Dental Adhesive Interfaces Reinforced with Magnetic Nanoparticles: Evaluation and Modeling with Micro-CT versus Optical Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
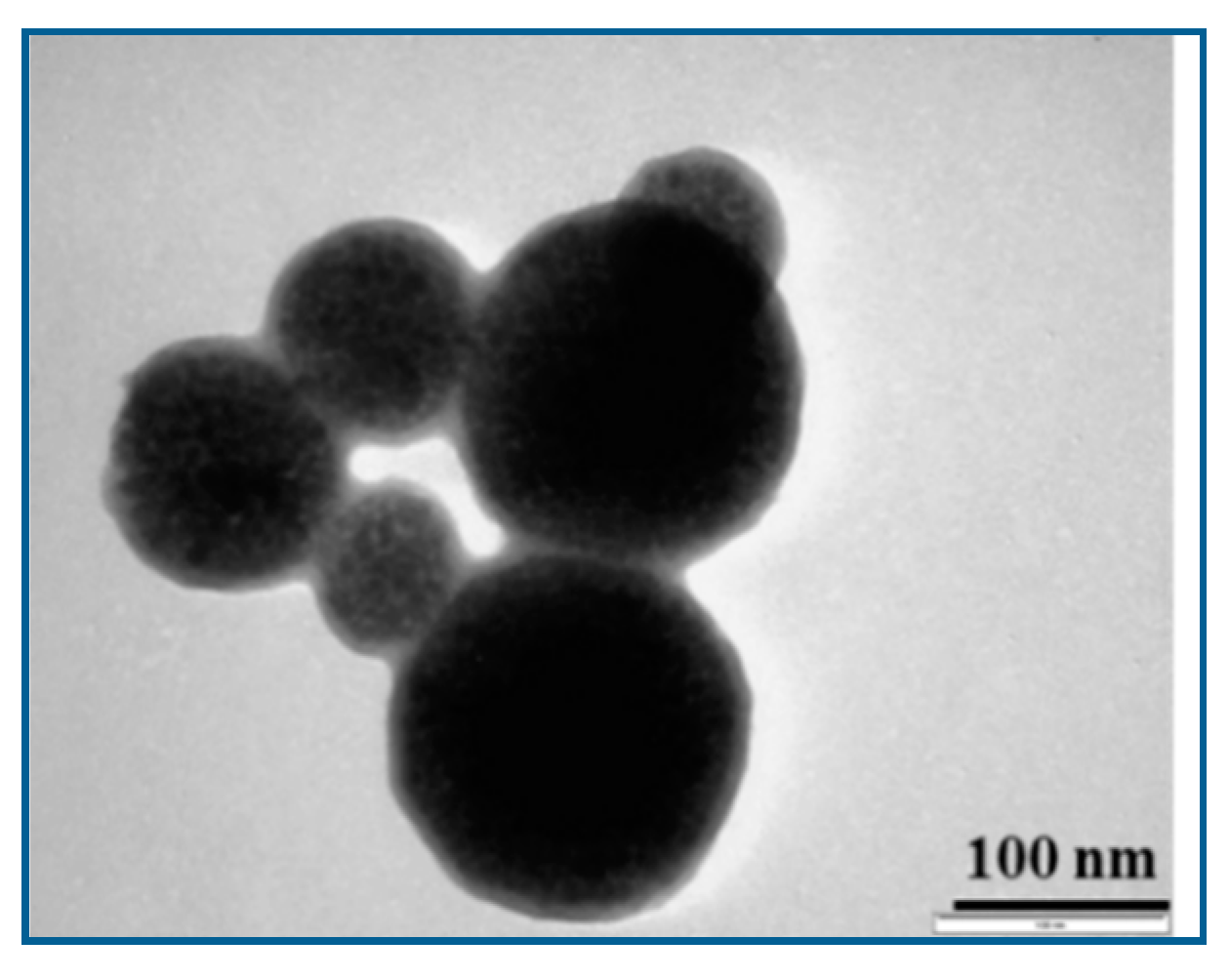
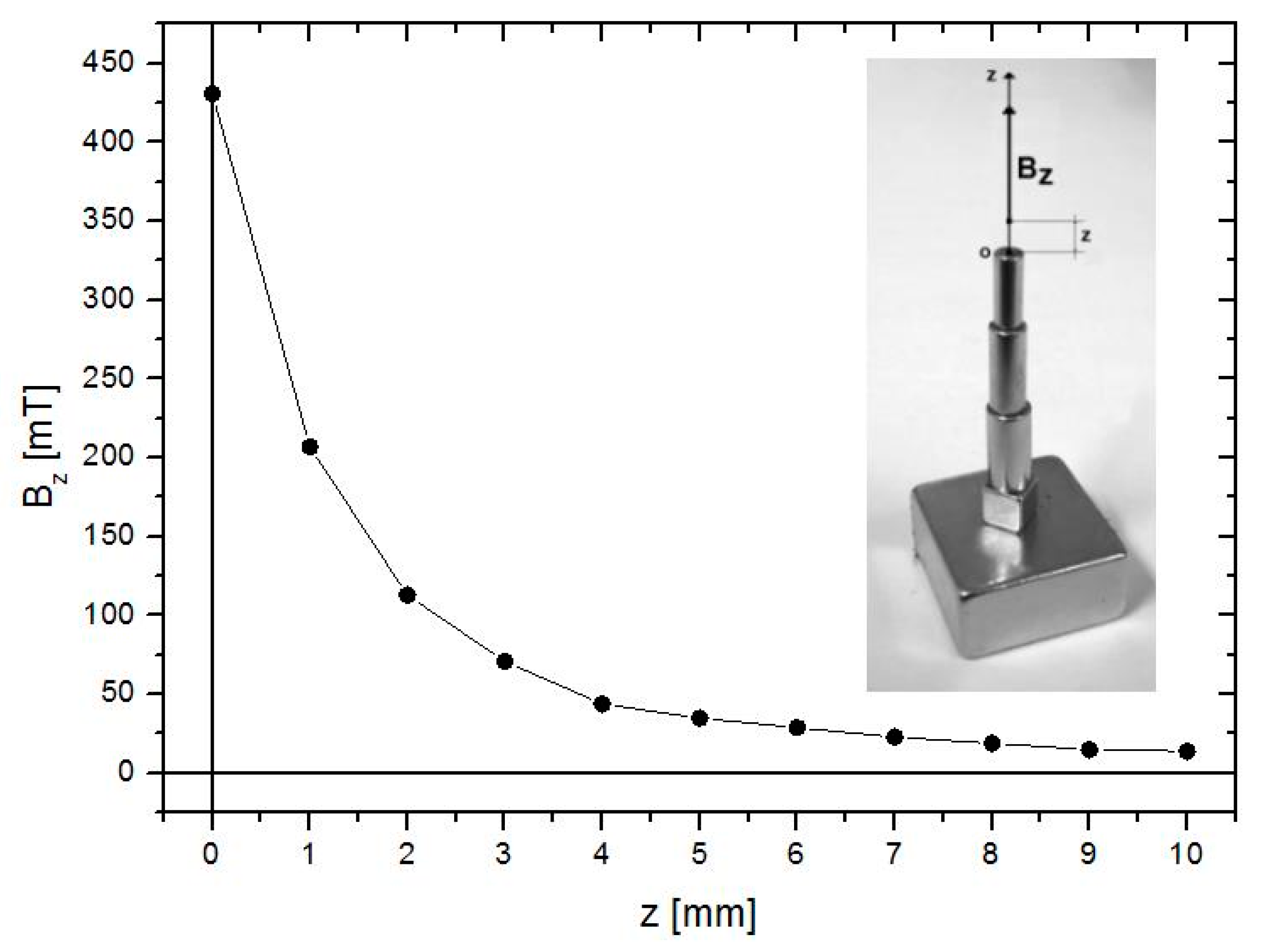
2.2. Preparation and Characterization of the MPs
2.3. Preparation of Samples
2.4. Optical Microscopy and Micro-CT Investigations
3. Results
3.1. Optical Microscopy Analysis
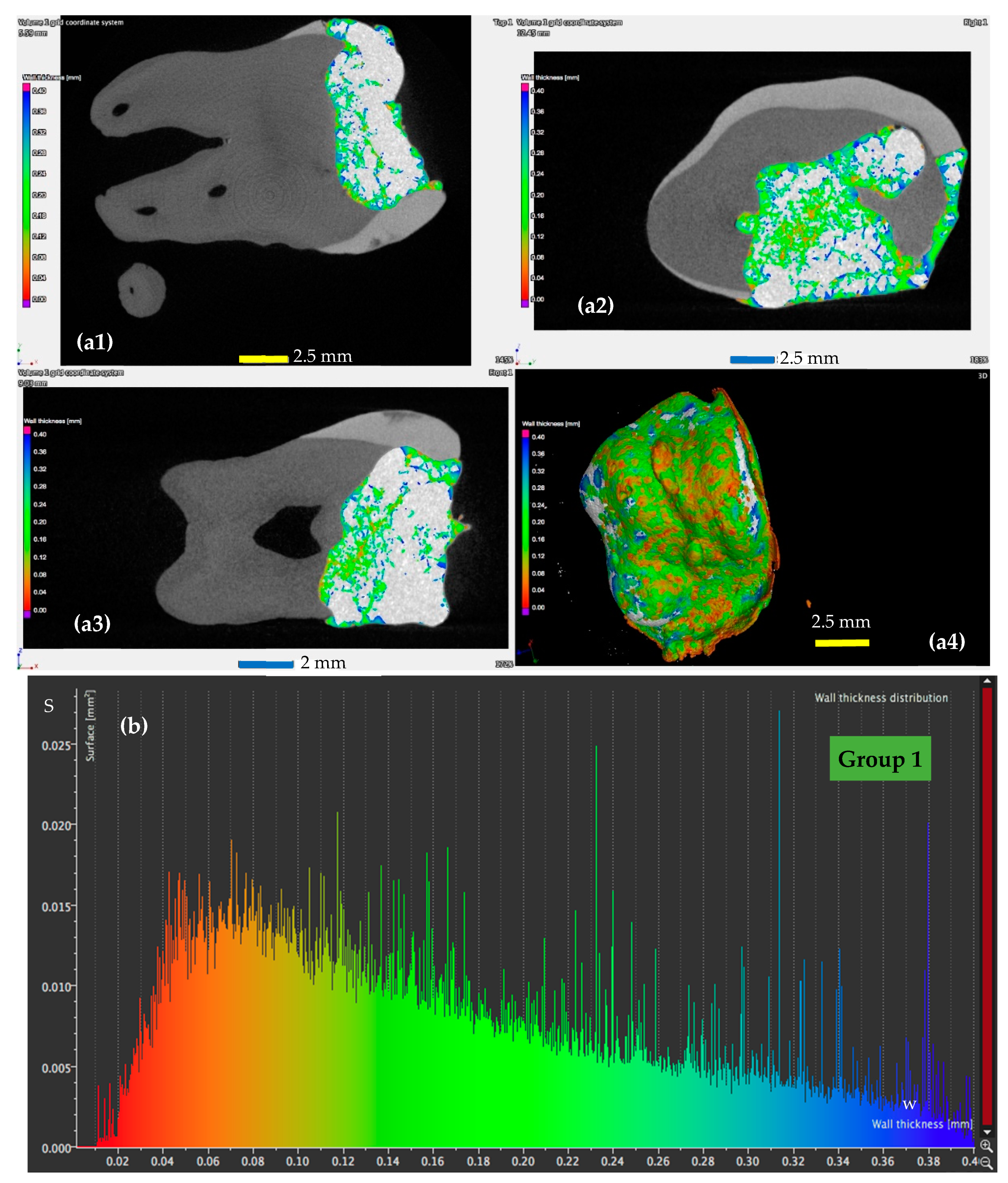
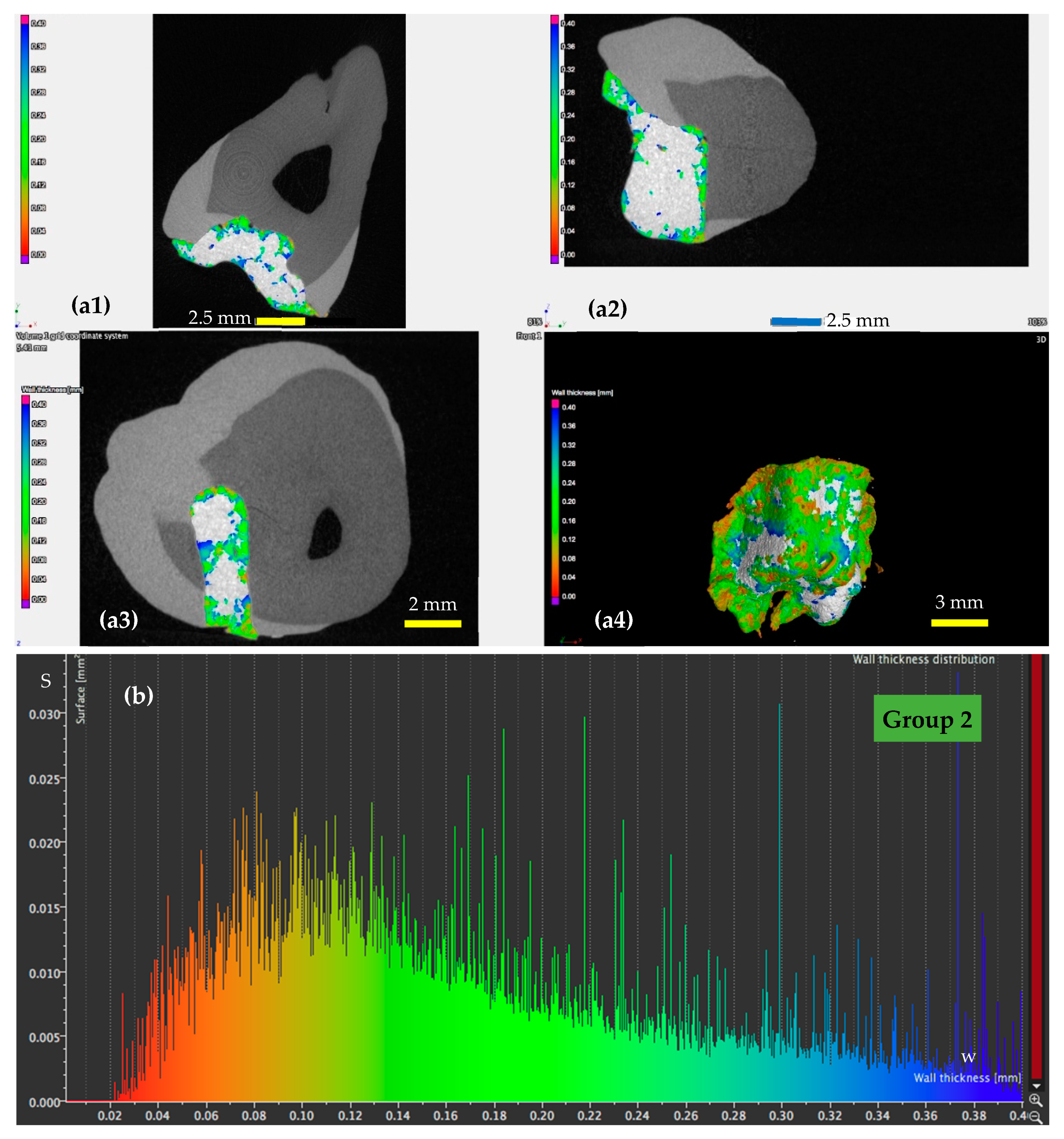
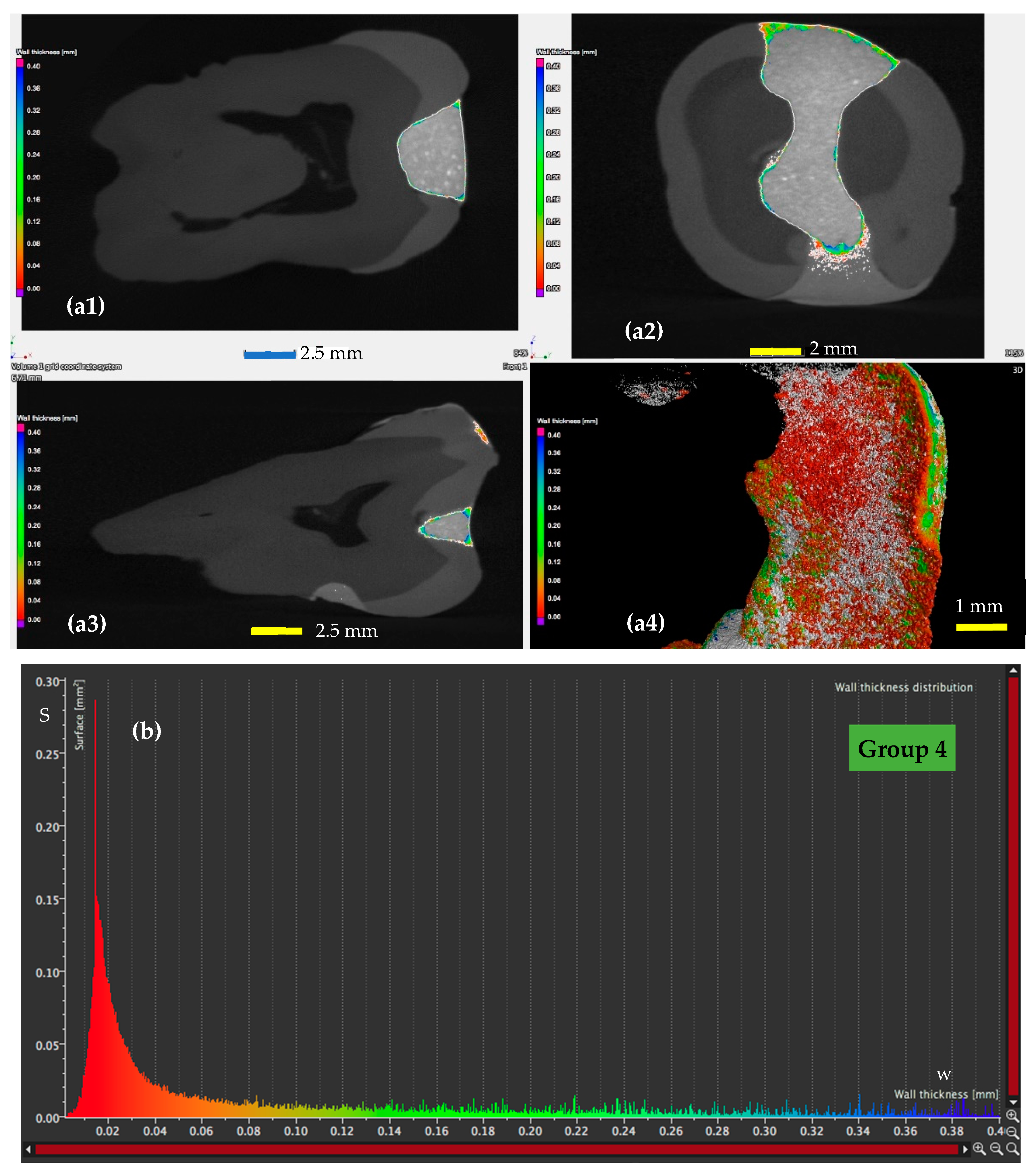
3.2. Micro-CT Analysis
- (i)
- For the control teeth of Group 1 (Figure 5), on the surface of which the dental adhesive was applied conventionally showed surfaces with w ranging from 0.01 to 0.4 mm. The largest area of the adhesive has a width of around 0.1 mm.
- (ii)
- For the samples of Group 2 (Figure 6), in which the dental adhesive was loaded with MPs in the absence of a magnetic field, the same range of w can be observed. A slight decrease of the areas of the surfaces w can be observed, which is predominant around a width w of 0.08 mm.
- (iii)
- For the samples of Group 3 (Figure 7), loaded with MPs on which a magnetic field was applied for 5 min, the areas of the adhesive with an increased width are getting much lower. Areas with adhesive thicknesses of 0.02 to 0.03 mm are in this case predominant.
- (iv)
- For the samples of Group 4 (Figure 8), loaded with MPs on which a magnetic field was applied for 10 min, the width of the adhesive layer decreases for the largest part of the considered areas. Thus, areas with adhesive thicknesses of 0.01 to 0.025 mm are predominant, while the area peaks that are still present in Figure 7 (for Group 3) do not appear anymore. The areas of the adhesive with increased width (towards the same peak value of area of 0.015 mm2) are very low.
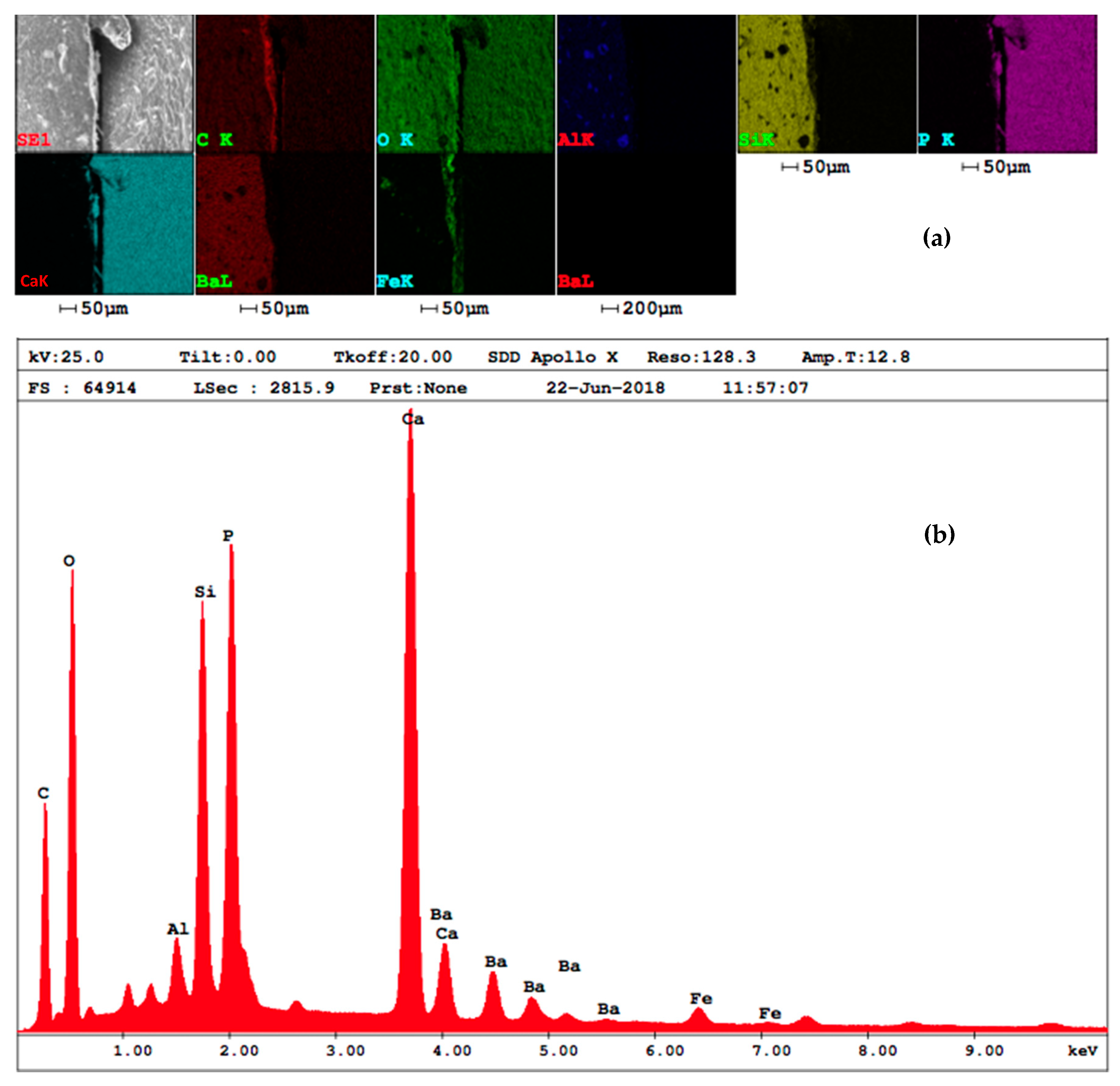
3.3. EDAX Analysis
4. Discussion
4.1. Development of the Novel Adhesive Material
4.2. Comparison between Optical Microscopy and Micro-CT Results
4.3. EDAX Analysis
4.4. Effect of MPs Inclusion in the Adhesives: Comparison between the Four Groups Using Micro-CT
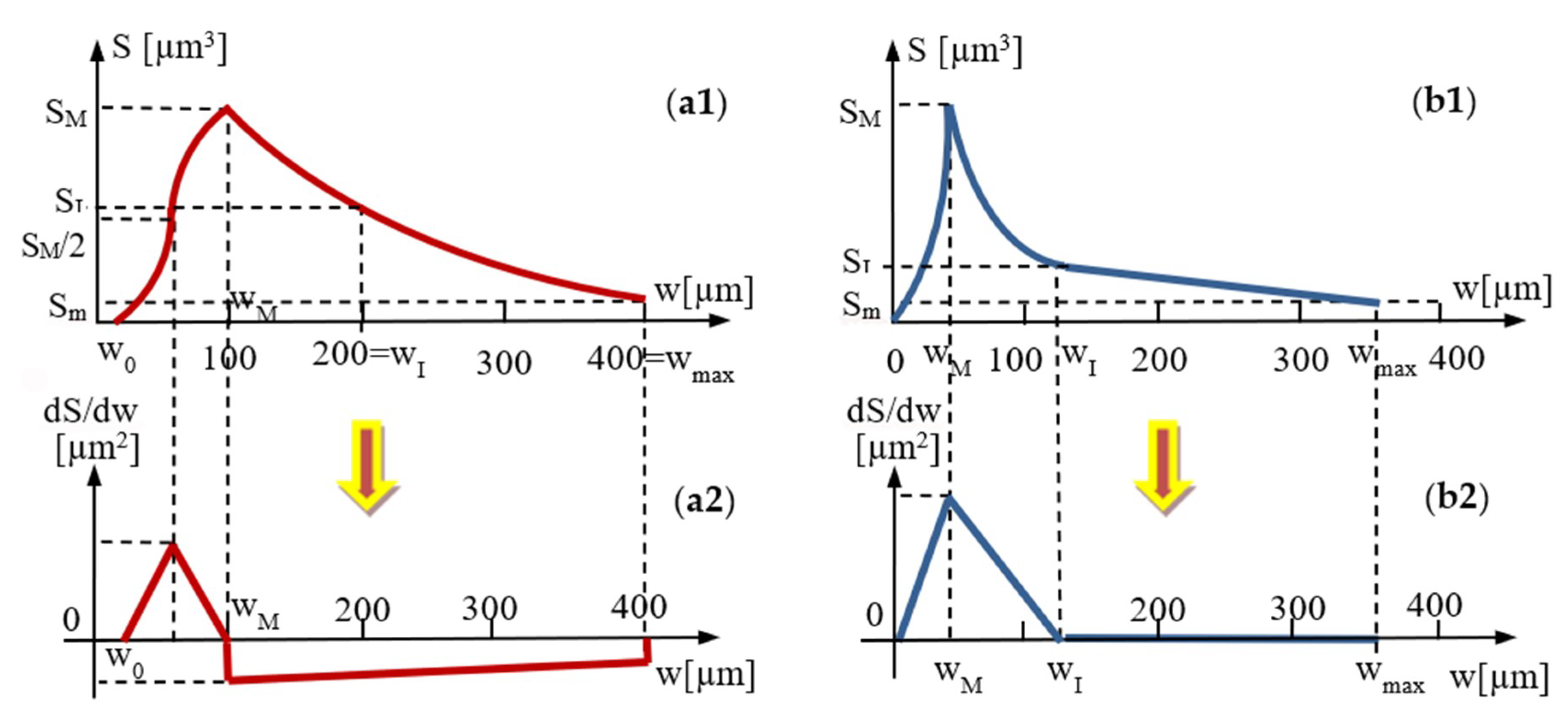
4.5. Mathematical Modeling of the Micro-CT Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
Appendix A
Appendix B
Ascertainment of the Functions of Areas of the Surfaces of Adhesives Regarding Their Widths–for Groups 3 and 4
References
- D’Arcangelo, C.; Vanini, L.; Casinelli, M.; Frascaria, M.; De Angelis, F.; Vadini, M.; D’Amario, M. Adhesive Cementation of Indirect Composite Inlays and Onlays: A Literature Review. Compendium 2015, 36, 570–577. [Google Scholar] [PubMed]
- Ramakrishna, S.; Mayer, J.; Wintemantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Comp. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Pinzon, L.M.; Watanabe, L.G.; Reis, A.F.; Powers, J.M.; Marshall, S.J.; Marshall, G.W. Analysis of interfacial structure and bond strength of self-etch adhesive systems. Am. J. Dent. 2013, 26, 335–340. [Google Scholar] [PubMed]
- Waldman, G.L.; Vaidyanathan, T.K.; Vaidyanathan, J. Microleakage and Resin-to-Dentin Interface Morphology of Pre-Etching versus Self-Etching Adhesive Systems. Open Dent. J. 2008, 2, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Sezinando, A. Looking for the ideal adhesive—A review. Rev. Port. Estomatol. Med. Dentária Cir. Maxilofac. 2014, 55, 194–206. [Google Scholar] [CrossRef]
- Borges, M.A.P.; Matos, I.C.; Dias, K.R.H.C. Influence of Two Self-Etching Primer Systems on Enamel Adhesion. Braz. Dent. J. 2007, 18, 113–118. [Google Scholar] [CrossRef]
- Hilton, T.J. Can modern restorative procedures and materials reliably seal cavities? In vitro investigations. Part 2. Am. J. Dent. 2002, 4, 279–289. [Google Scholar]
- Wu, W.; Cobb, E.; Dermann, K.; Rupp, W. Detecting margin leakage of dental composite restorations. J. Biomed. Mater. Res. 1983, 17, 37–43. [Google Scholar] [CrossRef]
- Mali, P.; Deshpande, S.; Singh, A. Microleakage of restorative materials: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2006, 24, 15–18. [Google Scholar]
- Carrera, C.A.; Lan, C.; Escobar-Sanabria, D.; Li, Y.; Rudney, J.; Aparicio, C.; Fok, A. The Use of Micro-CT with Image Segmentation to Quantify Leakage in Dental Restorations. Dent. Mater. 2015, 31, 382–390. [Google Scholar] [CrossRef]
- Kumar, M.; Lakshminarayanan, L. Methods of Detecting Microleakage. J. Conserv. Dent. 2004, 7, 79–88. [Google Scholar]
- Ozel, E.; Tuna, E.B.; Firatli, E. The effects of cavity-filling techniques on microleakage in class II resin restorations prepared with Er: YAG laser and diamond bur: A scanning electron microscopy study. J. Scanning Microsc. 2016, 38, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Milleding, P. Microleakage of indirect composite inlays. An in vitro comparison with the direct technique. Acta Odontol Scand. 1992, 50, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Hasanreisoglu, U.; Sonmez, H.; Uctasli, S.; Wilson, H.J. Microleakage of direct and indirect inlay/onlay systems. J. Oral. Rehabil. 1996, 23, 66–71. [Google Scholar] [CrossRef]
- Soares, C.J.; Celiberto, L.; Dechichi, P.; Fonseca, R.B.; Martins, L.R.M. Marginal integrity and microleakage of direct and indirect composite inlays—SEM and stereomicroscopic evaluation. Braz. Oral. Res. 2005, 19, 295–301. [Google Scholar] [CrossRef]
- Otis, L.; Everett, M.J.; Sathyam, U.S.; Colston, B.W., Jr. Optical Coherence Tomography: A New Imaging Technology for Dentistry. J. Am. Dent. Assoc. 2000, 131, 511–514. [Google Scholar] [CrossRef]
- Turki, A.; Bakhsha, B.; Sadrb, A.; Shimadaa, Y.; Junji Tagamia, B.; Yasunori, S. Non-invasive quantification of resin–dentin interfacial gaps using optical coherence tomography: Validation against confocal microscopy. Dent. Mat. 2011, 27, 915–925. [Google Scholar]
- Monteiro, G.Q.; Montes, M.A.; Gomes, A.S.; Mota, C.C.; Campello, S.L.; Freitas, A.Z. Marginal analysis of resin composite restorative systems using optical coherence tomography. Dent. Mater. 2011, 27, 213–223. [Google Scholar] [CrossRef]
- Hsieh, Y.-S.; Ho, Y.-C.; Lee, S.-Y.; Chuang, C.-C.; Tsai, J.; Lin, K.-F.; Sun, C.-W. Dental Optical Coherence Tomography. Sensors 2013, 13, 8928–8949. [Google Scholar] [CrossRef]
- Oancea, R.; Bradu, A.; Sinescu, C.; Negru, R.M.; Negrutiu, M.L.; Antoniac, I.; Duma, V.-F.; Podoleanu, A.G. Assessment of the sealant/tooth interface using optical coherence tomography. J Adhes. Sci. Technol. 2015, 29, 49–58. [Google Scholar] [CrossRef]
- Dsouza, R.; Subhash, H.; Neuhaus, K.; Kantamneni, R.; McNamara, P.M.; Hogan, J.; Wilson, C.; Leahy, M. Assessment of curing behavior of light-activated dental composites using intensity correlation based multiple reference optical coherence tomography. Lasers Surg. Med. 2016, 48, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Canjau, S.; Todea, C.; Negrutiu, M.L.; Sinescu, C.; Topala, F.I.; Marcauteanu, C.; Manescu, A.; Duma, V.-F.; Bradu, A.; Podoleanu, A. Optical Coherence Tomography for Non-Invasive ex vivo Investigations in Dental Medicine—A Joint Group Experience (Review). Mod. Technol. Med. 2015, 7, 97–115. [Google Scholar] [CrossRef]
- Cavalu, S.; Fritea, L.; Brocks, M.; Barbaro, K.; Murvai, G.; Costea, T.O.; Antoniac, I.; Verona, C.; Romani, M.; Latini, A.; et al. Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties. Materials 2020, 13, 2077. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Ren, J.; Zhang, C. TiO2-KH550 Nanoparticle-Reinforced PVA/xylan Composite Films with Multifunctional Properties. Materials 2018, 11, 1589. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matysek, D. 3D Hierarchical, Nanostructured Chitosan/PLA/HA Scaffolds Doped with TiO2/Au/Pt NPs with Tunable Properties for Guided Bone Tissue Engineering. Polymers 2020, 12, 792. [Google Scholar] [CrossRef]
- Chaitali, D.; Arup, G.; Manisha, A.; Ajay, G.; Madhuri, M. Improvement of anticancer drug release by cobalt ferrite magnetic nanoparticles through combined pH and temperature responsive technique. ChemPhysChem 2018, 19, 1–18. [Google Scholar]
- Antoniac, I.; Cernea, A.; Petcu, C.; Laptoiu, D.; Tabaras, D.; Tecu, C.; Antoniac, A.; Gradinaru, S. Synthesis and Characterization of Coated Iron Oxide Nanoparticles Produced for Drug Delivery in Viscoelastic Solution. Rev. Chim. 2020, 71, 145–154. [Google Scholar] [CrossRef]
- Covaliu, C.I.; Jitaru, I.; Paraschiv, G.; Vasile, E.; Biri¸s, S.-S.; Diamandescu, L.; Ionita, V.; Iovu, H. Core-shell hybrid nanomaterials based on CoFe2O4 particles coated with PVP or PEG biopolymers for applications in biomedicine. Powder Technol. 2013, 237, 415–426. [Google Scholar] [CrossRef]
- Szunerits, S.; Nait Saada, T.; Meziane, D.; Boukherroub, R. Magneto-Optical Nanostructures for Viral Sensing. Nanomaterials 2020, 10, 1271. [Google Scholar] [CrossRef]
- AlKahtani, R.N. The implications and applications of nanotechnology in dentistry: A review. Saudi. Dent. J. 2018, 30, 107–116. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Cojocaru, F.D.; Balan, V.; Popa, M.I.; Lobiuc, A.; Antoniac, A.; Antoniac, I.V.; Verestiuc, L. Biopolymers—Calcium phosphates composites with inclusions of magnetic nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2019, 125, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Zaharia, C.; Sinescu, C.; Gabor, A.; Socoliuc, V.; Talpos, S.; Hajaj, T.; Sfirloaga, P.; Oancea, R.; Miclau, M.; Negrutiu, M. Influences of Polymeric Magnetic Encapsulated Nanoparticles on the Adhesive Layer for Composite Materials Used for Class I Dental Fillings. Mater. Plast. 2019, 56, 399–404. [Google Scholar] [CrossRef]
- Oancea, R.; Zaharia, C.; Gabor, A.; Sinescu, C.; Mioc, M.; Vaduva, D.B.; Simon, C.P.; Socoliuc, V.; Rominu, M.; Negrutiu, M. Imagistic Analysis of Dental Adhesives Loaded with Nanoparticles Used on Teeth Sealing of Pits and Fissures with Resin Based Materials. Mater. Plast. 2019, 56, 449–453. [Google Scholar] [CrossRef]
- Zaharia, C.; Sinescu, C.; Gabor, A.; Cojocariu, A.C.; Socoliuc, V.; Szuhanek, C.; Negrutiu, M. MicroCT Analysis of Dental Adhesives Loaded with Nanoparticles. Rev. Chim. 2019, 70, 1769–1773. [Google Scholar] [CrossRef]
- Söderholm, K.J.; Geraldeli, S.; Shen, C. What do microtensile bond strength values of adhesives mean? J. Adhes. Dent. 2012, 14, 307–314. [Google Scholar]
- Neves, A.A.; Coutinho, E.; Poitevin, A.; Van der Sloten, J.; Van Merbeek, B.; Van Oosterwyck, H. Influence of joint component mechanical properties and adhesive layer thickness on stress distribution in micro-tensile bond strength specimens. Dent. Mater. 2008, 25, 4–12. [Google Scholar] [CrossRef]
- Buteica, D.; Borbath, I.; Nicolae, I.V.; Turcu, R.; Marinica, O.; Socoliuc, V. Optimization of Multicore-Shell Fe3O4-SiO2 Magnetic Nanocomposites Synthesis and Retention in Cellulose Pulp. AIP Proc. 2017, 1916, 040013. [Google Scholar]
- Bica, D. Preparation of magnetic fluids for various applications. Rom. Rep. Phys. 1995, 47, 265–672. [Google Scholar]
- Jaydeep, A.; Prateeti, C.; Balaram, D.; Arnab, D.; Sandeep, D.; Somenath, R.; Chen, J.; Tanmay, C. Preparation and characterization of ferromagnetic nickel oxide nanoparticles from three different precursors: Application in drug delivery. RSC Adv. 2015, 5, 35917–35928. [Google Scholar]
- Turcu, R.; Socoliuc, V.; Craciunescu, I.; Petran, A.; Paulus, A.; Franzreb, M.; Vasile, E.; Vekas, L. Magnetic microgels, a promising candidate for enhanced magnetic adsorbent particles in bioseparation: Synthesis, physicochemical characterization, and separation performance. Soft Matter 2015, 11, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, I.; Petran, A.; Liebscher, J.; Vekas, L.; Turcu, R. Synthesis and characterization of size-controlled magnetic clusters functionalized with polymer layer for wastewater depollution. Mater. Chem. Phys. 2017, 185, 91–97. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Coll. Interf. Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Hui, C.; Shen, C.M.; Yang, T.Z.; Bao, L.H.; Tian, J.F.; Ding, H.; Li, C.; Gao, H.J. Large-Scale Fe3O4 Nanoparticles Soluble in Water Synthesized by a Facile Method. J. Phys. Chem. C 2008, 112, 11336–11339. [Google Scholar] [CrossRef]
- Nicholson, J.W. Adhesive dental materials—A review. Int. J. Adhes. Adhes. 1998, 18, 229–236. [Google Scholar] [CrossRef]
- Caldas, I.P.; Alves, G.G.; Barbosa, I.B.; Scelza, P.; de Noronha, F.; Scelza, M.Z. In vitro cytotoxicity of dental adhesives: A systematic review. Dent. Mater. 2019, 35, 195–205. [Google Scholar] [CrossRef]
- Song, L.; Ye, Q.; Ge, X.; Misra, A.; Tamerler, C.; Spencer, P. New silyl-functionalized BisGMA provides autonomous strengthening without leaching for dental adhesives. Acta Biomater. 2019, 81, 130–139. [Google Scholar] [CrossRef]
- Heintze, S.D.; Rousson, V.; Mahn, E. Bond strength tests of dental adhesive systems and their correlation with clinical results—A meta-analysis. Dent. Mater. 2015, 31, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Rangreez, T.A.; Mobin, R. Polymer composites for dental fillings. Applications of Nanocomposite Materials in Dentistry. Woodhead Publ. Ser. Biomater. 2019, 205–224. [Google Scholar] [CrossRef]
- Foong, J.; Lee, K.; Nguyen, C.; Tang, G.; Austin, D.; Ch’ng, C.; Burrow, M.F.; Thomas, D.L. Comparison of microshear bond strengths of four self-etching bonding systems to enamel using two test methods. Aust. Dent. J. 2006, 51, 252–257. [Google Scholar] [CrossRef]
- Carrilho, E.; Cardoso, M.; Ferreira, M.M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP Based Dental Adhesives: Adhesive Interface Characterization and Adhesive Stability—A systematic Review. Curent Future Trends Dent. Mater. 2019, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.D.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Azad, E.; Atai, M.; Zandi, M.; Shokrollahi, P.; Solhi, L. Structure-properties relationships in dental adhesives: Effect of initiator, matrix monomer structure, and nano-filler incorporation. Dent. Mater. 2018, 34, 1263–1270. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.A.; Xu, H.H.K. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef]
- Lohbauer, U.; Wagner, A.; Belli, R.; Stoetzel, C.; Hilpert, A.; Kurland, H.D.; Grabow, J.; Müller, F.A. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010, 6, 4539–4546. [Google Scholar] [CrossRef]
- Jancar, J. Bond strength of five dental adhesives using a fracture mechanics approach. J. Mech. Behav. Biomed. Mater. 2011, 4, 245–254. [Google Scholar] [CrossRef]
- El Zohairy, A.A.; Saber, M.H.; Abdalla, A.I.; Feilzer, A.J. Efficacy of microtensile versus microshear bond testing for evaluation of bond strength of dental adhesive system. Dent. Mater. 2010, 26, 848–854. [Google Scholar] [CrossRef]
- Pashley, D.H. The evolution of dentin bonding. Dent. Today 2003, 22, 112–119. [Google Scholar] [PubMed]
- Betamar, N.; Cardew, G.; Van Noort, R. Influence of specimen designs on the microtensile bond strength to dentin. J. Adhes. Dent. 2007, 9, 159–168. [Google Scholar] [PubMed]
- Szep, S.; Kunkel, A.; Ronge, K.; Heidemann, D. Cytotoxicity of modern dentin adhesives—In vitro testing on gingival fibroblasts. J. Biomed. Mater. Res. 2002, 63, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Lombardo, G.; Balloni, S.; Bodo, M.; Cianetti, S.; Barbati, A.; Montaseri, A.; Marinucci, L. Cytotoxicity of universal dental adhesive systems: Assessment in vitro assays on human gingival fibroblasts. Toxicol. Vitr. 2019, 60, 252–260. [Google Scholar] [CrossRef]
- Khoroushi, M.; Etemadi, S.; Kheir, M.K. Marginal Leakage of Class V Composite Resin Restorations. Dent. Hypotheses 2018, 9, 11–15. [Google Scholar]
- Fogel, H.M.; Peikoff, M.D. Microleakage of root-end filling materials. J. Endod. 2001, 27, 456–458. [Google Scholar] [CrossRef]
- Shemesh, H.; Wu, M.K.; Wesselink, P.R. Leakage along apical root fillings with and without smear layer using two different leakage models: A two-month longitudinal ex vivo study. Int. Endod. J. 2006, 39, 968–976. [Google Scholar] [CrossRef]
- Çırakoğlu, S.; Baddal, B.; İslam, A. The Effectiveness of Laser-Activated Irrigation on the Apical Microleakage Qualities of MTA Repair HP and NeoMTA Plus in Simulated Immature Teeth: A Comparative Study. Materials 2020, 13, 3287. [Google Scholar] [CrossRef]
- Olejniczak, A.J.; Grine, F.E. Assessment of the accuracy of dental enamel thickness measurements using microfocal X-ray computed tomography. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006, 288, 263–275. [Google Scholar] [CrossRef]
- John, Ł.; Janeta, M.; Szafert, S. Designing of macroporous magnetic bioscaffold based on functionalized methacrylate network covered by hydroxyapatites and doped with nano-MgFe2O4 for potential cancer hyperthermia therapy. Mater. Sci. Eng. C 2017, 78, 901–911. [Google Scholar] [CrossRef]
- John, Ł.; Janeta, M.; Rajczakowska, M.; Ejfler, J.; Łydżba, D.; Szafert, S. Synthesis and microstructural properties of the scaffold based on a 3-(trimethoxysilyl)propyl methacrylate—POSS hybrid towards potential tissue engineering applications. RSC Adv. 2016, 6, 66037–66047. [Google Scholar] [CrossRef]
- Duma, V.-F.; Tankam, P.; Huang, J.; Won, J.J.; Rolland, J.P. Optimization of galvanometer scanning for Optical Coherence Tomography. Appl. Opt. 2015, 54, 5495–5507. [Google Scholar] [CrossRef] [PubMed]
- Duma, V.-F.; Dobre, G.; Demian, D.; Cernat, R.; Sinescu, C.; Topala, F.I.; Negrutiu, M.L.; Hutiu, G.; Bradu, A.; Podoleanu, A.G. Handheld scanning probes for optical coherence tomography. Rom. Rep. Phys. 2015, 67, 1346–1358. [Google Scholar]
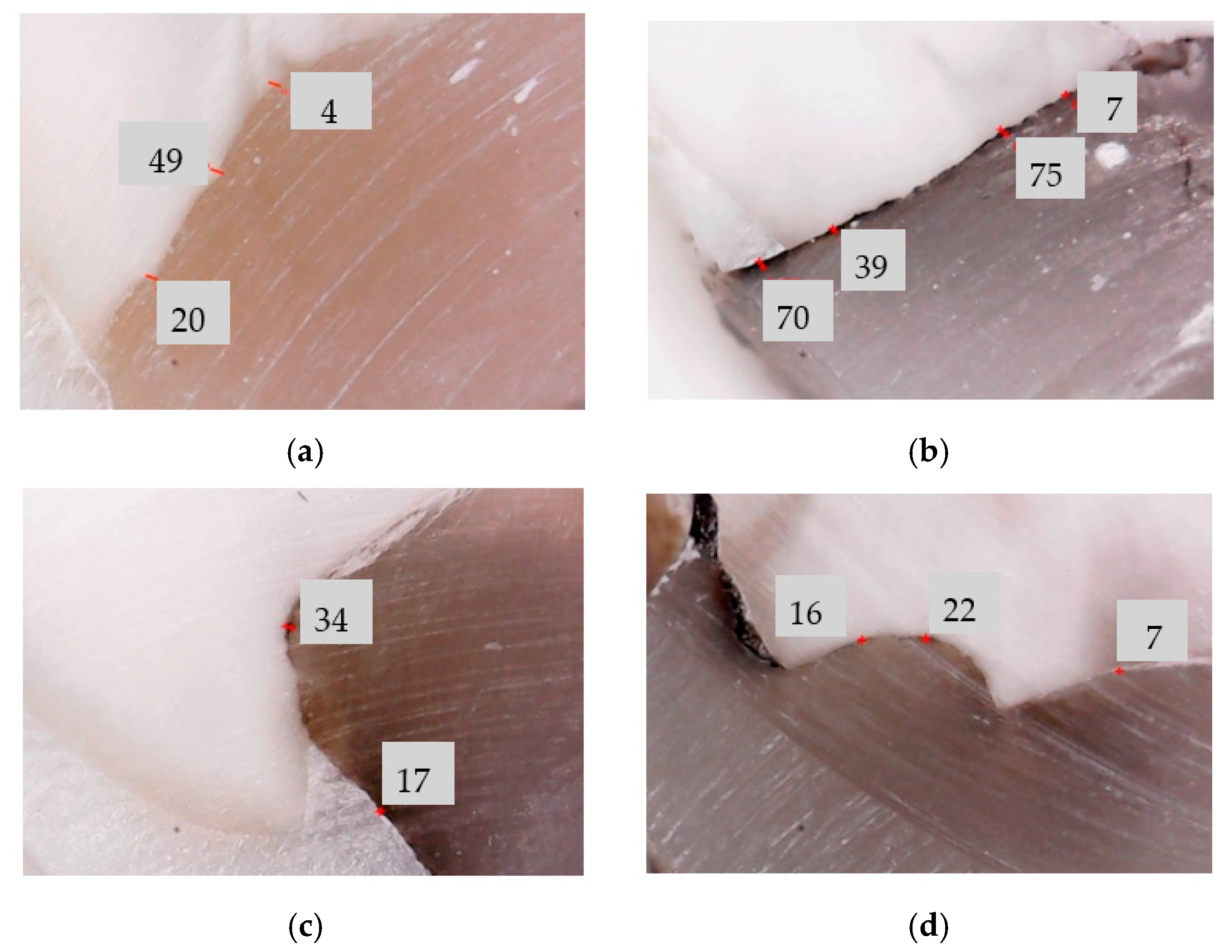

| Group | Sample | Average Values of the Width/Thickness of the Dental Adhesive Layer, w (μm) | |||||
|---|---|---|---|---|---|---|---|
| Vestibular Interface | Oral Interface | Pulpal Interface | |||||
| Microscopy | Micro-CT | Microscopy | Micro-CT | Microscopy | Micro-CT | ||
| 1 | 1 | 31 | 20 | 230 | 210 | 290 | 180 |
| 2 | 90 | 90 | 47 | 71 | 170 | 100 | |
| 3 | 240 | 31 | 210 | 47 | 90 | 170 | |
| 4 | 21 | 400 | 63 | 220 | 60 | 91 | |
| 5 | 50 | 72 | 37 | 61 | 59 | 20 | |
| 2 | 1 | 21 | 17 | 71 | 21 | 42 | 92 |
| 2 | 120 | 110 | 170 | 73 | 30 | 64 | |
| 3 | 53 | 400 | 160 | 56 | 16 | 270 | |
| 4 | 67 | 29 | 31 | 46 | 70 | 23 | |
| 5 | 59 | 190 | 130 | 210 | 310 | 77 | |
| 3 | 1 | 37 | 13 | 47 | 19 | 41 | 22 |
| 2 | 50 | 18 | 22 | 24 | 90 | 98 | |
| 3 | 29 | 32 | 30 | 38 | 54 | 29 | |
| 4 | 180 | 88 | 40 | 91 | 61 | 100 | |
| 5 | 13 | 36 | 190 | 66 | 170 | 310 | |
| 4 | 1 | 29 | 17 | 40 | 23 | 31 | 20 |
| 2 | 21 | 21 | 12 | 16 | 33 | 32 | |
| 3 | 31 | 30 | 37 | 21 | 17 | 11 | |
| 4 | 11 | 21 | 39 | 11 | 21 | 15 | |
| 5 | 37 | 14 | 29 | 24 | 22 | 27 | |
| Method | Area | n | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Micro-CT | Vestibular | 60 | 0.101 | 0.107 | 0.003 | 0.400 |
| Oral | 60 | 0.077 | 0.063 | 0.004 | 0.230 | |
| Pulpal | 60 | 0.104 | 0.079 | 0.011 | 0.310 | |
| Optical Microscopy | Vestibular | 60 | 0.071 | 0.055 | 0.004 | 0.240 |
| Oral | 60 | 0.094 | 0.065 | 0.005 | 0.240 | |
| Pulpal | 60 | 0.100 | 0.080 | 0.004 | 0.310 |
| Area | Ranks | n | Mean Rank | Sum of Ranks | psig |
|---|---|---|---|---|---|
| Vestibular | Negative Ranks | 26 | 39.02 | 1014.5 | 0.218 is |
| Positive Ranks | 32 | 21.77 | 696.5 | ||
| Oral | Negative Ranks | 16 | 25.28 | 404.5 | <0.001 s |
| Positive Ranks | 44 | 32.40 | 1425.5 | ||
| Pulpal | Negative Ranks | 25 | 35.54 | 888.5 | 0.845 is |
| Positive Ranks | 35 | 26.90 | 941.5 |
| Area | Group | n | Mean ± SD | Standard Error Mean | Mean Rank | Sum of Ranks | psig |
|---|---|---|---|---|---|---|---|
| Vestibular | 1 | 30 | 0.136 ± 0.096 | 0.017 | 32.32 | 969.5 | 0.420 is |
| 2 | 30 | 0.123 ± 0.102 | 0.019 | 28.68 | 860.5 | ||
| Oral | 1 | 30 | 0.139 ± 0.068 | 0.012 | 34.32 | 1029.5 | 0.090 is |
| 2 | 30 | 0.110 ± 0.053 | 0.010 | 26.68 | 800.5 | ||
| Pulpal | 1 | 30 | 0.138 ± 0.065 | 0.012 | 31.78 | 953.5 | 0.569 is |
| 2 | 30 | 0.129 ± 0.086 | 0.016 | 29.22 | 876.5 |
| Area | Group | n | Mean ± SD | Standard Error Mean | Mean Rank | Sum of Ranks | psig |
|---|---|---|---|---|---|---|---|
| Vestibular | 1 | 30 | 0.136 ± 0.096 | 0.017 | 38.10 | 1143.00 | 0.001 s |
| 3 | 30 | 0.062 ± 0.044 | 0.008 | 22.90 | 687.00 | ||
| Oral | 1 | 30 | 0.139 ± 0.068 | 0.012 | 39.33 | 1180.00 | <0.001 s |
| 3 | 30 | 0.068 ± 0.045 | 0.008 | 21.67 | 650.00 | ||
| Pulpal | 1 | 30 | 0.138 ± 0.065 | 0.012 | 33.55 | 1006.50 | 0.176 is |
| 3 | 30 | 0.117 ± 0.075 | 0.014 | 27.45 | 823.50 |
| Area | Group | n | Mean ± SD | Standard Error Mean | Mean Rank | Sum of Ranks | psig |
|---|---|---|---|---|---|---|---|
| Vestibular | 3 | 30 | 0.062 ± 0.044 | 0.008 | 39.87 | 1196.0 | <0.001 s |
| 4 | 30 | 0.024 ± 0.008 | 0.002 | 21.13 | 634.0 | ||
| Oral | 3 | 30 | 0.068 ± 0.045 | 0.008 | 41.02 | 1230.5 | <0.001 s |
| 4 | 30 | 0.026 ± 0.010 | 0.002 | 19.98 | 599.5 | ||
| Pulpal | 3 | 30 | 0.117 ± 0.075 | 0.014 | 43.67 | 1310.0 | <0.001 s |
| 4 | 30 | 0.024 ± 0.007 | 0.001 | 17.33 | 520.0 |
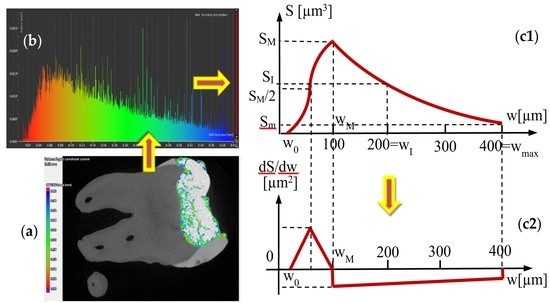
| Group | Parameters w (mm); S (mm2) | Area S of the Surface of Adhesive Layer with a Certain Width w | ||
|---|---|---|---|---|
| 1 | w0 = 0; | |||
| S0 = 0 | ||||
| wM = 0.07 | ||||
| SM = 0.014 | ||||
| wI = 0.2 | ||||
| SI = 0.008 | ||||
| wmax = 0.4 | ||||
| Sm = 0.002 | ||||
| 2 | w0 = 0.02; | |||
| S0 = 0 | ||||
| wM = 0.1 | ||||
| SM = 0.014 | ||||
| wI = 0.2 | ||||
| SI = 0.008 | ||||
| wmax = 0.4 | ||||
| Sm = 0.002 | ||||
| Group | Parameters w (mm); S (mm2) | Area S of the Surface of Adhesive Layer with a Certain Width w | ||
|---|---|---|---|---|
| 3 | wM = 0.02 | |||
| SM = 0.016 | ||||
| wI = 0.1 | ||||
| SI = 0.002 | ||||
| wmax = 0.4 | ||||
| Sm = 0.001 | ||||
| 4 | wM = 0.015 | |||
| SM = 0.015 | ||||
| wI = 0.18 | ||||
| SI = 0.002 | ||||
| wmax = 0.36 | ||||
| Sm = 0 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharia, C.; Duma, V.-F.; Sinescu, C.; Socoliuc, V.; Craciunescu, I.; Turcu, R.P.; Marin, C.N.; Tudor, A.; Rominu, M.; Negrutiu, M.-L. Dental Adhesive Interfaces Reinforced with Magnetic Nanoparticles: Evaluation and Modeling with Micro-CT versus Optical Microscopy. Materials 2020, 13, 3908. https://doi.org/10.3390/ma13183908
Zaharia C, Duma V-F, Sinescu C, Socoliuc V, Craciunescu I, Turcu RP, Marin CN, Tudor A, Rominu M, Negrutiu M-L. Dental Adhesive Interfaces Reinforced with Magnetic Nanoparticles: Evaluation and Modeling with Micro-CT versus Optical Microscopy. Materials. 2020; 13(18):3908. https://doi.org/10.3390/ma13183908
Chicago/Turabian StyleZaharia, Cristian, Virgil-Florin Duma, Cosmin Sinescu, Vlad Socoliuc, Izabell Craciunescu, Rodica Paula Turcu, Catalin Nicolae Marin, Anca Tudor, Mihai Rominu, and Meda-Lavinia Negrutiu. 2020. "Dental Adhesive Interfaces Reinforced with Magnetic Nanoparticles: Evaluation and Modeling with Micro-CT versus Optical Microscopy" Materials 13, no. 18: 3908. https://doi.org/10.3390/ma13183908
APA StyleZaharia, C., Duma, V.-F., Sinescu, C., Socoliuc, V., Craciunescu, I., Turcu, R. P., Marin, C. N., Tudor, A., Rominu, M., & Negrutiu, M.-L. (2020). Dental Adhesive Interfaces Reinforced with Magnetic Nanoparticles: Evaluation and Modeling with Micro-CT versus Optical Microscopy. Materials, 13(18), 3908. https://doi.org/10.3390/ma13183908