The Study of Reactive Ion Etching of Heavily Doped Polysilicon Based on HBr/O2/He Plasmas for Thermopile Devices
Abstract
1. Introduction
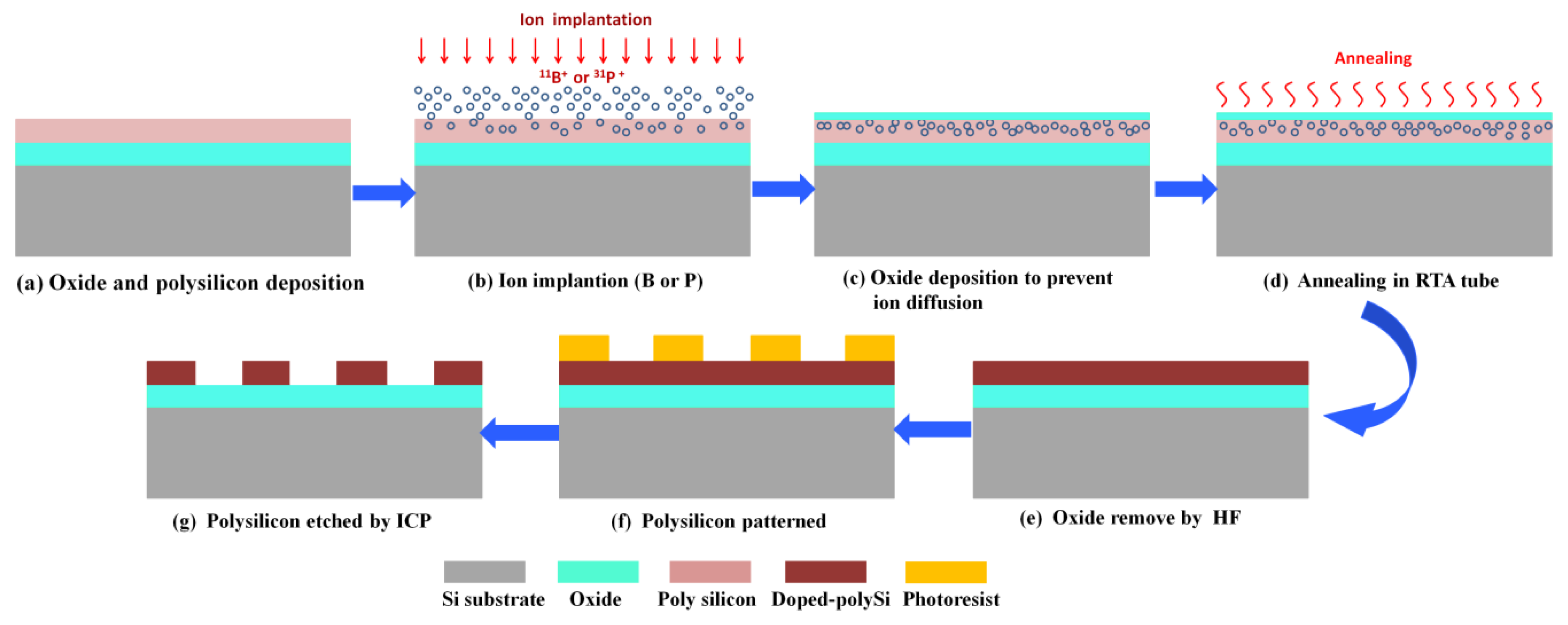
2. Materials and Methods
3. Results
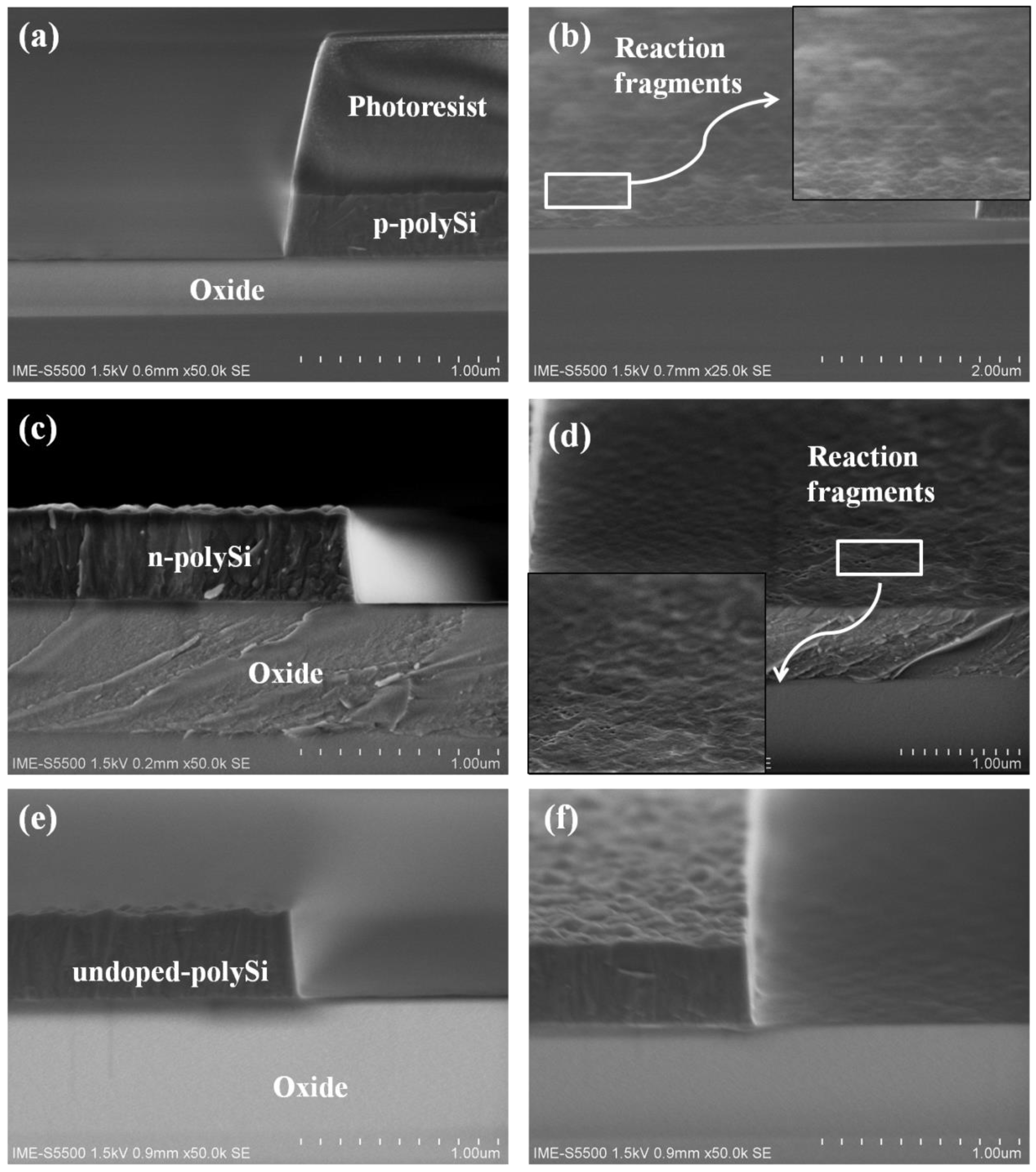
3.1. Study of Undoped and Heavily Doped Polysilicon Etching
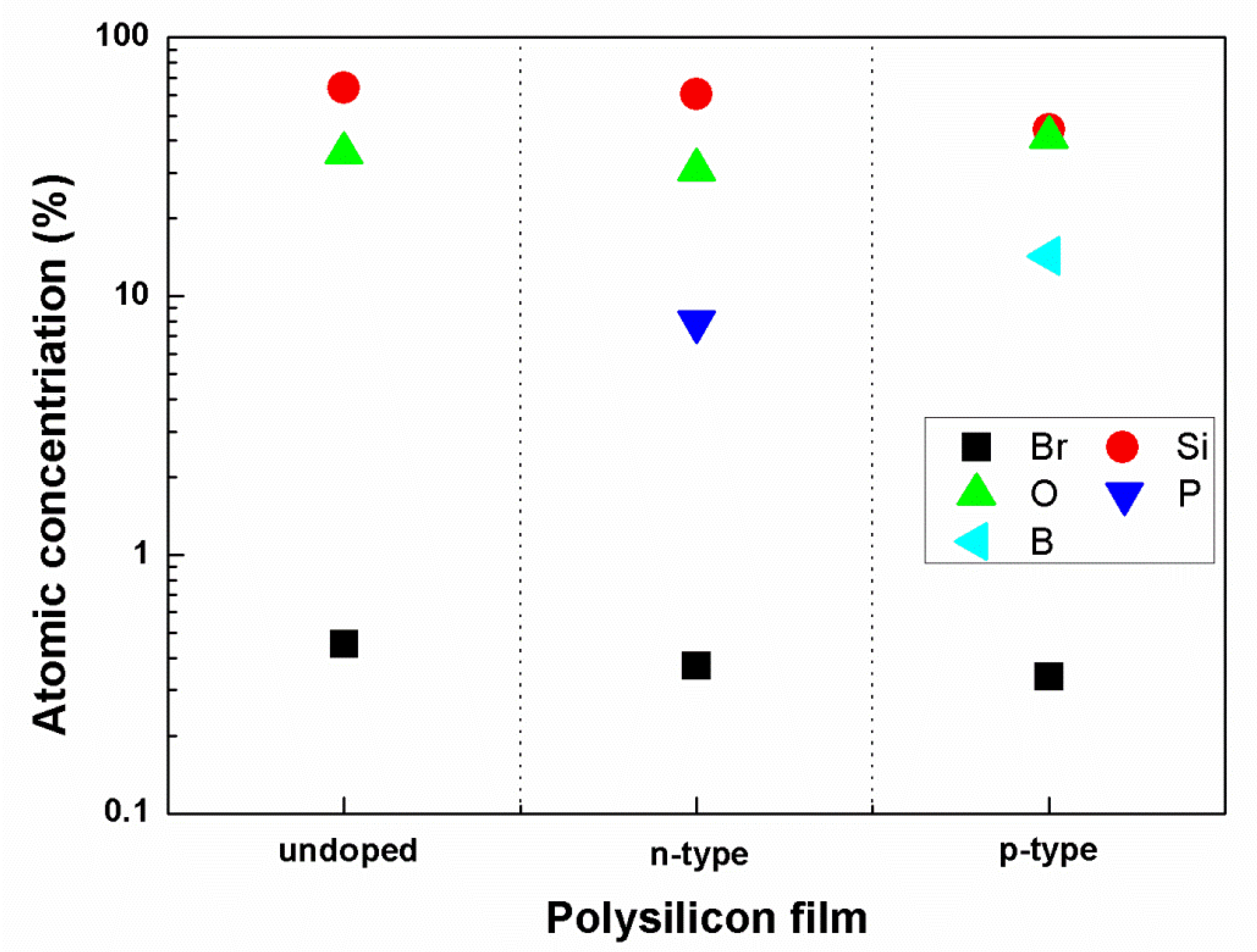
3.2. Study of O2 Effect and Reaction Fragments
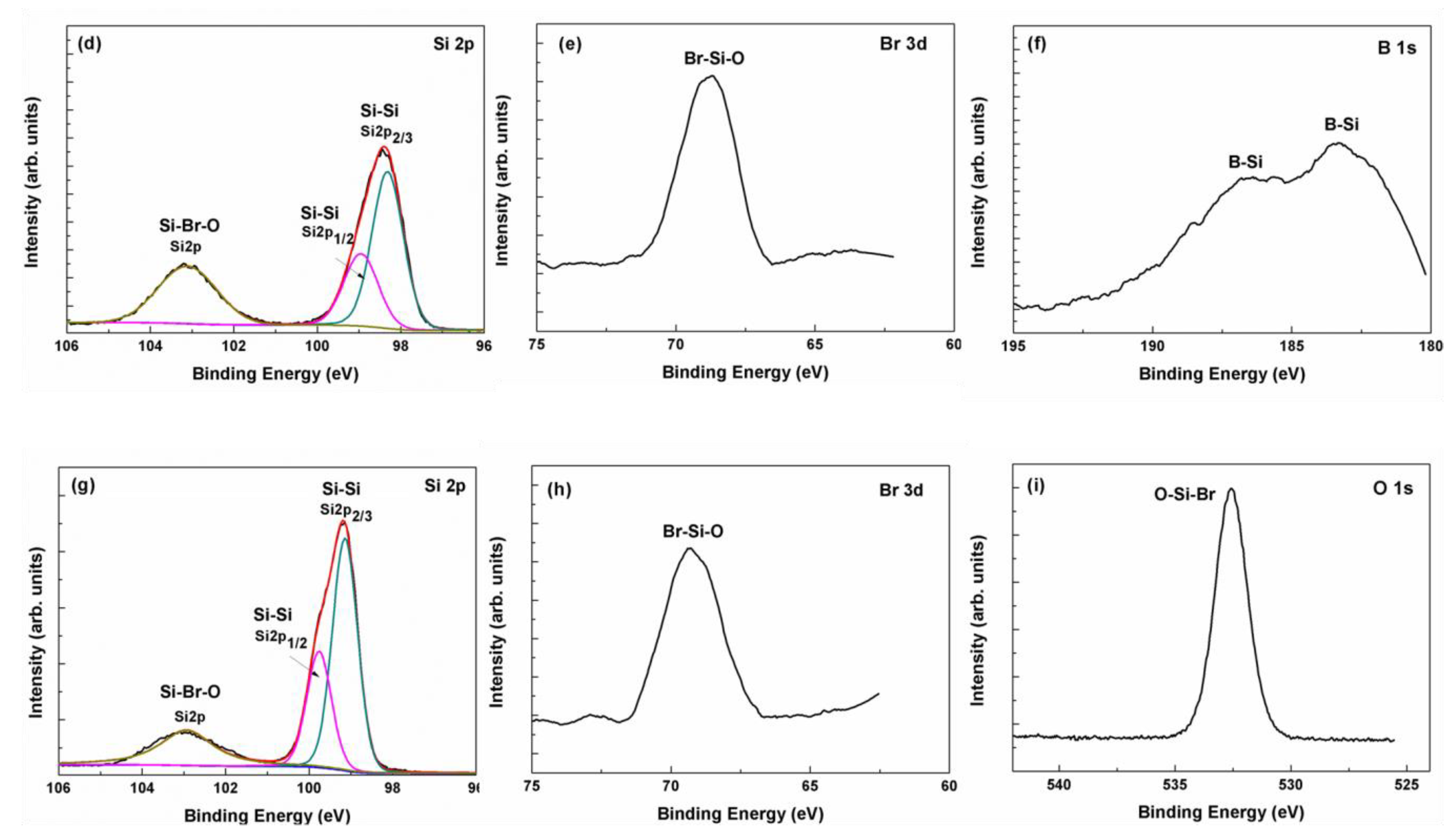
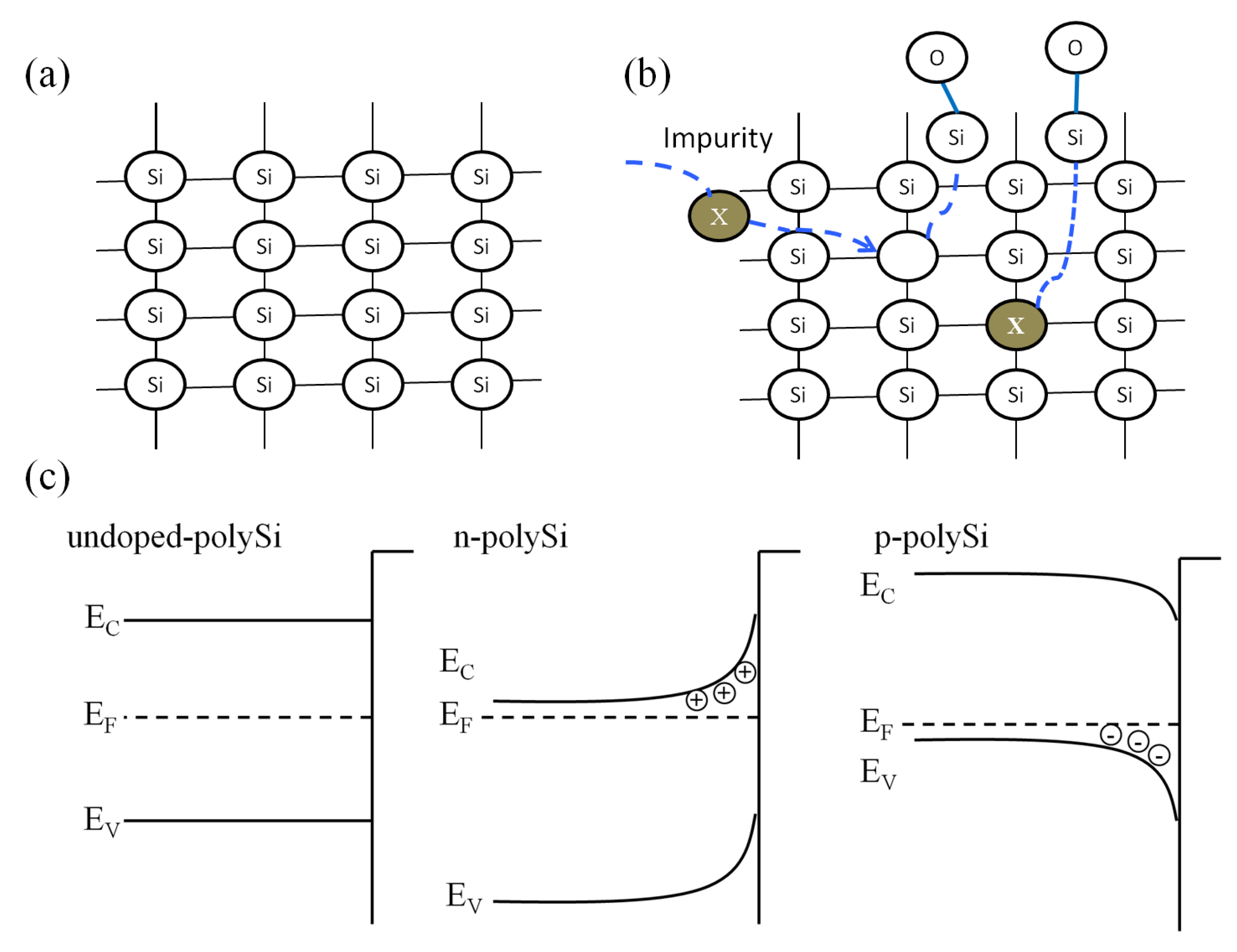
3.3. Study of Heavily Doped Polysilicon Etching Mechanism
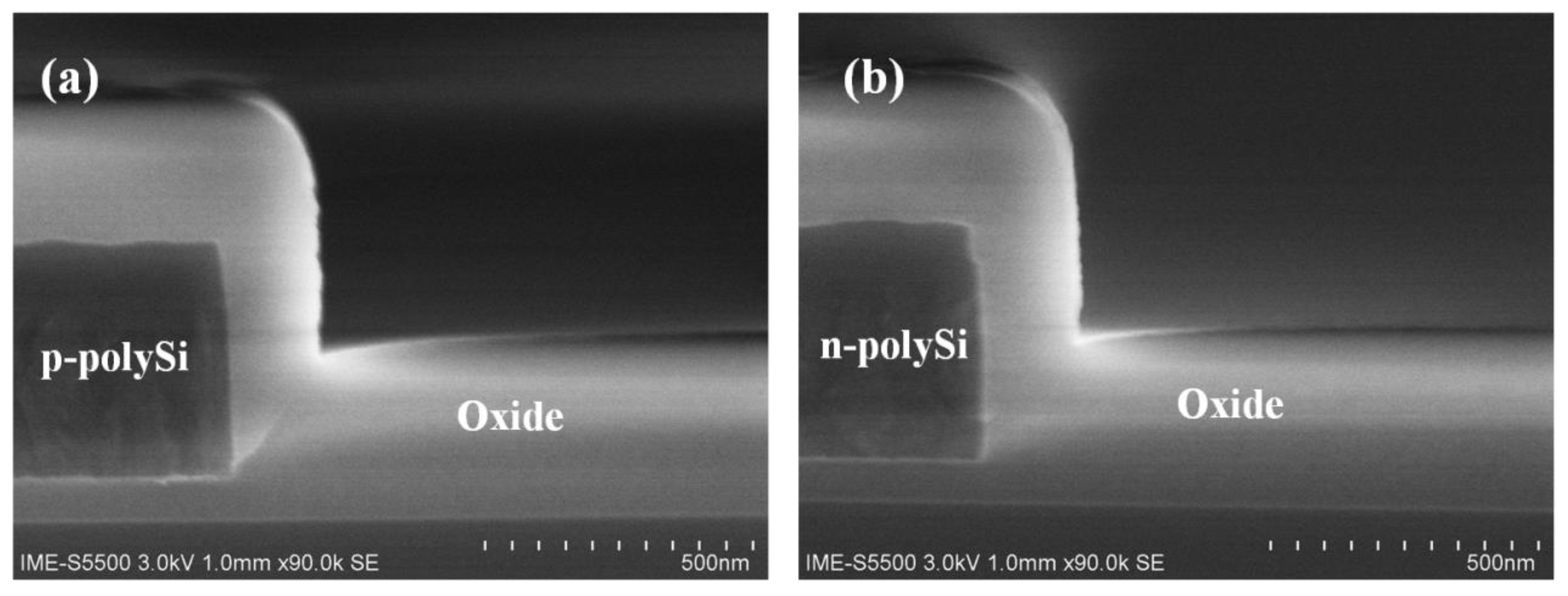
3.4. Material Structure and Resistance Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nagase, M.; Tokashiki, K. Advanced Gate Etching for Accurate CD Control for 130-nm Node ASIC Manufacturing. IEEE Trans. Semicond. Manuf. 2004, 17, 281–285. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Zhang, T.; Han, Z. A Study of Polycrystalline Silicon Damage Features Based on Nanosecond Pulse Laser Irradiation with Different Wavelength Effects. Materials 2017, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Ghisi, A.; Mariani, S. Effect of Imperfections Due to Material Heterogeneity on the Offset of Polysilicon MEMS Structures. Sensors 2019, 19, 3256. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Ghavifekr, H.B.; Shamsi, M. A Novel Biosensor Based on Micromechanical Resonator Array for Lab-On-a-Chip Applications. Sens. Imaging Int. J. 2019, 20, 39. [Google Scholar] [CrossRef]
- Ke, W.; Wang, Y.; Zhou, H.; Li, T.; Wang, Y. Design, fabrication, and characterization of a high-performance CMOS-compatible thermopile infrared detector with self-test function. J. Micromech. Microeng. 2018, 28, 125017. [Google Scholar] [CrossRef]
- Zhou, N.; Li, J.; Radamson, H.; Li, L.; Jiang, Q.; Li, J. Deep silicon etching for thermopile structures using a modified Bosch process. J. Micro/Nanolithogr. MEMS MOEMS 2019, 18, 024501. [Google Scholar] [CrossRef]
- Hung, Y.J.; Tang, C.; Chen, T.; Yen, T.; Tsai, M.; Lee, S. Low-loss polysilicon SWG-based narrowband waveguide Bragg reflector in bulk CMOS. Opt. Express 2020, 28, 6. [Google Scholar] [CrossRef]
- Xie, J.; Lee, C.; Wang, M.-F.; Liu, Y.; Feng, H. Characterization of heavily doped polysilicon films for CMOS-MEMS thermoelectric power generators. J. Micromech. Microeng. 2009, 19, 125029. [Google Scholar] [CrossRef]
- Bao, A.; Lei, C.; Mao, H.; Li, R.; Guan, Y. Study on a High Performance MEMS Infrared Thermopile Detector. Micromachines 2019, 10, 877. [Google Scholar] [CrossRef]
- Chang, K.-M.; Yeh, T.-H.; Deng, I.-C.; Lin, H.-C. Highly selective etching for polysilicon and etch-induced damage to gate oxide with halogen-bearing electron-cyclotron-resonance plasma. J. Appl. Phys. 1996, 80, 3048–3055. [Google Scholar] [CrossRef]
- Hedlund, C.; Jonsson, L.B.; Katardjiev, I.V.; Berg, S.; Blom, H.O. Angular dependence of the polysilicon etch rate during dry etching in SF[sub 6] and Cl[sub 2]. J. Vac. Sci. Technol. A 1997, 15, 686–691. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kim, Y.K.; Lee, H. A study of the role of HBr and oxygen on the etch selectivity and the post-etch profile in a polysilicon/oxide etch using HBr/O2 based high density plasma for advanced DRAMs. Mater. Sci. Semicond. Process. 2007, 10, 41–48. [Google Scholar] [CrossRef]
- Cheng, C.C.; Guinn, K.V.; Donnelly, V.M. Mechanism for anisotropic etching of photoresist-masked, polycrystalline silicon in HBr plasmas. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 1996, 14, 85. [Google Scholar] [CrossRef]
- Haass, M.; Darnon, M.; Cunge, G.; Joubert, O. Silicon etching in a pulsed HBr/O2 plasma. II. Pattern transfer. J. Vac. Sci. Technol. B 2015, 33, 3. [Google Scholar] [CrossRef]
- Berg, S.; Nender, C.; Buchta, R.; Norström, H. Dry etching of n- and p-type polysilicon: Parameters affecting the etch rate. J. Vac. Sci. Technol. A 1987, 5, 1600–1603. [Google Scholar] [CrossRef]
- Sato, M. Dopant-dependent Ion Assisted Etching Kinetics in Highly Doped Polysilicon Reactive Ion Etching. Jpn. J. Appl. Phys. 1998, 37, 5039–5046. [Google Scholar] [CrossRef]
- Weast, R.C. CRC Handbook of Chemistry and Physics; Chemical Rubber: West Palm Beach, FL, USA, 1989. [Google Scholar]
- Nakamura, M.; Iizuka, K.; Yano, H. Very High Selective N+Poly-Si RIE with Carbon Elimination. Jpn. J. Appl. Phys. 1989, 28, 2142–2146. [Google Scholar] [CrossRef]
- Nakagawa, O.S.; Ashok, S.; Krüger, J.K. A Schottky barrier study of HBr magnetron enhanced reactive ion etching damage in silicon. J. Appl. Phys. 1991, 69, 2057–2061. [Google Scholar] [CrossRef]
- Du, L.; Hirsch, E.W.; Economou, D.J.; Donnelly, V.M. Effects of O2 addition on in-plasma photo-assisted etching of Si with chlorine. J. Vac. Sci. Technol. A 2020, 38, 053003. [Google Scholar] [CrossRef]
- Mogab, C.J.; Adams, A.C.; Flamm, D.L. Plasma etching of Si and SiO2—The effect of oxygen additions to CF4 plasmas. J. Appl. Phys. 1978, 49, 3796–3803. [Google Scholar] [CrossRef]
- Iijima, Y.; Tazawa, T. Application of total reflection X-ray photoelectron spectroscopy to boron and phosphorus on Si wafer surface measurement. Spectrochim. Acta Part B 2004, 59, 1273–1276. [Google Scholar] [CrossRef]
- Mogab, C.J.; Levinstein, H.J. Anisotropic plasma etching of polysilicon. J. Vac. Sci. Technol. 1980, 17, 721–730. [Google Scholar] [CrossRef]
- Biersack, J.P.; Haggmark, L. Monte Carlo program for the transport of energetic ions in amorphous material. Nucl. Instrum. Methods 1980, 174, 257–269. [Google Scholar] [CrossRef]





| PolySi Type | Impurity Source | Dose (cm−2) | Energy (KeV) | Annealing Condition | Sheet Resistance (Ω/sq) |
|---|---|---|---|---|---|
| N-poly | PH3 | 1.0 × 1016 | 50 | 1050 °C, 60 s | 40 |
| P-poly | BF3 | 1.0 × 1016 | 50 | 1050 °C, 60 s | 60 |
| Step | Pressure (mTorr) | Source Power (W) | Bias Power (V) | Gas (sccm) | Time (s) |
|---|---|---|---|---|---|
| Breakthrough | 10 | 350 | −100 | 100 CF4 | 10 |
| Main Etch | 6 | 350 | −100 | 110 HBr/1 O2/50 He | Endpoint detection |
| Over Etch | 80 | 250 | −240 | 150 HBr/4 O2/120 He | 180 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, N.; Li, J.; Mao, H.; Liu, H.; Liu, J.; Gao, J.; Xiang, J.; Hu, Y.; Shi, M.; Ju, J.; et al. The Study of Reactive Ion Etching of Heavily Doped Polysilicon Based on HBr/O2/He Plasmas for Thermopile Devices. Materials 2020, 13, 4278. https://doi.org/10.3390/ma13194278
Zhou N, Li J, Mao H, Liu H, Liu J, Gao J, Xiang J, Hu Y, Shi M, Ju J, et al. The Study of Reactive Ion Etching of Heavily Doped Polysilicon Based on HBr/O2/He Plasmas for Thermopile Devices. Materials. 2020; 13(19):4278. https://doi.org/10.3390/ma13194278
Chicago/Turabian StyleZhou, Na, Junjie Li, Haiyang Mao, Hao Liu, Jinbiao Liu, Jianfeng Gao, Jinjuan Xiang, Yanpeng Hu, Meng Shi, Jiaxin Ju, and et al. 2020. "The Study of Reactive Ion Etching of Heavily Doped Polysilicon Based on HBr/O2/He Plasmas for Thermopile Devices" Materials 13, no. 19: 4278. https://doi.org/10.3390/ma13194278
APA StyleZhou, N., Li, J., Mao, H., Liu, H., Liu, J., Gao, J., Xiang, J., Hu, Y., Shi, M., Ju, J., Lei, Y., Yang, T., Li, J., & Wang, W. (2020). The Study of Reactive Ion Etching of Heavily Doped Polysilicon Based on HBr/O2/He Plasmas for Thermopile Devices. Materials, 13(19), 4278. https://doi.org/10.3390/ma13194278





