Assessing the Mechanical Weakness of Vertebrae Affected by Primary Tumors: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
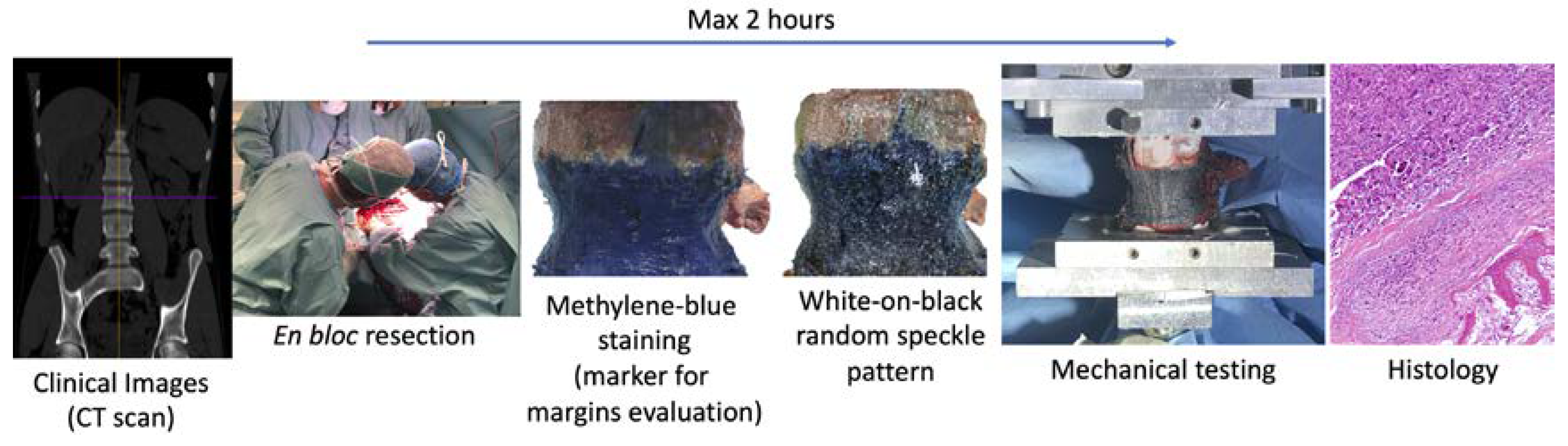
2.1. Protocol
- The specimen can stay in air only two hours to not modify the biological characteristics, after that the specimen must be fixed in formalin solution (also if the tests are not completed);
- The specimen must not be fractured or damaged to not compromise the evaluation of the margins of the tumor and the histological analysis.
2.2. Cases
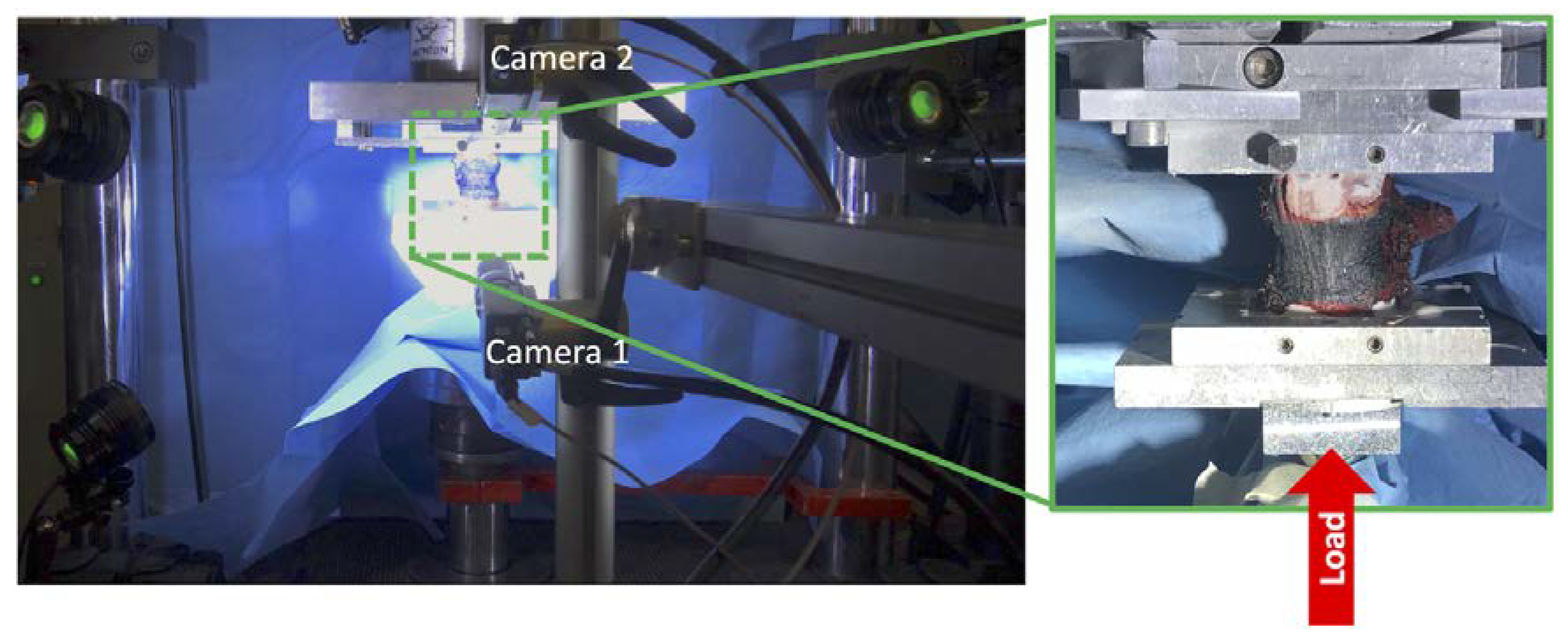
2.3. Mechanical Tests
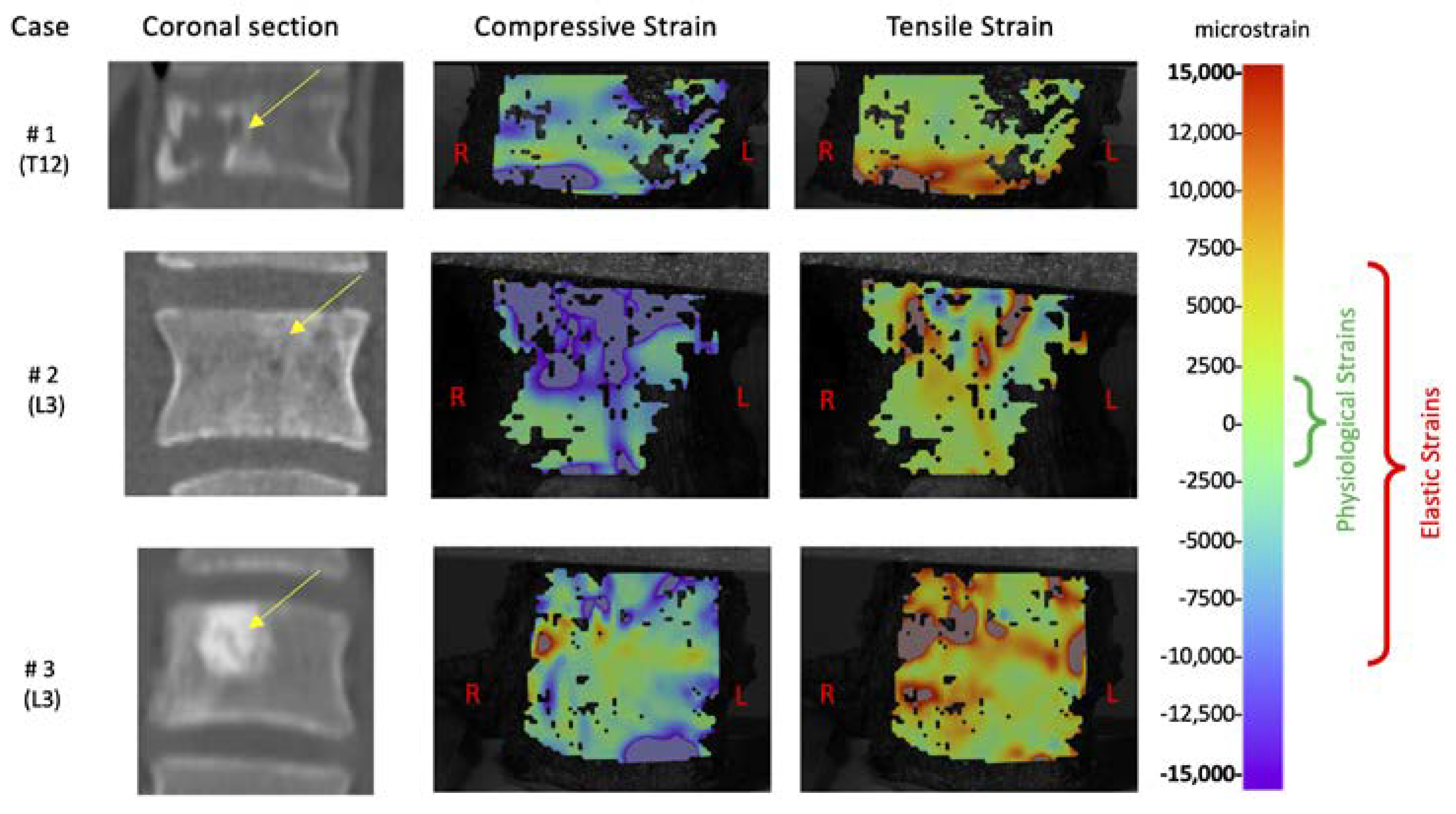
2.4. Strain Measurements
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dorfman, H.D.; Czerniak, B. Bone cancers. Cancer 1995, 75, 203–210. [Google Scholar] [CrossRef]
- Franchi, A. Epidemiology and classification of bone tumors. Clin. Cases Miner. Bone Metab. 2012, 9, 92–95. [Google Scholar] [PubMed]
- De Felice, F.; Piccioli, A.; Musio, D.; Tombolini, V. The role of radiation therapy in bone metastases management. Oncotarget 2017, 8. [Google Scholar] [CrossRef]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Dosani, M.; Lucas, S.; Wong, J.; Weir, L.; Lomas, S.; Cumayas, C.; Fisher, C.; Tyldesley, S. Impact of the spinal instability neoplastic score on surgical referral patterns and outcomes. Curr. Oncol. 2018, 25, 53. [Google Scholar] [CrossRef]
- Fox, S.; Spiess, M.; Hnenny, L.; Fourney, D.R. Spinal instability neoplastic score (SINS): Reliability among spine fellows and resident physicians in orthopedic surgery and neurosurgery. Glob. Spine J. 2017, 7, 744–748. [Google Scholar] [CrossRef]
- Costa, M.C.; Eltes, P.; Lazary, A.; Varga, P.P.; Viceconti, M.; Dall’Ara, E. Biomechanical assessment of vertebrae with lytic metastases with subject-specific finite element models. J. Mech. Behav. Biomed. Mater. 2019, 98, 268–290. [Google Scholar] [CrossRef]
- Charest-Morin, R.; Fisher, C.G.; Sahgal, A.; Boriani, S.; Gokaslan, Z.L.; Lazary, A.; Reynolds, J.; Bettegowda, C.; Rhines, L.D.; Dea, N. Primary bone tumor of the spine—An evolving field: What a general spine surgeon Should know. Glob. Spine J. 2019, 9, 108S–116S. [Google Scholar] [CrossRef]
- Galbusera, F.; Qian, Z.; Casaroli, G.; Bassani, T.; Costa, F.; Schlager, B.; Wilke, H.-J. The role of the size and location of the tumors and of the vertebral anatomy in determining the structural stability of the metastatically involved spine: A Finite element study. Transl. Oncol. 2018, 11, 639–646. [Google Scholar] [CrossRef]
- Palanca, M.; Barbanti-Bròdano, G.; Cristofolini, L. The size of simulated lytic metastases affects the strain distribution on the anterior surface of the vertebra. J. Biomech. Eng. 2018, 140, 111005. [Google Scholar] [CrossRef]
- Costa, M.C.; Campello, L.B.B.; Ryan, M.; Rochester, J.; Viceconti, M.; Dall’Ara, E. Effect of size and location of simulated lytic lesions on the structural properties of human vertebral bodies, a micro-finite element study. Bone Rep. 2020, 12, 100257. [Google Scholar] [CrossRef]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980, 153, 106–120. [Google Scholar] [CrossRef]
- Boriani, S.; Weinstein, J.N.; Biagini, R. Primary bone tumors of the spine. Spine 1997, 22, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Bilsky, M.H.; Laufer, I.; Fourney, D.R.; Groff, M.; Schmidt, M.H.; Varga, P.P.; Vrionis, F.D.; Yamada, Y.; Gerszten, P.C.; Kuklo, T.R. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine 2010, 13, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Picci, P.; Böhling, T.; Bacci, G.; Ferrari, S.; Sangiorgi, L.; Mercuri, M.; Ruggieri, P.; Manfrini, M.; Ferraro, A.; Casadei, R.; et al. Chemotherapy-induced tumor necrosis as a prognostic factor in localized Ewing’s sarcoma of the extremities. J. Clin. Oncol. 1997, 15, 1553–1559. [Google Scholar] [CrossRef]
- Girolami, M.; Pipola, V.; Ghermandi, R.; Tedesco, G.; Evangelisti, G.; Gasbarrini, A. Chiripa technique. Report of a novel technique to protect the spinal cord from incidental durotomies during complex spinal procedures. J. Clin. Neurosci. 2019, 59, 384–387. [Google Scholar] [CrossRef]
- Danesi, V.; Zani, L.; Scheele, A.; Berra, F.; Cristofolini, L. Reproducible reference frame for in vitro testing of the human vertebrae. J. Biomech. 2014, 47, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Dall’Ara, E.; Schmidt, R.; Pahr, D.; Varga, P.; Chevalier, Y.; Patsch, J.; Kainberger, F.; Zysset, P.; Dall’Ara, E.; Schmidt, R.; et al. A nonlinear finite element model validation study based on a novel experimental technique for inducing anterior wedge-shape fractures in human vertebral bodies in vitro. J. Biomech. 2010, 43, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.J.; Lanyon, L.E. Mechanical strain and bone cell function: A review. Osteoporos. Int. 2002, 13, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Cristofolini, L.; Brandolini, N.; Danesi, V.; Juszczyk, M.M.; Erani, P.; Viceconti, M. Strain distribution in the lumbar vertebrae under different loading configurations. Spine J. 2013, 13, 1281–1292. [Google Scholar] [CrossRef]
- Cristofolini, L. In vitro evidence of the structural optimization of the human skeletal bones. J. Biomech. 2015, 48, 787–796. [Google Scholar] [CrossRef]
- Lionello, G.; Sirieix, C.; Baleani, M. An effective procedure to create a speckle pattern on biological soft tissue for digital image correlation measurements. J. Mech. Behav. Biomed. Mater. 2014, 39, 1–8. [Google Scholar] [CrossRef]
- Lionello, G.; Cristofolini, L. A practical approach to optimizing the preparation of speckle patterns for digital-image correlation. Meas. Sci. Technol. 2014, 25, 107001. [Google Scholar] [CrossRef]
- Palanca, M.; Marco, M.; Ruspi, M.L.; Cristofolini, L. Full-field strain distribution in multi-vertebra spine segments: An in-vitro application of DIC. Med. Eng. Phys. 2018, 52, 76–83. [Google Scholar] [CrossRef]
- Palanca, M.; Brugo, T.M.M.; Cristofolini, L. Use of digital image correlation to understand the Biomechanics of the vertebra. J. Mech. Med. Biol. 2015, 15, 1540004–1540010. [Google Scholar] [CrossRef]
- Aamodt, A.; Lund-Larsen, J.; Eine, J.; Andersen, E.; Benum, P.; Husby, O.S. In vivo measuments show tensile axial strain in the proximal lateral aspect of the human femur. J. Orthop. Res. 1997, 15, 927–931. [Google Scholar] [CrossRef]
- Bayraktar, H.H.; Morgan, E.F.; Niebur, G.L.; Morris, G.E.; Wong, E.K.; Keaveny, T.M. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 2004, 37, 27–35. [Google Scholar] [CrossRef]
- Nazarian, A.; von Stechow, D.; Zurakowski, D.; Müller, R.; Snyder, B.D. Bone volume fraction explains the variation in strength and stiffness of cancellous bone affected by metastatic cancer and osteoporosis. Calcif. Tissue Int. 2008, 83, 368–379. [Google Scholar] [CrossRef]
- Dall’Ara, E.; Schmidt, R.; Zysset, P. Microindentation can discriminate between damaged and intact human bone tissue. Bone 2012, 50, 925–929. [Google Scholar] [CrossRef]
- Silva, M.J.; Hipp, J.A.; McGowan, D.P.; Takeuchi, T.; Hayes, W.C. Strength reductions of thoracic vertebrae in the presence of transcortical osseous defects: Effects of defect location, pedicle disruption, and defect size. Eur. Spine J. 1993, 2, 118–125. [Google Scholar] [CrossRef]
- Whyne, C.M.; Hu, S.S.; Lotz, J.C. Burst fracture in the metastatically involved spine: Development, validation, and parametric analysis of a three-dimensional poroelastic finite-element model. Spine 2003, 28, 652–660. [Google Scholar] [CrossRef]
- Windhagen, H.J.; Hipp, J.A.; Silva, M.J.; Lipson, S.J.; Hayes, W.C. Predicting failure of thoracic vertebrae with simulated and actual metastatic defects. Clin. Orthop. Relat. Res. 1997, 344, 313–319. [Google Scholar] [CrossRef]
- Alkalay, R.N.; Von Stechow, D.; Hackney, D.B. Augmentation of failed human vertebrae with critical un-contained lytic defect restores their structural competence under functional loading: An experimental study. Clin. Biomech. 2015, 30, 608–616. [Google Scholar] [CrossRef]
- Alkalay, R.N.; Harrigan, T.P. Mechanical assessment of the effects of metastatic lytic defect on the structural response of human thoracolumbar spine. J. Orthop. Res. 2016, 34, 1808–1819. [Google Scholar] [CrossRef]
- Bilsky, M.H.; Lis, E.; Raizer, J.; Lee, H.; Boland, P. The diagnosis and treatment of metastatic spinal tumor. Oncologist 1999, 4, 459–469. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef]
- Sahgal, A.; Whyne, C.M.; Ma, L.; Larson, D.A.; Fehlings, M.G. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol. 2013, 14, e310–e320. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Liverani, C.; Mercatali, L.; Cristofolini, L.; Giordano, E.; Minardi, S.; Porta, G.D.; De Vita, A.; Miserocchi, G.; Spadazzi, C.; Tasciotti, E.; et al. Investigating the mechanobiology of cancer cell–ECM interaction through collagen-based 3D scaffolds. Cell. Mol. Bioeng. 2017, 10, 223–234. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef] [PubMed]
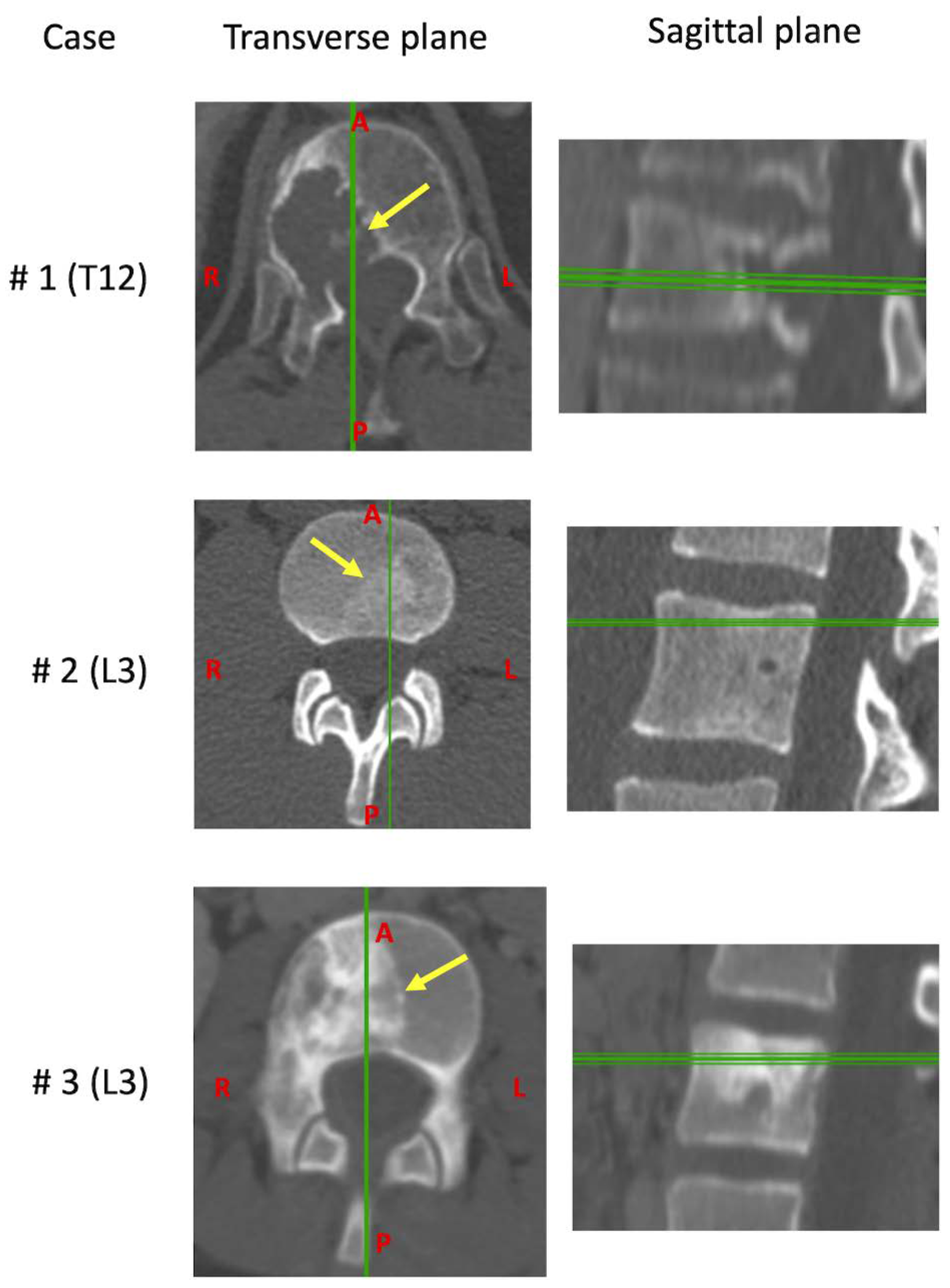
| Case | Age | Sex | Tumors | Vertebra | Grade | Local Extensions | 6-Point ESCC | SINS | Margins Classification | Presence of Metastasis | Months between Diagnosis and Surgery | Previous Therapy | Current Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 62 | M | Chordoma | T12 | IB | 7–10 | 1B | 11 | Focal margin, in the posterior part of the specimen. Wide, the other margins | NO | 2 | None | None |
| #2 | 22 | M | Ewing’s sarcoma | L3 | IIB | 2–6 | 0 | 4 | Wide (Necrosis 40%, Bologna system 1) | NO | 4 | Chemotherapy | Chemotherapy and Radiotherapy |
| #3 | 11 | F | Ewing’s sarcoma | L3 | IIB | 7–11 | 1A | 6 | Wide (Necrosis 80%, Bologna system 1) | NO | 6 | Chemotherapy | Chemotherapy and Radiotherapy |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palanca, M.; Cristofolini, L.; Gasbarrini, A.; Tedesco, G.; Barbanti-Bròdano, G. Assessing the Mechanical Weakness of Vertebrae Affected by Primary Tumors: A Feasibility Study. Materials 2020, 13, 3256. https://doi.org/10.3390/ma13153256
Palanca M, Cristofolini L, Gasbarrini A, Tedesco G, Barbanti-Bròdano G. Assessing the Mechanical Weakness of Vertebrae Affected by Primary Tumors: A Feasibility Study. Materials. 2020; 13(15):3256. https://doi.org/10.3390/ma13153256
Chicago/Turabian StylePalanca, Marco, Luca Cristofolini, Alessandro Gasbarrini, Giuseppe Tedesco, and Giovanni Barbanti-Bròdano. 2020. "Assessing the Mechanical Weakness of Vertebrae Affected by Primary Tumors: A Feasibility Study" Materials 13, no. 15: 3256. https://doi.org/10.3390/ma13153256
APA StylePalanca, M., Cristofolini, L., Gasbarrini, A., Tedesco, G., & Barbanti-Bròdano, G. (2020). Assessing the Mechanical Weakness of Vertebrae Affected by Primary Tumors: A Feasibility Study. Materials, 13(15), 3256. https://doi.org/10.3390/ma13153256







