Primary Stability of Revision Acetabular Reconstructions Using an Innovative Bone Graft Substitute: A Comparative Biomechanical Study on Cadaveric Pelvises
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Preparation of the Specimens
2.3. Biomechanical Testing
2.4. Measurement of Implant Motion
2.5. Statistical Analysis
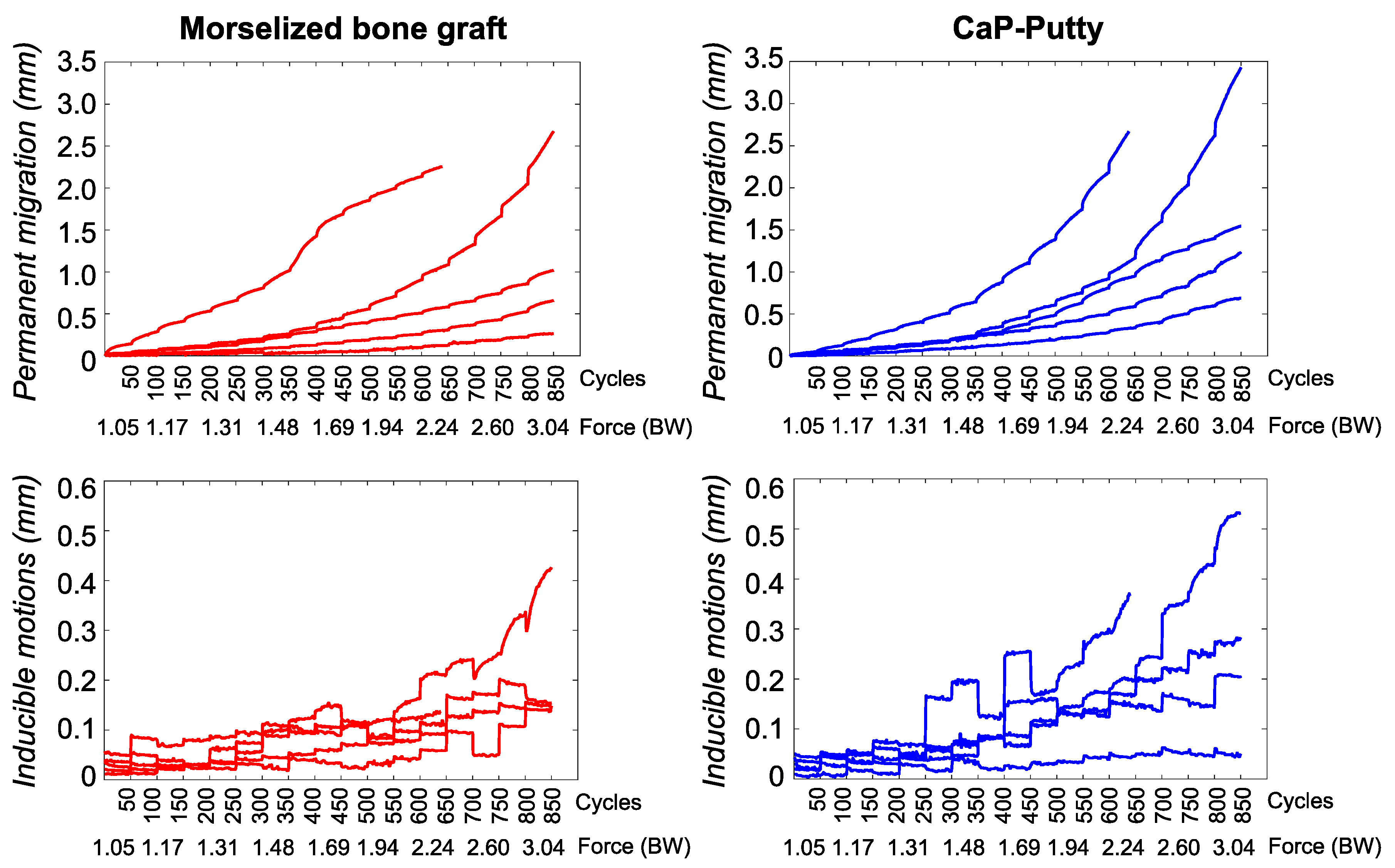
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohaddes, M.; Cnudde, P.H.J.; Rolfson, O.; Wall, A.; Kärrholm, J. Use of dual-mobility cup in revision hip arthroplasty reduces the risk for further dislocation: Analysis of seven hundred and ninety one first-time revisions performed due to dislocation, reported to the Swedish Hip Arthroplasty Register. Int. Orthop. 2017, 41, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Powers-Freeling, L. NJR 15th Annual Report; 2018. Available online: https://www.hqip.org.uk/resource/national-joint-registry-15th-annual-report-2018/#.X2nxphAzbIU (accessed on 22 September 2020).
- Clarnette, R.; Graves, S.; Lekkas, C. Overview of the AOA National Joint Replacement Registry. Orthop. J. Sports Med. 2016, 4. [Google Scholar] [CrossRef]
- Bergen, H. Norwegian Arthroplasty Register; 2019. Available online: http://nrlweb.ihelse.net/ (accessed on 22 September 2020).
- Bordini, B.; Stea, S.; Ancarani, C.; Toni, A. Regional Register of Orthopaedic Prosthetic Implantology. Overall Data: Hip Knee and Shoulder Arthroplasty in Emilia Romagna Region. Available online: https://ripo.cineca.it/authzssl/Reports.html (accessed on 22 September 2020).
- Johanson, N.A.; Driftmier, K.R.; Cerynik, U.L.; Stehman, C.C. Grading Acetabular Defects. J. Arthroplast. 2010, 25, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Pandit, H.; Grover, M.; Clarke, H. Impaction bone grafting in revision hip surgery. J. Arthroplast. 2003, 18, 852–859. [Google Scholar] [CrossRef]
- Gie, G.; Linder, L.; Ling, R.; Simon, J.; Slooff, T.; Timperley, A. Impacted cancellous allografts and cement for revision total hip arthroplasty. J. Bone Jt. Surgery. Br. Vol. 1993, 75, 14–21. [Google Scholar] [CrossRef]
- Slooff, T.J.J.H.; Huiskes, R.; Van Horn, J.; Lemmens, A.J. Bone grafting in total hip replacement for acetabular protrusion. Acta Orthop. Scand. 1984, 55, 593–596. [Google Scholar] [CrossRef]
- Abdullah, K.M.; Hussain, N.; Parsons, S.J.; Porteous, M.J.; Atrey, A. 11-Year Mean Follow-Up of Acetabular Impaction Grafting With a Mixture of Bone Graft and Hydroxyapatite Porous Synthetic Bone Substitute. J. Arthroplast. 2018, 33, 1481–1486. [Google Scholar] [CrossRef]
- Yoshimine, Y.; Akamine, A.; Mukai, M.; Maeda, K.; Matsukura, M.; Kimura, Y.; Makishima, T. Biocompatibility of tetracalcium phosphate cement when used as a bone substitute. Biomaterials 1993, 14, 403–406. [Google Scholar] [CrossRef]
- Arts, J.J.C.; Verdonschot, N.; Buma, P.; Schreurs, B.W. Larger bone graft size and washing of bone grafts prior to impaction enhances the initial stability of cemented cups: Experiments using a synthetic acetabular model. Acta Orthop. 2006, 77, 227–233. [Google Scholar] [CrossRef]
- Bolder, S.B.T.; Verdonschot, N.; Schreurs, B.W.; Buma, P. The initial stability of cemented acetabular cups can be augmented by mixing morsellized bone grafts with tricalciumphosphate/hydroxyapatite particles in bone impaction grafting. J. Arthroplast. 2003, 18, 1056–1063. [Google Scholar] [CrossRef]
- Goriainov, V.; Jones, A.; Briscoe, A.; New, A.; Dunlop, D. Do the Cup Surface Properties Influence the Initial Stability? J. Arthroplast. 2014, 29, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Plomiński, J.; Watral, Z.; Kwiatkowski, K. Testing the stability of the polyethylene acetabulum cemented on a frozen bone graft substrate on a model of an artificial hip joint. Acta Bioeng. Biomech. 2008, 10, 3–6. [Google Scholar] [PubMed]
- Walschot, L.H.B.; Aquarius, R.; Schreurs, B.W.; Buma, P.; Verdonschot, N. Better primary stability with porous titanium particles than with bone particles in cemented impaction grafting: Anin vitrostudy in synthetic acetabula. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Hettich, G.; Schierjott, R.A.; Epple, M.; Gbureck, U.; Heinemann, S.; Mozaffari-Jovein, H.; Grupp, T.M.; Jovein, M. Calcium Phosphate Bone Graft Substitutes with High Mechanical Load Capacity and High Degree of Interconnecting Porosity. Materials 2019, 12, 3471. [Google Scholar] [CrossRef]
- Schierjott, R.A.; Hettich, G.; Baxmann, M.; Morosato, F.; Cristofolini, L.; Grupp, T.M. Primary stability of a press-fit cup in combination with impaction grafting in an acetabular defect model. J. Orthop. Res. 2020. [Google Scholar] [CrossRef]
- Jacofsky, D.J.; McCamley, J.D.; Jaczynski, A.M.; Shrader, M.W.; Jacofsky, M.C. Improving Initial Acetabular Component Stability in Revision Total Hip Arthroplasty. J. Arthroplast. 2012, 27, 305–309. [Google Scholar] [CrossRef]
- Morosato, F.; Traina, F.; Cristofolini, L. Standardization of hemipelvis alignment for in vitro biomechanical testing. J. Orthop. Res. 2017, 36, 1645–1652. [Google Scholar] [CrossRef]
- Schierjott, R.A.; Hettich, G.; Graichen, H.; Jansson, V.; Rudert, M.; Traina, F.; Weber, P.; Grupp, T.M. Quantitative assessment of acetabular bone defects: A study of 50 computed tomography data sets. PLoS ONE 2019, 14, e0222511. [Google Scholar] [CrossRef]
- Morosato, F.; Traina, F.; Cristofolini, L. A reliable in vitro approach to assess the stability of acetabular implants using digital image correlation. Strain 2019, 55, e12318. [Google Scholar] [CrossRef]
- Damm, P.; Graichen, F.; Rohlmann, A.; Bender, A.; Bergmann, G. Total hip joint prosthesis for in vivo measurement of forces and moments. Med. Eng. Phys. 2010, 32, 95–100. [Google Scholar] [CrossRef]
- Pijls, B.G.; Nieuwenhuijse, M.J.; Fiocco, M.; Plevier, J.W.; Middeldorp, S.; Nelissen, R.G.; Valstar, E.R. Early proximal migration of cups is associated with late revision in THA. Acta Orthop. 2012, 83, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Pilliar, R.M.; Lee, J.M.; Maniatopoulos, C. Observations on the Effect of Movement on Bone Ingrowth into Porous-Surfaced Implants. Clin. Orthop. Relat. Res. 1986, 108–113. [Google Scholar] [CrossRef]
- Amirouche, F.; Solitro, G.; Broviak, S.; Goldstein, W.; Gonzalez, M.; Barmada, R. Primary cup stability in THA with augmentation of acetabular defect. A comparison of healthy and osteoporotic bone. Orthop. Traumatol. Surg. Res. 2015, 101, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.A.; Bitsch, R.G.; Janoszka, M.B.; Klotz, M.C.; Bruckner, T.; Jaeger, S. Treatment of High-Grade Acetabular Defects: Do Porous Titanium Cups Provide Better Stability Than Traditional Titanium Cups When Combined with an Augment? J. Arthroplast. 2018, 33, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Cartner, J.L.; Hartsell, Z.M.; Ricci, W.M.; Tornetta, P. Can We Trust Ex Vivo Mechanical Testing of Fresh–Frozen Cadaveric Specimens? The Effect of Postfreezing Delays. J. Orthop. Trauma 2011, 25, 459–461. [Google Scholar] [CrossRef]




| Donor | Cause of Death | Sex | Age (Years) | Height (cm) | Body Weight (kg) | BMI (kg/m2) | Side | Primary Cup Size (mm) | Revision Cup Size (mm) | Reconstruction Material |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | Sepsis | F | 83 | 164 | 63 | 23 | L | 56 | 58 | Bone graft |
| R | 56 | 58 | CaP-putty | |||||||
| #2 | Respiratory paralysis | M | 70 | 175 | 79 | 26 | L | 52 | 54 | Bone graft |
| R | 54 | 56 | CaP-putty | |||||||
| #3 | Unknown | M | 74 | 176 | 78 | 25 | L | 48 | 50 | CaP-putty |
| R | 48 | 50 | Bone graft | |||||||
| #4 | Coronary thrombosis | M | 71 | 187 | 92 | 26 | L | 60 | 62 | Bone graft |
| R | 62 | 64 | CaP-putty | |||||||
| #5 | Cardiac arrhythmia | M | 61 | 181 | 96 | 29 | L | 56 | 58 | Bone graft |
| R | 54 | 56 | CaP-putty | |||||||
| Median | - | 71 | 176 | 79 | 26 | - | 55 | 56.6 | 5 vs. 5 | |
| SD | - | 7.9 | 8.5 | 13.2 | 2.2 | - | 4.5 | 4.3 | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morosato, F.; Traina, F.; Schierjott, R.A.; Hettich, G.; Grupp, T.M.; Cristofolini, L. Primary Stability of Revision Acetabular Reconstructions Using an Innovative Bone Graft Substitute: A Comparative Biomechanical Study on Cadaveric Pelvises. Materials 2020, 13, 4312. https://doi.org/10.3390/ma13194312
Morosato F, Traina F, Schierjott RA, Hettich G, Grupp TM, Cristofolini L. Primary Stability of Revision Acetabular Reconstructions Using an Innovative Bone Graft Substitute: A Comparative Biomechanical Study on Cadaveric Pelvises. Materials. 2020; 13(19):4312. https://doi.org/10.3390/ma13194312
Chicago/Turabian StyleMorosato, Federico, Francesco Traina, Ronja A. Schierjott, Georg Hettich, Thomas M. Grupp, and Luca Cristofolini. 2020. "Primary Stability of Revision Acetabular Reconstructions Using an Innovative Bone Graft Substitute: A Comparative Biomechanical Study on Cadaveric Pelvises" Materials 13, no. 19: 4312. https://doi.org/10.3390/ma13194312
APA StyleMorosato, F., Traina, F., Schierjott, R. A., Hettich, G., Grupp, T. M., & Cristofolini, L. (2020). Primary Stability of Revision Acetabular Reconstructions Using an Innovative Bone Graft Substitute: A Comparative Biomechanical Study on Cadaveric Pelvises. Materials, 13(19), 4312. https://doi.org/10.3390/ma13194312







