Impact of Bio-Carrier Immobilized with Marine Bacteria on Self-Healing Performance of Cement-Based Materials
Abstract
1. Introduction
2. Experimental Procedure
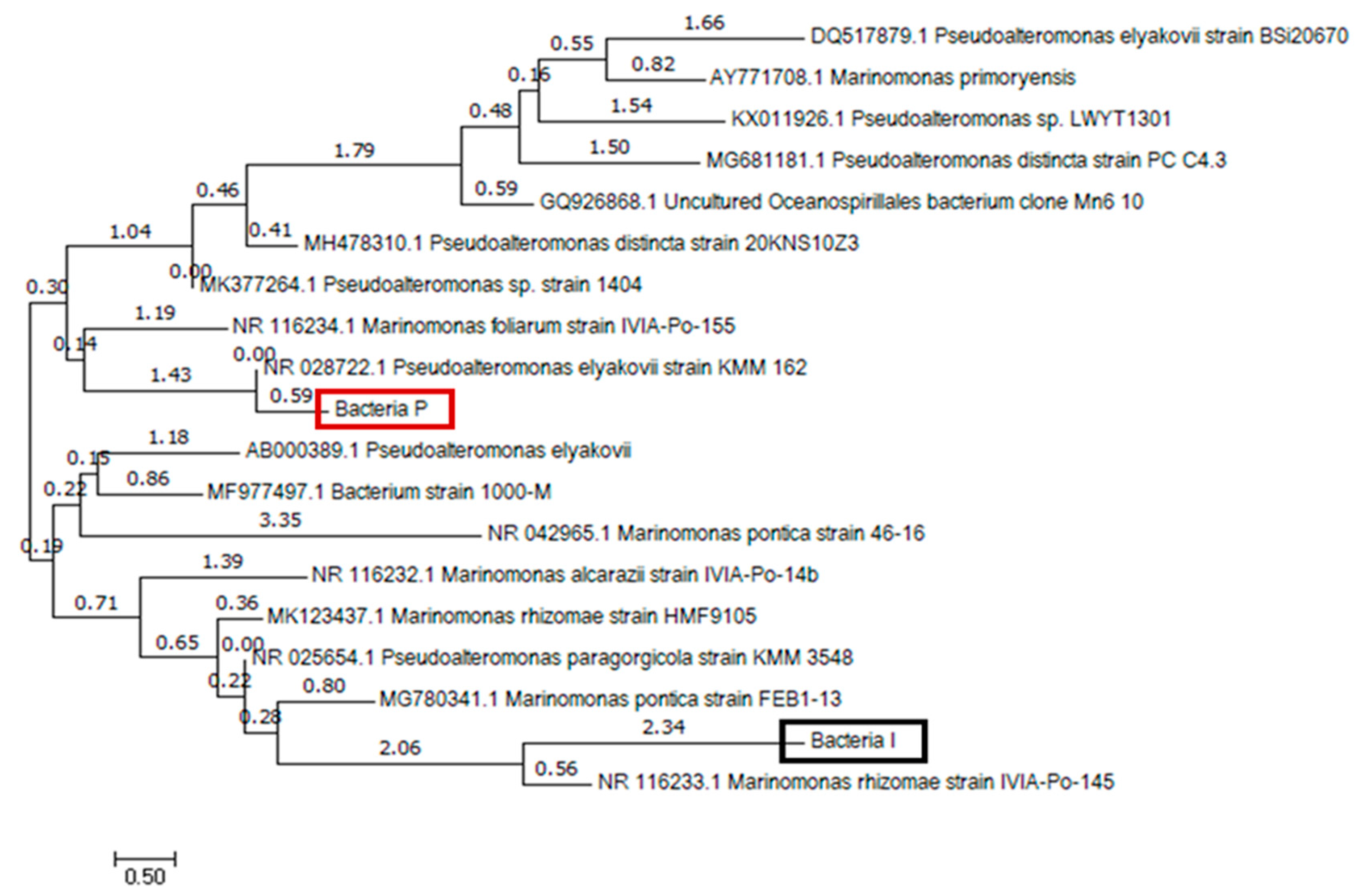
2.1. Identification of Ureolytic Bacteria Isolated in Seawater
2.2. Material and Specimen Preparation
2.3. Test
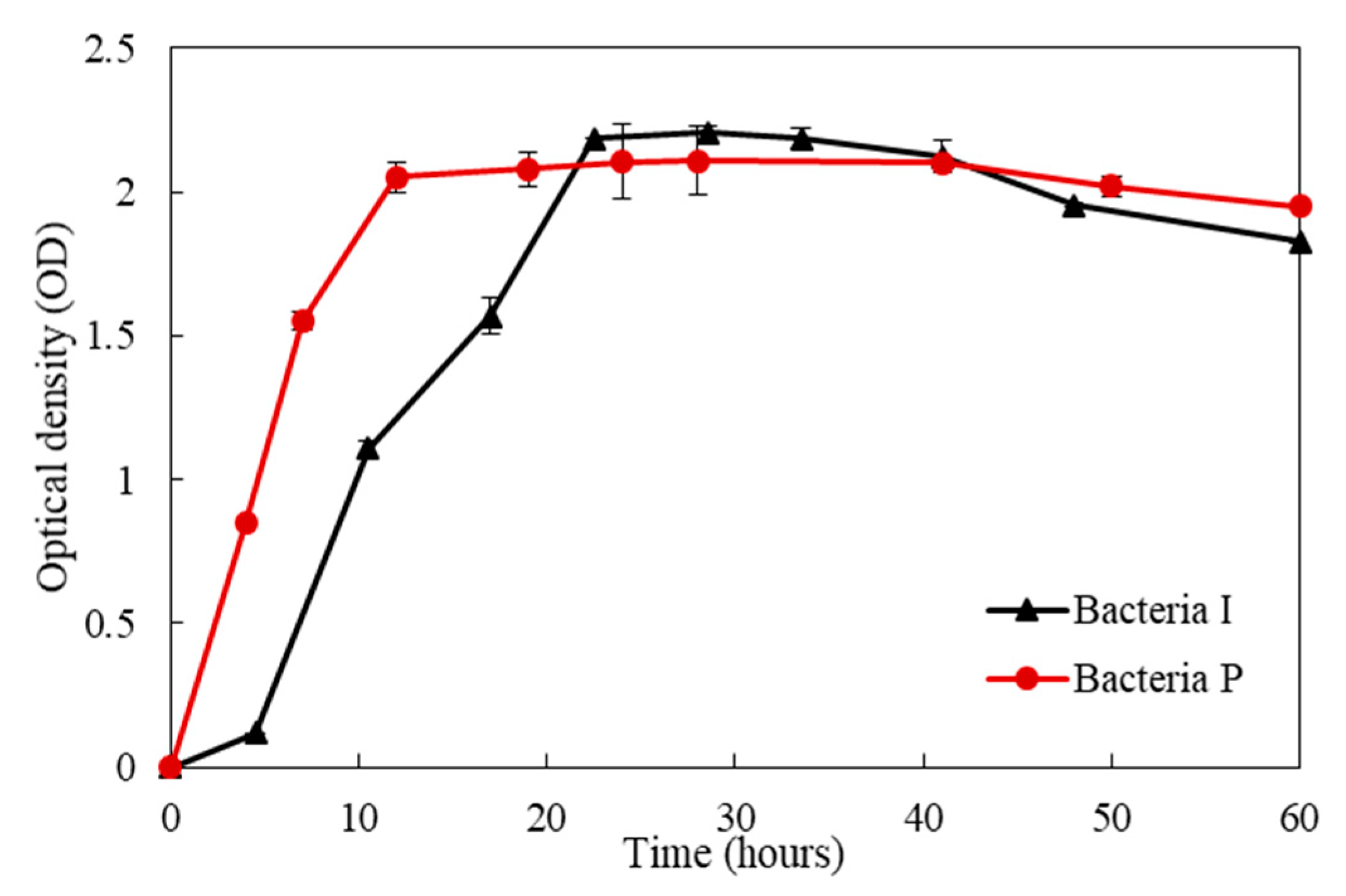
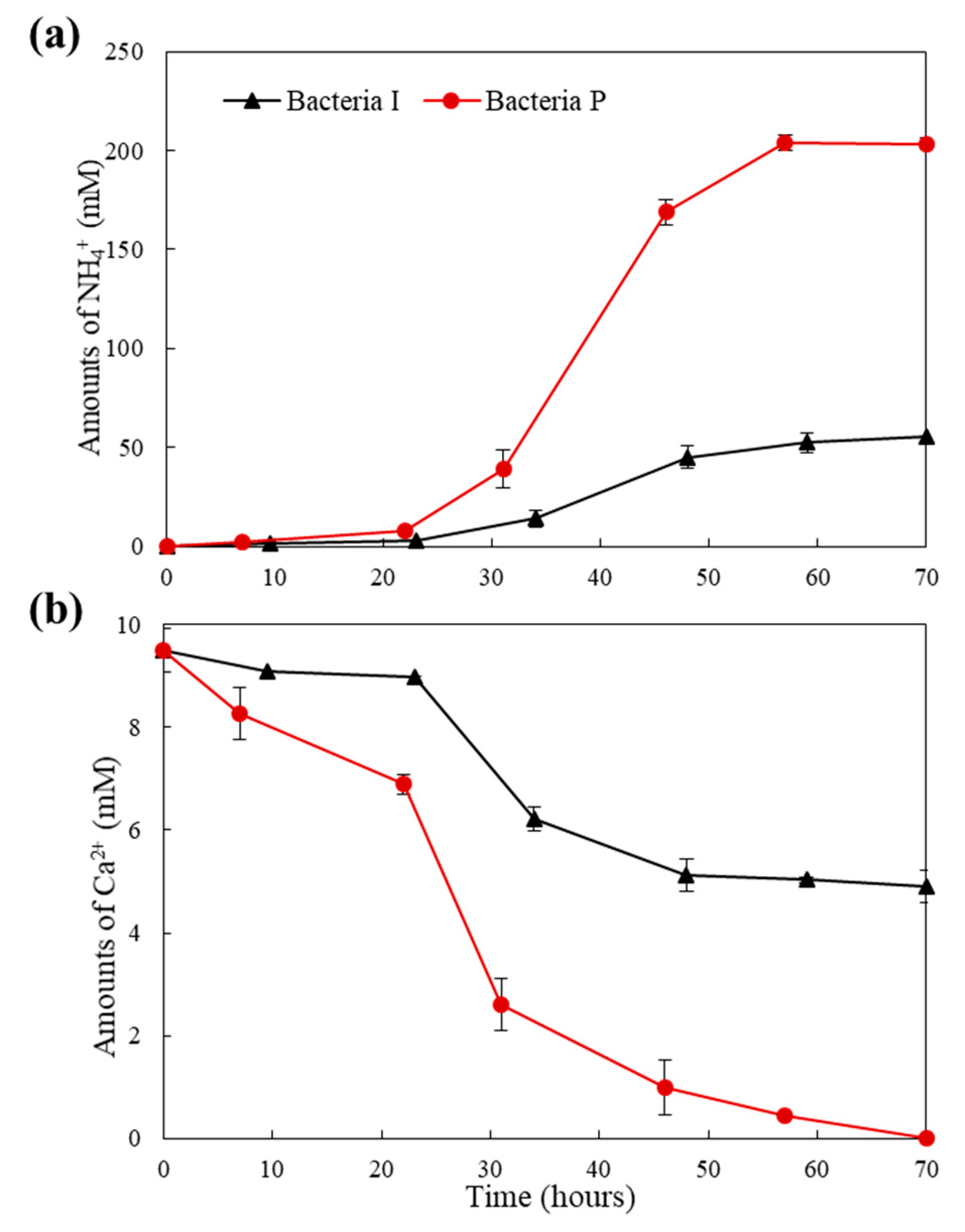
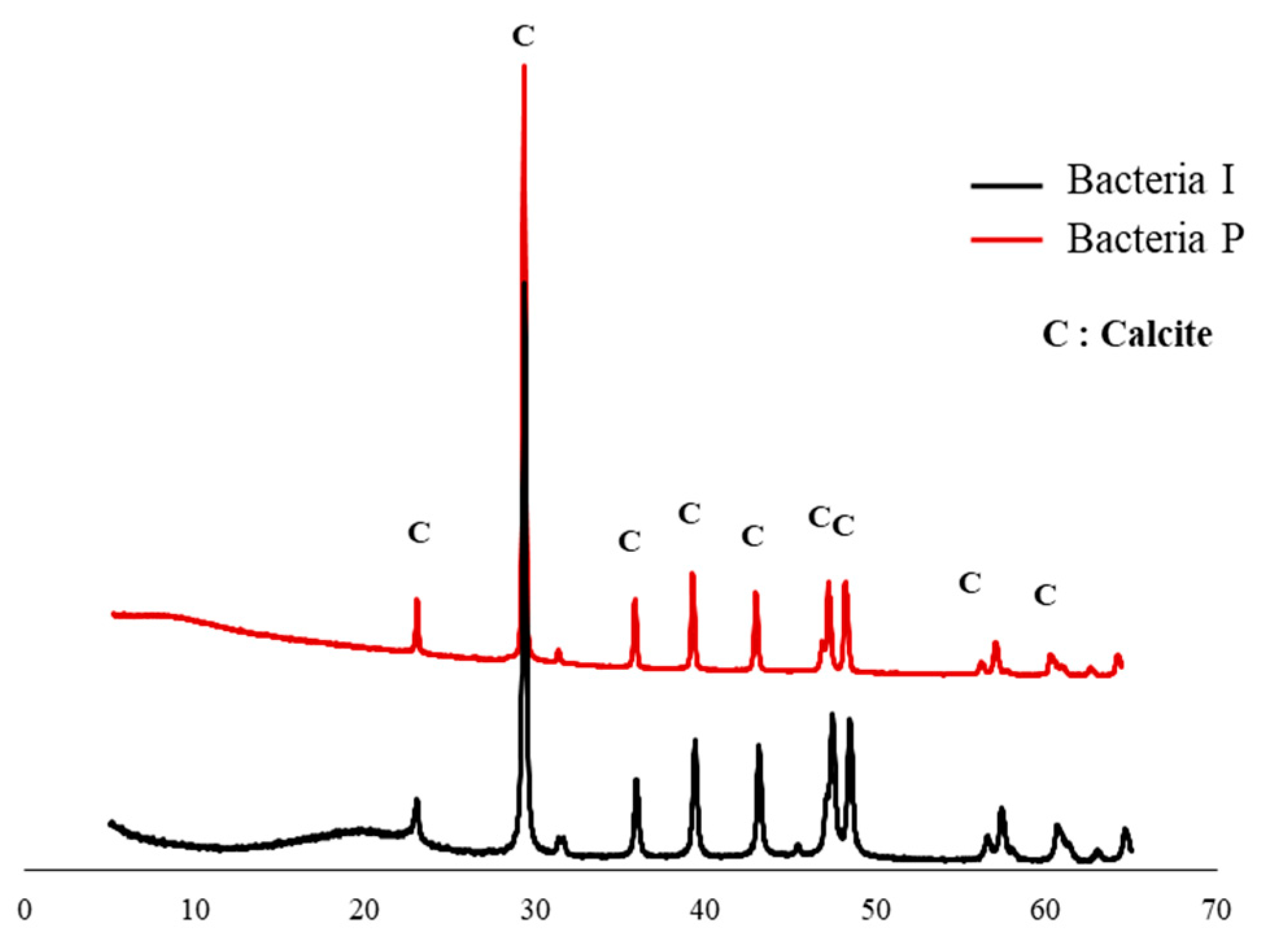
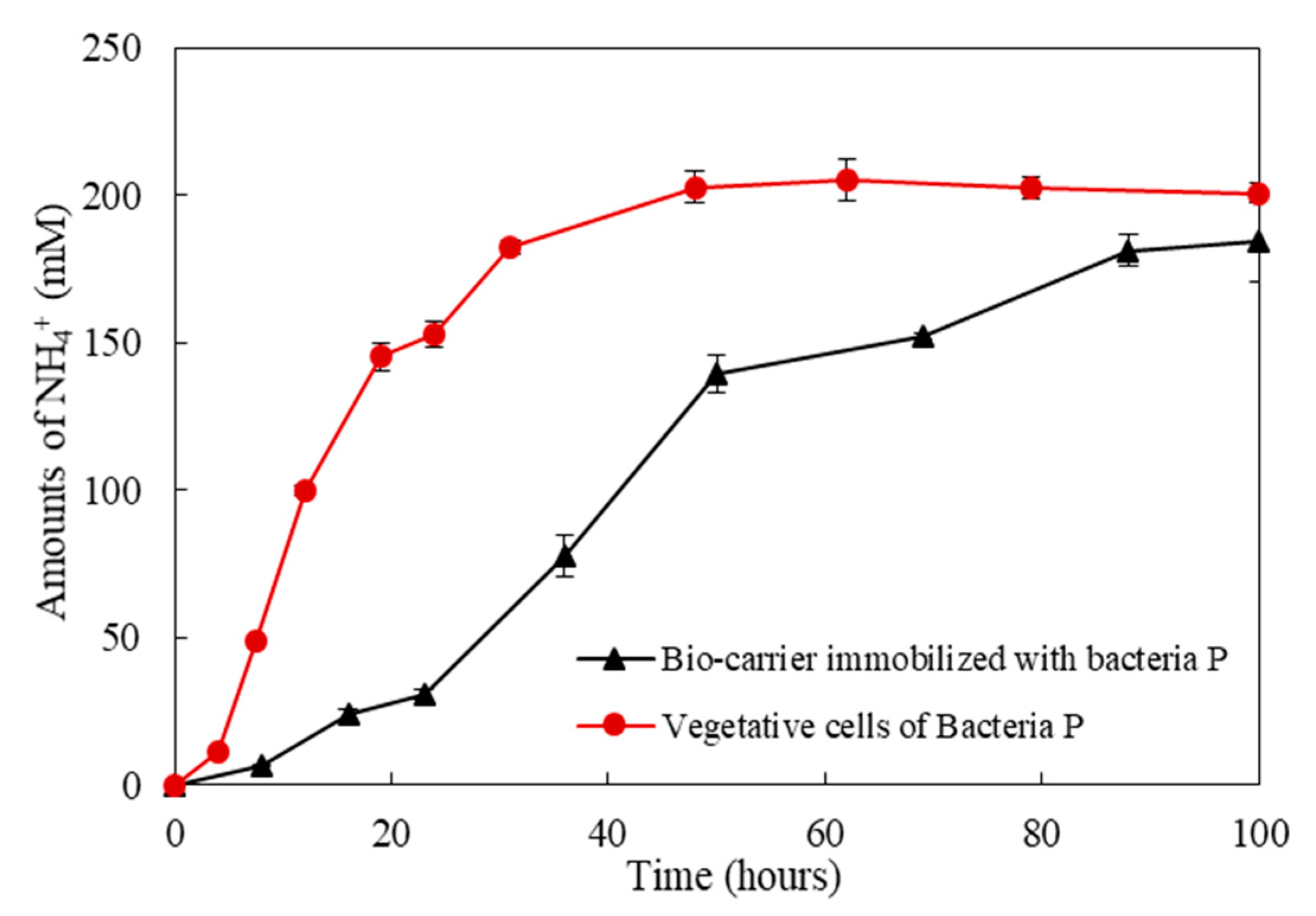
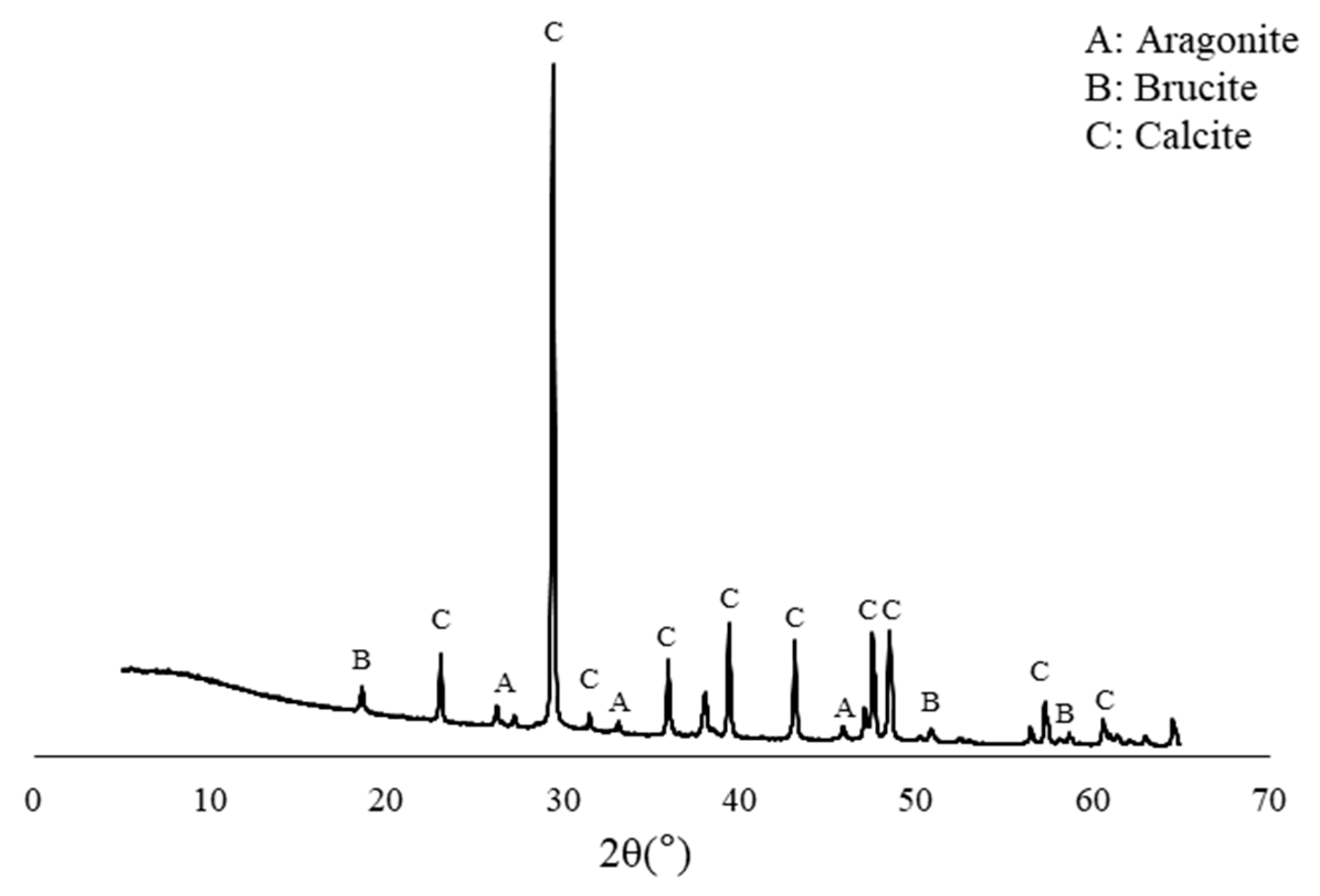
3. Metabolic Characteristics of the Isolated Bacteria
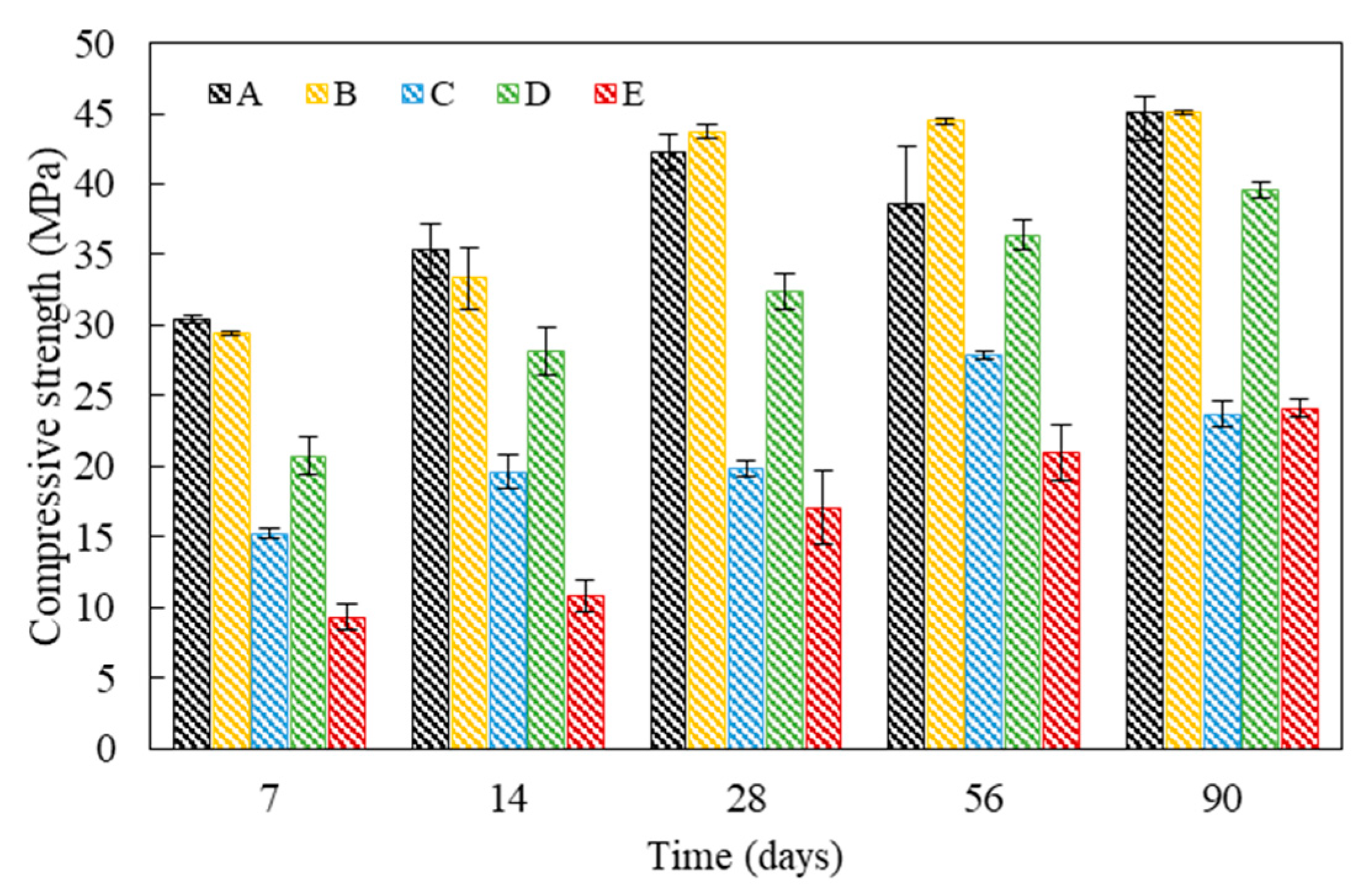
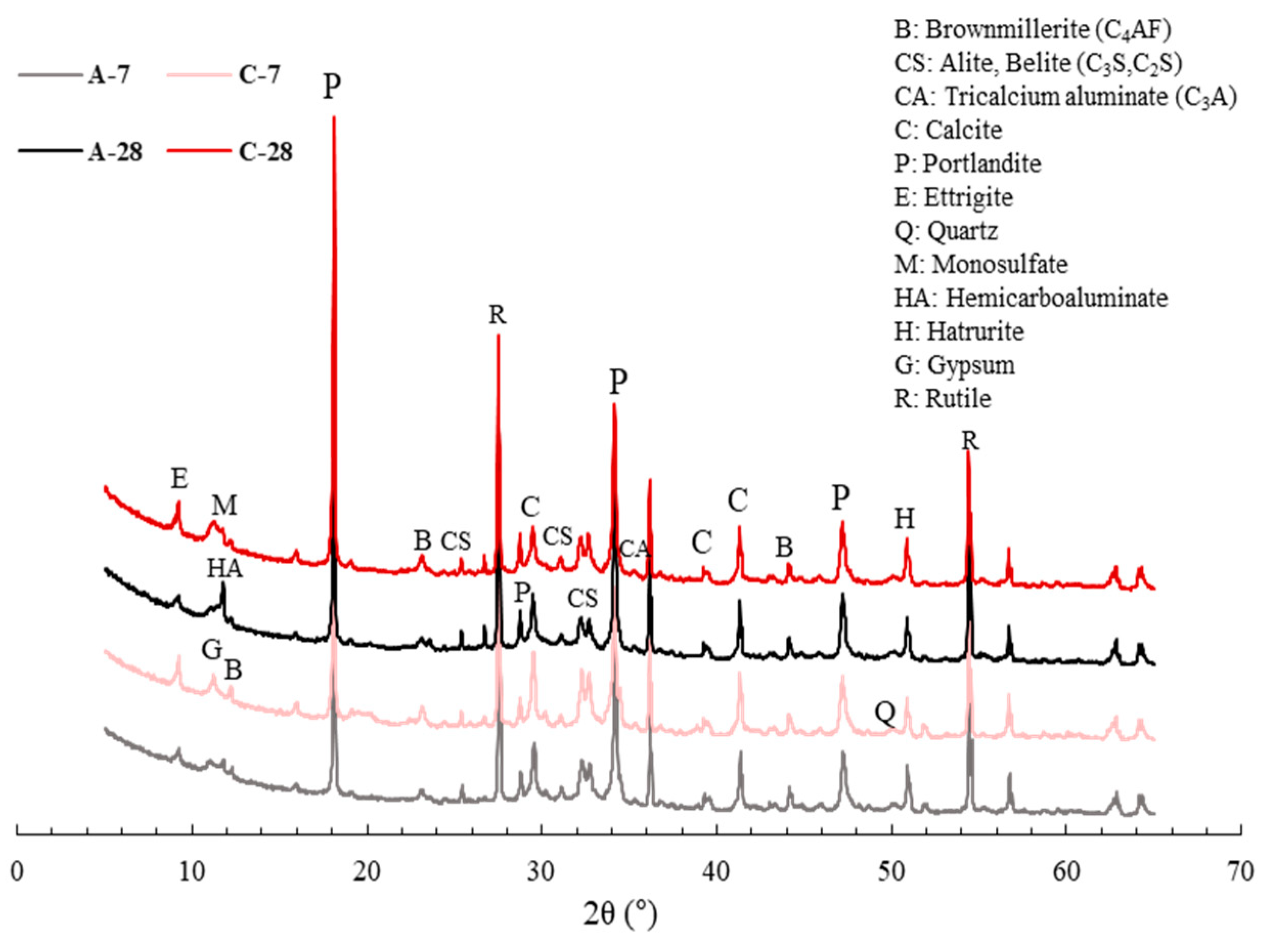
4. Physico-Mechanical Properties and Pore Characteristics of Mortar Specimens with Bio-Carrier
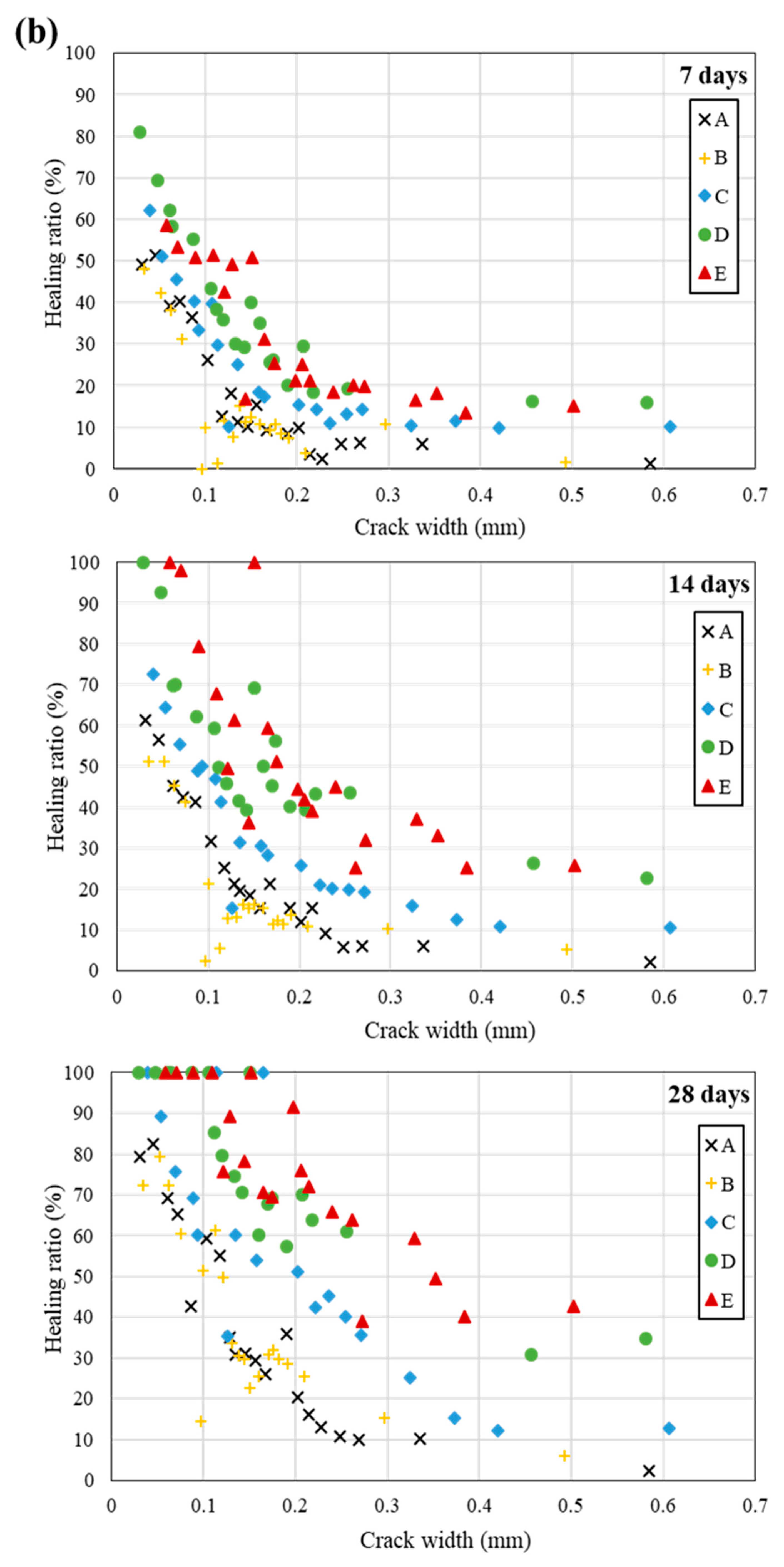
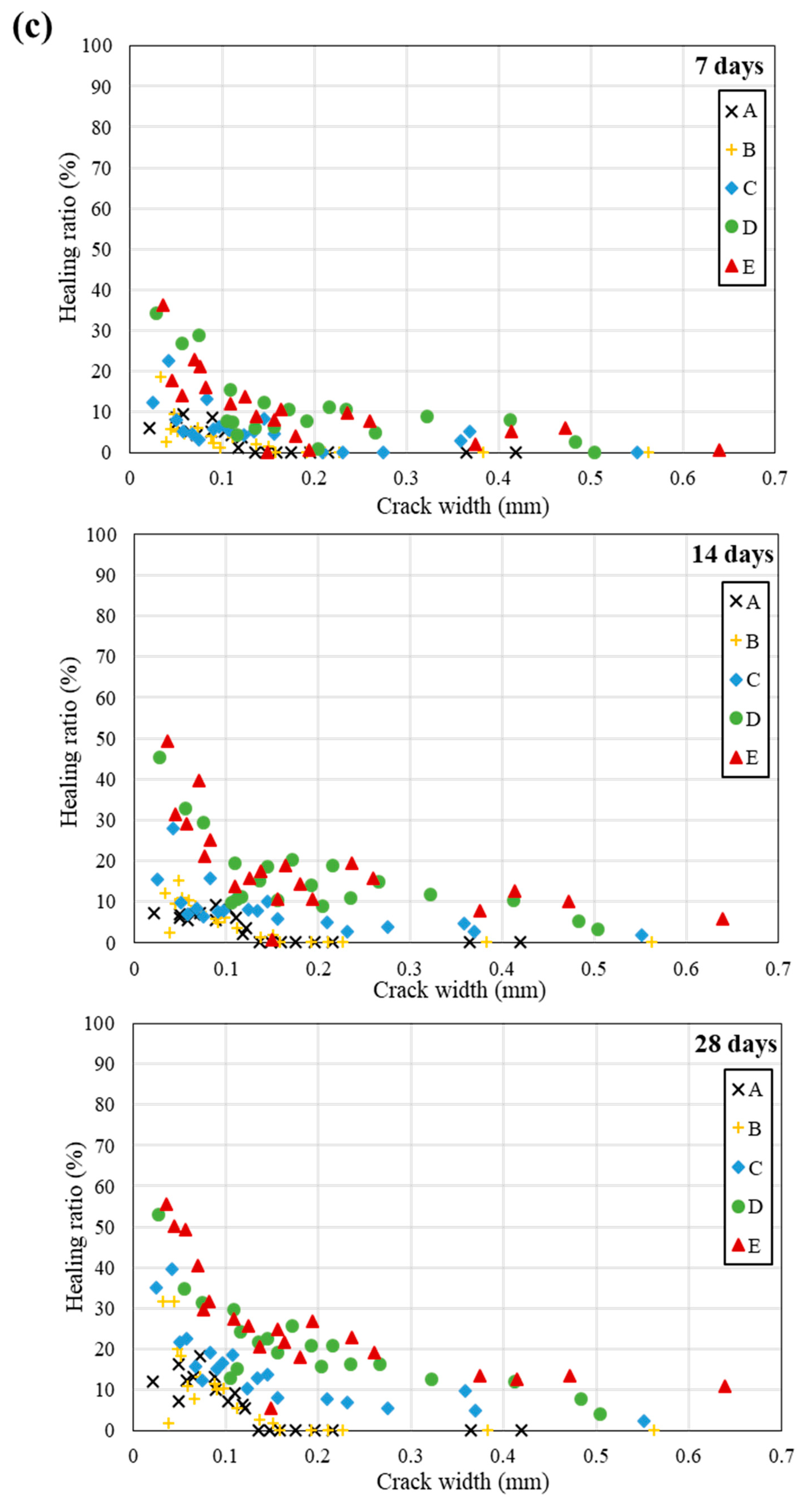
5. Self-Healing Performance of Mortar Specimens with Bio-Carrier
6. Concluding Remarks
- (1)
- The rate of urea degradation in vegetative cells was higher than that of bacteria immobilized in carriers. This finding indicates that the bio-carrier could retard the metabolic activity, yet it could protect the bacteria from the extreme environment of the concrete.
- (2)
- The highest healing ratio was observed in the specimen incorporating the bio-carrier. It is suggested that the bio-carrier not only created a space for bacteria in concrete, but also stored moisture and nutrients, thereby improving the long-term self-healing efficiency.
- (3)
- The specimens incorporating bacteria and the bio-carrier had a higher healing ratio when treated in seawater than those treated in tap water or in air environment. It can be said that the various ions present in the seawater have a positive effect on promoting the metabolism of bacteria isolated in the seawater.
- (4)
- The crack healing ratio of mortar specimens was determined by measuring the surface crack width through microscopic observation in this paper, although self-healing efficiency could influenced not only by crack width but also by depth. Further studies on the healing ratio of crack depths are needed to secure long-term durability.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Safiuddin, M.; Kaish, A.B.M.A.; Woon, C.-O.; Raman, S.N. Early-age cracking in concrete: Causes, consequences, remedial measures, and recommendations. Appl. Sci. 2018, 8, 1730. [Google Scholar] [CrossRef]
- Xue, C.; Li, W.; Li, J.; Wang, K. Numerical investigation on interface crack initiation and propagation behaviour of self-healing cementitious materials. Cem. Concr. Res. 2019, 122, 1–16. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, S.; Wang, S.; Huang, Y.; Yang, Q.; Liu, S.; Wang, J.; Du, P.; Cheng, X.; Zhou, Z. Research on the Improvement of Concrete Autogenous Self-healing Based on the Regulation of Cement Particle Size Distribution (PSD). Materials 2019, 12, 2818. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, A.R.; Nelson, A.J.; Nehdi, M.L. Visualization and quantification of crack self-healing in cement-based materials incorporating different minerals. Cem. Concr. Compos. 2019, 103, 49–58. [Google Scholar] [CrossRef]
- Son, H.M.; Kim, H.Y.; Park, S.M.; Lee, H.K. Ureolytic/non-ureolytic bacteria co-cultured self-healing agent for cementitious materials crack repair. Materials 2018, 11, 782. [Google Scholar] [CrossRef]
- Wang, J.; Basheer, P.A.M.; Nanukuttan, S.V.; Long, A.E.; Bai, Y. Influence of service loading and the resulting micro-cracks on chloride resistance of concrete. Constr. Build. Mater. 2016, 108, 56–66. [Google Scholar] [CrossRef]
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Van Belleghem, B.; Kessler, S.; van den Heede, P.; van Tittelboom, K.; de Belie, N. Chloride induced reinforcement corrosion behavior in self-healing concrete with encapsulated polyurethane. Cem. Concr. Res. 2018, 113, 130–139. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Yu, T.; Qu, F.; Tam, V.W.Y. Investigation on early-age hydration, mechanical properties and microstructure of seawater sea sand cement mortar. Constr. Build. Mater. 2020, 249, 118776. [Google Scholar] [CrossRef]
- Belie, N.; Gruyaert, E.; Al-Tabbaa, A.; Antonaci, P.; Baera, C.; Bajare, D.; Darquennes, A.; Davies, R.; Ferrara, L.; Jefferson, A.; et al. A review of self-healing concrete for damage management of structures. Adv. Mater. Interfaces 2018, 5, 1800074. [Google Scholar] [CrossRef]
- Sangadji, S. Can self-healing mechanism helps concrete structures sustainable? Procedia Eng. 2017, 171, 238–249. [Google Scholar] [CrossRef]
- Schlangen, E.; Sangadji, S. Addressing infrastructure durability and sustainability by self healing mechanisms—Recent advances in self healing concrete and asphalt. Procedia Eng. 2013, 54, 39–57. [Google Scholar] [CrossRef]
- Luo, M.; Qian, C.-X.; Li, R.-Y. Factors affecting crack repairing capacity of bacteria-based self-healing concrete. Constr. Build. Mater. 2015, 87, 1–7. [Google Scholar] [CrossRef]
- Nielsen, S.; Koren, K.; Löbmann, K.; Hinge, M.; Scoma, A.; Kjeldsen, K.; Røy, H. Constraints on CaCO3 precipitation in superabsorbent polymer by aerobic bacteria. Appl. Microbiol. Biot. 2020, 104. [Google Scholar] [CrossRef]
- Palin, D.; Wiktor, V.; Jonkers, H.M. A bacteria-based self-healing cementitious composite for application in low-temperature marine environments. Biomimetics 2017, 2, 13. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Ghorbel, E.; Fares, H.; Cousture, A. Bacterial self-healing of concrete and durability assessment. Cem. Concr. Compos. 2019, 104, 103340. [Google Scholar] [CrossRef]
- Seifan, S.; Sarabadani, Z.; Berenjian, A. Microbially induced calcium carbonate precipitation to design a new type of bio self-healing dental composite. Appl. Microbiol. Biot. 2020, 104, 2029–2037. [Google Scholar] [CrossRef]
- Lu, S.; Chen, M.; Dang, Y.; Cao, L.; He, J.; Zhong, J. Bacterial self healing cement based materials: Mechanism at nanoscale. AIP Adv. 2019, 9, 105312. [Google Scholar] [CrossRef]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb. Tech. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.; Stahl, D.A. Brock Biology of Microorganisms, 13th ed.; Pearson: London, UK, 2012. [Google Scholar]
- Seifan, M.; Sarmah, A.K.; Samani, A.K.; Ebrahiminezhad, A.; Ghasemi, Y.; Berenjian, A. Mechanical properties of bio self-healing concrete containing immobilized bacteria with iron oxide nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 4489–4498. [Google Scholar] [CrossRef]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Wang, J.; van Tittelboom, K.; de Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Gao, M.; Guo, J.; Cao, H.; Wang, H.; Xiong, X.; Krastev, R.; Nie, K.; Xu, H.; Liu, L. Immobilized bacteria with pH-response hydrogel for self-healing of concrete. J. Environ. Manage. 2020, 261, 110225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Belie, N.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. Biot. 2011, 39, 567–577. [Google Scholar] [CrossRef]
- Lucas, S.S.; Moxham, C.; Tziviloglou, E.; Jonkers, H. Study of self-healing properties in concrete with bacteria encapsulated in expanded clay. Sci. Technol. Mater. 2018, 30, 93–98. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X. Self-healing of concrete cracks by use of bacteria-containing low alkali cementitious material. Constr. Build. Mater. 2018, 167, 1–14. [Google Scholar] [CrossRef]
- Xu, H.; Lian, J.; Gao, M.; Fu, D.; Yan, Y. Self-healing concrete using rubber particles to immobilize bacterial spores. Materials 2019, 12, 2313. [Google Scholar] [CrossRef]
- Miller, C.S.; Handley, K.M.; Wrighton, K.C.; Frischkorn, K.R.; Thomas, B.C.; Banfield, J.F. Short-read assembly of full-length 16S amplicons reveals bacterial diversity in subsurface sediments. PLoS ONE 2013, 8, e56018. [Google Scholar] [CrossRef]
- Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in, or [50-mm] Cube Specimens) ASTM C109/C109M-16a; ASTM International: West Conshohocken, PA, USA, 2016.
- Snellings, R.; Chwast, J.; Cizer, Ö.; de Belie, N.; Dhandapani, Y.; Durdzinski, P.; Elsen, J.; Haufe, J.; Hooton, D.; Patapy, C.; et al. RILEM TC-238 SCM recommendation on hydration stoppage by solvent exchange for the study of hydrate assemblages. Mater. Struct. 2018, 51, 172. [Google Scholar] [CrossRef]
- Aranda, M.; de la Torre, A.; Leon-Reina, L. Rietveld quantitative phase analysis of OPC clinkers, cements and hydration products. Rev. Mineral. Geochem. 2012, 74, 169–209. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Füllmann, T.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative study of Portland cement hydration by X-ray diffraction/Rietveld analysis and independent methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Feng, T.; Zhou, M.; Zhao, L.; Zhou, A.; Li, Z. Immobilizing bacteria in expanded perlite for the crack self-healing in concrete. Constr. Build. Mater. 2017, 148, 610–617. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, L. Controllable synthesis of calcium carbonate polymorphs at different temperatures. Powder Technol. 2009, 189, 64–69. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; González-Muñoz, M.T. Influence of substrate mineralogy on bacterial mineralization of calcium carbonate: Implications for stone conservation. Appl. Environ. Microbiol. 2012, 78, 4017. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Son, H.M.; Park, S.M.; Seo, J.H.; Lee, H.K. Effects of temperature and salinity on concrete-surface rreatment by bacteria in marine environment. ACI Mater. J. 2020, 117. [Google Scholar]
- Ivanova, E.P.; Onyshchenko, O.M.; Christen, R.; Lysenko, A.M.; Zhukova, N.V.; Shevchenko, L.S.; Kiprianova, E.A. Marinomonas pontica sp. nov., isolated from the Black Sea. Int. J. Syst. Evol. Micr. 2005, 55, 275–279. [Google Scholar] [CrossRef]
- Al Khudary, R.; Stößer, N.I.; Qoura, F.; Antranikian, G. Pseudoalteromonas arctica sp. nov., an aerobic, psychrotolerant, marine bacterium isolated from Spitzbergen. Int. J. Syst. Evol. Micr. 2008, 58, 2018–2024. [Google Scholar] [CrossRef]
- Han, S.; Choi, E.K.; Park, W.; Yi, C.; Chung, N. Effectiveness of expanded clay as a bacteria carrier for self-healing concrete. Appl. Biol. Chem. 2019, 62, 19. [Google Scholar] [CrossRef]
- Pungrasmi, W.; Intarasoontron, J.; Jongvivatsakul, P.; Likitlersuang, S. Evaluation of Microencapsulation techniques for MICP bacterial spores applied in self-healing concrete. Sci. Rep. 2019, 9, 12484. [Google Scholar] [CrossRef]
- Wang, J.; Mignon, A.; Snoeck, D.; Wiktor, V.; van Vliergerghe, S.; Boon, N.; de Belie, N. Application of modified-alginate encapsulated carbonate producing bacteria in concrete: A promising strategy for crack self-healing. Front. Microbiol. 2015, 6, 1088. [Google Scholar] [CrossRef]
- Seifan, M.; Ebrahiminezhad, A.; Younes, G.; Berenjian, A. Microbial calcium carbonate precipitation with high affinity to fill the concrete pore space: Nanobiotechnological approach. Bioproc. Biosystems Eng. 2018, 42. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Biswas, M.; Chattopadhyay, B.D.; Mandal, S. Microbial activity on the microstructure of bacteria modified mortar. Cem. Concr. Compos. 2009, 31, 93–98. [Google Scholar] [CrossRef]
- Chaurasia, L.; Bisht, V.; Singh, L.P.; Gupta, S. A novel approach of biomineralization for improving micro and macro-properties of concrete. Constr. Build. Mater. 2019, 195, 340–351. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, H.K. Hydration kinetics of high-strength concrete with untreated coal bottom ash for internal curing. Cem. Concr. Compos. 2018, 91, 67–75. [Google Scholar] [CrossRef]
- Jang, J.G.; Kim, H.J.; Kim, H.K.; Lee, H.K. Resistance of coal bottom ash mortar against the coupled deterioration of carbonation and chloride penetration. Mater. Des. 2016, 93, 160–167. [Google Scholar] [CrossRef]
- Aggarwal, Y.; Siddique, R. Microstructure and properties of concrete using bottom ash and waste foundry sand as partial replacement of fine aggregates. Constr. Build. Mater. 2014, 54, 210–223. [Google Scholar] [CrossRef]
- Kim, H.Y.; Son, H.M.; Park, S.M.; Lee, H.K. Effects of biological admixtures on hydration and mechanical properties of Portland cement paste. Constr. Build. Mater. 2020, 235, 117461. [Google Scholar] [CrossRef]
- Bundur, Z.B.; Kirisits, M.J.; Ferron, R.D. Biomineralized cement-based materials: Impact of inoculating vegetative bacterial cells on hydration and strength. Cem. Concr. Res. 2015, 67, 237–245. [Google Scholar] [CrossRef]
- Jang, J.G.; Kim, G.M.; Kim, H.J.; Lee, H.K. Review on recent advances in CO2 utilization and sequestration technologies in cement-based materials. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Palin, D.; Wiktor, V.; Jonkers, H.M. Autogenous healing of marine exposed concrete: Characterization and quantification through visual crack closure. Cem. Concr. Res. 2015, 73, 17–24. [Google Scholar] [CrossRef]
- Alazhari, M.; Sharma, T.; Heath, A.; Cooper, R.; Paine, K. Application of expanded perlite encapsulated bacteria and growth media for self-healing concrete. Constr. Build. Mater. 2018, 160, 610–619. [Google Scholar] [CrossRef]
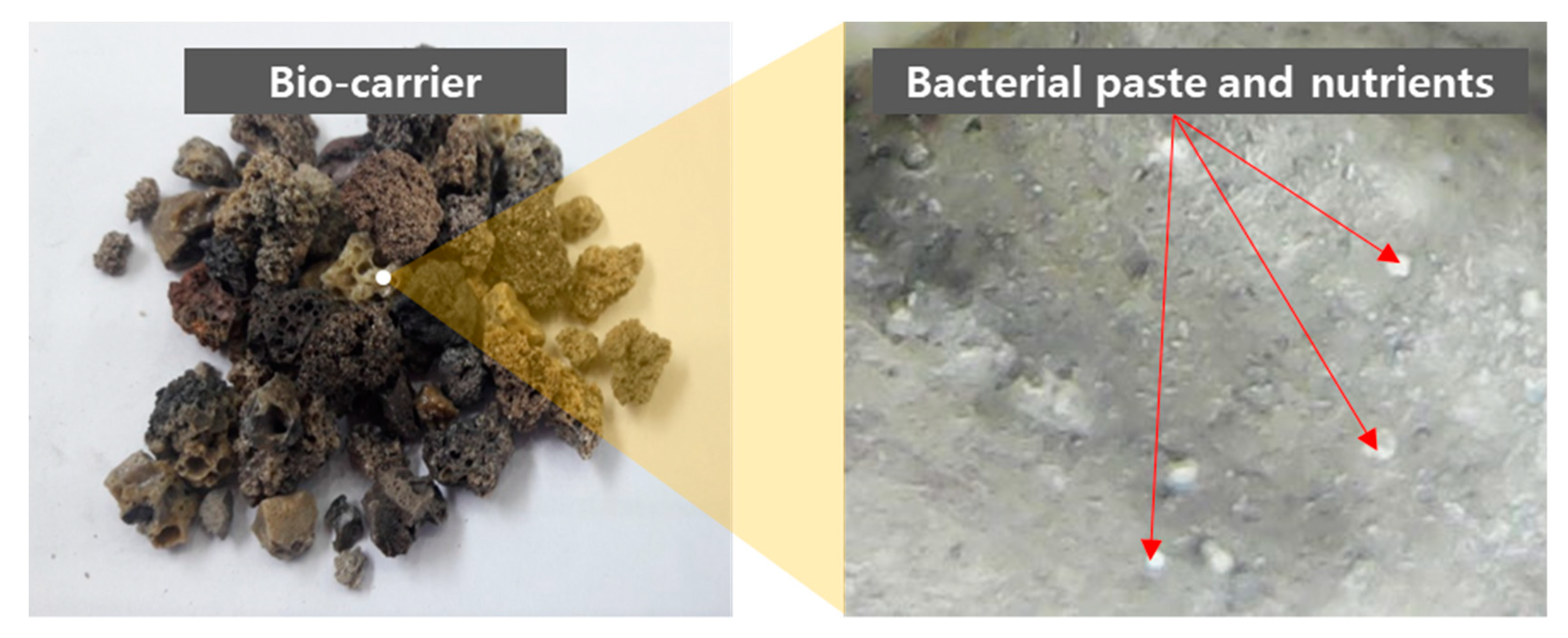




| Specimen ID | Cement | Water | Bacterial Solution * | Sand | Bottom Ash | Bio-Carrier |
|---|---|---|---|---|---|---|
| A | 1.0 | 0.5 | 1.5 | |||
| B | 1.0 | 0.5 | 1.5 | 0.2 | ||
| C | 1.0 | 0.5 | 1.5 | |||
| D | 1.0 | 0.5 | 1.5 | 0.2 | ||
| E | 1.0 | 0.5 | 1.5 | 0.2 |
| A | C | |||
|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | |
| Average pore diameter | 14.50 nm | 13.24 nm | 16.77 nm | 11.71 nm |
| Median pore diameter * | 24.19 nm | 15.33 nm | 30.91 nm | 13.08 nm |
| Porosity | 32.16% | 27.09% | 38.60% | 24.50% |
| Phase Name | A | C | ||
|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | |
| C3S | 3.2 | 1.2 | 7.8 | 2.1 |
| β-C2S | 8.9 | 5.9 | 9.3 | 8.6 |
| C3A | 0 | 0 | 2.3 | 0 |
| C4AF | 2.6 | 0.1 | 4.9 | 2.1 |
| Quartz | 1.1 | 2.8 | 2.2 | 3.1 |
| Portlandite | 15.4 | 16.1 | 14.5 | 17.3 |
| Calcite | 5.8 | 6.1 | 6.5 | 8.3 |
| Gypsum | 1.4 | 0.8 | 2.5 | 0.4 |
| Ettringite | 3.9 | 3.4 | 7.7 | 6.2 |
| Anhydrite | 0.1 | 0 | 0.6 | 0.5 |
| Lime | 3.3 | 0.8 | 4.8 | 0.6 |
| Amorphous | 54.3 | 62.8 | 36.9 | 50.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Son, H.; Seo, J.; Lee, H.K. Impact of Bio-Carrier Immobilized with Marine Bacteria on Self-Healing Performance of Cement-Based Materials. Materials 2020, 13, 4164. https://doi.org/10.3390/ma13184164
Kim H, Son H, Seo J, Lee HK. Impact of Bio-Carrier Immobilized with Marine Bacteria on Self-Healing Performance of Cement-Based Materials. Materials. 2020; 13(18):4164. https://doi.org/10.3390/ma13184164
Chicago/Turabian StyleKim, Hayeon, Hyeongmin Son, Joonho Seo, and H. K. Lee. 2020. "Impact of Bio-Carrier Immobilized with Marine Bacteria on Self-Healing Performance of Cement-Based Materials" Materials 13, no. 18: 4164. https://doi.org/10.3390/ma13184164
APA StyleKim, H., Son, H., Seo, J., & Lee, H. K. (2020). Impact of Bio-Carrier Immobilized with Marine Bacteria on Self-Healing Performance of Cement-Based Materials. Materials, 13(18), 4164. https://doi.org/10.3390/ma13184164





