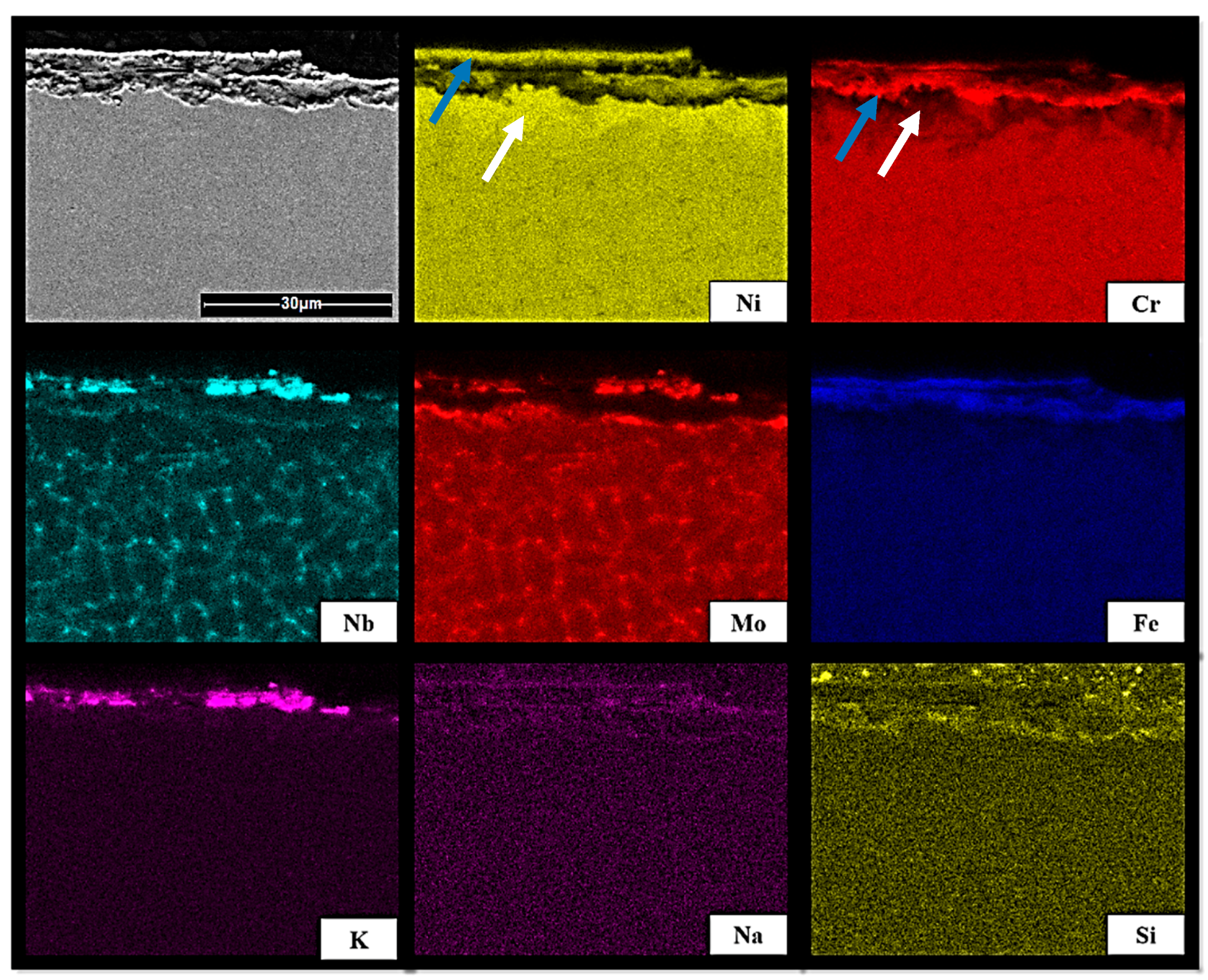
3.4. SEM Analysis of Inconel Sample Cross-Section after Exposure Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martins, F.; Felgueiras, C.; Smitková, M. Fossil Fuel Energy Consumption in European Countries. Energy Procedia 2018, 153, 107–111. [Google Scholar] [CrossRef]
- Kibria, A.; Akhundjanov, S.B.; Oladi, R. Fossil Fuel Share in the Energy Mix and Economic Growth. Int. Rev. Econ. Financ. 2019, 59, 253–264. [Google Scholar] [CrossRef]
- EU Directive. Available online: https://eur-lex.europa.eu/legal-content/pl/LSU/?uri=CELEX:32018L2001 (accessed on 4 September 2020).
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An Overview of the Organic and Inorganic Phase Composition of Biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. A Review of the Use of Composted Municipal Solid Waste in Agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Basu. Biomass Gasification, Pyrolysis and Torrefacation Practical Design and Theory, Chapter 3 Biomass Characteristics; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and Disadvantages of Composition and Properties of Biomass in Comparison with Coal: An Overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Bashir, M.S.; Jensen, P.A.; Frandsen, F.; Wedel, S.; Dam-Johansen, K.; Wadenbäck, J.; Pedersen, S.T. Ash Transformation and Deposit Build-up during Biomass Suspension and Grate Firing: Full-Scale Experimental Studies. Fuel Process. Technol. 2012, 97, 93–106. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Durham, R.N.; Galetz, M.C. Corrosion Behavior of Stainless and Low-Chromium Steels and IN625 in Molten Nitrate Salts at 600 °C. Sol. Energy Mater. Sol. Cells 2016, 144, 109–116. [Google Scholar] [CrossRef]
- Olson, L.; Sridharan, K.; Anderson, M.; Allen, T. Intergranular Corrosion of High Temperature Alloys in Molten Fluoride Salts. Mater. High Temp. 2010, 27, 145–149. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Kalemba-Rec, I.; Nowak, W. The Influence of Potassium-Rich Biomass Ashes on Steel Corrosion above 550 °C. Energy Convers. Manag. 2019, 187, 15–28. [Google Scholar] [CrossRef]
- Solecka, M.; Radziszewska, A.; Rutkowski, B. New Insight on Study of Ni-Base Alloy Clad Layer after Oxidation at 650 °C. Corros. Sci. 2019, 149, 244–248. [Google Scholar] [CrossRef]
- Liu, B.; Wei, X.; Wang, W.; Lu, J.; Ding, J. Corrosion Behavior of Ni-Based Alloys in Molten NaCl-CaCl2-MgCl2 Eutectic Salt for Concentrating Solar Power. Sol. Energy Mater. Sol. Cells 2017, 170, 77–86. [Google Scholar] [CrossRef]
- Kawahara, Y. Application of High Temperature Corrosion-Resistant Materials and Coatings under Severe Corrosive Environment in Waste-to-Energy Boilers. J. Therm. Spray Technol. 2007, 16, 202–213. [Google Scholar] [CrossRef]
- Naghiyan Fesharaki, M.; Shoja-Razavi, R.; Mansouri, H.A.; Jamali, H. Evaluation of the Hot Corrosion Behavior of Inconel 625 Coatings on the Inconel 738 Substrate by Laser and TIG Cladding Techniques. Opt. Laser Technol. 2019, 111, 744–753. [Google Scholar] [CrossRef]
- Mohammadi Zahrani, E.; Alfantazi, A.M. High Temperature Corrosion and Electrochemical Behavior of INCONEL 625 Weld Overlay in PbSO4-Pb3O4-PbCl2-CdO-ZnO Molten Salt Medium. Corros. Sci. 2014, 85, 60–76. [Google Scholar] [CrossRef]
- Xu, X.; Mi, G.; Chen, L.; Xiong, L.; Jiang, P.; Shao, X.; Wang, C. Research on Microstructures and Properties of Inconel 625 Coatings Obtained by Laser Cladding with Wire. J. Alloys Compd. 2017, 715, 362–373. [Google Scholar] [CrossRef]
- Pickin, C.G.; Williams, S.W.; Lunt, M. Characterisation of the Cold Metal Transfer (CMT) Process and Its Application for Low Dilution Cladding. J. Mater. Process. Technol. 2011, 211, 496–502. [Google Scholar] [CrossRef]
- Rozmus-Górnikowska, M.; Cieniek, Ł.; Blicharski, M.; Kusiński, J. Microstructure and Microsegregation of an Inconel 625 Weld Overlay Produced on Steel Pipes by the Cold Metal Transfer Technique. Arch. Metall. Mater. 2014, 59, 1081–1084. [Google Scholar] [CrossRef]
- Rozmus-Górnikowska, M. Mikrostruktura i Mikrosegregacja Warstwy Ze Stopu Inconel 625 Napawanej Łukowo Na Rury Kotłowe. Inżynieria Mater. 2015, 1, 100–103. [Google Scholar] [CrossRef]
- Dhinakaran, V.; Ajith, J.; Fathima Yasin Fahmidha, A.; Jagadeesha, T.; Sathish, T.; Stalin, B. Wire Arc Additive Manufacturing (WAAM) Process of Nickel Based Superalloys—A Review. Mater. Today Proc. 2019, 21, 920–925. [Google Scholar] [CrossRef]
- Varghese, P.; Vetrivendan, E.; Dash, M.K.; Ningshen, S.; Kamaraj, M.; Kamachi Mudali, U. Weld Overlay Coating of Inconel 617 M on Type 316 L Stainless Steel by Cold Metal Transfer Process. Surf. Coat. Technol. 2019, 357, 1004–1013. [Google Scholar] [CrossRef]
- Yangfan, W.; Xizhang, C.; Chuanchu, S. Microstructure and Mechanical Properties of Inconel 625 Fabricated by Wire-Arc Additive Manufacturing. Surf. Coat. Technol. 2019, 374, 116–123. [Google Scholar] [CrossRef]
- de Sousa Malafaia, A.M.; de Oliveira, R.B.; Latu-Romain, L.; Wouters, Y.; Baldan, R. Isothermal Oxidation of Inconel 625 Superalloy at 800 and 1000 °C: Microstructure and Oxide Layer Characterization. Mater. Charact. 2020, 161, 110160. [Google Scholar] [CrossRef]
- Solecka, M.; Kopia, A.; Radziszewska, A.; Rutkowski, B. Microstructure, Microsegregation and Nanohardness of CMT Clad Layers of Ni-Base Alloy on 16Mo3 Steel. J. Alloys Compd. 2018, 751, 86–95. [Google Scholar] [CrossRef]
- Selvi, S.; Vishvaksenan, A.; Rajasekar, E. Cold Metal Transfer (CMT) Technology—An Overview. Defence Technol. 2018, 14, 28–44. [Google Scholar] [CrossRef]
- Kusiński, J. Struktura i Właściwości Powłok Ze Stopów Inconel 625 i 686 Napawanych Metodą CMT Na Rury i Ściany Szczelne Kotłów Energetycznych. Inżynieria Mater. 2015, 1, 13–17. [Google Scholar] [CrossRef]
- ASTM G1-03. Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- DIN EN ISO 18122:2016-03. Standard: Solid Biofuels—Determination of Ash Content; German Institute for Standarisation (Deutsches Institut für Normung): Berlin, Germany, 2016. [Google Scholar]
- DIN 51733:2016-04. Standard: Testing of Solid Mineral Fuels—Ultimate Analysis and Calculation of Oxygen Content; German Institute for Standarisation (Deutsches Institut für Normung): Berlin, Germany, 2016. [Google Scholar]
- Reinmöller, M.; Schreiner, M.; Guhl, S.; Neuroth, M.; Meyer, B. Ash Behavior of Various Fuels: The Role of the Intrinsic Distribution of Ash Species. Fuel 2019, 253, 930–940. [Google Scholar] [CrossRef]
- Smol, M.; Kulczycka, J.; Kowalski, Z. Sewage Sludge Ash (SSA) from Large and Small Incineration Plants as a Potential Source of Phosphorus—Polish Case Study. J. Environ. Manag. 2016, 184, 617–628. [Google Scholar] [CrossRef]
- Benassi, L.; Zanoletti, A.; Depero, L.E.; Bontempi, E. Sewage Sludge Ash Recovery as Valuable Raw Material for Chemical Stabilization of Leachable Heavy Metals. J. Environ. Manag. 2019, 245, 464–470. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, C.; Yan, F.; Shao, N.; Tang, Y.; Zhang, Z. Product Characteristics and Kinetics of Sewage Sludge Pyrolysis Driven by Alkaline Earth Metals. Energy 2018, 153, 921–932. [Google Scholar] [CrossRef]

| Element | C | Si | Mn | Cr | Ni | Mo | Nb | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Mass wt % | 0.1 | 0.4 | 0.4 | 21.0–23.0 | By balance | 8.0–10.0 | 3.2–3.8 | 0.4 | <1.0 |
| Composition/wt% | Mixed Wood Ash (WA) | Oat Straw Ash (OA) | Sewage Sludge Ash (SA) |
|---|---|---|---|
| CO2 | 19.80 | 7.00 | 0.00 |
| Na2O | 0.00 | 0.90 | 0.53 |
| MgO | 8.29 | 1.98 | 5.86 |
| Al2O3 | 1.89 | 0.86 | 7.55 |
| SiO2 | 8.57 | 33.27 | 21.00 |
| P2O5 | 7.24 | 9.67 | 29.09 |
| SO3 | 1.29 | 2.04 | 1.85 |
| Cl | 0.05 | 0.57 | 0.00 |
| K2O | 15.18 | 36.80 | 2.26 |
| CaO | 34.84 | 6.47 | 20.55 |
| TiO2 | 0.05 | 0.05 | 0.97 |
| Mn | 1.91 | 0.10 | 0.08 |
| Fe2O3 | 0.90 | 0.28 | 10.27 |
| Sum | 100.0 | 100.0 | 100.0 |
| B/A (mass basis) | 3.28 | 1.05 | 0.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błoniarz, A.; Schreiner, M.; Reinmöller, M.; Kopia, A. Corrosion Resistance of Inconel 625 CMT-Cladded Layers after Long-Term Exposure to Biomass and Waste Ashes in High-Temperature Conversion Processes. Materials 2020, 13, 4374. https://doi.org/10.3390/ma13194374
Błoniarz A, Schreiner M, Reinmöller M, Kopia A. Corrosion Resistance of Inconel 625 CMT-Cladded Layers after Long-Term Exposure to Biomass and Waste Ashes in High-Temperature Conversion Processes. Materials. 2020; 13(19):4374. https://doi.org/10.3390/ma13194374
Chicago/Turabian StyleBłoniarz, Aleksandra, Marcus Schreiner, Markus Reinmöller, and Agnieszka Kopia. 2020. "Corrosion Resistance of Inconel 625 CMT-Cladded Layers after Long-Term Exposure to Biomass and Waste Ashes in High-Temperature Conversion Processes" Materials 13, no. 19: 4374. https://doi.org/10.3390/ma13194374
APA StyleBłoniarz, A., Schreiner, M., Reinmöller, M., & Kopia, A. (2020). Corrosion Resistance of Inconel 625 CMT-Cladded Layers after Long-Term Exposure to Biomass and Waste Ashes in High-Temperature Conversion Processes. Materials, 13(19), 4374. https://doi.org/10.3390/ma13194374







