Expanding NIST Calibration of Fluorescent Microspheres for Flow Cytometry to More Fluorescence Channels and Smaller Particles
Abstract
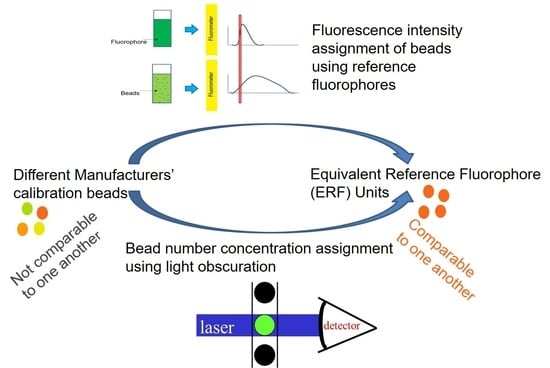
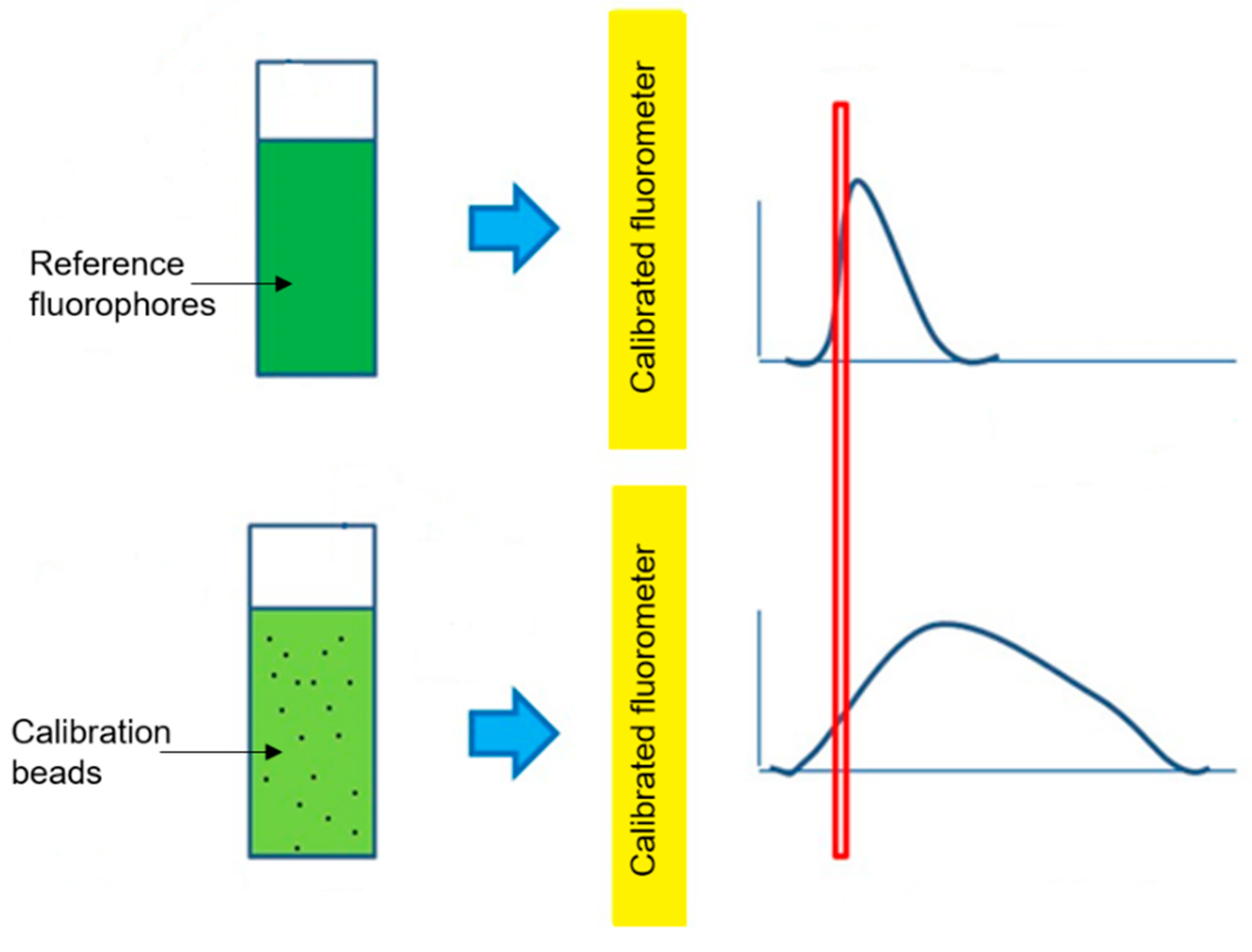
1. Introduction
2. Materials and Methods
2.1. New Reference Fluorophores
2.1.1. qNMR
2.1.2. HPLC
2.1.3. Reference Fluorophore Solutions
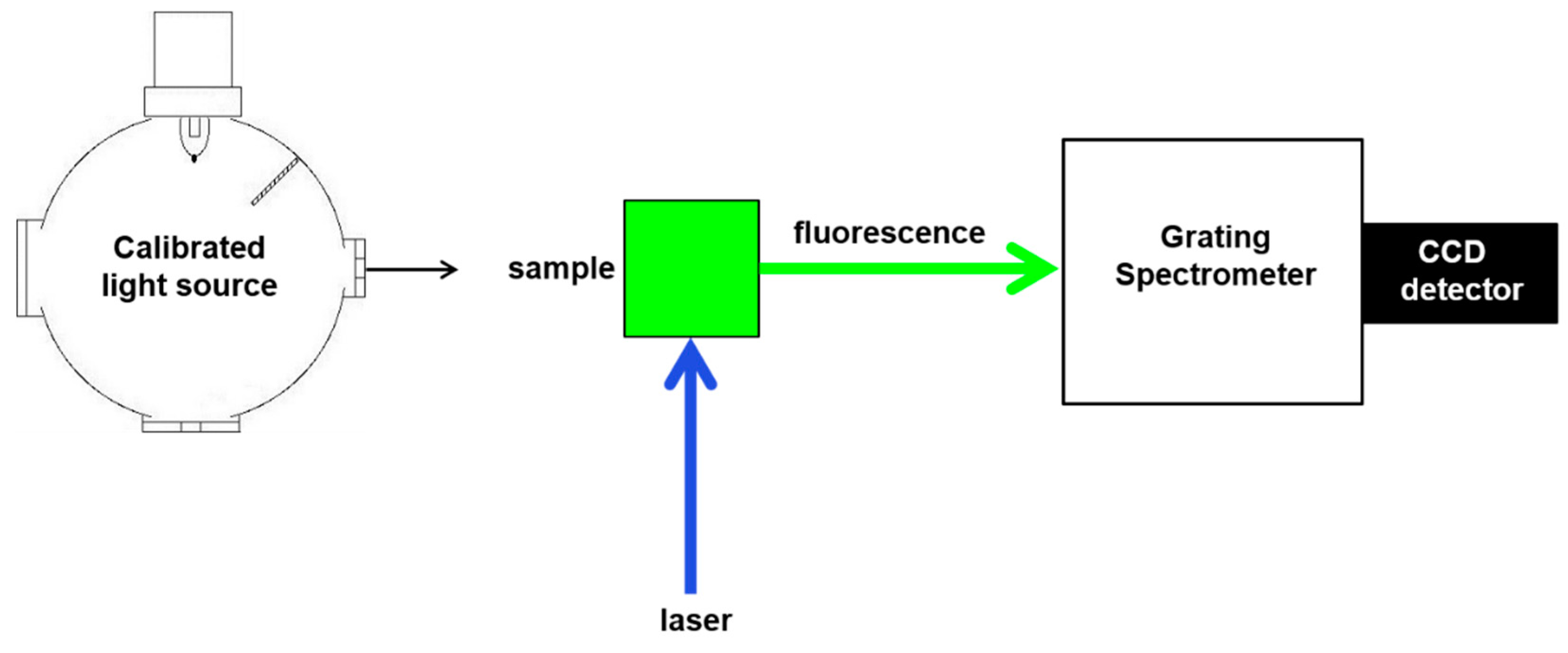
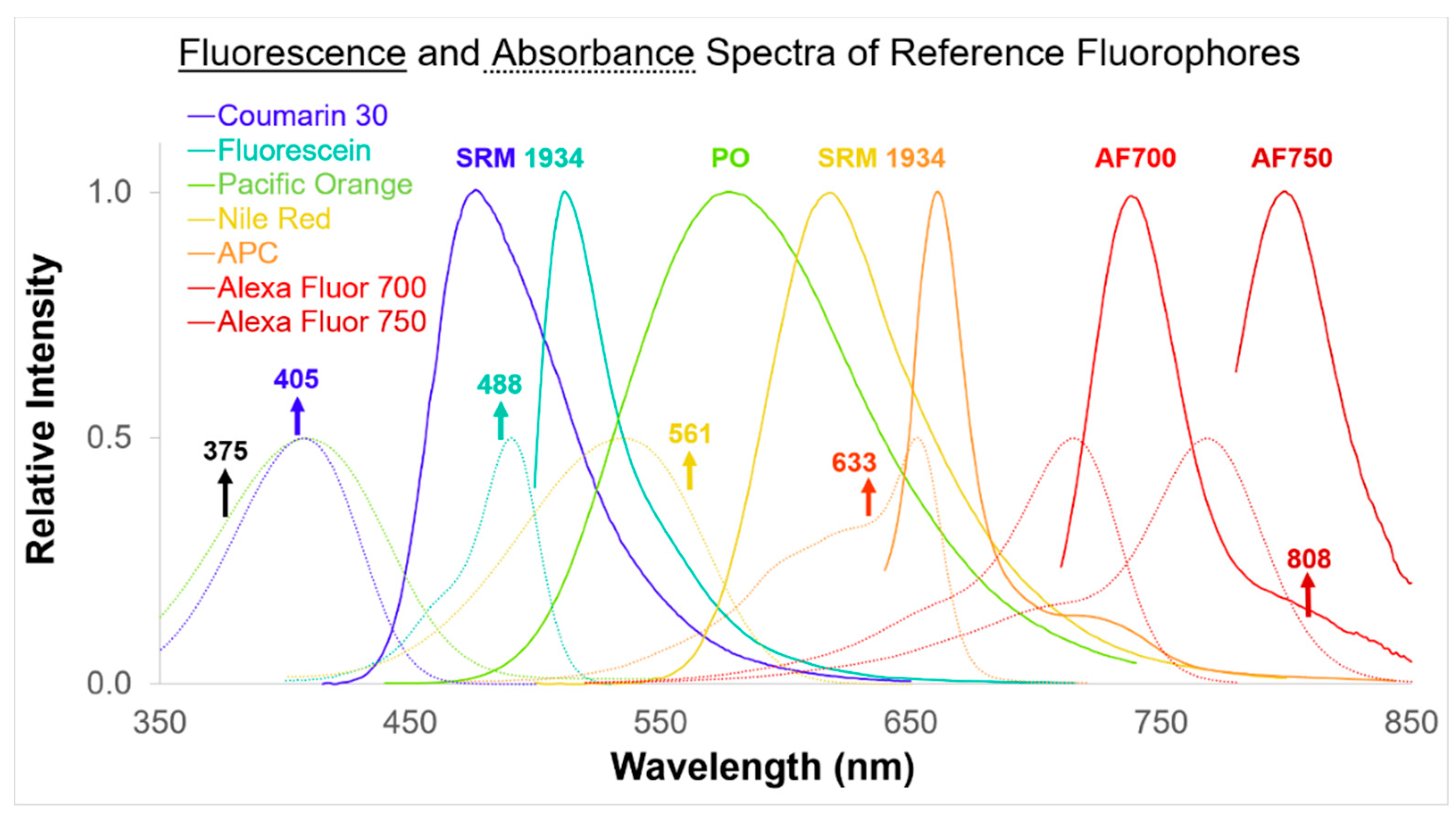
2.2. Fluorescence Spectra
2.3. Bead Number Concentration
2.3.1. Micrometer-Sized Particles
2.3.2. Submicrometer Particles
3. Results
3.1. New Reference Fluorophores—Purity and Concentration Determinations
3.1.1. qNMR Purity Determination
3.1.2. HPLC Purity Determination
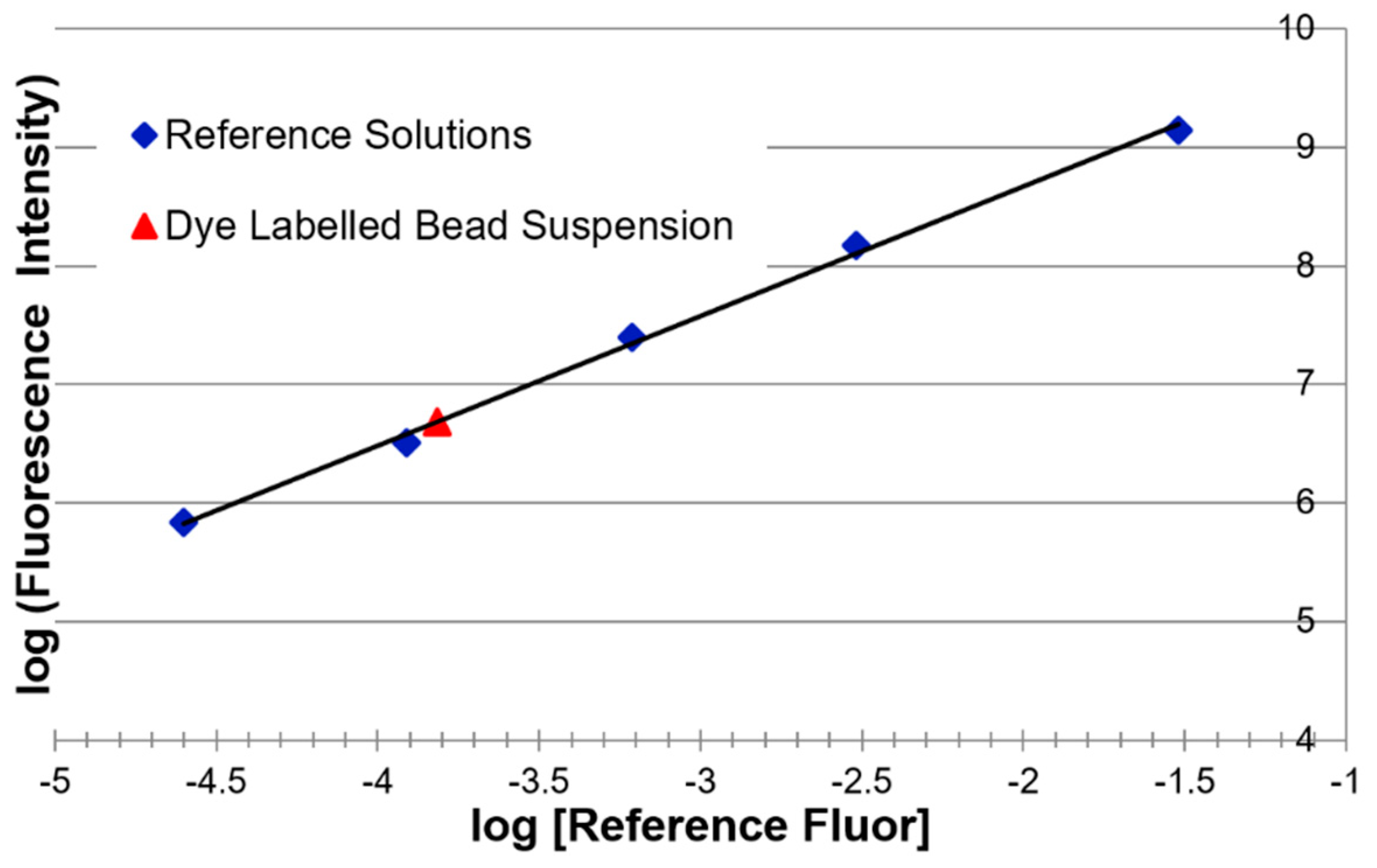
3.1.3. Reference Fluorophore Solutions—Concentration Determination
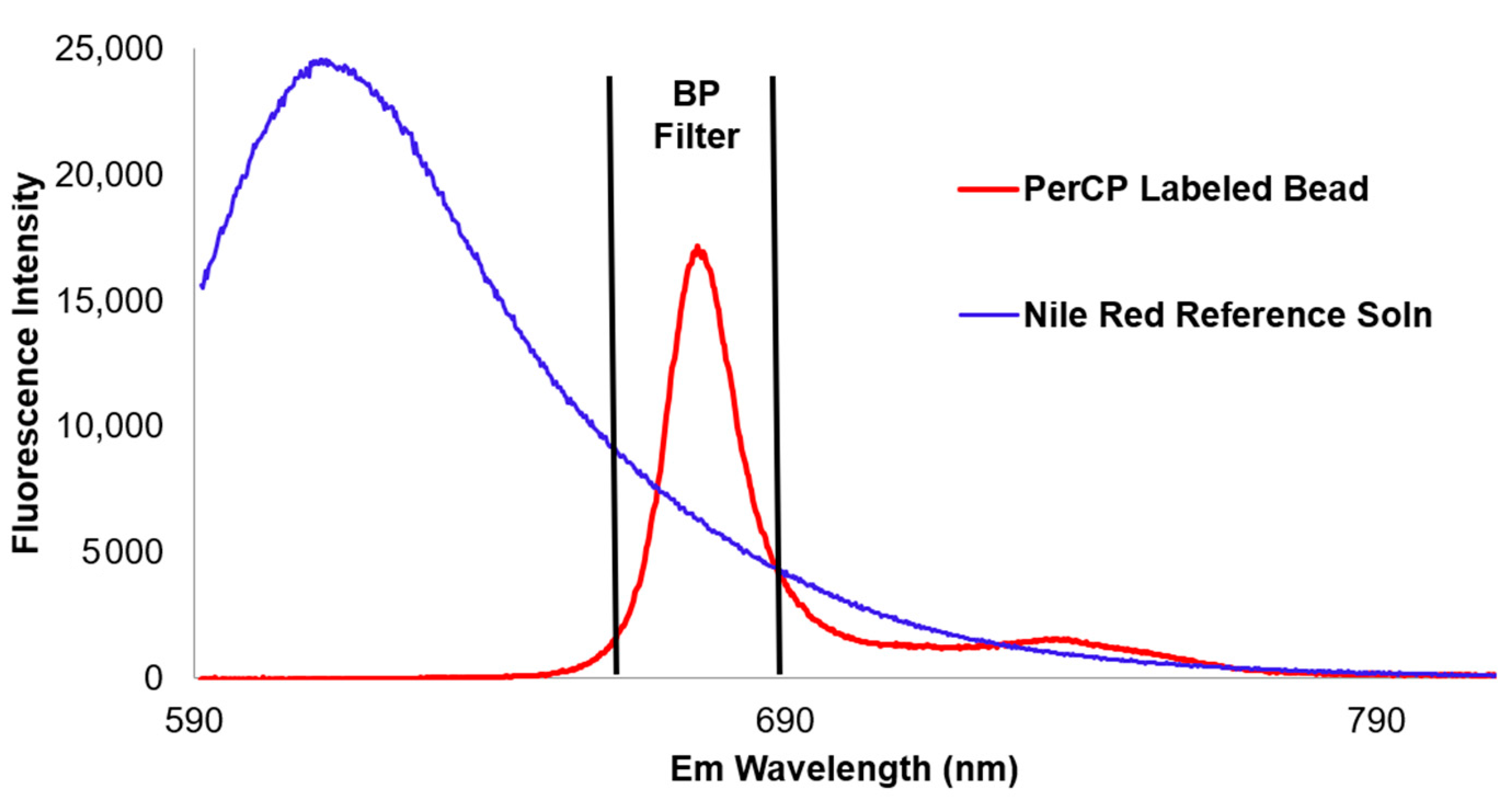
3.2. Fluorescence Intensity Measurements
3.3. Bead Concentration Measurements
3.3.1. Micrometer-sized Particle Counting
3.3.2. Submicrometer Particle Counting
3.4. ERF Assignments Per Bead
4. Discussion
4.1. New Reference Fluorophores—Purity and Concentration Analyses
4.2. ERF Assignments Per Bead—Fluorescence Channels
4.3. Submicrometer Particle Counting
| Technique | Size Limit/Range (nm) | Sample Volume | Sample Concentration mL−1 | Caveats | Ref. |
|---|---|---|---|---|---|
| LO | 2000 | 15 mL | 103 to 104 | size limit dilution error | [15] |
| EM | 1–100 | 10 mL | 1010 to 1012 | unknown volume collection time | [27,28] |
| AF4-DLS | 2 | 10 mL | 10 mg | not accurate | [20,26] |
| NTA | 10–1000 | 12 mL | 106 to 109 | need standard | [22,23] |
| RPS | 200 | 15 mL | 104 to 106 | need standard size limit dilution error | [24] |
| Next Gen RPS | 60 | 10 mL | 106 to 1010 | need standard | [18,19] |
| FCM | 80 | 100 mL | 105 to 107 | need standard | [17] |
| Quantum FCM | 30 | N/D † | N/D † | N/D † | [29] |
| Virus Counter | 25–300 | 200 mL | 105 to 109 | virus specific | [25] |
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
NIST Disclaimer
References
- NIST. Flow Cytometry Quantitation Consortium. Fed. Regist. 2016, 81, 46054–46055. [Google Scholar]
- Wang, L.; DeRose, P.C.; Gaigalas, A.K. Assignment of the Number of Equivalent Reference Fluorophores to Dyed Microspheres. J. Res. NIST 2016, 121, 264–281. [Google Scholar] [CrossRef]
- Wang, L.; Degheidy, H.; Abbasi, F.; Mostowski, H.; Marti, G.; Bauer, S.; Hoffman, R.A.; Gaigalas, A.K. Quantitative Flow Cytometry Measurements in Antibodies Bound per Cell Based on a CD4 Reference. Curr. Protoc. Cytom. 2016, 75, 1.29.1–1.29.14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gaigalas, A.K.; DeRose, P.C. A Model for the Binding of Fluorescently Labeled Anti-Human CD4 Monoclonal Antibodies to CD4 Receptors on Human Lymphocytes. J. Res. Natl. Inst. Stand. Technol. 2018, 123, 123022. [Google Scholar] [CrossRef]
- Hoffman, R.A.; Wang, L. Standardization, Calibration, and Control in Flow Cytometry. Curr. Protoc. Cytom. 2017, 79, 1.3.1–1.3.27. [Google Scholar]
- NIST. Certificate of Analysis, Standard Reference Material 1934, Fluorescent Dyes for Quantitative Flow Cytometry (Visible Spectral Range). 2016. Available online: https://www-s.nist.gov/srmors/view_cert.cfm?srm=1934 (accessed on 14 September 2020).
- NIST. Certificate of Analysis, NIST PS1, Primary Standard for Quantitative NMR (Benzoic Acid). 2018. Available online: https://www.nist.gov/system/files/documents/2020/07/16/NIST_PS1_COA.pdf (accessed on 14 September 2020).
- Duewer, D.L.; Parris, R.M.; White, E.W.; May, W.E.; Elbaum, H. Metrologically Sound Traceable Assessment of the Chemical Purity of Organic Reference Materials. In NIST Special Publication 1012; NIST: Gaithersburg, MD, USA, 2004. [Google Scholar]
- Nelson, M.A.; Waters, J.F.; Toman, B.; Lang, B.E.; Rück, A.; Breitruck, K.; Obkircher, M.; Windust, A.; Lippa, K.A. A New Realization of SI for Organic Chemical Measurement: NIST PS1 Primary Standard for Quantitative NMR (Benzoic Acid). Anal. Chem. 2018, 90, 10510–10517. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.H.; Saunders, R.D.; Hattenburg, A.T. National Bureau of Standards (U.S.) Special Publication 250-1; U.S. GPO: Washington, DC, USA, 1987.
- Yoon, H.W.; Gibson, C.E. NIST (U.S.) Special Publication 250-89; U.S. GPO: Washington, DC, USA, 2011.
- Larason, T.C.; Houston, J.M. NIST (U.S.) Special Publication 250-41; U.S. GPO: Washington, DC, USA, 2008.
- Larason, S.S.; Bruce, C.L. Cromer. J. Res. Natl. Inst. Stand. Technol. 1996, 101, 133. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.Y.; Early, E.A.; Parr, A.C. NIST (U.S.) Special Publication 250-48; U.S. GPO: Washington, DC, USA, 1998.
- Ripple, D.; DeRose, P.C. Primary Determination of Particle Number Concentration with Light Obscuration and Dynamic Imaging Particle Counters. J. Res. NIST 2018, 123, 002. [Google Scholar] [CrossRef]
- DeRose, P.C.; Wang, L. NIST Fluorescence-based Measurement Services. BioPharm Int. 2018, 31, 24–29. [Google Scholar]
- Brittain, G.C.; Chen, Y.Q.; Martinez, E.; Tang, V.A.; Renner, T.M.; Langlois, M.-A.; Gulnik, S. A Novel Semiconductor-Based Flow Cytometer with Enhanced Light-Scatter Sensitivity for the Analysis of Biological Nanoparticles. Sci. Rep. 2016, 9, 16039. [Google Scholar] [CrossRef]
- Varga, Z.; Feher, B.; Kitka, D.; Wacha, A.; Bota, A.; Berenyi, S.; Pipich, V.; Fraikin, J.-L. Size Measurement of Extracellular Vesicles and Synthetic Liposomes: The Impact of the Hydration Shell and the Protein Corona. Colloids Surf. B Biointerfaces 2020, 192, 111053. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; van der Pol, E.; Boing, A.N.; Hajji, N.; Sturk, G.; van Leeuwen, T.G.; Nieuwland, R. Reproducible Extracellular Vesicle Size and Concentration Determination with Tunable Resistive Pulse Sensing. J. Extracell. Vesicles 2014, 3, 25922. [Google Scholar] [CrossRef]
- Zhang, H.; Lyden, D. Asymmetrical flow field-flow fractionation Technology for Exomere and Small Extracellular Vesicle Separation and Characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- Gercel-Taylor, C.; Atay, S.; Tullis, R.H.; Kesimer, M.; Taylor, D.D. Nanoparticle Analysis of Circulating Cell-Derived Vesicles in Ovarian Cancer Patients. Anal. Biochem. 2012, 428, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Soo, C.Y.; Song, Y.; Zheng, Y.; Campbell, E.C.; Riches, A.C.; Gunn-Moore, F.; Powis, S.J. Nanoparticle Tracking Analysis Monitors Microvesicle and Exosome Secretion from Immune Cells. Immunology 2012, 136, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Desgeorges, A.; Hollerweger, J.; Lassacher, T.; Rohde, E.; Helmbrecht, C.; Gimona, M. Differential Fluorescence Nanoparticle Tracking Analysis for Enumeration of the Extracellular Vesicle Content in Mixed Particulate Solutions. Methods 2020, 177, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rhyner, M.N. The Coulter Principle for Analysis of Subvisible Particles in Protein Formulations. AAPS J. 2011, 13, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Gates, T.; Montange, R.; Schickert, A.; Shives, K.D.; Steaffens, J. Application Note: Rapid Real Time Quantification of Lentivirus Particles Using Antibody-based Detection on the Virus Counter 3100 Platform; Sartorius Stedim North America: Arvada, CO, USA, 2018. [Google Scholar]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Hoekstra, A.G.; Sturk, A.; Otto, C.; van Leeuwen, T.G.; Nieuwland, R. Optical and Non-optical Methods for Detection and Characterization of Microparticles and Exosomes. J. Thromb. Haemost. 2010, 8, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Kurokawa, A. Measurement of the Number Concentration of Gold Nanoparticle Suspension by Scanning Electron Microscopy. Metrologia 2019, 56, 044001. [Google Scholar] [CrossRef]
- Burenkov, I.A.; Cheng, Y.-H.; Polyakov, S. Towards Quantum-Enabled Flow Cytometry. In Proceedings of the CLEO: QELS_Fundamental Science 2018, San Jose, CA, USA, 13–18 May 2018. [Google Scholar]
| Units | PO | U95 | AF700 | U95 | AF750 | U95 |
|---|---|---|---|---|---|---|
| mg/kg | 20.10 | 0.46 | 20.15 | 0.34 | 15.99 | 0.43 |
| mol/kg | 3.724 × 10−5 | 8.6 × 10−7 | 2.044 × 10−5 | 3.4 × 10−7 | 1.812 × 10−5 | 4.9 × 10−7 |
| mol/L | 4.091 × 10−5 | 9.4 × 10−7 | 2.245 × 10−5 | 3.8 × 10−7 | 1.991 × 10−5 | 5.3 × 10−7 |
| Reference Fluor (nm) | λEX (nm)/λEm (nm) (nm) | Δλ (nm) (nm) | Reference Fluor (nm) | λEX (nm)/λEm (nm) | Δλ (nm) (nm) |
|---|---|---|---|---|---|
| C30 | 375/450 | 45 | NR | 488/610 | 20 |
| C30 | 375/525 | 40 | NR | 488/660 | 50 |
| PO | 375/675 | 30 | NR | 488/690 | 50 |
| C30 | 405/440 | 50 | NR | 488/695 | 40 |
| C30 | 405/450 | 45 | NR | 488/780 | 60 |
| C30 | 405/450 | 50 | NR | 561/585 | 42 |
| C30 | 405/512 | 25 | NR | 561/590 | 16 |
| C30 | 405/525 | 40 | NR | 561/610 | 20 |
| C30 | 405/525 | 50 | NR | 561/620 | 15 |
| C30 | 405/530 | 30 | NR | 561/670 | 30 |
| C30 | 405/605 | 40 | NR | 561/675 | 30 |
| PO | 405/610 | 20 | NR | 561/710 | 50 |
| PO | 405/615 | 24 | NR | 561/720 | 60 |
| PO | 405/660 | 10 | NR | 561/763 | 43 |
| PO | 405/670 | 30 | NR | 561/789 | 78 |
| PO | 405/763 | 43 | APC | 633/660 | 10 |
| FL | 488/525 | 20 | APC | 633/665 | 20 |
| FL | 488/525 | 35 | APC | 633/670 | 14 |
| FL | 488/525 | 40 | APC | 633/670 | 30 |
| FL | 488/525 | 50 | APC | 633/710 | 50 |
| FL | 488/530 | 30 | AF 700 | 633/712 | 25 |
| FL | 488/530 | 40 | AF 700 | 633/720 | 30 |
| NR | 488/574 | 26 | AF 700 | 633/763 | 43 |
| FL | 488/585 | 40 | APC | 633/780 | 60 |
| FL | 488/585 | 42 | AF 700 | 633/780 | 60 |
| NR | 488/593 | 52 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeRose, P.; Tian, L.; Elsheikh, E.; Urbas, A.; Zhang, Y.-Z.; Wang, L. Expanding NIST Calibration of Fluorescent Microspheres for Flow Cytometry to More Fluorescence Channels and Smaller Particles. Materials 2020, 13, 4111. https://doi.org/10.3390/ma13184111
DeRose P, Tian L, Elsheikh E, Urbas A, Zhang Y-Z, Wang L. Expanding NIST Calibration of Fluorescent Microspheres for Flow Cytometry to More Fluorescence Channels and Smaller Particles. Materials. 2020; 13(18):4111. https://doi.org/10.3390/ma13184111
Chicago/Turabian StyleDeRose, Paul, Linhua Tian, Elzafir Elsheikh, Aaron Urbas, Yu-Zhong Zhang, and Lili Wang. 2020. "Expanding NIST Calibration of Fluorescent Microspheres for Flow Cytometry to More Fluorescence Channels and Smaller Particles" Materials 13, no. 18: 4111. https://doi.org/10.3390/ma13184111
APA StyleDeRose, P., Tian, L., Elsheikh, E., Urbas, A., Zhang, Y.-Z., & Wang, L. (2020). Expanding NIST Calibration of Fluorescent Microspheres for Flow Cytometry to More Fluorescence Channels and Smaller Particles. Materials, 13(18), 4111. https://doi.org/10.3390/ma13184111