Comparative Study on Chloride Binding Capacity of Cement-Fly Ash System and Cement-Ground Granulated Blast Furnace Slag System with Diethanol-Isopropanolamine
Abstract
1. Introduction
2. Materials and Test Methods
2.1. Materials
2.1.1. Cement
2.1.2. Chemicals
2.1.3. Preparation of the Samples
2.2. Test Methods
2.2.1. Chloride Binding Ratio
2.2.2. Compressive Strength
2.2.3. Hydration Heat
2.2.4. Pore Structure
2.2.5. Phase Analysis
3. Results and Discussion
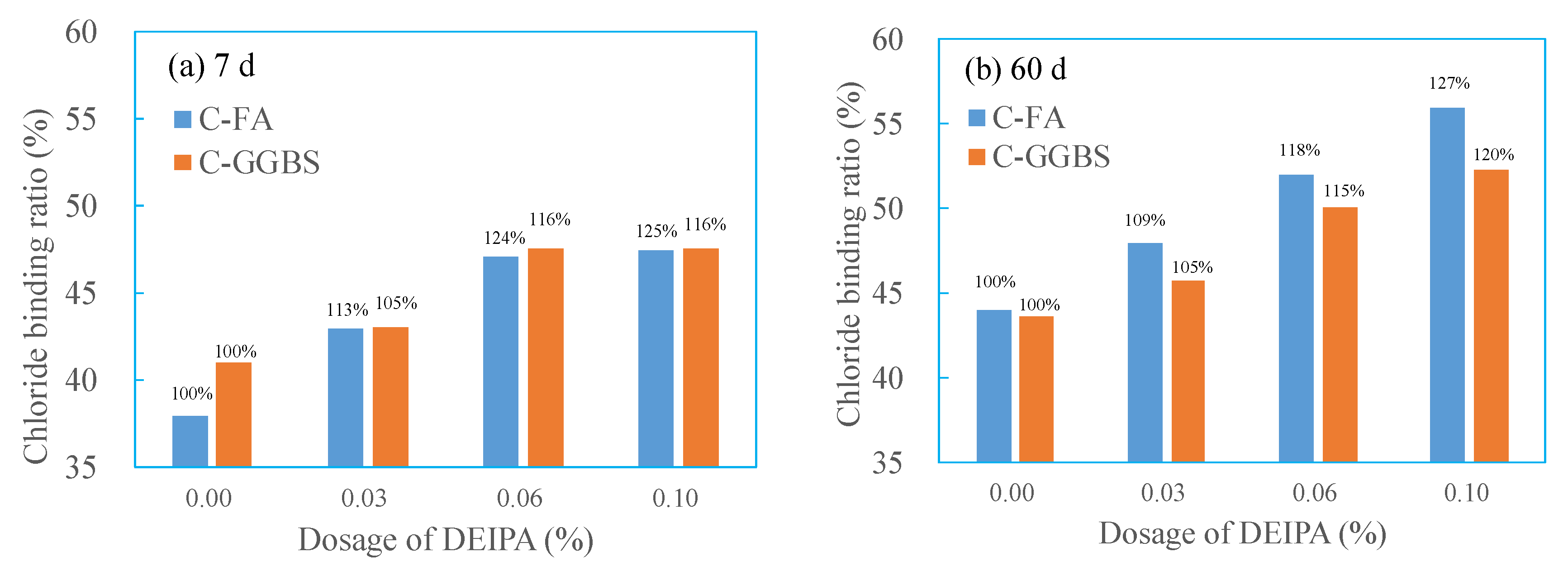
3.1. Effect of DEIPA on Chloride Binding Capacity
3.2. Hydration Process
3.2.1. Hydration Heat
3.2.2. Compressive Strength
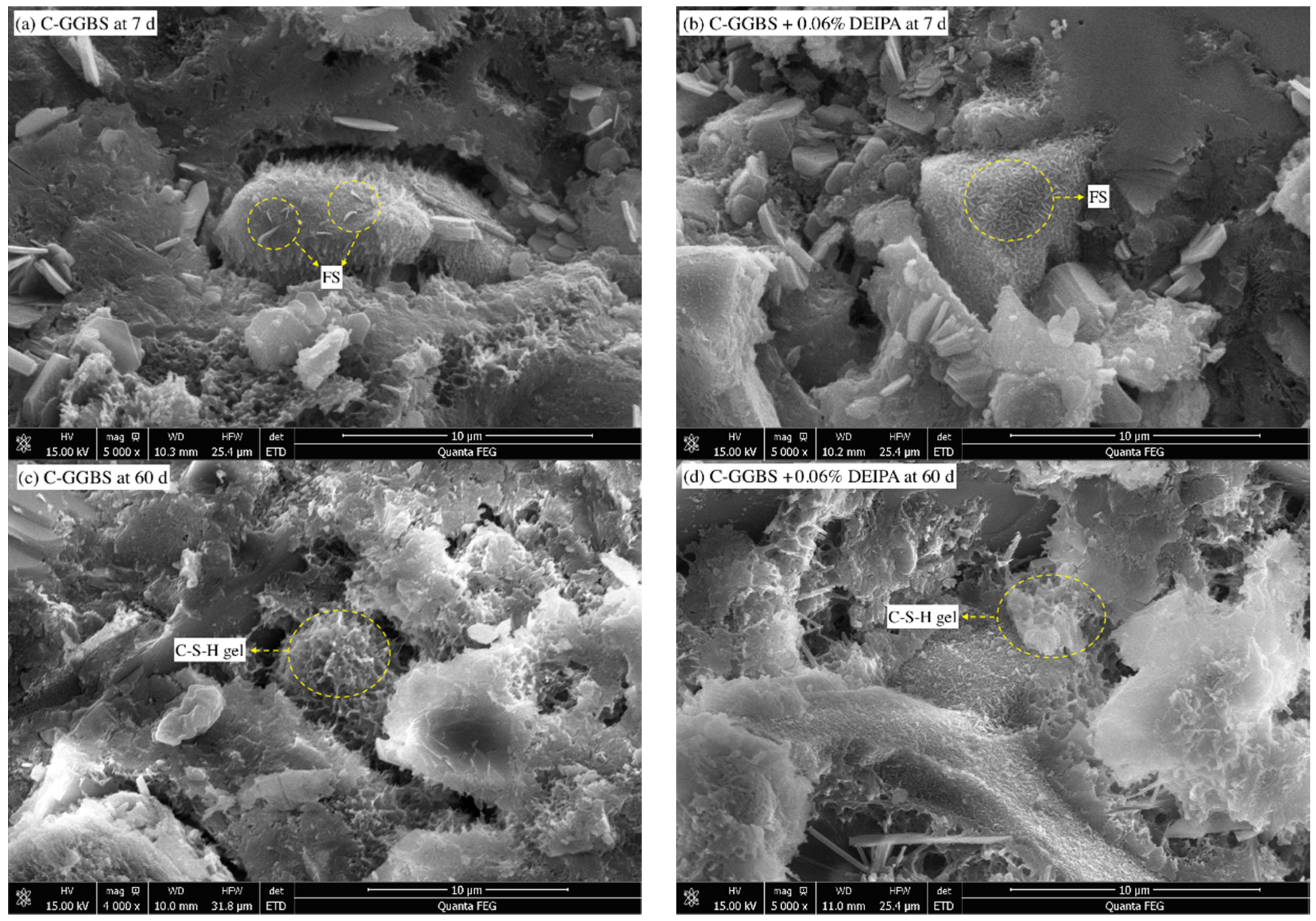
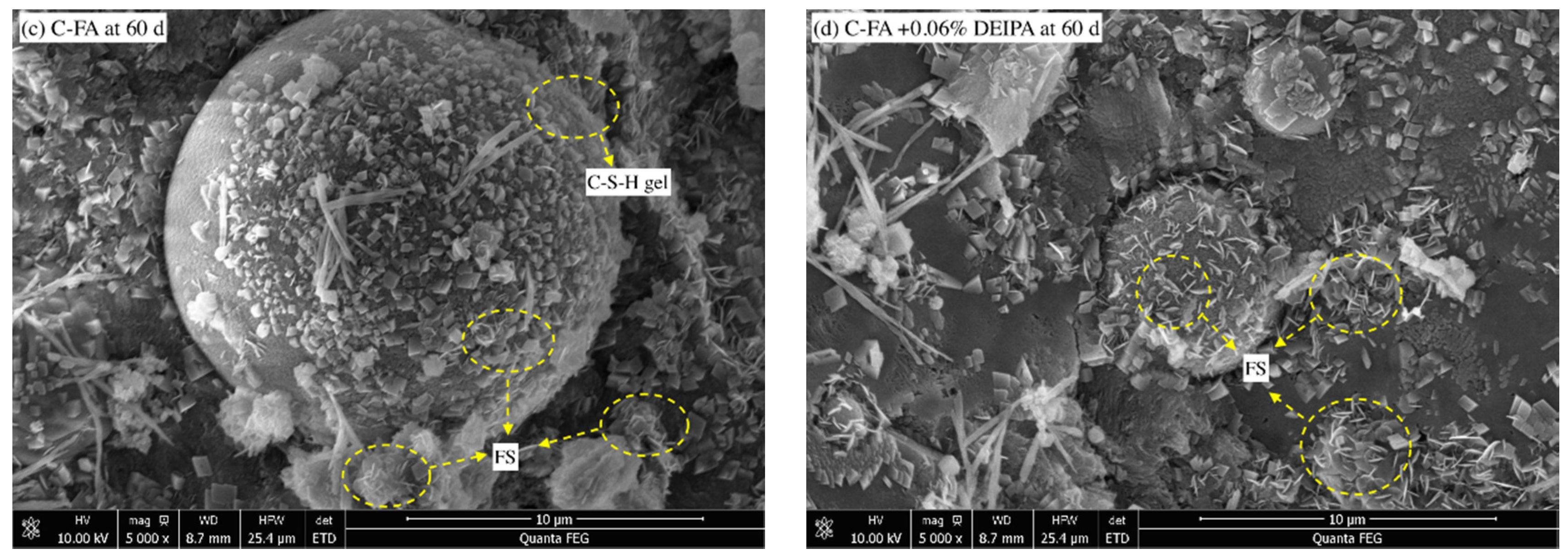
3.3. Hydrates Analysis
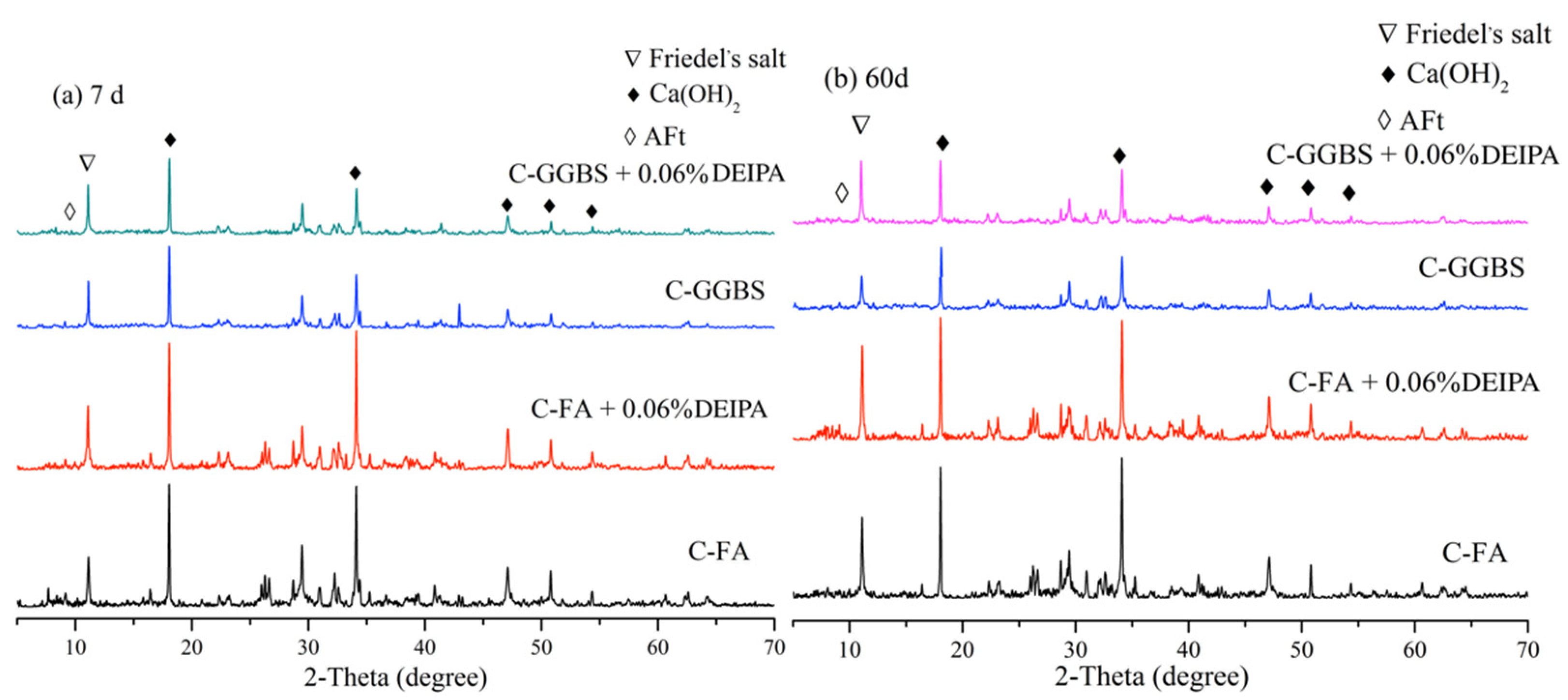
3.3.1. XRD
3.3.2. TG
3.3.3. SEM
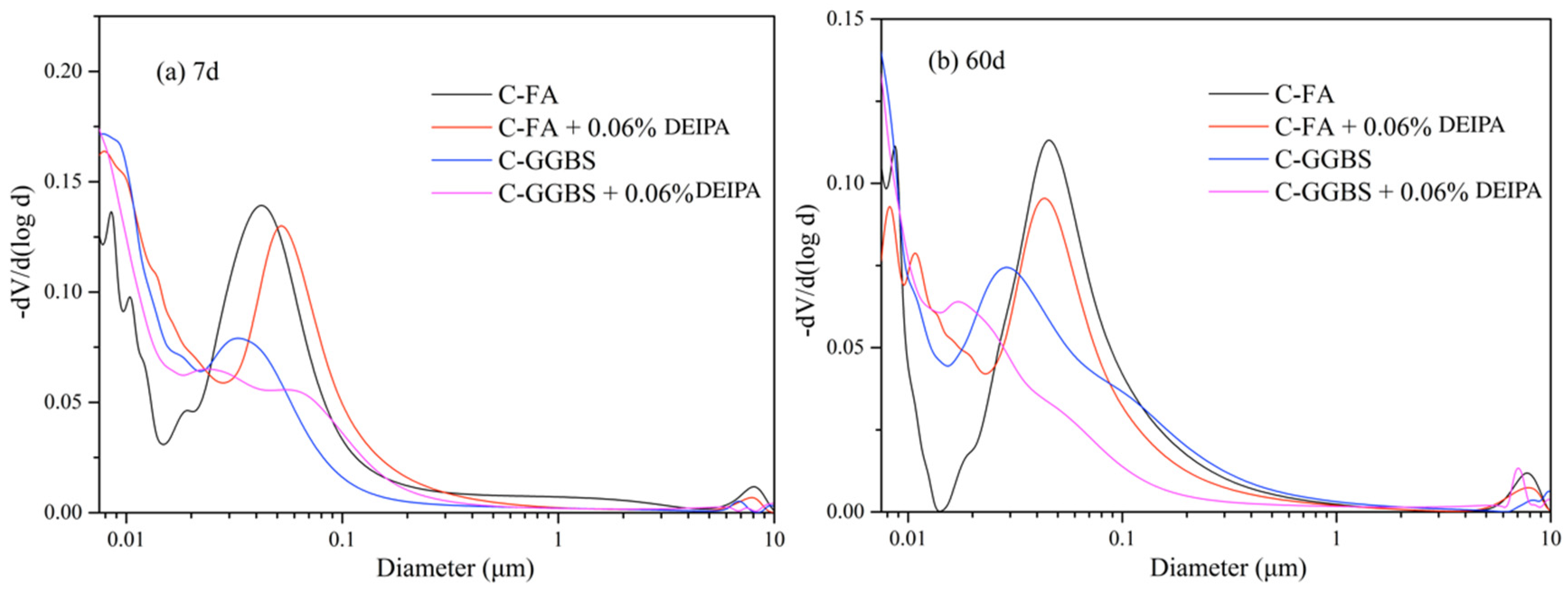
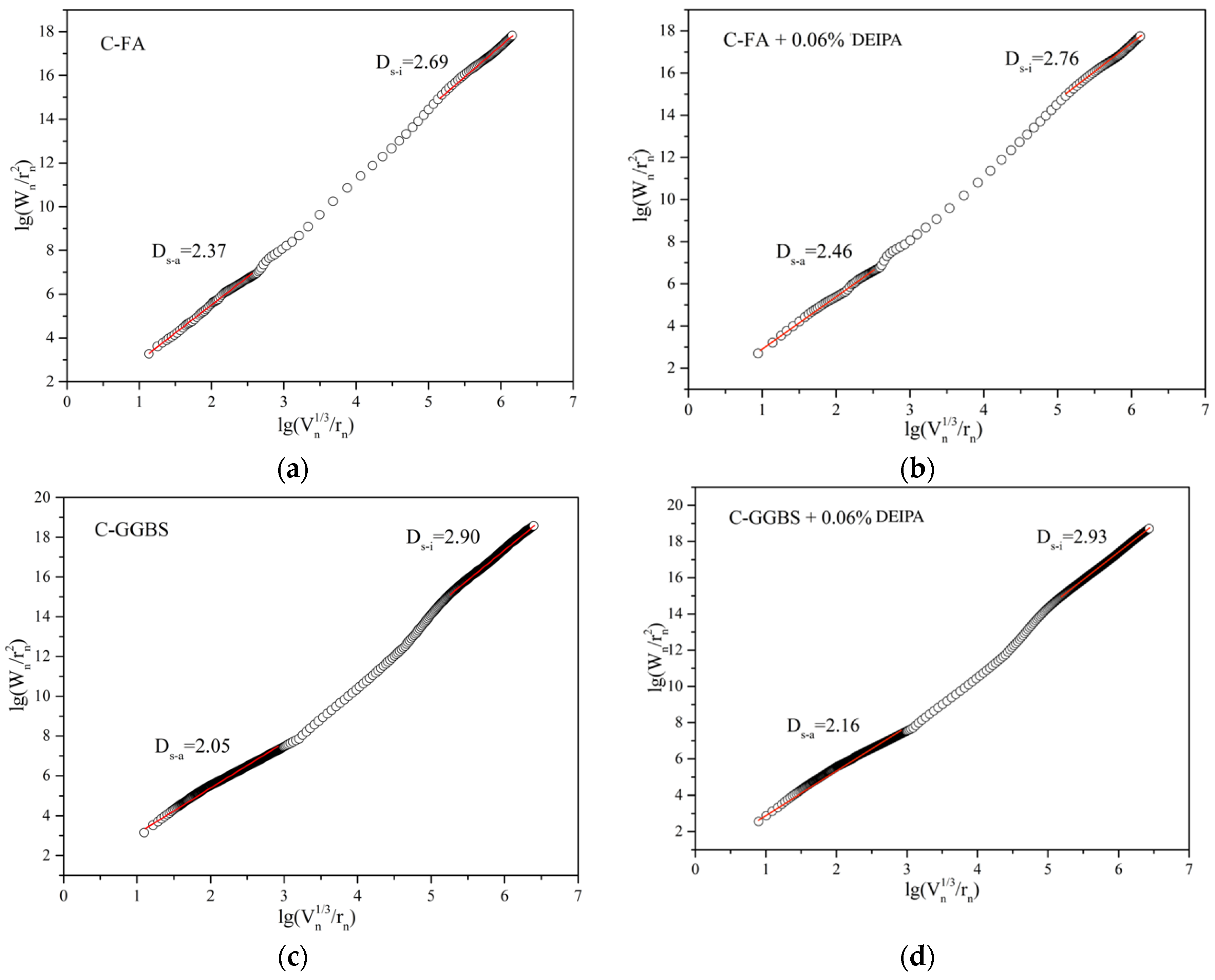
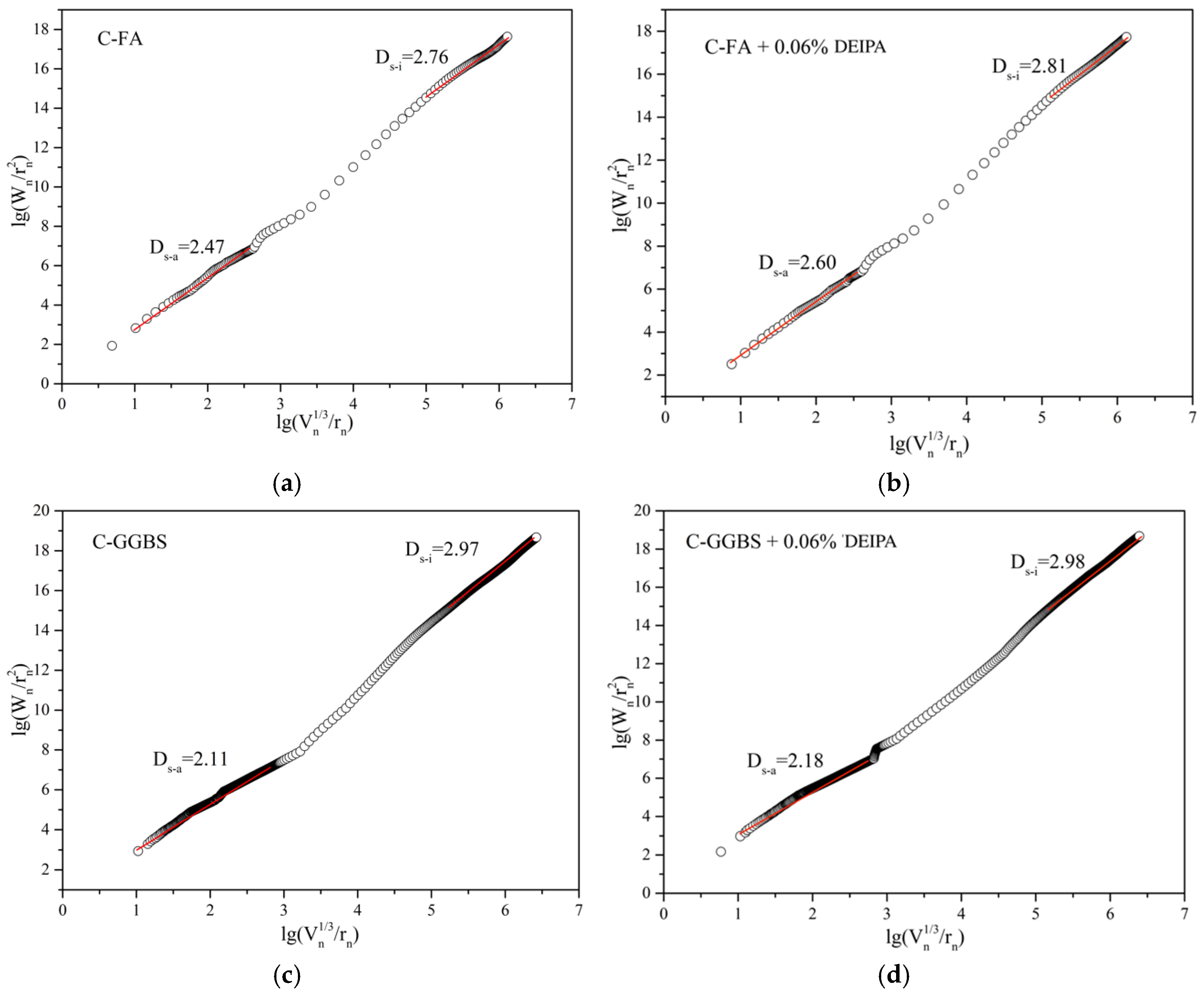
3.4. Pore Structure
3.5. Discussion
3.5.1. Effect of DEIPA on Hydration Process
3.5.2. Effect of DEIPA on Chloride Binding Capacity
4. Conclusions
- (1)
- DEIPA facilitated the hydration and increased the compressive strength of both C-FA and C-GGBS systems, and the main reason was that DEIPA facilitate the pozzolanic reaction of FA and GGBS.
- (2)
- DEIPA increased the chloride binding capacity of both the C-FA and C-GGBS systems. One reason was that DEIPA facilitated the dissolution of aluminate to hasten the formation of FS, with contribution to the chemical binding. Other reasons were that DEIPA increased the complex of pore structure, which increased the migration resistance and expedited the hydration of the system to produce more amount of C-S-H gel which benefited to the physical binding.
- (3)
- By contrast, DEIPA presented greater ability to elevate the chloride binding capacity in the C-FA system. The reason for this was that DEIPA showed stronger ability to expedite the dissolution of aluminate of FA than that of GGBS.
Author Contributions
Funding
Conflicts of Interest
References
- Bendixen, M.; Best, J.; Hackney, C.; Iversen, L.L. Time is running out for sand. Nature 2019, 571, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zou, C. CT study on meso-crack propagation of gradient composite concrete subjected to sulfate erosion. Mag. Concr. Res. 2015, 67, 1127–1134. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Zhang, P.; Ma, Y.; Zhao, T.; Wang, H.; Zhang, Z. Water absorption and chloride diffusivity of concrete under the coupling effect of uniaxial compressive load and freeze–thaw cycles. Constr. Build. Mater. 2019, 209, 566–576. [Google Scholar] [CrossRef]
- Bao, J.; Li, S.; Zhang, P.; Ding, X.; Xue, S.; Cui, Y.; Zhao, T. Influence of the incorporation of recycled coarse aggregate on water absorption and chloride penetration into concrete. Constr. Build. Mater. 2020, 239, 117845. [Google Scholar] [CrossRef]
- Hyatt, N.C.; Ojovan, M.I. Special Issue: Materials for Nuclear Waste Immobilization. Materials 2019, 12, 3611. [Google Scholar] [CrossRef] [PubMed]
- Orlova, A.I.; Ojovan, M.I. Ceramic Mineral Waste-Forms for Nuclear Waste Immobilization. Materials 2019, 12, 2638. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Glassy Wasteforms for Nuclear Waste Immobilization. Metall. Mater. Trans. Phys. Metall. Mater. Sci. 2011, 42, 837–851. [Google Scholar] [CrossRef]
- Yang, Z.; Sui, S.; Wang, L.; Feng, T.; Gao, Y.; Mu, S.; Tang, L.; Jiang, J. Improving the chloride binding capacity of cement paste by adding nano-Al2O3: The cases of blended cement pastes. Constr. Build Mater. 2020, 232, 784–795. [Google Scholar] [CrossRef]
- Mesbah, A.; Cau-dit-Coumes, C.; Renaudin, G.; Frizon, F.; Leroux, F. Uptake of chloride and carbonate ions by calcium monosulfoaluminate hydrate. Cem. Concr. Res. 2012, 42, 1157–1165. [Google Scholar] [CrossRef]
- Gbozee, M.; Zheng, K.; He, F.; Zeng, X. The influence of aluminum from metakaolin on chemical binding of chloride ions in hydrated cement pastes. Appl. Clay Sci. 2018, 158, 186–194. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Zhang, T.; Mei, J.; Qi, H.; Chen, P.; Wang, J. Effects of colloidal nano-SiO2 on the immobilization of chloride ions in cement-fly ash system. Cem. Concr. Compos. 2020, 110, 103596. [Google Scholar] [CrossRef]
- Li, C.; Ma, B.; Tan, H.; Zhang, T.; Liu, X.; Chen, P. Effect of triisopropanolamine on chloride binding of cement paste with ground-granulated blast furnace slag. Constr. Build. Mater. 2020, 256, 119494. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, T.; Tan, H.; Liu, X.; Mei, J.; Jiang, W.; Qi, H.; Gu, B. Effect of TIPA on Chloride Immobilization in Cement-Fly Ash Paste. Adv. Mater. Sci. Eng. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X. How carbonation curing influences ca leaching of Portland cement paste: Mechanism and mathematical modeling. J. Am. Ceram. Soc. 2019, 102, 7755–7767. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Gao, X.; Zhang, J. Effect of pulverized fuel ash, ground granulated blast-furnace slag and CO2 curing on performance of magnesium oxysulfate cement. Constr. Build. Mater. 2020, 230, 116990. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Gao, X.; Li, Y.; Cai, J.; Wang, J. Influence of salt freeze-thaw cycles on the damage and the following electrical and self-sensing performance of carbon nanofibers concrete. Mater. Res. Express 2019, 6, 025705. [Google Scholar] [CrossRef]
- Yousefi Oderji, S.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, S.; Xiao, Y.; Zeng, W.; Yi, M.; Wan, J. Effect of hydration and silicone resin on Basic Oxygen Furnace slag and its asphalt mixture. J. Clean. Prod. 2016, 112, 392–400. [Google Scholar] [CrossRef]
- Tao, G.; Xiao, Y.; Yang, L.; Cui, P.; Kong, D.; Xue, Y. Characteristics of steel slag filler and its influence on rheological properties of asphalt mortar. Constr. Build. Mater. 2019, 201, 439–446. [Google Scholar] [CrossRef]
- Pan, P.; Wu, S.; Xiao, Y.; Liu, G. A review on hydronic asphalt pavement for energy harvesting and snow melting. Renew. Sustain. Energ. Rev. 2015, 48, 624–634. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Li, L.; Ren, Q.; Yang, X.; Jiang, Z.; Zhang, Z. Utilization of low-quality desulfurized ash from semi-dry flue gas desulfurization by mixing with hemihydrate gypsum. Fuel 2019, 255, 115783. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Liu, J.; Ren, M.; Lu, A. Multi-functional properties of carbon nanofiber reinforced reactive powder concrete. Constr. Build. Mater. 2018, 187, 699–707. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Dong, B.; Chen, Q.; Gu, L.; Yang, X.; Wang, W. Differences between their influences of TEA and TEA·HCl on the properties of cement paste. Constr. Build. Mater. 2020, 239, 117797. [Google Scholar] [CrossRef]
- Heinz, D.; Göbel, M.; Hilbig, H.; Urbonas, L.; Bujauskaite, G. Effect of TEA on fly ash solubility and early age strength of mortar. Cem. Concr. Res. 2010, 40, 392–397. [Google Scholar] [CrossRef]
- Ma, S.H.; Li, W.F.; Zhang, S.B.; Hu, Y.Y.; Shen, X.D. Study on the hydration and microstructure of Portland cement containing diethanol-isopropanolamine. Cem. Concr. Res. 2015, 67, 122–130. [Google Scholar] [CrossRef]
- Riding, K.; Silva, D.A.; Scrivener, K. Early age strength enhancement of blended cement systems by CaCl2 and diethanol-isopropanolamine. Cem. Concr. Res. 2010, 40, 935–946. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; He, X.; Zhao, R.; Chen, P.; Su, Y.; Yang, J. Preparation of ultrafine fly ash by wet grinding and its utilization for immobilizing chloride ions in cement paste. Waste Manag. 2020, 113, 456–468. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Li, H.; Mei, J.; Zhang, T.; Chen, P.; Gu, B. Chloride immobilization of cement-based material containing nano-Al2O3. Constr. Build. Mater. 2019, 220, 43–52. [Google Scholar] [CrossRef]
- Chen, P.; Ma, B.; Tan, H.; Liu, X.; Zhang, T.; Qi, H.; Peng, Y.; Yang, Q.; Wang, J. Effects of amorphous aluminum hydroxide on chloride immobilization in cement-based materials. Constr. Build. Mater. 2020, 231, 117171. [Google Scholar] [CrossRef]
- Zhang, B.; Li, S. Determination of the surface fractal dimension for porous media by mercury porosimetry. Ind. Eng. Chem. Res. 1995, 34, 1383–1386. [Google Scholar] [CrossRef]
- Yang, J.; Su, Y.; He, X.; Tan, H.; Jiang, Y.; Zeng, L.; Strnadel, B. Pore structure evaluation of cementing composites blended with coal by-products: Calcined coal gangue and coal fly ash. Fuel Process. Technol. 2018, 181, 75–90. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-Chong, T.; Dangla, P. Surface fractal analysis of pore structure of high-volume fly-ash cement pastes. Appl. Surf. Sci. 2010, 257, 762–768. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.; Liu, X. Scale-dependent nature of the surface fractal dimension for bi- and multi-disperse porous solids by mercury porosimetry. Appl. Surf. Sci. 2006, 253, 1349–1355. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Gu, B.; Zhang, T.; Chen, P.; Li, H.; Mei, J. Effect of aluminum sulfate on the hydration of Portland cement, tricalcium silicate and tricalcium aluminate. Constr. Build. Mater. 2020, 232, 117179. [Google Scholar] [CrossRef]
- Tan, H.; Li, M.; Ren, J.; Deng, X.; Zhang, X.; Nie, K.; Zhang, J.; Yu, Z. Effect of aluminum sulfate on the hydration of tricalcium silicate. Constr. Build. Mater. 2019, 205, 414–424. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Gao, L.; Chen, P. Improvement in chloride immobilization of cement-metakaolin system by triisopropanolamine. Appl. Clay. Sci. 2020, 193, 105656. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.; Yang, X. Effect and characterization of the nucleation C-S-H seed on the reactivity of granulated blast furnace slag powder. Constr. Build. Mater. 2020, 238, 117726. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Jiang, Z.; Suo, Z.; Zhang, G.; Wu, J.; Yu, L. Effect of different grinding aids on property of granulated blast furnace slag powder. Mater. Struct. 2015, 48, 3885–3893. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Li, H.; Jiang, Z.; Gong, Z.; Yang, X.; Chen, Q. Utilization of the black tea powder as multifunctional admixture for the hemihydrate gypsum. J. Clean. Prod. 2019, 210, 231–237. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Li, H.; Ren, Q. Performance and ITZ of pervious concrete modified by vinyl acetate and ethylene copolymer dispersible powder. Constr. Build. Mater. 2020, 235, 117532. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; He, X.; Su, Y. Pore structure of affected zone around saturated and large superabsorbent polymers in cement paste. Cem. Concr. Compos. 2019, 97, 54–67. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Su, Y.; He, X.; Tan, H.; Yang, W.; Strnadel, B. Eco-friendly treatment of low-calcium coal fly ash for high pozzolanic reactivity: A step towards waste utilization in sustainable building material. J. Clean. Prod. 2019, 238, 117962. [Google Scholar] [CrossRef]
- Gu, L.; Li, H.; Yang, X.; Dong, B.; Wen, Z. Leakage behavior of toxic substances of naphthalene sulfonate-formaldehyde condensation from cement based materials. J. Environ. Manag. 2020, 255, 109934. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, T.; Tan, H.; Liu, X.; Mei, J.; Qi, H.; Jiang, W.; Zou, F. Effect of triisopropanolamine on compressive strength and hydration of cement-fly ash paste. Constr. Build. Mater. 2018, 179, 89–99. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Li, H.; Xu, L.; Ren, Q.; Li, L. Effect of triethanolamine hydrochloride on the performance of cement paste. Constr. Build. Mater. 2019, 200, 218–225. [Google Scholar] [CrossRef]
- Hu, M.; Guo, J.; Xu, Y.; Fan, J.; Cao, L.; Wang, M.; Feng, Y. Influence of triethanolamine on reactivity of hydrated matrix in sodium silicate self-healing system and the mechanism. Constr. Build. Mater. 2018, 185, 445–452. [Google Scholar] [CrossRef]
- Gartner, E.; Myers, D. Influence of Tertiary Alkanolamines on Portland Cement Hydration. J. Am. Ceram. Soc. 2010, 76, 1521–1530. [Google Scholar] [CrossRef]
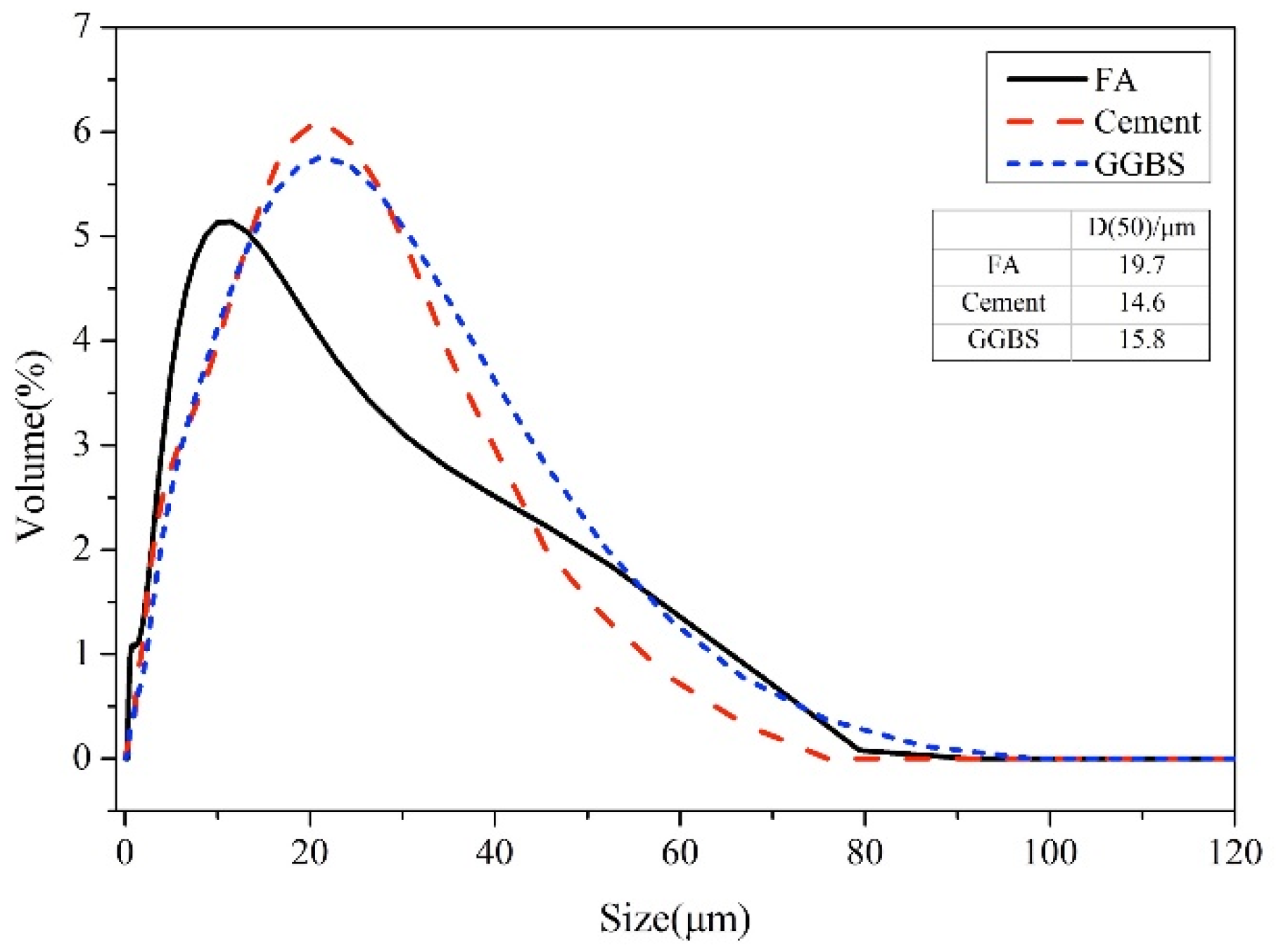
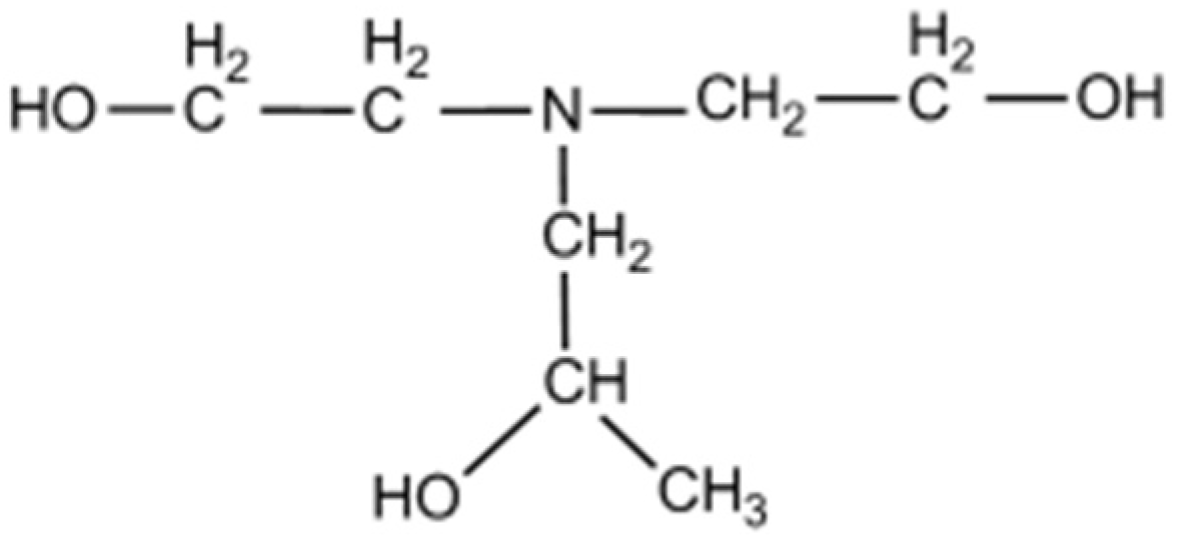



| Raw Materials | LOI. | CaO | MgO | SiO2 | Al2O3 | Fe2O3 | SO3 | K2O | Na2O | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | wt% | 3.81 | 58.24 | 1.95 | 24.08 | 4.72 | 2.46 | 2.31 | 1.02 | 0.27 |
| FA | wt% | 5.97 | 4.12 | 0.50 | 48.33 | 31.69 | 4.14 | 1.37 | 1.34 | 0.37 |
| GGBS | wt% | 4.35 | 39.37 | 10.84 | 30.01 | 14.02 | 0.42 | 2.49 | 0.30 | 0.43 |
| System | Cement | GGBS | FA | NaCl | DEIPA |
|---|---|---|---|---|---|
| C-GGBS | 70 | 30 | - | 1.11 | 0 |
| 70 | 30 | - | 1.11 | 0.03 | |
| 70 | 30 | - | 1.11 | 0.05 | |
| 70 | 30 | - | 1.11 | 0.10 | |
| C-FA | 70 | - | 30 | 1.11 | 0 |
| 70 | - | 30 | 1.11 | 0.03 | |
| 70 | - | 30 | 1.11 | 0.05 | |
| 70 | - | 30 | 1.11 | 0.10 |
| Time (h) | Hydration Heat (J/g) | |||||
|---|---|---|---|---|---|---|
| C-FA | C-GGBS | |||||
| 0.0% | 0.06%DEIPA | 0.10%DEIPA | 0.0% | 0.06%DEIPA | 0.10%DEIPA | |
| 6 | 8.20 | 9.02 | 8.83 | 23.00 | 30.06 | 24.90 |
| 12 | 24.04 | 24.21 | 23.78 | 53.00 | 58.11 | 51.84 |
| 24 | 91.03 | 93.49 | 96.19 | 126.00 | 144.38 | 136.61 |
| 72 | 179.05 | 187.23 | 194.27 | 218.29 | 241.97 | 229.35 |
| 168 | 225.00 | 234.85 | 244.07 | 308.16 | 344.10 | 325.29 |
| Temperature/°C | C-FA | C-GGBS | ||
|---|---|---|---|---|
| Blank | 0.06% DEIPA | Blank | 0.06% DEIPA | |
| 50–200 °C | 8.49 | 9.25 | 10.55 | 11.71 |
| 280–390 °C | 1.54 | 1.61 | 1.74 | 1.95 |
| 400–500 °C | 2.64 | 2.71 | 2.40 | 2.42 |
| CH content | 10.85 | 11.14 | 9.87 | 9.95 |
| Temperature/°C | C-FA | C-GGBS | ||
|---|---|---|---|---|
| Blank | 0.06% DEIPA | Blank | 0.06% DEIPA | |
| 50–200 °C | 9.61 | 10.13 | 10.91 | 12.26 |
| 280–390 °C | 1.98 | 2.13 | 1.82 | 1.95 |
| 400–500 °C | 2.58 | 2.39 | 2.56 | 2.43 |
| CH content | 10.61 | 9.83 | 10.52 | 9.99 |
| Age | C-FA (mL/g) | C-GGBS (mL/g) | ||
|---|---|---|---|---|
| Blank | 0.06% DEIPA | Blank | 0.06% DEIPA | |
| 7 d | 0.1207 | 0.1336 | 0.1516 | 0.1510 |
| 60 d | 0.0888 | 0.0902 | 0.1327 | 0.1133 |
| Age | FA | GGBS | ||||
|---|---|---|---|---|---|---|
| Blank | 20 g/L DEIPA | Increase Ratio by DEIPA (%) | Blank | 20 g/L DEIPA | Increase Ratio by DEIPA (%) | |
| 7 d | 150 | 430 | 187 | 80 | 230 | 92 |
| 60 d | 400 | 1400 | 367 | 150 | 700 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, Y.; Liu, J.; Feng, Z.; Kong, S. Comparative Study on Chloride Binding Capacity of Cement-Fly Ash System and Cement-Ground Granulated Blast Furnace Slag System with Diethanol-Isopropanolamine. Materials 2020, 13, 4103. https://doi.org/10.3390/ma13184103
Liu H, Zhang Y, Liu J, Feng Z, Kong S. Comparative Study on Chloride Binding Capacity of Cement-Fly Ash System and Cement-Ground Granulated Blast Furnace Slag System with Diethanol-Isopropanolamine. Materials. 2020; 13(18):4103. https://doi.org/10.3390/ma13184103
Chicago/Turabian StyleLiu, Huaqing, Yan Zhang, Jialong Liu, Zixia Feng, and Sen Kong. 2020. "Comparative Study on Chloride Binding Capacity of Cement-Fly Ash System and Cement-Ground Granulated Blast Furnace Slag System with Diethanol-Isopropanolamine" Materials 13, no. 18: 4103. https://doi.org/10.3390/ma13184103
APA StyleLiu, H., Zhang, Y., Liu, J., Feng, Z., & Kong, S. (2020). Comparative Study on Chloride Binding Capacity of Cement-Fly Ash System and Cement-Ground Granulated Blast Furnace Slag System with Diethanol-Isopropanolamine. Materials, 13(18), 4103. https://doi.org/10.3390/ma13184103




