The Formation Mechanism of a Self-Organized Periodic-Layered Structure at the Solid-(Cr, Fe)2B/Liquid-Al Interface
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Samples
2.2. Immersion Test
2.3. Material Characterization
3. Results and Discussion
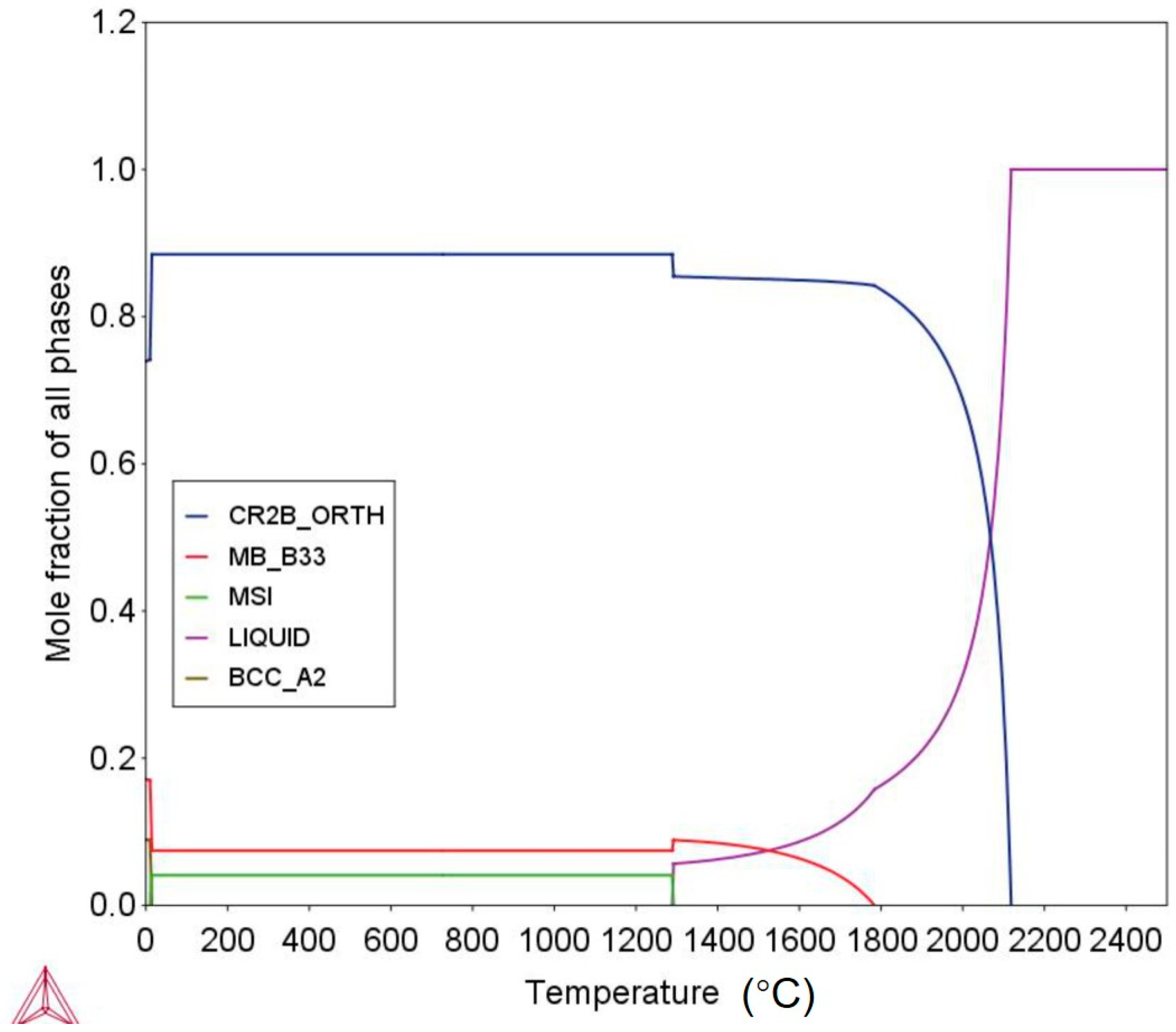
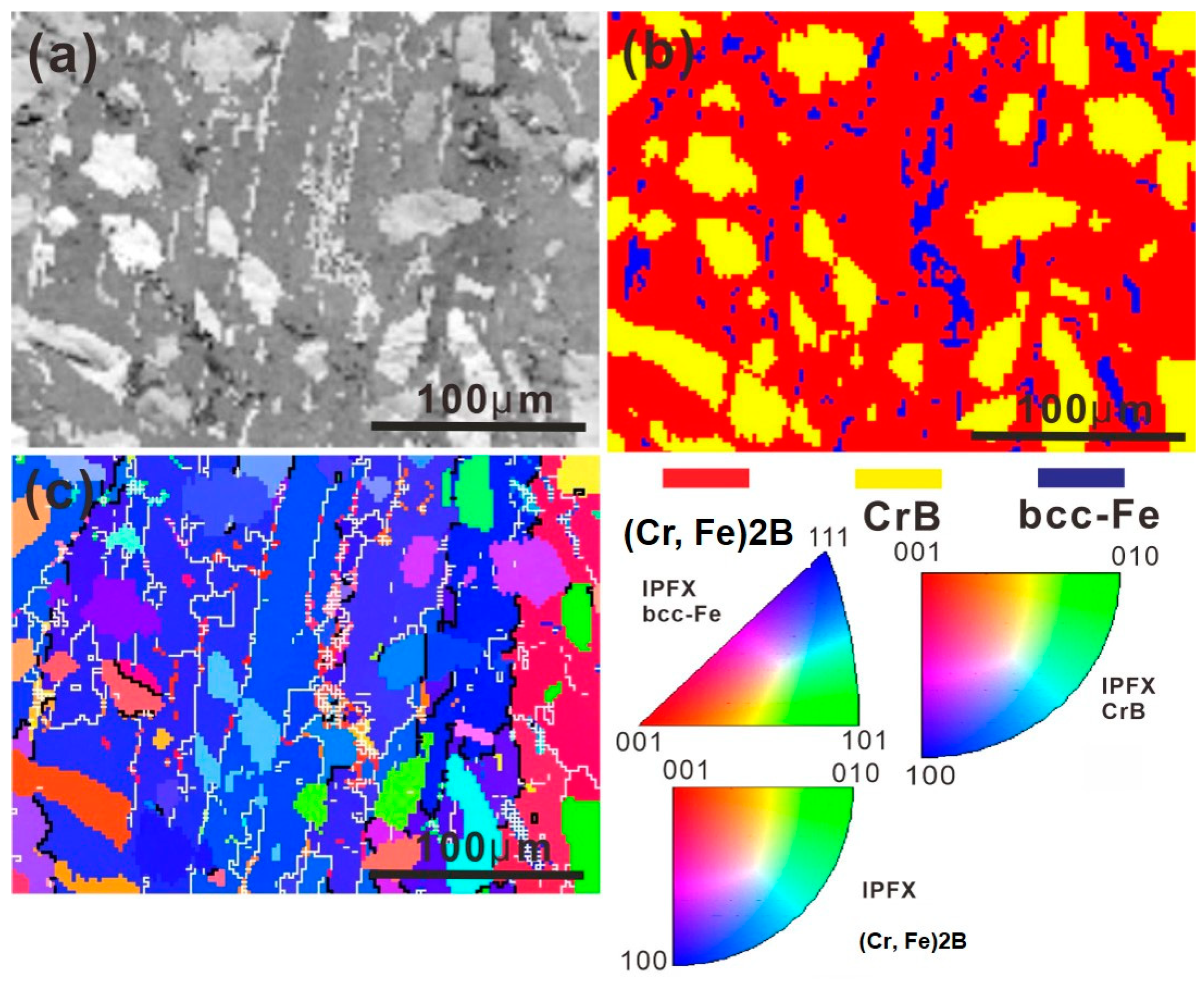
3.1. As-Cast Microstructure
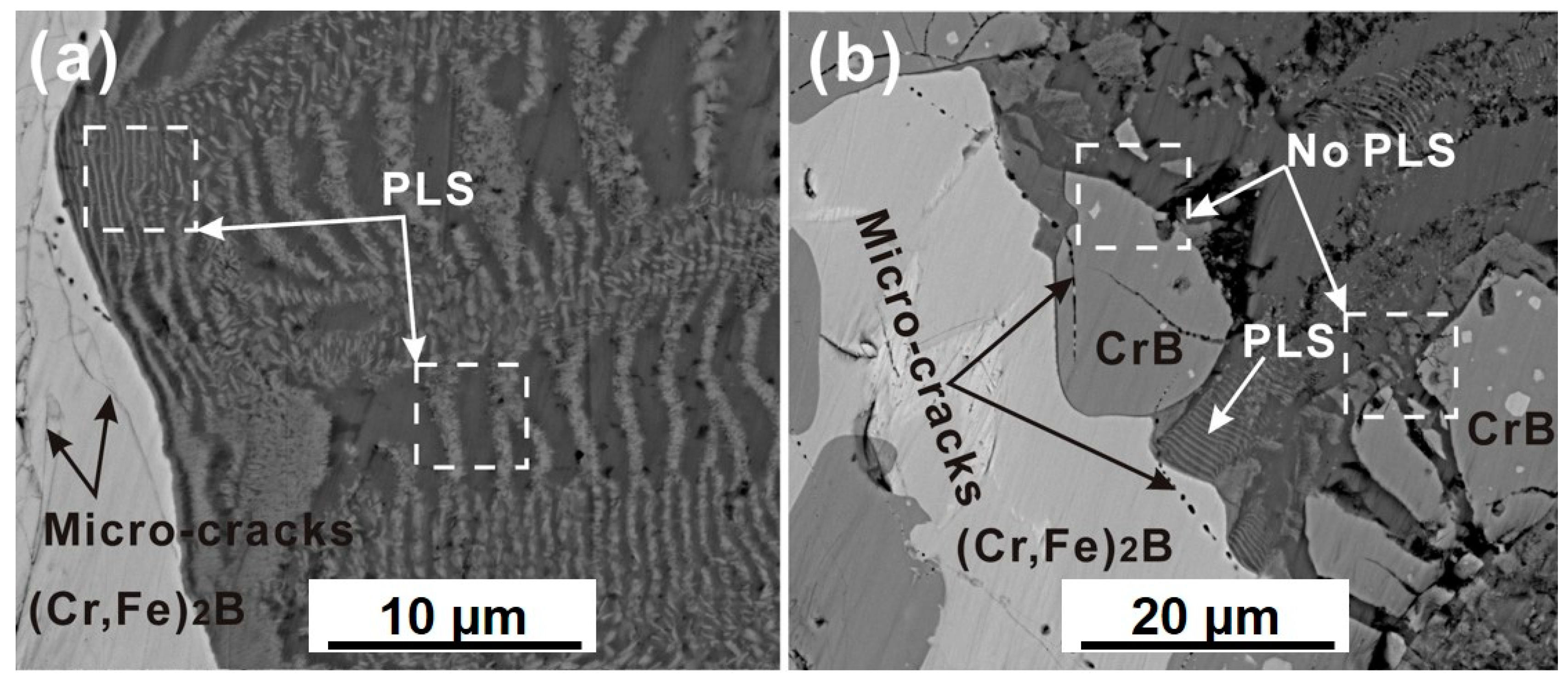
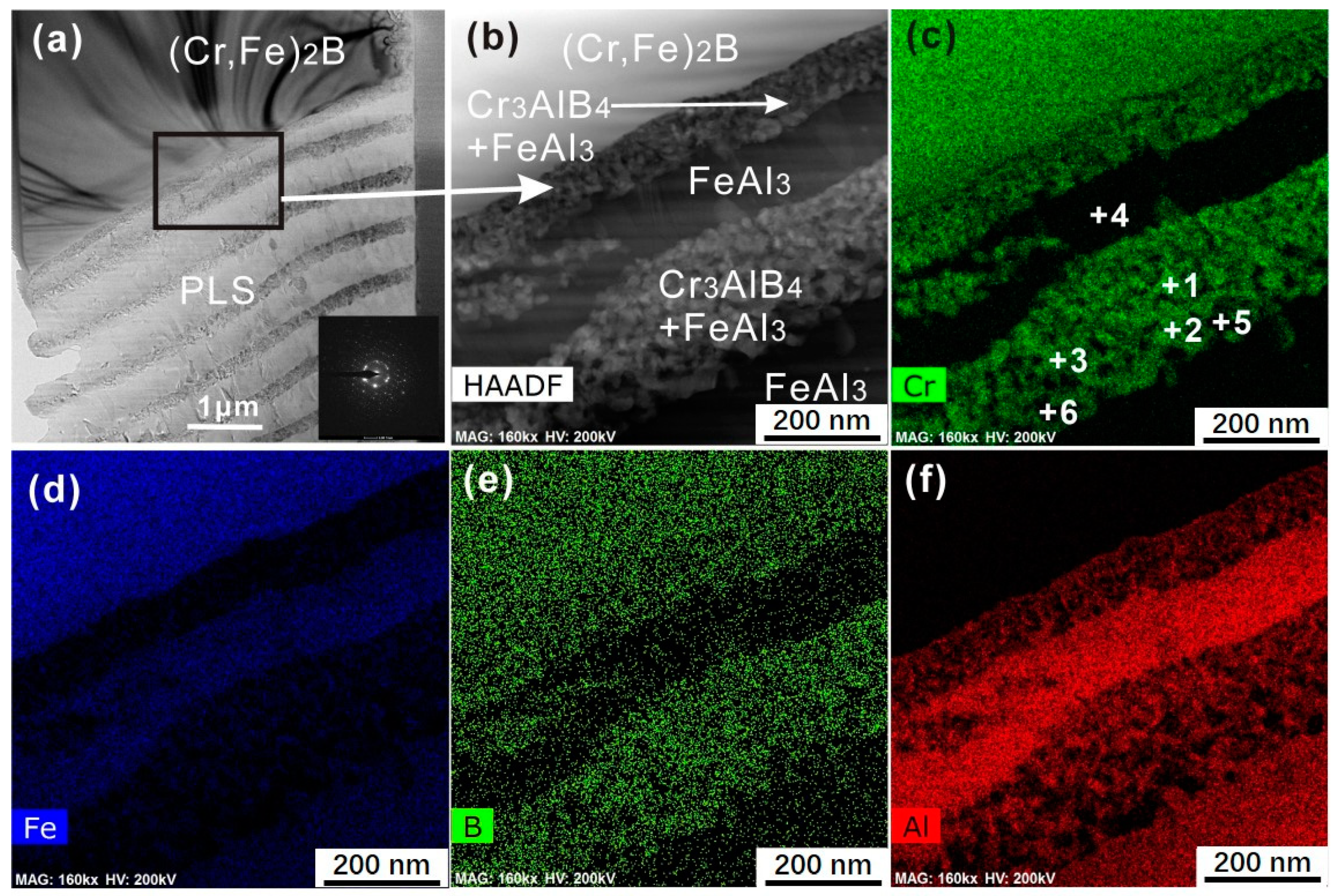
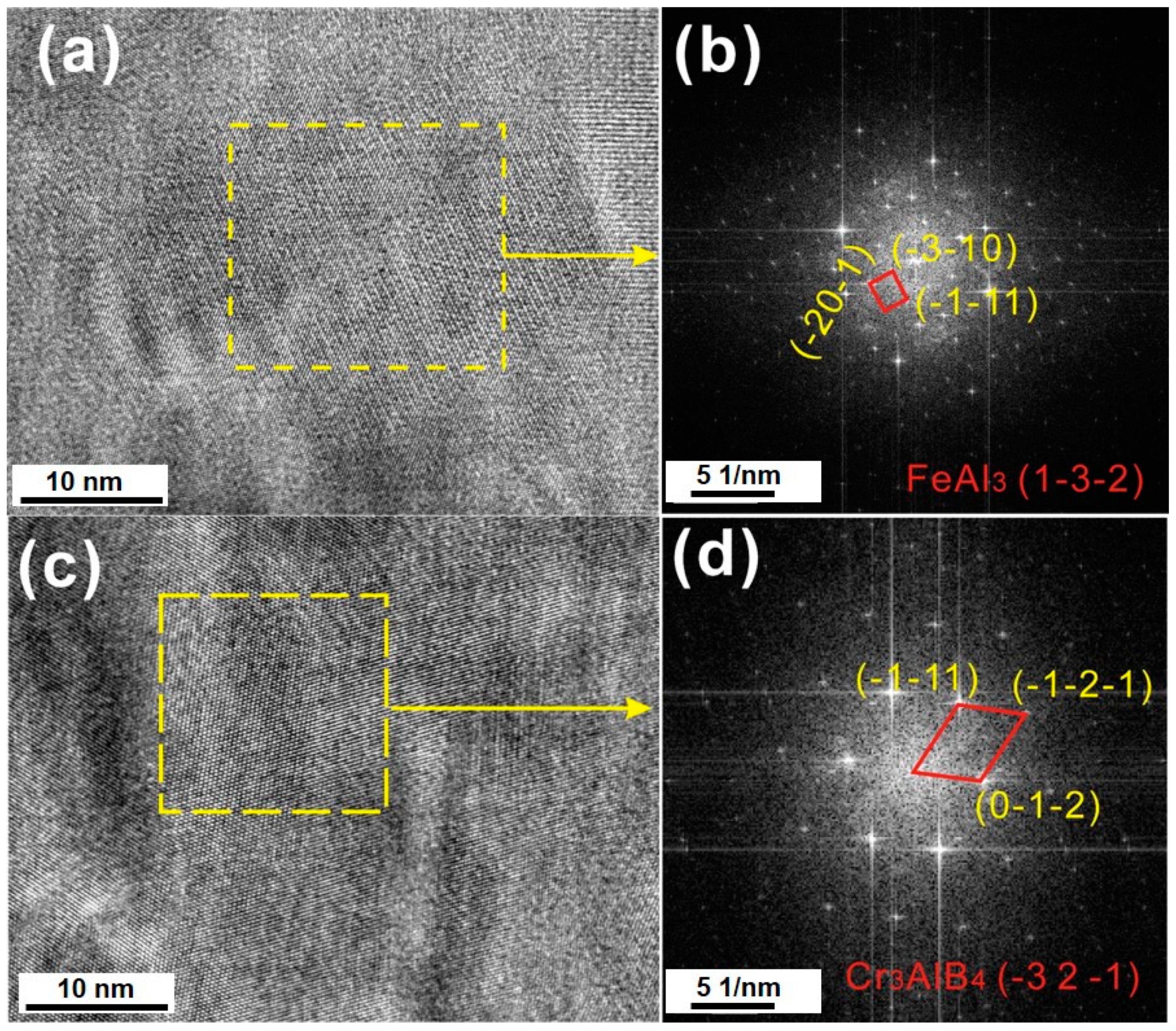
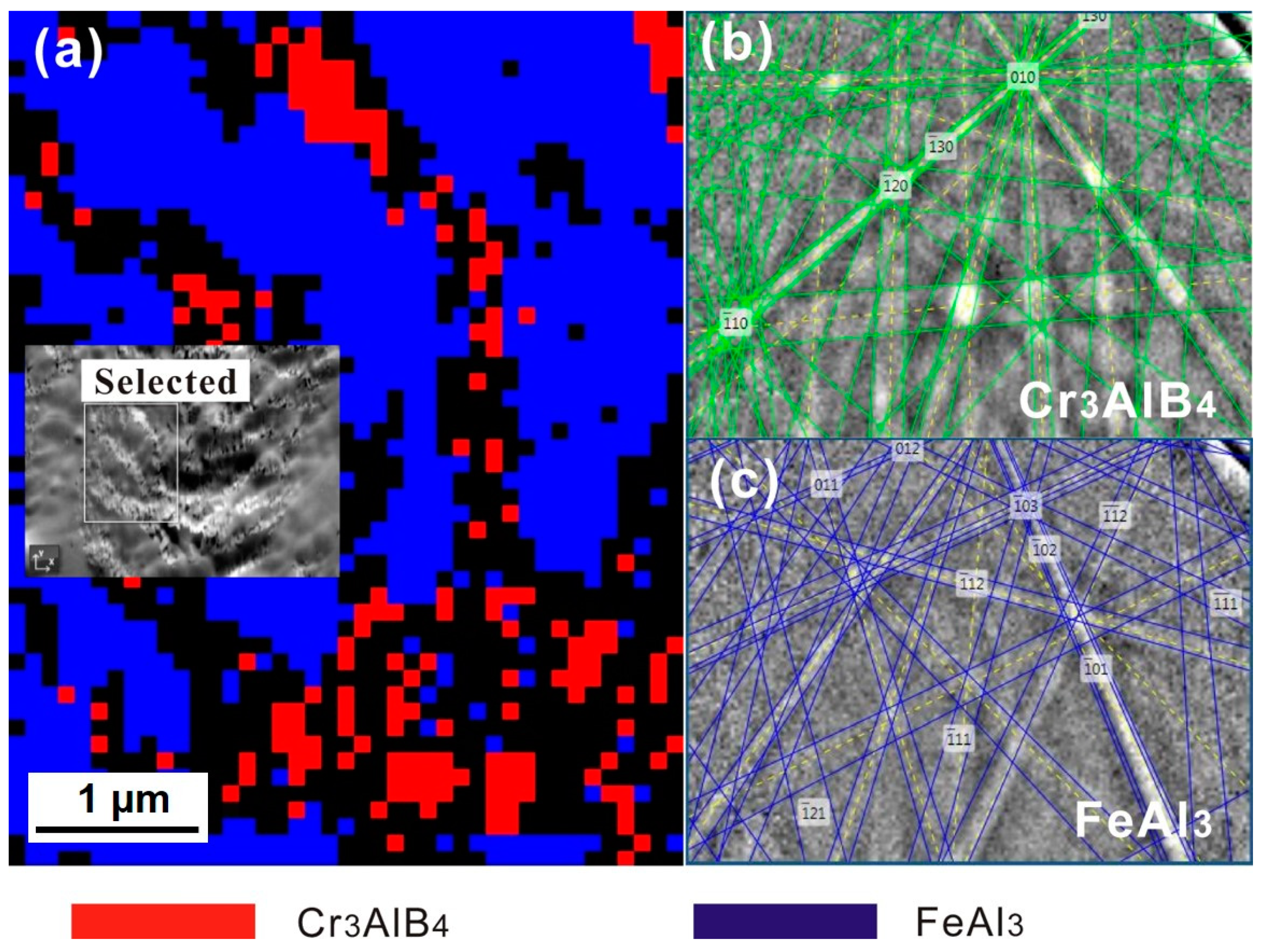
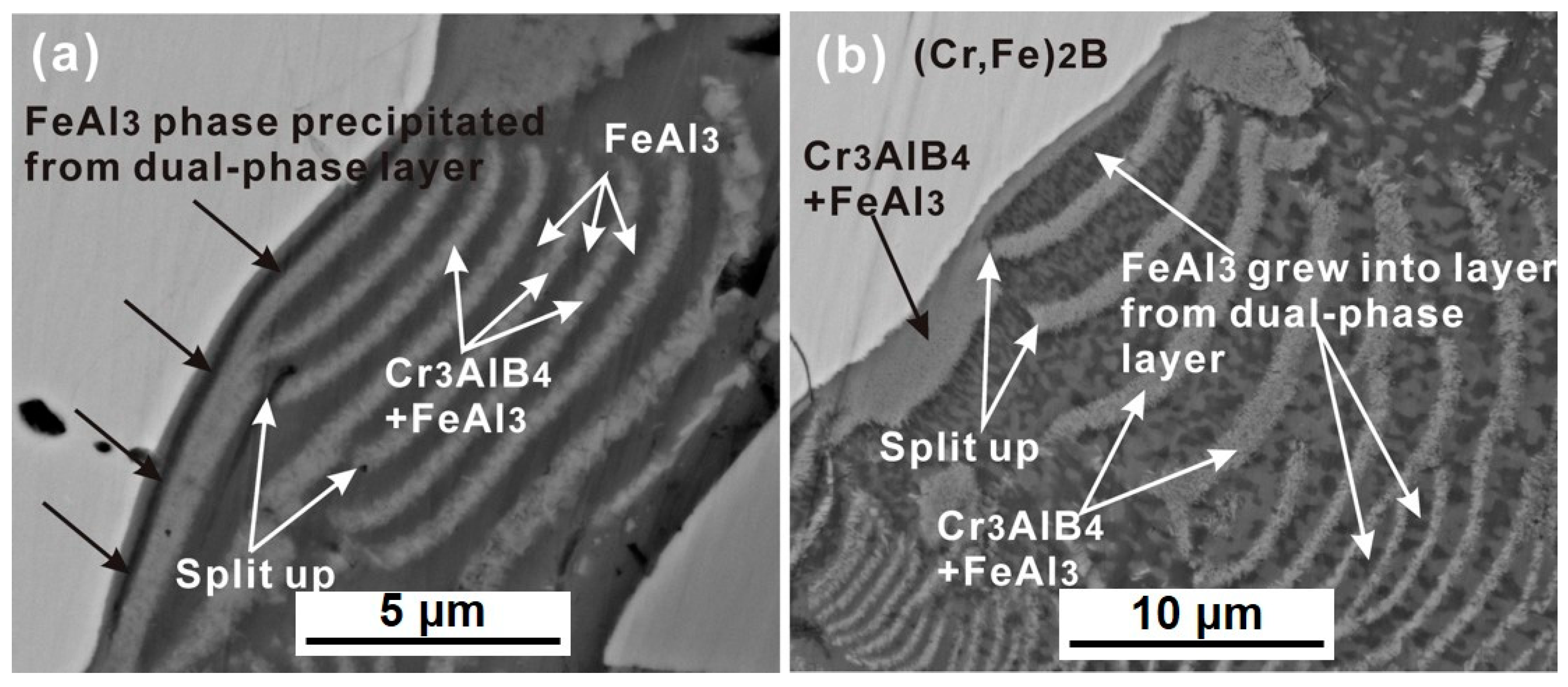
3.2. Interface Morphology and Reaction Products
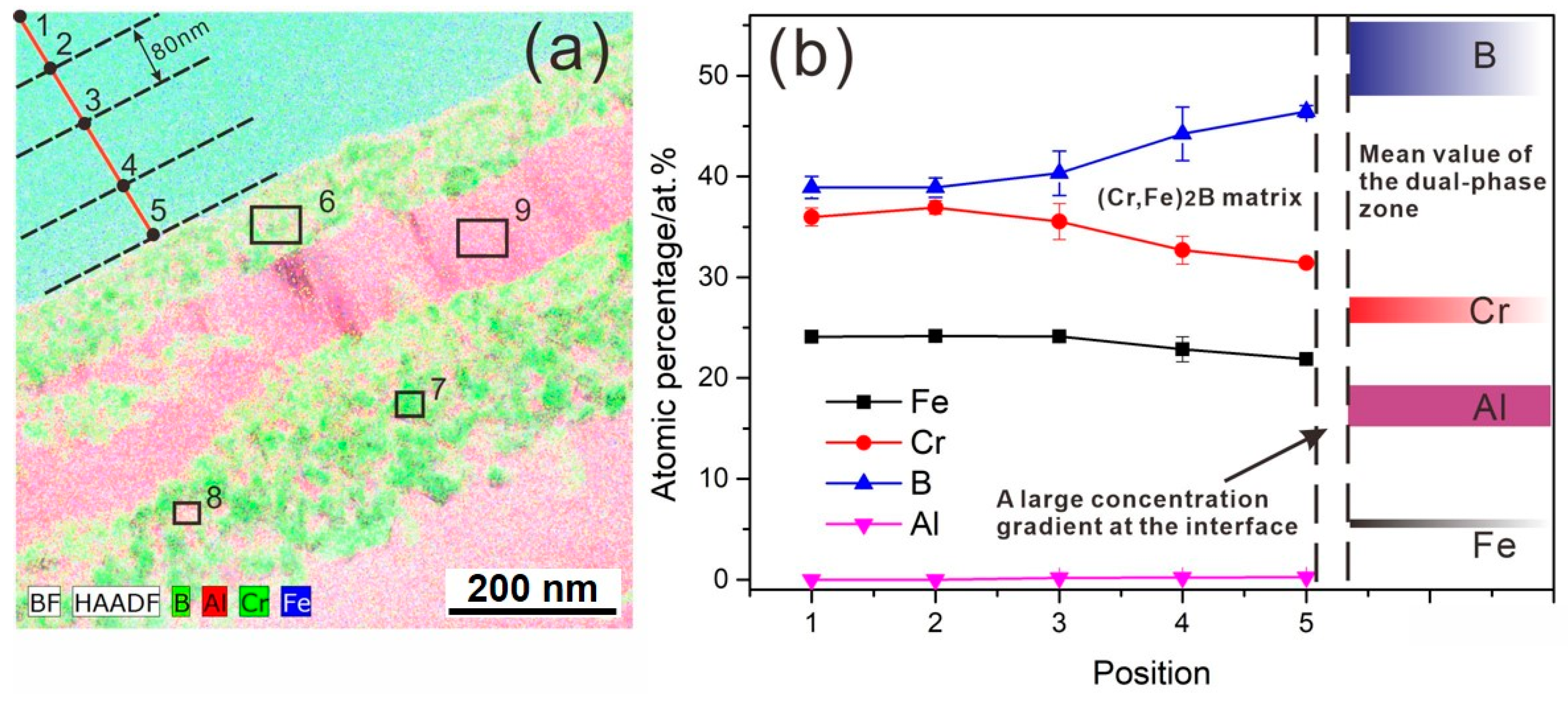
3.3. Diffusion Kinetics of an Individual Element
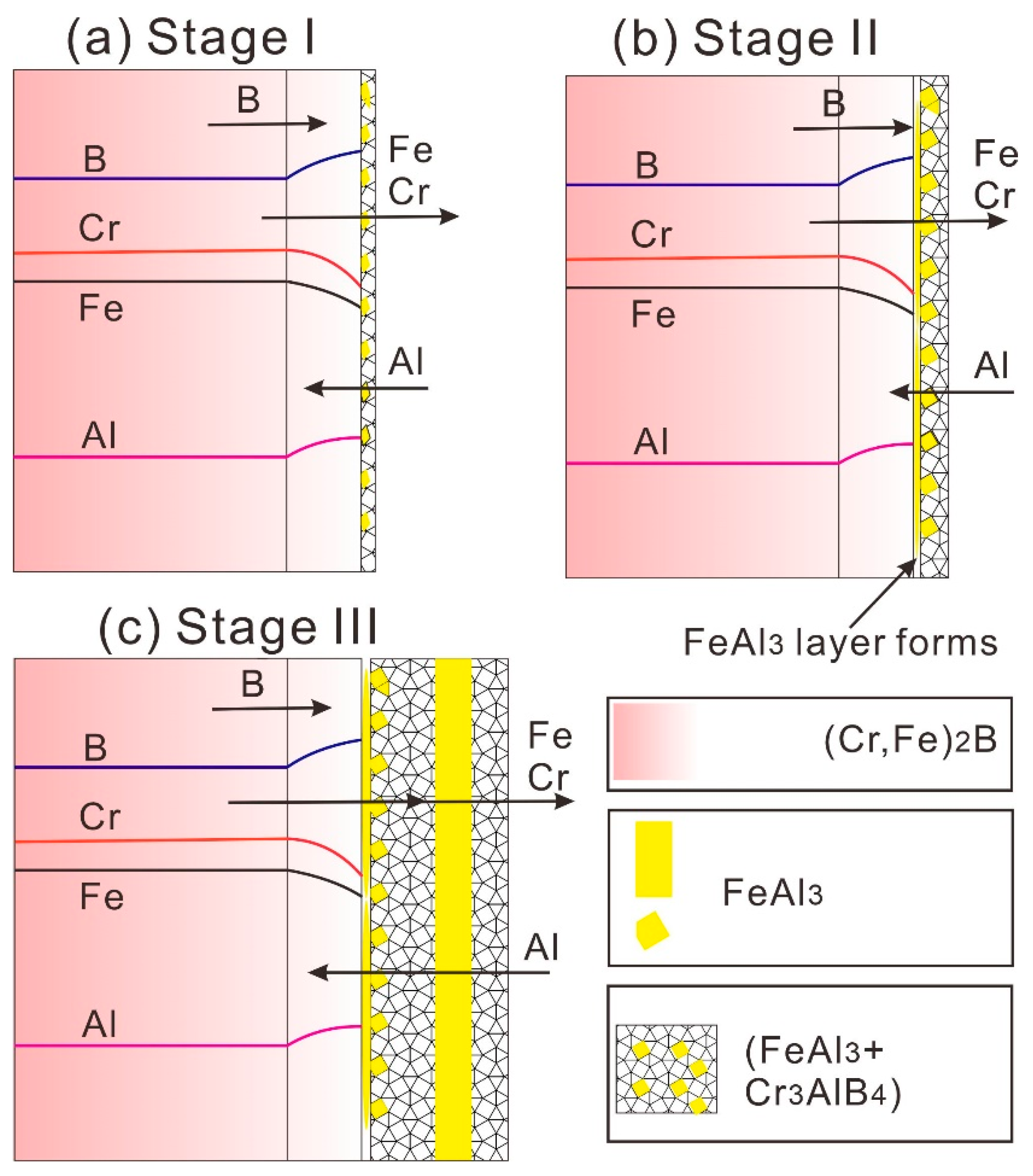
3.4. The Potential Formation Mechanism
4. Conclusions
- (i)
- A novel Fe-Cr-B alloy was prepared, consisting of (Cr, Fe)2B boride, the globular CrB phase, and the grainy α-Fe phase.
- (ii)
- The PLS structure was observed at the Cr, Fe)2B/Al interface at 750 °C for 8 h, and was not found in that of CrB and α-Fe phases. The PLS within the reaction zone of the solid-(Cr, Fe)2B/liquid-Al couple was alternated by a single FeAl3 layer and a (Cr3AlB4 + FeAl3) dual-phases layer.
- (iii)
- Based on the present experimental results, the periodic layer formation in the (Cr, Fe)2B/Al couple supports the diffusion-controlled precipitation and growth model. The formation of PLS undergoes three stages. At first, the preferential leaching of Fe and Cr elements from the (Cr, Fe)2B phase, accompanied by the inward diffusion of Al and fast diffusion of B toward the interface, resulting in the initial formation of the (Cr3AlB4 + FeAl3) dual-phase layer. As time goes on, the FeAl3 crystals precipitated from the Cr3AlB4 phase and growth into the new FeAl3 layer. Finally, the Cr-rich layer was split up to form new pairs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Nash, P.; Liu, T.; Zhao, N.; Zhu, S. The Large Scale Synthesis of Aligned Plate Nanostructures. Sci. Rep. 2016, 6, 29972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Luo, H.F.; Shi, L.Y. Periodic Layered Structure Formed During Interfacial Reaction. J. Iron. Steel. Res. Int. 2016, 23, 1127–1133. [Google Scholar] [CrossRef]
- He, M.; Su, X.P.; Yin, F.C.; Wang, J.H.; Li, Z. Periodic layered structure in Ni3Si/Zn diffusion couples. Scr. Mater. 2008, 59, 411–413. [Google Scholar] [CrossRef]
- Su, X.P.; Gao, C.P.; Li, Z.F.; Wang, J.H.; Liu, S.P.; Liu, Y.; Peng, H.P. The Mechanism of Periodic Layered Structure Formation in Ni3Si/Zn System. J. Phase. Equilib. Diff. 2013, 34, 416–420. [Google Scholar] [CrossRef]
- Su, X.P.; Liu, C.; Yang, S.; Yin, F.C.; Wang, J.H. A general qualitative description for the initial stages of periodic layer formation in Ni3Si/Zn diffusion couples. Scr. Mater. 2010, 62, 485–487. [Google Scholar] [CrossRef]
- Chen, Y.C.; Zhang, X.F.; Ren, Y.K.; Han, L.; Lin, D.Y.; Wang, Q.P. Microstructure evolution of periodic layers formed during solid state reaction between Zn and Ni3Si. Intermetallics 2013, 36, 8–11. [Google Scholar] [CrossRef]
- Chen, Y.C.; Zhang, X.F.; Li, Y.J.; Ren, Y.K.; Lin, D.Y.; Wang, Q.P. Feathery structure discovered in the reaction zone of diffusion couple Zn/CuTi2. Mater. Lett. 2012, 85, 142–145. [Google Scholar] [CrossRef]
- Gutman, I.; Klinger, L.; Gotman, I.; Shapiro, M. Experimental observation of periodic structure formation in SiO2-Mg system. Scr. Mater. 2001, 45, 363–367. [Google Scholar] [CrossRef]
- Klinger, L.; Gotman, I.; Gutman, I. A switch-over model of periodic structure formation in ternary diffusion couples. Scr. Mater. 2001, 45, 1221–1226. [Google Scholar] [CrossRef]
- Gutman, I.; Gotman, I.; Shapiro, M. Kinetics and mechanism of periodic structure formation at SiO2/Mg interface. Acta Mater. 2006, 54, 4677–4684. [Google Scholar] [CrossRef]
- Oberhauser, S.; Strobl, C.; Schreiber, G.; Wuestefeld, C.; Rafaja, D. Formation of periodic patterns in the (Ni,W)/Al diffusion couples. Surf. Coat. Technol. 2010, 204, 2307–2315. [Google Scholar] [CrossRef]
- Perez, E.; Keiser, D.D.J.; Sohn, Y.H. Phase Constituents and Microstructure of Interaction Layer Formed in U-Mo Alloys vs. Al Diffusion Couples Annealed at 873 K (600 °C). Metall. Mater. Trans. A 2011, 42, 3071–3083. [Google Scholar] [CrossRef]
- Zhang, X.M.; Chen, W.P.; Luo, H.F.; Zhou, T. Formation of periodic layered structure between novel Fe-Cr-B cast steel and molten aluminum. Scr. Mater. 2017, 130, 288–291. [Google Scholar] [CrossRef]
- Bhanumurthy, K.; Schmid-Fetzer, R. Interface reactions between silicon carbide and metals (Ni, Cr, Pd, Zr). Compos. Part A Appl. S 2011, 32, 569–574. [Google Scholar] [CrossRef]
- Chen, S.W.; Chen, C.C.; Chang, C.H. Interfacial reactions in Sn/Ni-7 wt %V. couple. Scr. Mater. 2007, 56, 453–456. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Luo, H. Corrosion-Wear Resistance and Interfacial Morphologies of Novel Fe-Cr-B Cast Steels in Molten Aluminum. Tribol. Lett. 2018, 66, 112. [Google Scholar] [CrossRef]
- Lentz, J.; Röttger, A.; Großwendt, F.; Theisen, W. Enhancement of hardness, modulus and fracture toughness of the tetragonal (Fe, Cr)2B and orthorhombic (Cr, Fe)2B phases with addition of Cr. Mater. Des. 2018, 156, 113–124. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Luo, H.; Li, S.; Zhou, T.; Shi, L. Corrosion resistance and interfacial morphologies of novel Fe-Cr-Mo-B cast steels in molten aluminum. Corros. Sci. 2017, 125, 20–28. [Google Scholar] [CrossRef]
- Wang, M.M.; Xue, J.; Gao, R.; Gao, H.Y.; Zhou, Y.; Zhao, Y.Y.; Liu, Y.H.; Kang, M.D.; Wang, J. Interface morphology and corrosion behavior of bulk Fe2B in liquid Al. Mater. Charact. 2019, 152, 1–11. [Google Scholar] [CrossRef]
- Wang, M.M.; Gao, R.; Gao, H.Y.; Zhou, Y.; Fan, Y.Y.; Zhao, Y.Y.; Ju, J.; Liu, Y.H.; Kang, M.D.; Wang, J. Improved corrosion resistance of Ni-modified Fe-Cr-B steel in molten zinc via phase transformation and microstructure control. Surf. Coat. Technol. 2019, 374, 975–986. [Google Scholar] [CrossRef]
- Ma, S.Q.; Xing, J.D.; Fu, H.G.; Gao, Y.M.; Zhang, J.J. Microstructure and crystallography of borides and secondary precipitation in 18 wt % Cr-4 wt % Ni-1 wt % Mo-3.5 wt % B-0.27 wt % C steel. Acta Mater. 2012, 60, 831–843. [Google Scholar] [CrossRef]
- Guo, C.; Kelly, P. Boron solubility in Fe-Cr-B. cast irons. Mater. Sci. Eng. A 2003, 352, 40–45. [Google Scholar] [CrossRef]
- Brown, B.E.; Beerntsen, D. Refinement of an iron chromium boride with the Mn4B structure. Acta Crystallogr. 1964, 17, 448–450. [Google Scholar] [CrossRef]
- Okada, S.; Atoda, T.; Higashi, I. Structural investigation of Cr2B3, Cr3B4, and CrB by single-crystal diffractometry. J. Solid. State Chem. 1987, 68, 61–67. [Google Scholar] [CrossRef]
- Basinski, Z.S.; Rothery, W.H.; Sutton, A. Proceedings of the Royal Society of London. Ser. A Math. Phys. Sci. 1955, 229, 459–467. [Google Scholar]
- Grin, J. Refinement of the Fe4Al13 structure and its relationship to the quasi homological hometypical structures. Crys. Mater. 1994, 209, 479–487. [Google Scholar]
- Burkhardt, U.; Grin, Y.; Ellner, M.; Peters, K. Structure refinement of the iron–aluminium phase with the approximate composition Fe2Al5. Acta Crys. Sect. B Struct. Sci. 1994, 50, 313–316. [Google Scholar] [CrossRef]
- Cooper, A.S. Precise lattice constants of germanium, aluminum, gallium arsenide, uranium, sulphur, quartz and sapphire. Acta Crystallogr. 1962, 15, 578–582. [Google Scholar] [CrossRef]
- Ade, M.; Hillebrecht, H. Ternary borides Cr2AlB2, Cr3AlB4, and Cr4AlB6: The first members of the series (CrB2) n CrAl with n = 1, 2, 3 and a unifying concept for ternary borides as MAB-phases. Inorg. Chem. 2015, 54, 6122–6135. [Google Scholar] [CrossRef]
- Kota, S.; Wang, W.; Lu, J.; Natu, V.; Opagiste, C.; Ying, G.; Hultman, L.; May, S.J.; Barsoum, M.W. Magnetic properties of Cr2AlB2, Cr3AlB4, and CrB powders. J. Alloys Compd. 2018, 767, 474–482. [Google Scholar] [CrossRef]
- Li, X.L.; Scherf, A.; Heilmaier, M.; Stein, F. The Al-Rich Part of the Fe-Al Phase Diagram. J. Phase. Equilib. Diff. 2016, 37, 162–173. [Google Scholar] [CrossRef]
- Campbell, C.E.; Kattner, U.R. Assessment of the Cr-B system and extrapolation to the Ni-Al-Cr-B quaternary system. Calphad 2002, 26, 477–490. [Google Scholar] [CrossRef]
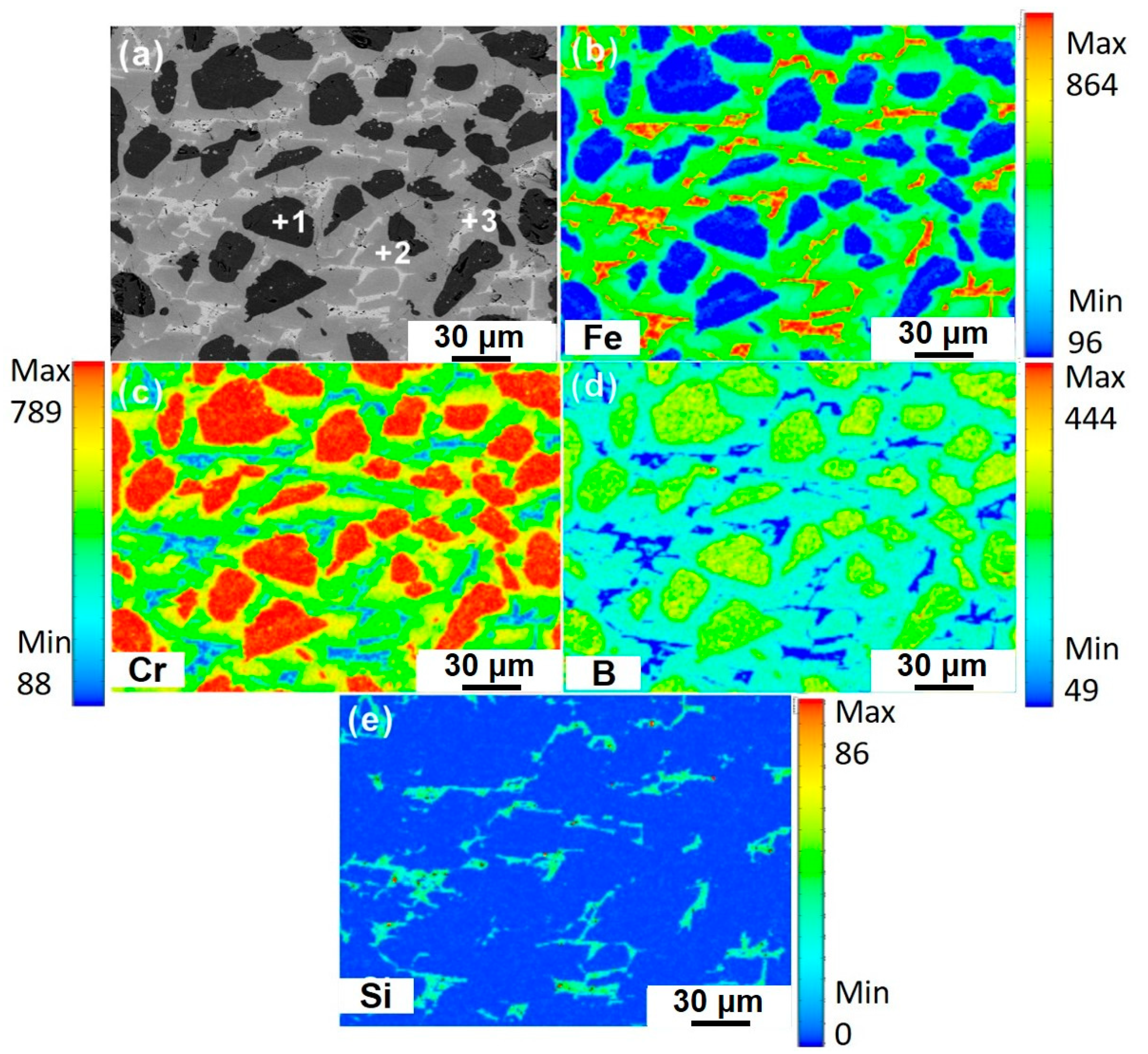
| Points | Fe | Cr | B | Si |
|---|---|---|---|---|
| 1 | 5.23(0.41) | 46.82(0.34) | 47.95(0.37) | - |
| 2 | 31.11(2.11) | 37.35(2.39) | 31.54(0.29) | - |
| 3 | 78.55(1.12) | 9.81(1.49) | 3.35(0.22) | 8.29(0.85) |
| Phase | Cell Parameters | Structure | ||||||
|---|---|---|---|---|---|---|---|---|
| a(Å) | b(Å) | c(Å) | α | β | γ | Structure | Space Group | |
| CrFeB [23] | 14.57 | 7.32 | 4.22 | 90° | 90° | 90° | Orthorhombic | 70 |
| CrB [24] | 2.98 | 7.87 | 2.93 | 90° | 90° | 90° | Orthorhombic | 63 |
| Fe(bcc) [25] | 2.87 | 2.87 | 2.87 | 90° | 90° | 90° | Cubic | 229 |
| FeAl3 [26] | 15.49 | 8.08 | 12.47 | 90° | 90° | 90° | Monoclinic | 12 |
| Fe2Al5 [27] | 7.66 | 6.42 | 4.22 | 90° | 90° | 90° | Orthorhombic | 63 |
| Al [28] | 4.05 | 4.05 | 4.05 | 90° | 90° | 90° | Cubic | 225 |
| Cr3AlB4 [29] | 2.96 | 2.98 | 8.05 | 90° | 90° | 90° | Orthorhombic | 47 |
| Cr4AlB6 [29] | 2.95 | 21.28 | 3.01 | 90° | 90° | 90° | Orthorhombic | 65 |
| Cr2AlB2 [30] | 2.94 | 2.94 | 2.94 | 90° | 90° | 90° | Orthorhombic | 65 |
| Points | Fe | Cr | B | Al |
|---|---|---|---|---|
| 1 | 6.15(0.35) | 24.89(1.16) | 52.50(3.28) | 16.46(1.68) |
| 2 | 4.18(0.41) | 29.49(1.35) | 51.45(3.09) | 14.88(2.88) |
| 3 | 5.08(0.38) | 22.65(1.45) | 55.13(2.86) | 17.14(1.98) |
| 4 | 22.63(2.18) | 3.41(0.28) | - | 73.96(2.45) |
| 5 | 22.51(2.25) | 5.42(0.22) | 1.55(0.22) | 70.52(3.2) |
| 6 | 24.39(2.12) | 4.14(0.19) | 1.37(0.25) | 70.10(2.66) |
| Points | Fe | Cr | B | Al |
|---|---|---|---|---|
| 1 | 24.10(0.33) | 35.98(0.89) | 38.92(1.08) | 0 |
| 2 | 24.16(0.28) | 36.93(0.67) | 38.91(0.97) | 0 |
| 3 | 24.13(0.42) | 35.54(1.79) | 40.33(2.20) | 0.17(0.05) |
| 4 | 22.85(1.25) | 32.7(1.39) | 44.24(2.65) | 0.21(0.02) |
| 5 | 21.89(0.42) | 31.41(0.15) | 46.47(0.58) | 0.23(0.03) |
| 6 | 5.17(0.42) | 26.51(1.28) | 51.44(3.72) | 16.88(2.07) |
| 7 | 2.31(0.60) | 29.52(0.51) | 61.04(1.08) | 7.13(0.87) |
| 8 | 20.24(3.89) | 3.17(0.23) | - | 76.59(3.70) |
| 9 | 23.31(2.30) | 3.87(0.23) | - | 72.82(2.58) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Ju, J.; Li, J.; Zhou, Y.; Lv, H.; Gao, H.; Wang, J. The Formation Mechanism of a Self-Organized Periodic-Layered Structure at the Solid-(Cr, Fe)2B/Liquid-Al Interface. Materials 2020, 13, 3869. https://doi.org/10.3390/ma13173869
Wang M, Ju J, Li J, Zhou Y, Lv H, Gao H, Wang J. The Formation Mechanism of a Self-Organized Periodic-Layered Structure at the Solid-(Cr, Fe)2B/Liquid-Al Interface. Materials. 2020; 13(17):3869. https://doi.org/10.3390/ma13173869
Chicago/Turabian StyleWang, Mengmeng, Jiang Ju, Jingjing Li, Yang Zhou, Haiyang Lv, Haiyan Gao, and Jun Wang. 2020. "The Formation Mechanism of a Self-Organized Periodic-Layered Structure at the Solid-(Cr, Fe)2B/Liquid-Al Interface" Materials 13, no. 17: 3869. https://doi.org/10.3390/ma13173869
APA StyleWang, M., Ju, J., Li, J., Zhou, Y., Lv, H., Gao, H., & Wang, J. (2020). The Formation Mechanism of a Self-Organized Periodic-Layered Structure at the Solid-(Cr, Fe)2B/Liquid-Al Interface. Materials, 13(17), 3869. https://doi.org/10.3390/ma13173869







