Quantifying 3D Strain in Scaffold Implants for Regenerative Medicine
Abstract
1. Introduction
2. Materials and Methods
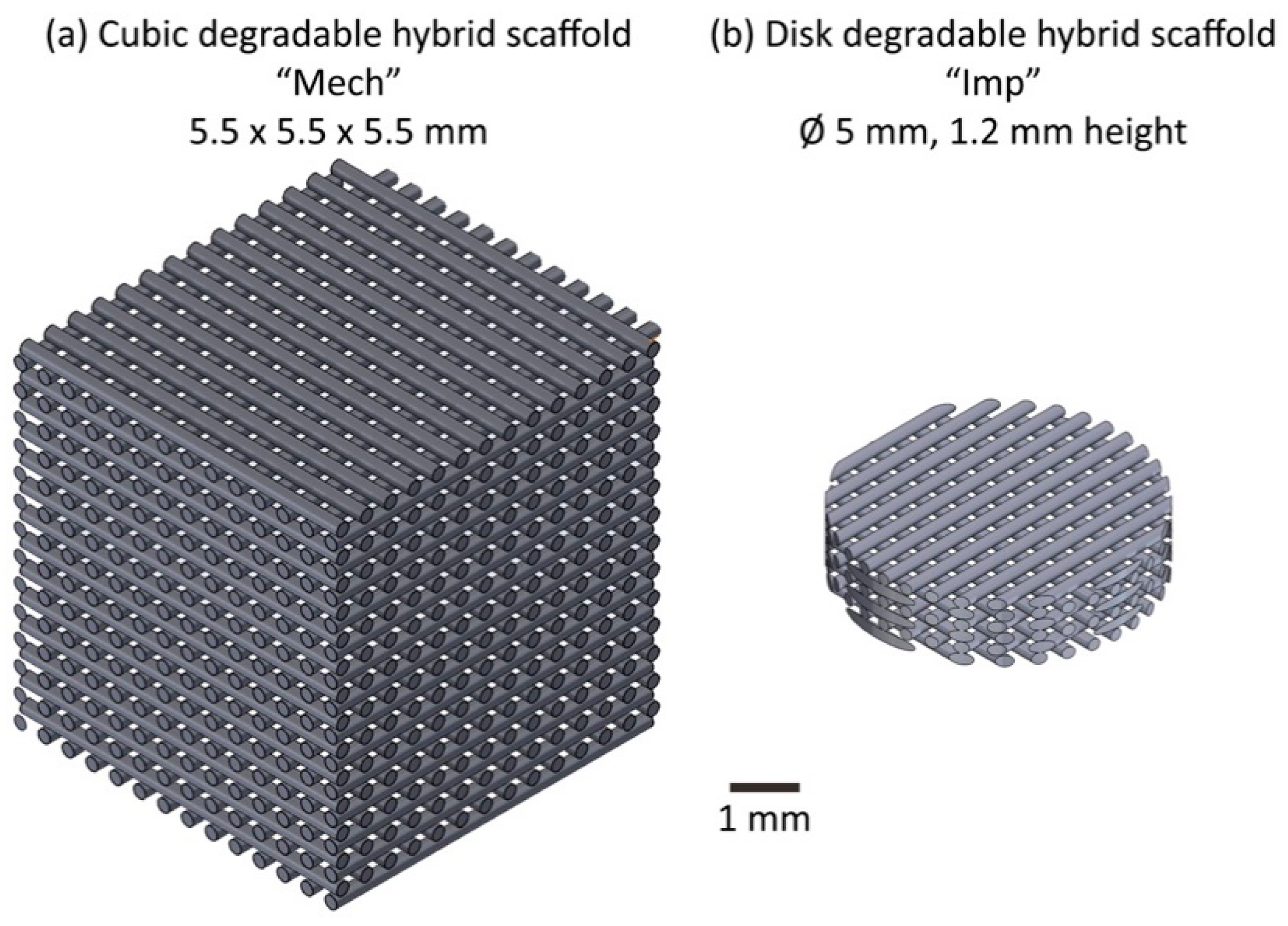
2.1. Scaffold Implant Manufacture
2.2. Imaging
2.2.1. Three-Dimensional Micro-Computed Tomography
2.2.2. Two-Dimensional Imaging
2.3. Feature Correlation
2.3.1. Digital Volume Correlation (DVC)
2.3.2. Digital Image Correlation (DIC)
2.3.3. Error Quantification
2.3.4. Ground-Truth Validation
3. Results
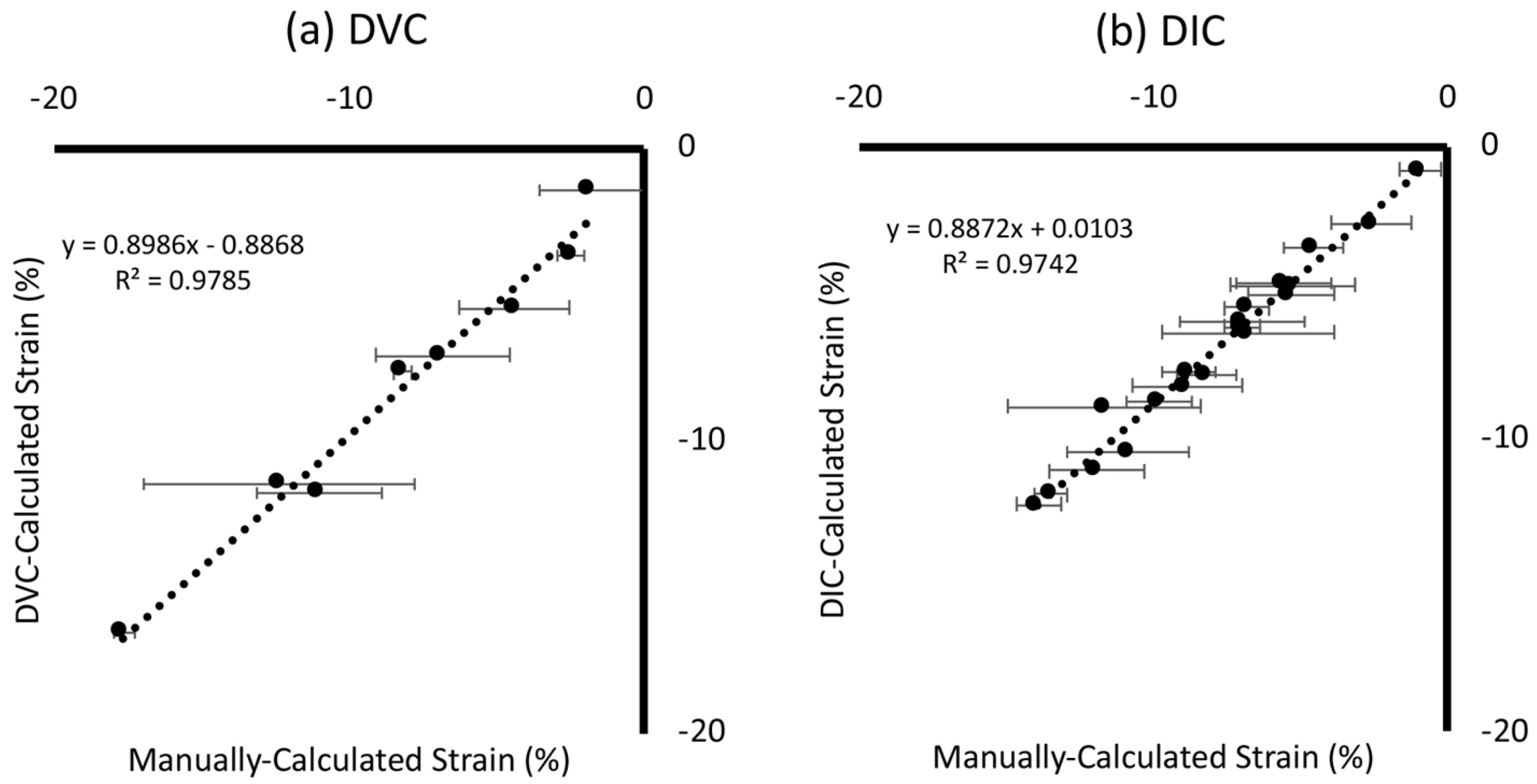
3.1. Ground-Truth Validation
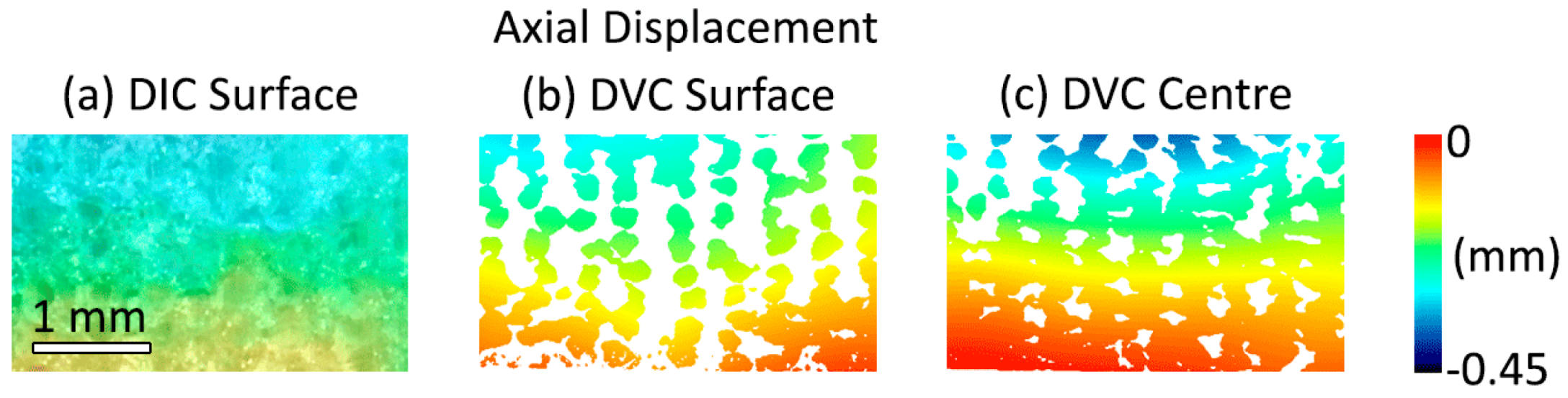
3.1.1. Global Strain
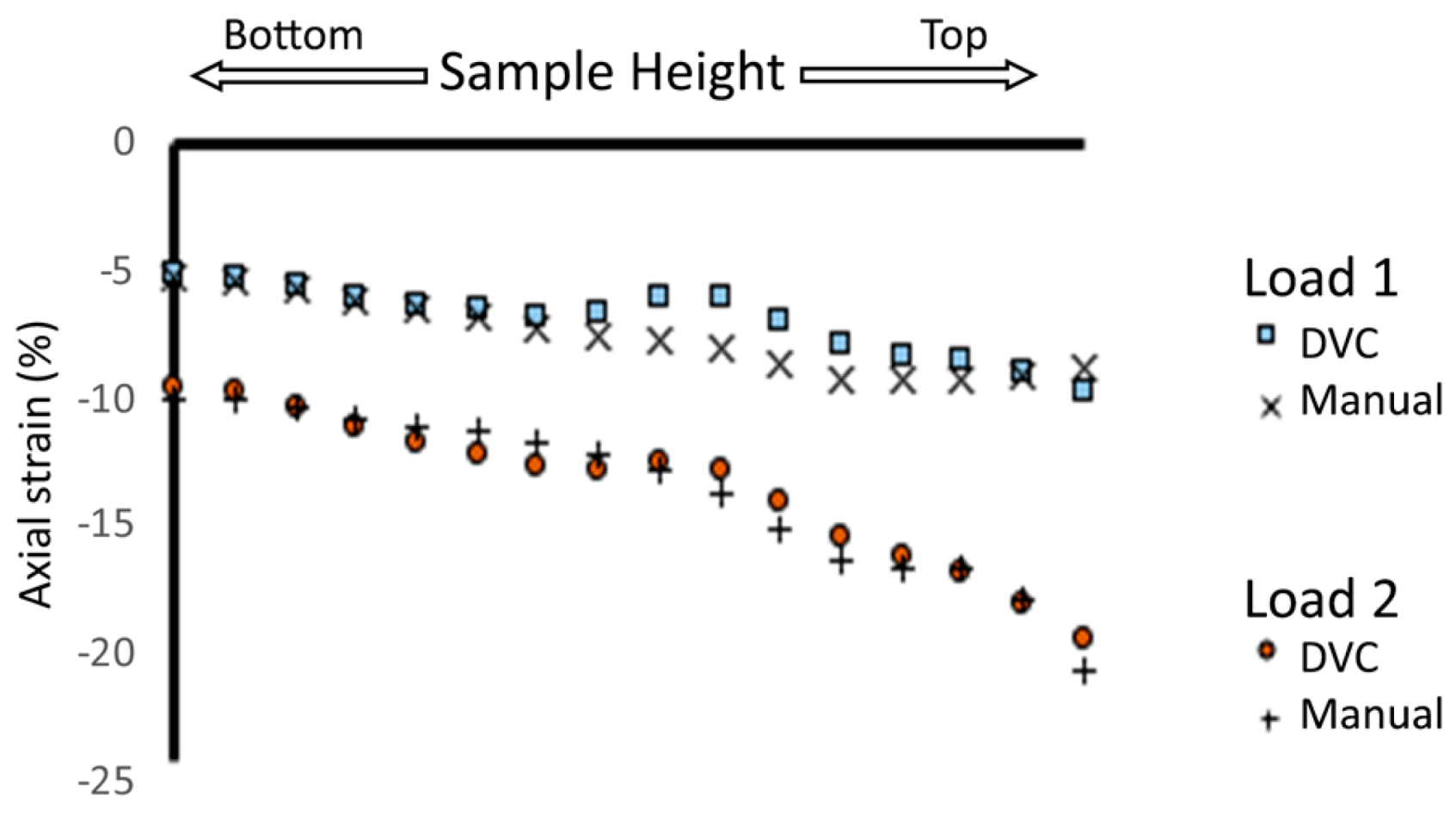
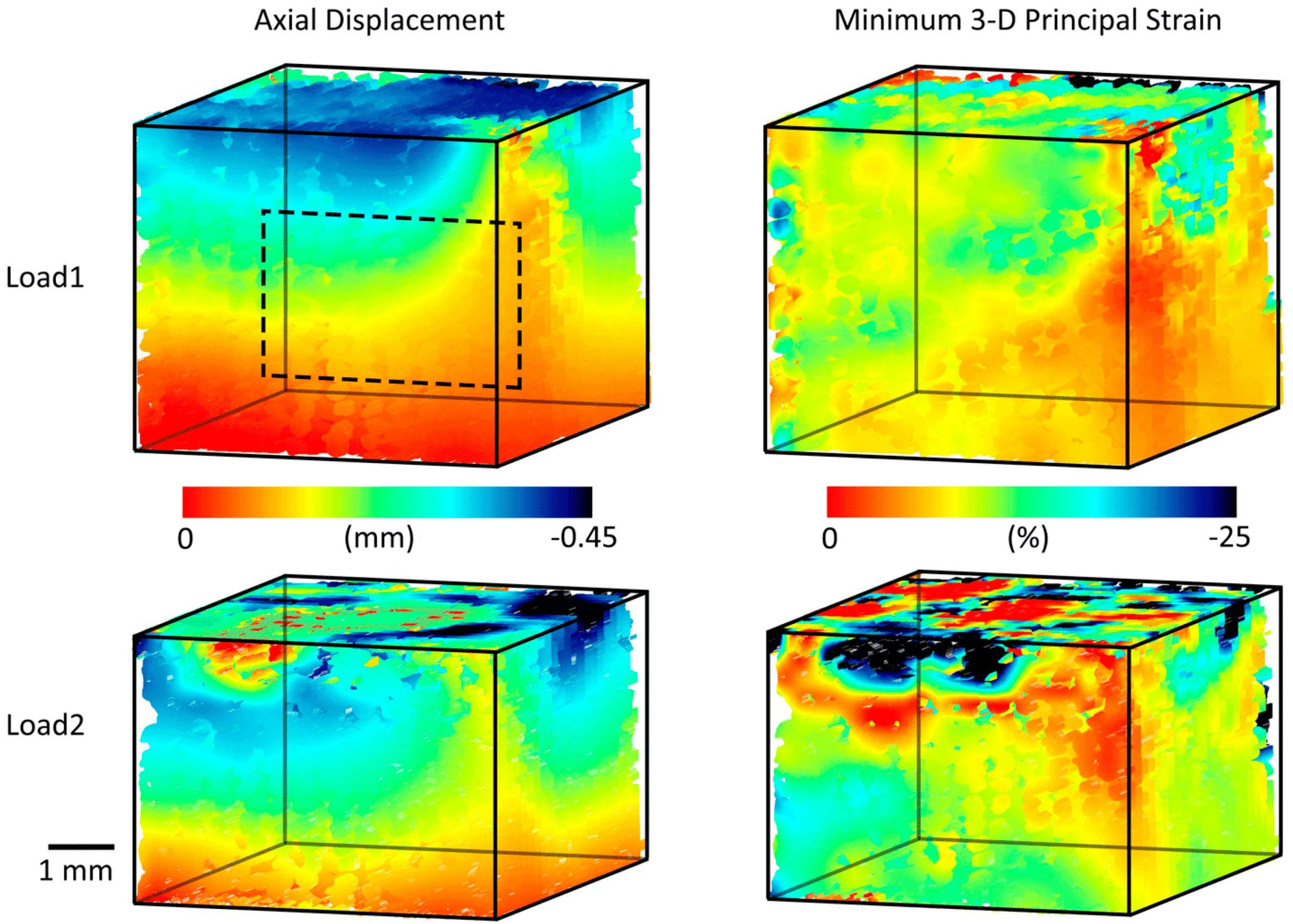
3.1.2. Intra-Sample Strain
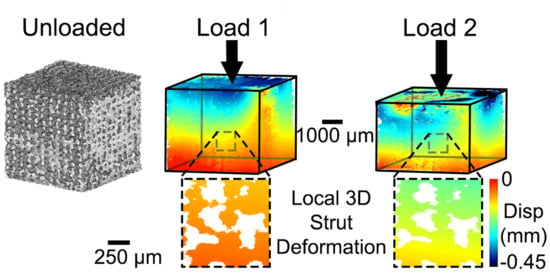
3.2. Three-Dimensional Implant Imaging and Strain Visualisation
3.3. Error Quantification
3.3.1. Digital Volume Correlation
3.3.2. Digital Image Correlation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Byrne, D.P.; Lacroix, D.; Planell, J.A.; Kelly, D.J.; Prendergast, P.J. Simulation of tissue differentiation in a scaffold as a function of porosity, Young’s modulus and dissolution rate: Application of mechanobiological models in tissue engineering. Biomaterials 2007, 28, 5544–5554. [Google Scholar] [CrossRef]
- Beaupré, G.S.; Stevens, S.S.; Carter, D.R. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J. Rehabilitation Res. Dev. 2000, 37, 145–151. [Google Scholar]
- Panadero, J.; Lanceros-Méndez, S.; Ribelles, J.L.G. Differentiation of mesenchymal stem cells for cartilage tissue engineering: Individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Behrens, P.; Bitter, T.; Kurz, B.; Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 2006, 13, 194–202. [Google Scholar] [CrossRef]
- Disney, C.M.; Lee, P.; Hoyland, J.; Sherratt, M.; Bay, B. A review of techniques for visualising soft tissue microstructure deformation and quantifying strain Ex Vivo. J. Microsc. 2018, 272, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Palanca, M.; Tozzi, G.; Cristofolini, L. The use of digital image correlation in the biomechanical area: A review. Int. Biomech. 2015, 3, 1–21. [Google Scholar] [CrossRef]
- Idaszek, J.; Brynk, T.; Jaroszewicz, J.; Vanmeert, F.; Bruinink, A.; Święszkowski, W. Investigation of mechanical properties of porous composite scaffolds with tailorable degradation kinetics after in vitro degradation using digital image correlation. Polym. Compos. 2015, 38, 2402–2410. [Google Scholar] [CrossRef]
- Bay, B.K.; Smith, T.S.; Fyhrie, D.P.; Saad, M. Digital volume correlation: Three-dimensional strain mapping using X-ray tomography. Exp. Mech. 1999, 39, 217–226. [Google Scholar] [CrossRef]
- Roberts, B.; Perilli, E.; Reynolds, K.J. Application of the digital volume correlation technique for the measurement of displacement and strain fields in bone: A literature review. J. Biomech. 2014, 47, 923–934. [Google Scholar] [CrossRef]
- Palanca, M.; Bodey, A.J.; Giorgi, M.; Viceconti, M.; Lacroix, D.; Cristofolini, L.; Dall’Ara, E. Local displacement and strain uncertainties in different bone types by digital volume correlation of synchrotron microtomograms. J. Biomech. 2017, 58, 27–36. [Google Scholar] [CrossRef]
- Palanca, M.; Cristofolini, L.; Dall’Ara, E.; Curto, M.; Innocente, F.; Danesi, V.; Tozzi, G. Digital volume correlation can be used to estimate local strains in natural and augmented vertebrae: An organ-level study. J. Biomech. 2016, 49, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.P.; Barber, A.H.; Blunn, G.; Tozzi, G. Optimization of digital volume correlation computation in SR-microCT images of trabecular bone and bone-biomaterial systems. J. Microsc. 2018, 272, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Madi, K.; Staines, K.A.; Bay, B.K.; Javaheri, B.; Geng, H.; Bodey, A.J.; Cartmell, S.A.; Pitsillides, A.; Lee, P.D. In situ characterization of nanoscale strains in loaded whole joints via synchrotron X-ray tomography. Nat. Biomed. Eng. 2019, 4, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Benoit, A.; Guérard, S.; Gillet, B.; Guillot, G.; Hild, F.; Mitton, D.; Périé, J.-N.; Roux, S. 3D analysis from micro-MRI during in situ compression on cancellous bone. J. Biomech. 2009, 42, 2381–2386. [Google Scholar] [CrossRef]
- Ridzwan, M.I.Z.; Sukjamsri, C.; Pal, B.; Arkel, R.J.; Bell, A.; Khanna, M.; Baskaradas, A.; Abel, R.; Boughton, O.R.; Cobb, J.; et al. Femoral fracture type can be predicted from femoral structure: A finite element study validated by digital volume correlation experiments. J. Orthop. Res. 2017, 36, 993–1001. [Google Scholar] [CrossRef]
- Disney, C.M.; Eckersley, A.; McConnell, J.C.; Geng, H.; Bodey, A.J.; Hoyland, J.A.; Lee, P.D.; Sherratt, M.J.; Bay, B.K. Synchrotron tomography of intervertebral disc deformation quantified by digital volume correlation reveals microstructural influence on strain patterns. Acta Biomater. 2019, 92, 290–304. [Google Scholar] [CrossRef]
- Arora, H.; Nila, A.; Vitharana, K.; Sherwood, J.M.; Nguyen, T.-T.N.; Karunaratne, A.; Mohammed, I.K.; Bodey, A.J.; Hellyer, P.J.; Overby, D.R.; et al. Microstructural Consequences of Blast Lung Injury Characterized with Digital Volume Correlation. Front. Mater. 2017, 4, 4. [Google Scholar] [CrossRef]
- Tavana, S.; Clark, J.; Prior, J.; Baxan, N.; Masouros, S.; Newell, N.; Hansen, U. Quantifying deformations and strains in human intervertebral discs using Digital Volume Correlation combined with MRI (DVC-MRI). J. Biomech. 2020, 102, 109604. [Google Scholar] [CrossRef]
- Sukjamsri, C.; Geraldes, D.M.; Gregory, T.; Ahmed, F.; Hollis, D.; Schenk, S.; Amis, A.; Emery, R.; Hansen, U.N. Digital volume correlation and micro-CT: An in-vitro technique for measuring full-field interface micromotion around polyethylene implants. J. Biomech. 2015, 48, 3447–3454. [Google Scholar] [CrossRef]
- Rapagna, S.; Berahmani, S.; Wyers, C.E.; Bergh, J.P.W.V.D.; Reynolds, K.J.; Tozzi, G.; Janssen, D.; Perilli, E. Quantification of human bone microarchitecture damage in press-fit femoral knee implantation using HR-pQCT and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2019, 97, 278–287. [Google Scholar] [CrossRef]
- Fernández, M.P.; Dall’Ara, E.; Bodey, A.J.; Parwani, R.; Barber, A.H.; Blunn, G.W.; Tozzi, G. Full-Field Strain Analysis of Bone–Biomaterial Systems Produced by the Implantation of Osteoregenerative Biomaterials in an Ovine Model. ACS Biomater. Sci. Eng. 2019, 5, 2543–2554. [Google Scholar] [CrossRef]
- Madi, K.; Tozzi, G.; Zhang, Q.; Tong, J.; Cossey, A.; Au, A.; Hollis, D.; Hild, F. Computation of full-field displacements in a scaffold implant using digital volume correlation and finite element analysis. Med. Eng. Phys. 2013, 35, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Jandejsek, I.; Jiroušek, O.; Vavrik, D. Precise strain measurement in complex materials using Digital Volumetric Correlation and time lapse micro-CT data. Procedia Eng. 2011, 10, 1730–1735. [Google Scholar] [CrossRef]
- Tallia, F.; Russo, L.; Li, S.; Orrin, A.L.H.; Shi, X.; Chen, S.; Steele, J.A.M.; Meille, S.; Chevalier, J.; Lee, P.D.; et al. Bouncing and 3D printable hybrids with self-healing properties. Mater. Horizons 2018, 5, 849–860. [Google Scholar] [CrossRef]
- Li, S.; Tallia, F.; Mohammed, A.A.; Stevens, M.M.; Jones, J.R. Scaffold channel size influences stem cell differentiation pathway in 3-D printed silica hybrid scaffolds for cartilage regeneration. Biomater. Sci. 2020, 8, 4458–4466. [Google Scholar] [CrossRef] [PubMed]
- Novak, B.M. Hybrid Nanocomposite Materials–between inorganic glasses and organic polymers. Adv. Mater. 1993, 5, 422–433. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Liu, L.; Morgan, E.F. Accuracy and precision of digital volume correlation in quantifying displacements and strains in trabecular bone. J. Biomech. 2007, 40, 3516–3520. [Google Scholar] [CrossRef]
- Tozzi, G.; Fernández, M.P.; Davis, S.; Karali, A.; Kao, A.P.; Blunn, G. Full-Field Strain Uncertainties and Residuals at the Cartilage-Bone Interface in Unstained Tissues Using Propagation-Based Phase-Contrast XCT and Digital Volume Correlation. Materials 2020, 13, 2579. [Google Scholar] [CrossRef]
- Franck, C.; Hong, S.; Maskarinec, S.A.; Tirrell, D.A.; Ravichandran, G. Three-dimensional Full-field Measurements of Large Deformations in Soft Materials Using Confocal Microscopy and Digital Volume Correlation. Exp. Mech. 2007, 47, 427–438. [Google Scholar] [CrossRef]
- Fu, J.; Pierron, F.; Ruiz, P.D. Elastic stiffness characterization using three-dimensional full-field deformation obtained with optical coherence tomography and digital volume correlation. J. Biomed. Opt. 2013, 18, 121512. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pan, B.; Lubineau, G. Morphological evolution and internal strain mapping of pomelo peel using X-ray computed tomography and digital volume correlation. Mater. Des. 2018, 137, 305–315. [Google Scholar] [CrossRef]
- Bornert, M.; Chaix, J.-M.; Doumalin, P.; Dupré, J.-C.; Fournel, T.; Jeulin, D.; Maire, E.; Moreaud, M.; Moulinec, H. Mesure tridimensionnelle de champs cinématiques par imagerie volumique pour l’analyse des matériaux et des structures. Instrum. Mes. Métrologie 2004, 4, 43–88. [Google Scholar]
- Dall’Ara, E.; Peña-Fernández, M.; Palanca, M.; Giorgi, M.; Cristofolini, L.; Tozzi, G. Precision of Digital Volume Correlation Approaches for Strain Analysis in Bone Imaged with Micro-Computed Tomography at Different Dimensional Levels. Front. Mater. 2017, 4, 31. [Google Scholar] [CrossRef]
- Tozzi, G.; Dall’Ara, E.; Palanca, M.; Curto, M.; Innocente, F.; Cristofolini, L. Strain uncertainties from two digital volume correlation approaches in prophylactically augmented vertebrae: Local analysis on bone and cement-bone microstructures. J. Mech. Behav. Biomed. Mater. 2017, 67, 117–126. [Google Scholar] [CrossRef]
- Morgeneyer, T.; Helfen, L.; Mubarak, H.; Hild, F. 3D Digital Volume Correlation of Synchrotron Radiation Laminography Images of Ductile Crack Initiation: An Initial Feasibility Study. Exp. Mech. 2012, 53, 543–556. [Google Scholar] [CrossRef]
- Leclerc, H.; Perie, J.-N.; Roux, S.; Hild, F. Voxel-Scale Digital Volume Correlation. Exp. Mech. 2010, 51, 479–490. [Google Scholar] [CrossRef]
- Dall’Ara, E.; Barber, D.; Viceconti, M. About the inevitable compromise between spatial resolution and accuracy of strain measurement for bone tissue: A 3D zero-strain study. J. Biomech. 2014, 47, 2956–2963. [Google Scholar] [CrossRef]
- Palanca, M.; Tozzi, G.; Cristofolini, L.; Viceconti, M.; Dall’Ara, E. Three-Dimensional Local Measurements of Bone Strain and Displacement: Comparison of Three Digital Volume Correlation Approaches. J. Biomech. Eng. 2015, 137, 071006. [Google Scholar] [CrossRef]
- Buljac, A.; Taillandier-Thomas, T.; Helfen, L.; Morgeneyer, T.F.; Hild, F. Evaluation of measurement uncertainties of digital volume correlation applied to laminography data. J. Strain Anal. Eng. Des. 2017, 53, 49–65. [Google Scholar] [CrossRef]
- Roux, S.; Hild, F.; Viot, P.; Bernard, D. Three-dimensional image correlation from X-ray computed tomography of solid foam. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1253–1265. [Google Scholar] [CrossRef]
- Fernández, M.P.; Cipiccia, S.; Dall’Ara, E.; Bodey, A.J.; Parwani, R.; Pani, M.; Blunn, G.W.; Barber, A.H.; Tozzi, G. Effect of SR-microCT radiation on the mechanical integrity of trabecular bone using in situ mechanical testing and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2018, 88, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.P.; Black, C.I.; Dawson, J.; Gibbs, D.; Kanczler, J.M.; Oreffo, R.O.C.; Tozzi, G. Exploratory Full-Field Strain Analysis of Regenerated Bone Tissue from Osteoinductive Biomaterials. Materials 2020, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.M.; Milot, L.; Parry, C.; Williams, R.; Burns, P.N. Inter- and Intra-Operator Reliability and Repeatability of Shear Wave Elastography in the Liver: A Study in Healthy Volunteers. Ultrasound Med. Biol. 2013, 39, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Pan, B. Thermal error analysis and compensation for digital image/volume correlation. Opt. Lasers Eng. 2018, 101, 1–15. [Google Scholar] [CrossRef]
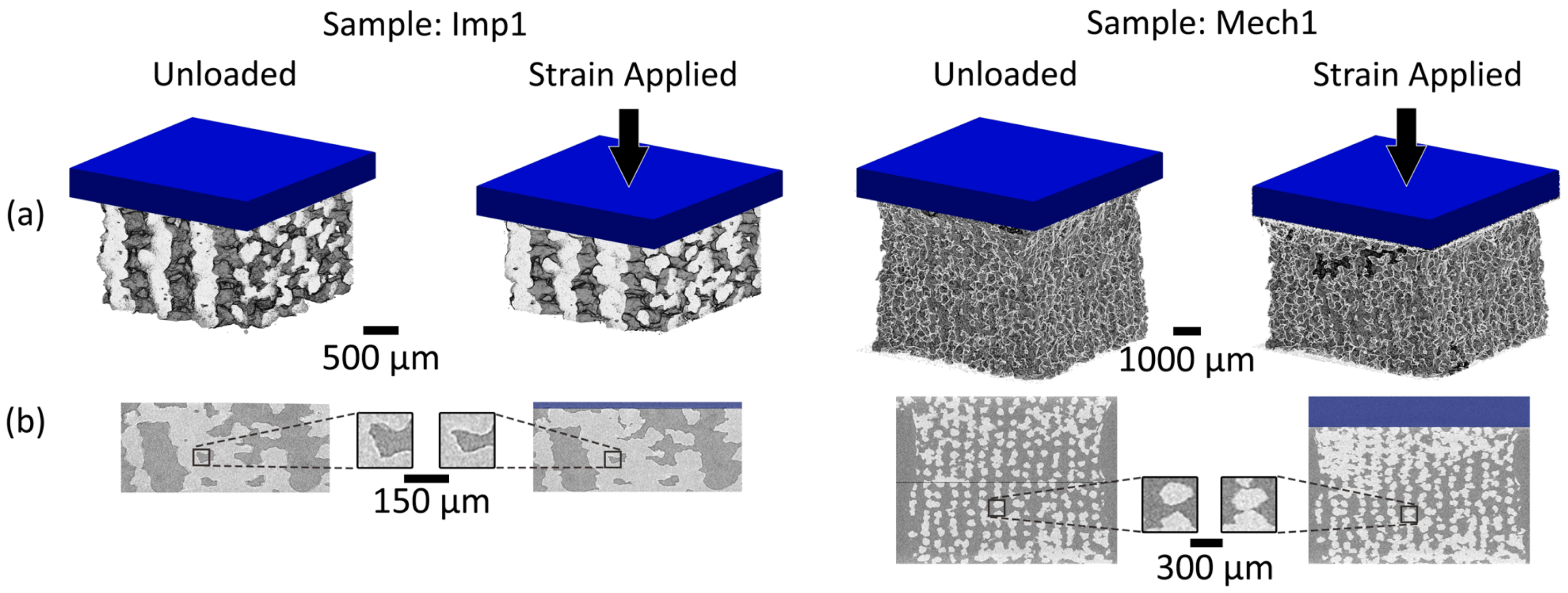
| Sample | Global Overall Strain (%) at Loading Step | Voxel Size (µm) | Projections | Exposure Time Per Projection (s) | Sample Height (µm) |
|---|---|---|---|---|---|
| Imp1 | −2 | 3.9 | 1201 | 1.5 | 930 |
| −12 | |||||
| Imp2 | −4 | 3.9 | 1201 | 1.5 | 910 |
| Imp3 | −7 | 3.9 | 1201 | 1.5 | 925 |
| Imp4 | −2 | 4.1 | 1201 | 1.5 | 1200 |
| −11 | |||||
| Mech1 | −8 | 10.6 | 801 | 1 | 4750 |
| −18 | |||||
| Mech2 | −8 | 4.1 | 1201 | 1.5 | 3375 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, J.N.; Tavana, S.; Heyraud, A.; Tallia, F.; Jones, J.R.; Hansen, U.; Jeffers, J.R.T. Quantifying 3D Strain in Scaffold Implants for Regenerative Medicine. Materials 2020, 13, 3890. https://doi.org/10.3390/ma13173890
Clark JN, Tavana S, Heyraud A, Tallia F, Jones JR, Hansen U, Jeffers JRT. Quantifying 3D Strain in Scaffold Implants for Regenerative Medicine. Materials. 2020; 13(17):3890. https://doi.org/10.3390/ma13173890
Chicago/Turabian StyleClark, Jeffrey N., Saman Tavana, Agathe Heyraud, Francesca Tallia, Julian R. Jones, Ulrich Hansen, and Jonathan R. T. Jeffers. 2020. "Quantifying 3D Strain in Scaffold Implants for Regenerative Medicine" Materials 13, no. 17: 3890. https://doi.org/10.3390/ma13173890
APA StyleClark, J. N., Tavana, S., Heyraud, A., Tallia, F., Jones, J. R., Hansen, U., & Jeffers, J. R. T. (2020). Quantifying 3D Strain in Scaffold Implants for Regenerative Medicine. Materials, 13(17), 3890. https://doi.org/10.3390/ma13173890