Radiation-Induced Defects and Effects in Germanate and Tellurite Glasses
Abstract
:1. Introduction
2. Description of the Tellurite and Germanate Glasses
2.1. Elaboration Methods of HMO Glasses
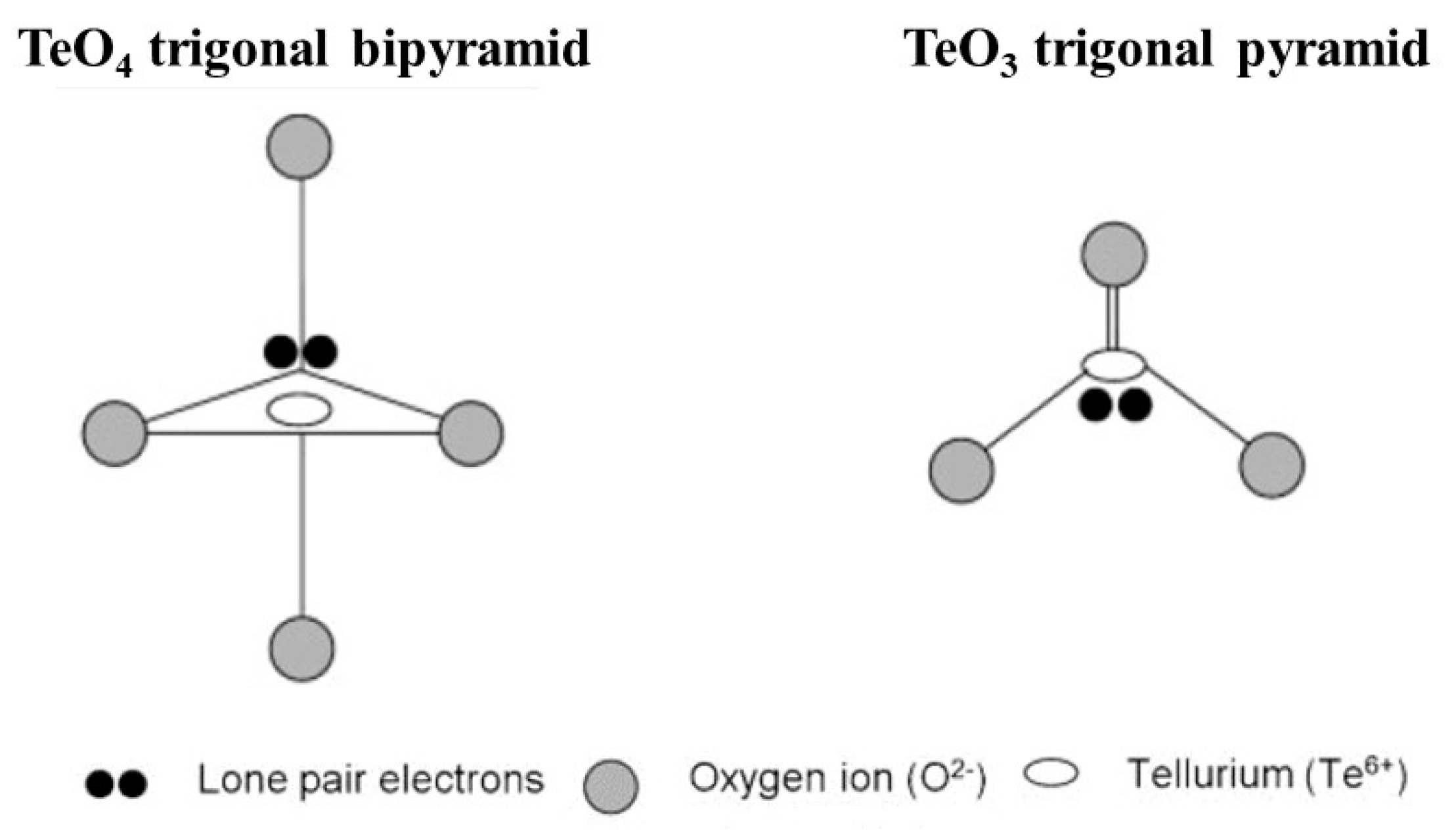
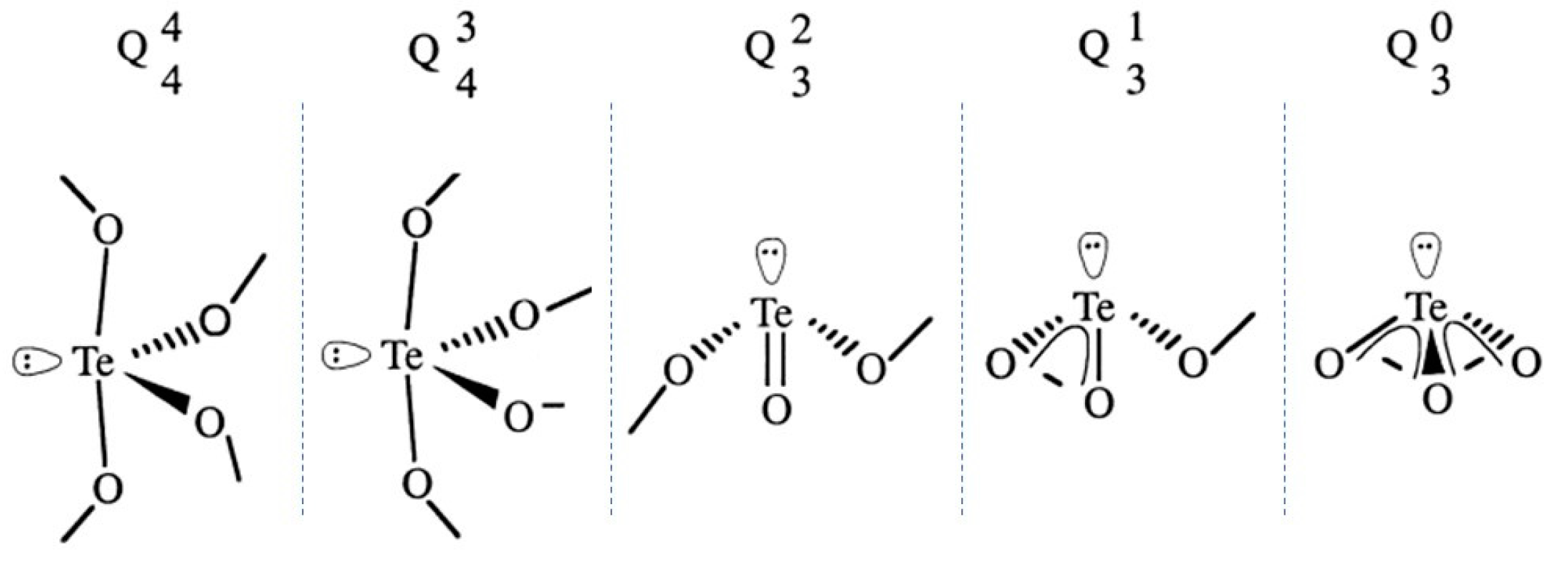
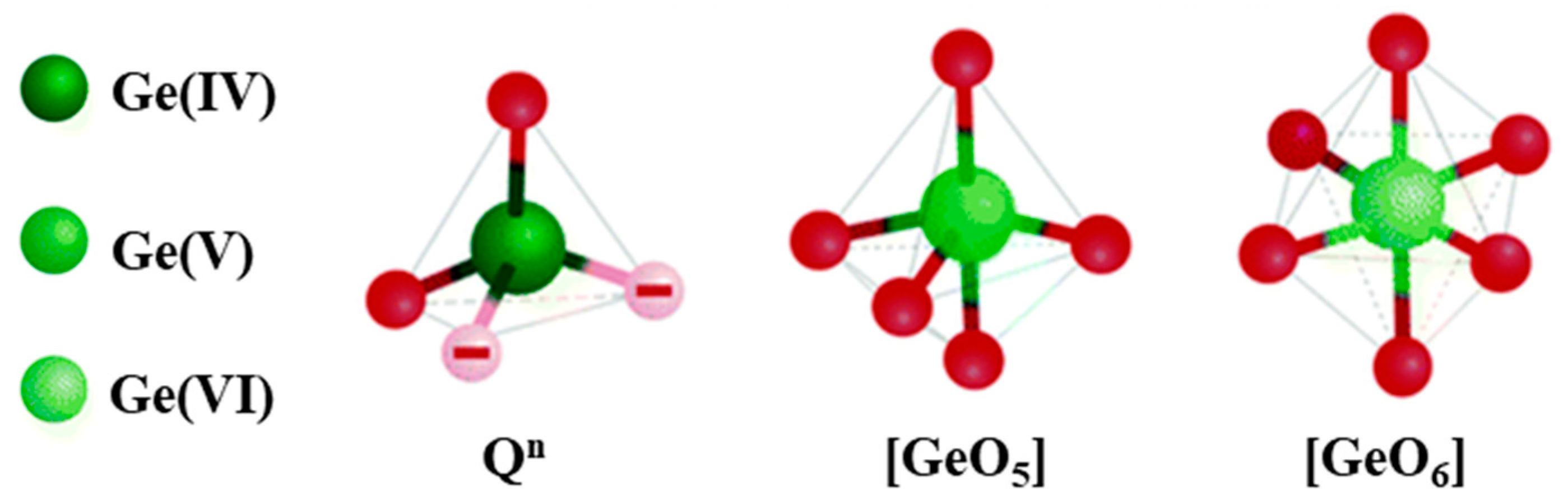
2.2. Glass Network Structure of HMO Glasses
3. Defects Formation in Germanate and Tellurite Glass Due to Radiation Treatment
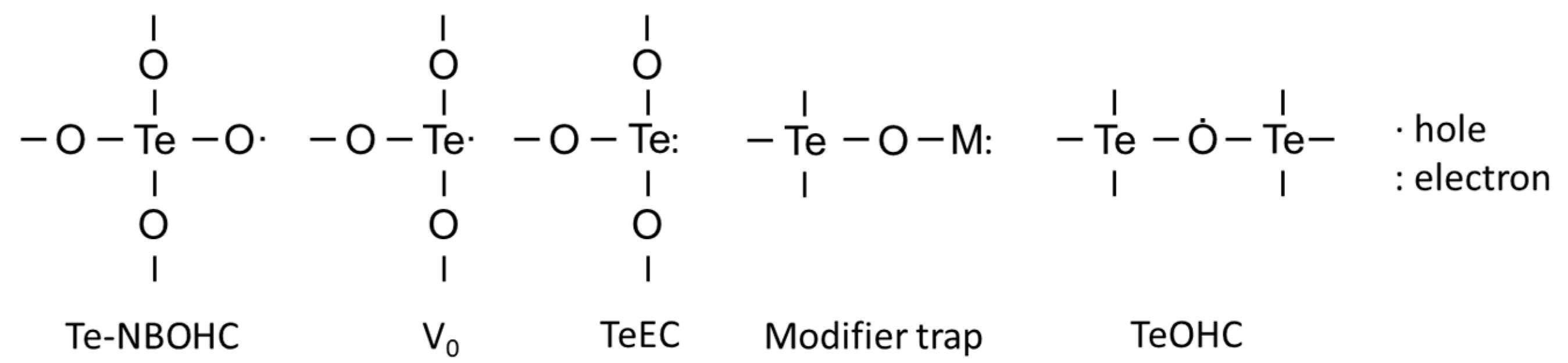
3.1. Types of Defects and Their Characterization
3.2. Impact of Glass Composition on Defects Formation
4. Local Changes in the Glasses Due to Radiation Treatment Using Short Pulsed Lasers
4.1. Laser-Induced Structural Modifications
4.2. Photostructuring
5. Conclusions and Future Opportunities
Author Contributions
Funding
Conflicts of Interest
References
- Tomashuk, A.L. Performance of special radiation-hardened optical fibers intended for use in the telecom spectral windows at a megagray level. IEEE Trans. Nucl. Sci. 1998, 45, 1558–1565. [Google Scholar] [CrossRef]
- Lotarev, S.V.; Gelmanova, T.O.; Priseko, Y.S.; Paleari, A.; Sigaev, V.N. Local laser-induced crystallization of lanthanum boron germanate glass near LaBGeO5 composition. In Photonics, Devices, and Systems V, Proceedings of the 7th International Conference on Photonics, Devices and Systems, Prague, Czech Republic, 24–26 August 2011; Tománek, P., Senderáková, D., Páta, P., Eds.; Spie: London, UK, 2011; Volume 8306, p. 830619. [Google Scholar]
- Yang, C.; Shinozaki, K.; Honma, T.; Komatsu, T. Nano-crystallization and highly oriented crystal line patterning of Sm3+-doped Bi2GeO5 and Bi4Ge3O12 in bismuth germanate-based glasses. J. Non Cryst. Solids 2017, 459, 116–122. [Google Scholar] [CrossRef]
- Shpotyuk, O.I. Radiation-induced effects in chalcogenide glasses: Topological mechanisms and application. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2000, 166, 525–528. [Google Scholar] [CrossRef]
- Bishay, A. Radiation induced color centers in multicomponent glasses. J. Non Cryst. Solids 1970, 3, 54–114. [Google Scholar] [CrossRef]
- Elkholy, M.M. Thermoluminescence for rare-earths doped tellurite glasses. Mater. Chem. Phys. 2003, 77, 321–330. [Google Scholar] [CrossRef]
- Griscom, D.L. A minireview of the natures of radiation-induced point defects in pure and doped silica glasses and their visible/near-ir absorption bands, with emphasis on self-trapped holes and how they can be controlled. Phys. Res. Int. 2013, 2013, 379041. [Google Scholar] [CrossRef]
- Girard, S.; Alessi, A.; Richard, N.; Martin-Samos, L.; De Michele, V.; Giacomazzi, L.; Agnello, S.; Francesca, D.D.; Morana, A.; Winkler, B.; et al. Overview of radiation induced point defects in silica-based optical fibers. Rev. Phys. 2019, 4, 100032. [Google Scholar] [CrossRef]
- Petit, L.; Carlie, N.; Anderson, T.; Choi, J.; Richardson, M.; Richardson, K.C. Progress on the photoresponse of chalcogenide glasses and films to near-infrared femtosecond laser irradiation: A review. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 1323–1334. [Google Scholar] [CrossRef]
- Petit, L. Radiation effects on phosphate glasses: Review. Int. J. Appl. Glass Sci. 2019. [Google Scholar] [CrossRef]
- Möncke, D.; Ehrt, D. Radiation-induced defects in CoO- and NiO-doped fluoride, phosphate, silicate and borosilicate glasses. Glas. Sci. Technol. Glass Berichte 2002, 75, 243–253. [Google Scholar]
- Petit, Y.; Danto, S.; Guérineau, T.; Khalil, A.A.; Le Camus, A.; Fargin, E.; Duchateau, G.; Bérubé, J.P.; Vallée, R.; Messaddeq, Y.; et al. On the femtosecond laser-induced photochemistry in silver-containing oxide glasses: Mechanisms, related optical and physico-chemical properties, and technological applications. Adv. Opt. Technol. 2018, 7, 291–309. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, D.M.; Kassab, L.R.P.; Olivero, M.; Lemos, T.B.N.; da Silva, D.V.; Gomes, A.S.L. Er3+ doped waveguide amplifiers written with femtosecond laser in germanate glasses. Opt. Mater. 2011, 33, 1902–1906. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.M.; Jesus-Silva, A.J.; Silva, A.C.A.; Dantas, N.O.; Fonseca, E.J.S. Waveguides written in silver-doped tellurite glasses. Opt. Mater. 2020, 101, 109767. [Google Scholar] [CrossRef]
- Tomashuk, A.L.; Dianov, E.M.; Golant, K.M.; Khrapko, R.R.; Spinov, D.E. Performance of special radiation-hardened optical fibers intended for use in the telecom spectral windows at a megagray level. In Proceedings of the RADECS 97 Fourth European Conference on Radiation and its Effects on Components and Systems (Cat. No.97TH8294), Cannes, France, 15–19 September 1997; pp. 453–456. [Google Scholar] [CrossRef]
- Wang, J.S.; Vogel, E.M.; Snitzer, E.; Jackel, J.L.; da Silva, V.L.; Silberberg, Y. 1.3 μm emission of neodymium and praseodymium in tellurite-based glasses. J. Non Cryst. Solids 1994, 178, 109–113. [Google Scholar] [CrossRef]
- Mori, A.; Ohishi, Y.; Sudo, S. Erbium-doped tellurite glass fibre laser and amplifier. Electron. Lett. 1997, 33, 863–864. [Google Scholar] [CrossRef]
- Heo, J.; Shin, Y.B.; Jang, J.N. Spectroscopic analysis of Tm^3+ in PbO-Bi_2O_3-Ga_2O_3 glass. Appl. Opt. 1995, 34, 4284. [Google Scholar] [CrossRef]
- Pan, Z.; Morgan, S.H.; Loper, A.; King, V.; Long, B.H.; Collins, W.E. Infrared to visible upconversion in Er3+-doped-lead-germanate glass: Effects of Er3+ ion concentration. J. Appl. Phys. 1995, 77, 4688–4692. [Google Scholar] [CrossRef]
- Pierce, M.C.; Jackson, S.D.; Dickinson, M.R.; King, T.A.; Sloan, P. Laser-tissue interaction with a continuous wave 3-μm fibre laser: Preliminary studies with soft tissue. Lasers Surg. Med. 2000, 26, 491–495. [Google Scholar] [CrossRef]
- Kassab, L.R.P.; Da Silva, D.S.; De Araújo, C.B. Influence of metallic nanoparticles on electric-dipole and magnetic-dipole transitions of Eu3+ doped germanate glasses. J. Appl. Phys. 2010, 107, 113506. [Google Scholar] [CrossRef]
- De Assumpção, T.A.A.; Da Silva, D.M.; Camilo, M.E.; Kassab, L.R.P.; Gomes, A.S.L.; De Araújo, C.B.; Wetter, N.U. Frequency upconversion properties of Tm3+ doped TeO2-ZnO glasses containing silver nanoparticles. Proc. J. Alloys Compd. 2012, 536, S504–S506. [Google Scholar]
- Wang, G.; Wu, X.; Cen, D.; He, H.; Fu, Y.; Ren, Z.; Wang, Y.; Cai, X.; Li, X.; Han, G. A bifunctional scaffold for tissue regeneration and photothermal therapy. J. Biomed. Nanotechnol. 2018, 14, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Moirangthem, R.S.; Yaseen, M.T.; Wei, P.-K.; Cheng, J.-Y.; Chang, Y.-C. Enhanced localized plasmonic detections using partially-embedded gold nanoparticles and ellipsometric measurements. Biomed. Opt. Express 2012, 3, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, R.; Felix, J.F.; Moura, L.G.; Morais, P.C. One step fabrication of glass-silver@core-shell fibers: Silver-doped phosphate glasses as precursors of SERS substrates. J. Mater. Chem. C 2014, 2, 9021–9027. [Google Scholar] [CrossRef]
- De Araujo, C.B.; Silvério da Silva, D.; Alves de Assumpção, T.A.; Kassab, L.R.P.; Mariano da Silva, D. Enhanced optical properties of germanate and tellurite glasses containing metal or semiconductor nanoparticles. Sci. World J. 2013, 2013, 385193. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.M.P.; Almeida, G.F.B.; Boni, L.; Mendonça, C.R. Nonlinear optical properties and femtosecond laser micromachining of special glasses. J. Braz. Chem. Soc. 2015, 26, 2418–2429. [Google Scholar] [CrossRef]
- Naranjo, L.P.; De Araújo, C.B.; Malta, O.L.; Cruz, P.A.S.; Kassab, L.R.P. Enhancement of Pr3+ luminescence in PbO- GeO2 glasses containing silver nanoparticles. Appl. Phys. Lett. 2005, 87, 241914. [Google Scholar] [CrossRef]
- De Araújo, C.B.; Kassab, L.R.P.; Kobayashi, R.A.; Naranjo, L.P.; Santa Cruz, P.A. Luminescence enhancement of Pb2+ ions in TeO2-PbO- GeO2 glasses containing silver nanostructures. J. Appl. Phys. 2006, 99, 123522. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, J.; Cheng, M.; Zhang, S.; Jia, Y.; Zhao, J. Radiative transition, local field enhancement and energy transfer microcosmic mechanism of tellurite glasses containing Er3+, Yb3+ ions and Ag nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2015, 159, 39–52. [Google Scholar] [CrossRef]
- Amjad, R.J.; Sahar, M.R.; Dousti, M.R.; Ghoshal, S.K.; Jamaludin, M.N.A. Surface enhanced raman scattering and plasmon enhanced fluorescence in zinc-tellurite glass. Opt. Express 2013, 21, 14282. [Google Scholar] [CrossRef]
- Martins, M.M.; Kassab, L.R.P.; da Silva, D.M.; de Araújo, C.B. Tm3+ doped Bi2O3-GeO2 glasses with silver nanoparticles for optical amplifiers in the short-wave-infrared-region. J. Alloys Compd. 2019, 772, 58–63. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, X.; Dai, S.; Xu, Y.; Chen, F.; Lin, C.; Xu, T.; Nie, Q. Silver nanoparticles enhanced upconversion luminescence in Er 3+/Yb 3+ codoped bismuth-germanate glasses. J. Phys. Chem. C 2011, 115, 25040–25045. [Google Scholar] [CrossRef]
- Ghoshal, S.K.; Awang, A.; Sahar, M.R.; Arifin, R. Gold nanoparticles assisted surface enhanced raman scattering and luminescence of Er3+ doped zinc-sodium tellurite glass. J. Lumin. 2015, 159, 265–273. [Google Scholar] [CrossRef]
- Churbanov, M.F.; Moiseev, A.N.; Chilyasov, A.V.; Dorofeev, V.V.; Kraev, I.A.; Lipatova, M.M.; Kotereva, T.V.; Dianov, E.M.; Plotnichenko, V.G.; Kryukova, E.B. Production of high-purity TeO2-ZnO and TeO2-WO3 glasses with the reduced content of OH-groups. J. Optoelectron. Adv. Mater. 2007, 9, 3229–3234. [Google Scholar]
- Mori, A.; Kobayashi, K.; Yamada, M.; Kanamori, T.; Oikawa, K.; Nishida, Y.; Ohishi, Y. Low noise broadband tellurite-based Er3+-doped fibre amplifiers. Electron. Lett. 1998, 34, 887–888. [Google Scholar] [CrossRef]
- Désévédavy, F.; Strutynski, C.; Lemière, A.; Mathey, P.; Gadret, G.; Jules, J.; Kibler, B.; Smektala, F. Review of tellurite glasses purification issues for mid-IR optical fiber applications. J. Am. Ceram. Soc. 2020, 103, 4017–4034. [Google Scholar] [CrossRef]
- Wilding, M.; Benmore, C.; Weber, R.; Alderman, O.; Tamalonis, A.; McMillan, P.F.; Wilson, M.; Ribiero, M.C.C.; Parise, J. Exploring the structure of glass-forming liquids using high energy X-ray diffraction, containerless methodology and molecular dynamics simulation. J. Non Cryst. Solids X 2019, 3, 100027. [Google Scholar] [CrossRef]
- Stanworth, J.E. Tellurite glasses. Nature 1952, 169, 581–582. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry, 3rd ed.; Clarendon Press: Oxford, UK, 1962; ISBN 9780199657636. [Google Scholar]
- Jha, A.; Richards, B.D.O.; Jose, G.; Toney Fernandez, T.; Hill, C.J.; Lousteau, J.; Joshi, P. Review on structural, thermal, optical and spectroscopic properties of tellurium oxide based glasses for fibre optic and waveguide applications. Int. Mater. Rev. 2012, 57, 357–382. [Google Scholar] [CrossRef]
- McLaughlin, J.C.; Tagg, S.L.; Zwanziger, J.W.; Haeffner, D.R.; Shastri, S.D. Structure of tellurite glass: A combined NMR, neutron diffraction, and X-ray diffraction study. J. Non Cryst. Solids 2000, 274, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Neov, S.; Kozhukharov, V.; Gerasimova, I.; Krezhov, K.; Sidzhimov, B. A model for structural recombination in tellurite glasses. J. Phys. C Solid State Phys. 1979, 12, 2475–2485. [Google Scholar] [CrossRef]
- Philippot, E. Force de la liaison Te-O: Coordination et localisation de la paire libre de l’atome de tellure IV dans les tellurites. J. Solid State Chem. 1981, 38, 26–33. [Google Scholar] [CrossRef]
- Sabadel, J.C.; Armand, P.; Cachau-Herreillat, D.; Baldeck, P.; Doclot, O.; Ibanez, A.; Philippot, E. Structural and nonlinear optical characterizations of tellurium oxide-based glasses: TeO2-BaO-TiO2. J. Solid State Chem. 1997, 132, 411–419. [Google Scholar] [CrossRef]
- Koroleva, O.N.; Shtenberg, M.V.; Zainullina, R.T.; Lebedeva, S.M.; Nevolina, L.A. Vibrational spectroscopy and density of K2O-B2O3-GeO2 glasses with variable B/Ge ratio. Phys. Chem. Chem. Phys. 2019, 21, 12676–12684. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Evstrop’ev, K.S. On the structure of simple germanate glass. Dokl. Akad. Nauk SSSR 1962, 145, 797–800. [Google Scholar]
- Murthy, M.K.; Ip, J. Some physical properties of alkali germanate glasses. Nature 1964, 201, 285–286. [Google Scholar] [CrossRef]
- Matusita, K.; Sakka, S.; Kamiya, K. Kinetics study of the crystallization of glass by differential scanning calorimetry. Phys. Chem. Glass 1979, 20, 81–84. [Google Scholar]
- Ueno, M.; Misawa, M.; Suzuki, K. On the change in coordination of Ge atoms in Na2OGeO2 glassess. Phys. B + C 1983, 120, 347–351. [Google Scholar] [CrossRef]
- Sakka, S.; Kamiya, K. Structure of alkali germanate glasses studied by spectroscopic techniques. J. Non Cryst. Solids 1982, 49, 103–116. [Google Scholar] [CrossRef]
- Lapeyre, C.; Petiau, J.; Calas, G.; Gauthier, F.; Gombert, J. Ordre local autour du germanium dans les verres du systeme SiO2-GeO2-B2O3-Na2O: Etude par spectrometrie d’absorption X. Bull. Mineral. 1983, 106, 77–85. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Almeida, R.M. Structural anomaly in sodium germanate glasses by molecular dynamics simulation. J. Non Cryst. Solids 2001, 281, 152–161. [Google Scholar] [CrossRef]
- Hannon, A.C.; Di Martino, D.; Santos, L.F.; Almeida, R.M. A model for the Ge-O coordination in germanate glasses. J. Non Cryst. Solids 2007, 353, 1688–1694. [Google Scholar] [CrossRef]
- Henderson, G.S.; Wang, H.M. Germanium coordination and the germanate anomaly. Eur. J. Mineral. 2002, 14, 733–744. [Google Scholar] [CrossRef]
- Soltay, L.G.; Henderson, G.S. Structural differences between lithium silicate and lithium germanate glasses by Raman spectroscopy. Phys. Chem. Glass 2005, 46, 381–384. [Google Scholar]
- Zhang, W.J.; Wang, W.C.; Zhang, Q.Y.; Jiang, Z.H. New insights into the structure and physical properties of sodium and potassium germanate glass via the phase diagram approach. J. Non Cryst. Solids 2017, 475, 108–115. [Google Scholar] [CrossRef]
- Alderman, O.L.G.; Hannon, A.C.; Feller, S.; Beanland, R.; Holland, D. The germanate anomaly in alkaline earth germanate glasses. J. Phys. Chem. C 2017, 121, 9462–9479. [Google Scholar] [CrossRef]
- Tikhomirov, V.K.; Jha, A.; Perakis, A.; Sarantopoulou, E.; Naftaly, M.; Krasteva, V.; Li, R.; Seddon, A.B. Interpretation of the Boson peak in rare-earth ion doped glasses. J. Non Cryst. Solids 1999, 256, 89–94. [Google Scholar] [CrossRef]
- Huston, A.L.; Justus, B.L.; Falkenstein, P.L.; Miller, R.W.; Ning, H.; Altemus, R. Remote optical fiber dosimetry. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2001, 184, 55–67. [Google Scholar] [CrossRef]
- Ueda, J.; Hashimoto, A.; Tanabe, S. Orange persistent luminescence and photodarkening related to paramagnetic defects of Nondoped CaO-Ga2O3-GeO2 glass. J. Phys. Chem. C 2019, 123, 29946–29953. [Google Scholar] [CrossRef]
- Giehl, J.M.; Pontuschka, W.M.; Barbosa, L.C.; Ludwig, Z.M.C.C. EPR of γ-induced paramagnetic centers in tellurite glasses. J. Non Cryst. Solids 2010, 356, 1762–1767. [Google Scholar] [CrossRef]
- Prohaska, J.D.; Li, J.; Wang, J.S.; Bartram, R.H. Electron spin resonance observations of excimer-laser-induced paramagnetic centers in tellurite glasses. Appl. Phys. Lett. 1995, 67, 1841–1843. [Google Scholar] [CrossRef]
- Nishida, T.; Yamada, M.; Ichii, T.; Takashima, Y. Structural change of the IR-transmitting tellurite, gallate and aluminate glasses caused by the 60Co-Gamma Ray irradiation. Jpn. J. Appl. Phys. 1991, 30, 768–774. [Google Scholar] [CrossRef]
- Goutaland, F.; Mortier, M.; Capoen, B.; Turrell, S.; Bouazaoui, M.; Boukenter, A.; Ouerdane, Y. UV-assisted crystallisation of tellurite and germanate-based glasses. Opt. Mater. 2006, 28, 1276–1279. [Google Scholar] [CrossRef]
- Watterich, A.; Bartram, R.H.; Gilliam, O.R.; Kappers, L.A.; Edwards, G.J.; Földvari, I.; Voszka, R. ESR identification of radiation-induced oxygen vacancy centers in paratellurite. Phys. Rev. B 1985, 32, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Hosono, H.; Abe, Y.; Kinser, D.L.; Weeks, R.A.; Muta, K.; Kawazoe, H. Nature and origin of the 5-eV band in SiO2:GeO2 glasses. Phys. Rev. B 1992, 46, 11445–11451. [Google Scholar] [CrossRef]
- Nishii, J.; Fukumi, K.; Yamanaka, H.; Kawamura, K.I.; Hosono, H.; Kawazoe, H. Photochemical reactions in GeO2-SiO2 glasses induced by ultraviolet irradiation: Comparison between Hg lamp and excimer laser. Phys. Rev. B 1995, 52, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Janer, C.L.; Carballar, A.; Navarro, L.; Galo, J.L.; Rubio, R.M. Photosensitivity color-center model for ge-doped silica preforms. IEEE Photonics J. 2013, 5, 6100511. [Google Scholar] [CrossRef] [Green Version]
- Fujimaki, M.; Watanabe, T.; Katoh, T.; Kasahara, T.; Miyazaki, N.; Ohki, Y.; Nishikawa, H. Structures and generation mechanisms of paramagnetic centers and absorption bands responsible for Ge-doped optical-fiber gratings. Phys. Rev. B Condens. Matter Mater. Phys. 1998, 57, 3920–3926. [Google Scholar] [CrossRef]
- Di Francesca, D.; Boukenter, A.; Agnello, S.; Girard, S.; Alessi, A.; Paillet, P.; Marcandella, C.; Richard, N.; Gelardi, F.M.; Ouerdane, Y. X-ray irradiation effects on fluorine-doped germanosilicate optical fibers. Opt. Mater. Express 2014, 4, 1683. [Google Scholar] [CrossRef]
- Chen, X.; Heng, X.; Tang, G.; Zhu, T.; Sun, M.; Shan, X.; Wen, X.; Guo, J.; Qian, Q.; Yang, Z. Gamma radiation induced darkening in barium gallo-germanate glass. Opt. Express 2016, 24, 9149. [Google Scholar] [CrossRef]
- Padlyak, B.V.; Jungner, H.; Fabisiak, K.; Dubelt, S.P. Radiation-induced defects in glasses and ceramics of the CaO-Ga 2 O 3-GeO 2 system. Rev. Adv. Mater. Sci. 2006, 12, 97–105. [Google Scholar]
- Friebele, E.J.; Griscom, D.L.; Sigel, G.H. Defect centers in a germanium-doped silica-core optical fiber. J. Appl. Phys. 1974, 45, 3424–3428. [Google Scholar] [CrossRef]
- Weeks, R.A.; Purcell, T. Electron spin resonance and optical absorption in GeO2. J. Chem. Phys. 1965, 43, 483–491. [Google Scholar] [CrossRef]
- Padlyak, B. V Radiation-induced paramagnetic centers in the glasses Of CaO-Ga2O3-GeO2 system. Radiat. Eff. Defects Solids 2003, 158, 411–418. [Google Scholar] [CrossRef]
- Azzoni, C.B.; Di Martino, D.; Paleari, A.; Almeida, R.M. Paramagnetic sites in alkali germanate glasses. J. Non Cryst. Solids 2000, 278, 19–23. [Google Scholar] [CrossRef]
- Skuja, L.; Hirano, M.; Hosono, H.; Kajihara, K. Defects in oxide glasses. Phys. Status Solidi 2005, 2, 15–24. [Google Scholar] [CrossRef]
- Henderson, G.S.; Fleet, M.E. The structure of glasses along the Na2O-GeO2 join. J. Non Cryst. Solids 1991, 134, 259–269. [Google Scholar] [CrossRef]
- Baccaro, S.; Cecilia, A.; Chen, G.; Du, Y.; Nencini, L.; Wang, S. Optical transmittance and irradiation resistance of rare-earth (Ce 3+, Tb3+, Pr3+) doped heavy germanate glasses. Radiat. Eff. Defects Solids 2003, 158, 451–456. [Google Scholar] [CrossRef]
- Baccaro, S.; Cecilia, A.; Chen, G.; Du, Y.; Montecchi, M.; Wang, H.; Wang, S. Effects of irradiation on transmittance of cerium doped germanate glasses in the ultraviolet and visible regions. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2002, 191, 352–355. [Google Scholar] [CrossRef]
- Zhou, Y.; Baccaro, S.; Cemmi, A.; Yang, Y.; Chen, G. Study on optical properties and γ-ray irradiation resistance of heavy metal oxide tellurite glasses: Study on optical properties and γ-ray irradiation resistance of heavy metal oxide tellurite glasses. Phys. Status Solidi 2015, 12, 76–79. [Google Scholar] [CrossRef]
- Stroud, J.S. Color centers in a cerium-containing silicate glass. J. Chem. Phys. 1962, 37, 836–841. [Google Scholar] [CrossRef]
- Stroud, J.S. Color-center kinetics in cerium-containing glass. J. Chem. Phys. 1965, 43, 2442–2450. [Google Scholar] [CrossRef]
- Jetschke, S.; Unger, S.; Schwuchow, A.; Leich, M.; Jäger, M. Role of Ce in Yb/Al laser fibers: Prevention of photodarkening and thermal effects. Opt. Express 2016, 24, 13009. [Google Scholar] [CrossRef] [PubMed]
- Jetschke, S.; Unger, S.; Schwuchow, A.; Leich, M.; Kirchhof, J. Efficient Yb laser fibers with low photodarkening by optimization of the core composition. Opt. Express 2008, 16, 15540. [Google Scholar] [CrossRef] [PubMed]
- Baccaro, S.; Cecilia, A.; Chen, G.; Du, Y.; Montecchi, M.; Wang, H.; Wang, S. Transmission properties of heavy-germanate glasses as hosts for scintillating rare earths. Nuclear Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2002, 486, 321–324. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Geddes, C.D.; Cao, H.; Gryczynski, I.; Gryczynski, Z.; Fang, J.; Lakowicz, J.R. Metal-enhanced fluorescence (MEF) due to silver colloids on a planar surface: Potential applications of indocyanine green to in vivo imaging. J. Phys. Chem. A 2003, 107, 3443–3449. [Google Scholar] [CrossRef]
- Dianov, E.M.; Starodubov, D.S. Efficient photobleaching of 390-nm luminescence in germanosilicate preforms by the third harmonic of a Nd:YAG laser. Opt. Lett. 1996, 21, 635. [Google Scholar] [CrossRef] [Green Version]
- Shimotsuma, Y.; Sakakura, M.; Miura, K.; Qiu, J.; Kazansky, P.G.; Fujita, K.; Hirao, A. Application of Femtosecond-Laser Induced Nanostructures in Optical Memory. J. Nanosci. Nanotechnol. 2007, 7, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Qiu, J.; Mitsuyu, T.; Hirao, K. Space-selective growth of frequency-conversion crystals in glasses with ultrashort infrared laser pulses. Opt. Lett. 2000, 25, 408. [Google Scholar] [CrossRef]
- Ayiriveetil, A.; Sabapathy, T.; Varma, G.S.; Ramamurty, U.; Asokan, S. Structural and mechanical characterization on ultrafast laser written chalcogenide glass waveguides. Opt. Mater. Express 2016, 6, 2530. [Google Scholar] [CrossRef]
- Fernandez, T.T.; Hernandez, M.; Sotillo, B.; Eaton, S.M.; Jose, G.; Osellame, R.; Jha, A.; Fernandez, P.; Solis, J. Role of ion migrations in ultrafast laser written tellurite glass waveguides. Opt. Express 2014, 22, 15298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandi, P.; Jose, G.; Jayakrishnan, C.; Debbarma, S.; Chalapathi, K.; Alti, K.; Dharmadhikari, A.K.; Dharmadhikari, J.A.; Mathur, D. Femtosecond laser written channel waveguides in tellurite glass. Opt. Express 2006, 14, 12145. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Song, J.; Hu, X.; Sun, H.; Lin, G.; Pan, H.; Cheng, Y.; Liu, L.; Qiu, J.; Zhao, Q.; et al. Femtosecond laser-induced inverted microstructures inside glasses by tuning refractive index of objective’s immersion liquid. Opt. Lett. 2011, 36, 2125. [Google Scholar] [CrossRef]
- Bressel, L.; de Ligny, D.; Sonneville, C.; Martinez, V.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Femtosecond laser induced density changes in GeO_2 and SiO_2 glasses: Fictive temperature effect [Invited]. Opt. Mater. Express 2011, 1, 605. [Google Scholar] [CrossRef]
- Bressel, L.; de Ligny, D.; Sonneville, C.; Martinez-Andrieux, V.; Juodkazis, S. Laser-induced structural changes in pure GeO2 glasses. J. Non Cryst. Solids 2011, 357, 2637–2640. [Google Scholar] [CrossRef]
- Liu, Y.; Shimizu, M.; Wang, X.; Zhu, B.; Sakakura, M.; Shimotsuma, Y.; Qiu, J.; Miura, K.; Hirao, K. Confocal Raman imaging of femtosecond laser induced microstructures in germanate glasses. Chem. Phys. Lett. 2009, 477, 122–125. [Google Scholar] [CrossRef]
- Bressel, L.; de Ligny, D.; Gamaly, E.G.; Rode, A.V.; Juodkazis, S. Observation of O2 inside voids formed in GeO2 glass by tightly-focused fs-laser pulses. Opt. Mater. Express 2011, 1, 1150–1158. [Google Scholar] [CrossRef]
- Tu, Z.; Teng, Y.; Zhou, J.; Zhou, S.; Zeng, H.; Qiu, J. Raman spectroscopic investigation on femtosecond laser induced residual stress and element distribution in bismuth germanate glasses. J. Raman Spectrosc. 2013, 44, 307–311. [Google Scholar] [CrossRef]
- Wang, X.; Sakakura, M.; Liu, Y.; Qiu, J.; Shimotsuma, Y.; Hirao, K.; Miura, K. Modification of long range order in germanate glass by ultra fast laser. Chem. Phys. Lett. 2011, 511, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Yu, Y.; Zhang, F.; Zhang, L.; Zhou, S.; Qiu, J. Precipitation of bismuth nanoparticles and elements distribution in bismuth germanate glass induced by femtosecond laser. Mater. Lett. 2014, 128, 204–207. [Google Scholar] [CrossRef]
- Inoue, S.; Nukui, A.; Yamamoto, K.; Yano, T.; Shibata, S.; Yamane, M. Refractive index patterning of tellurite glass surfaces by ultra short pulse laser spot heating. J. Mater. Sci. 2002, 37, 3459–3465. [Google Scholar] [CrossRef]
- Da Silva, D.S.; Ursus Wetter, N.; Kassab, L.R.P.; De Rossi, W.; De Araujo, M.S. Double line waveguide amplifiers written by femtosecond laser irradiation in rare-earth doped germanate glasses. J. Lumin. 2020, 217, 116789. [Google Scholar] [CrossRef]
- Da Silva, D.S.; Wetter, N.U.; de Rossi, W.; Kassab, L.R.P.; Samad, R.E. Production and characterization of femtosecond laser-written double line waveguides in heavy metal oxide glasses. Opt. Mater. 2018, 75, 267–273. [Google Scholar] [CrossRef]
- Berneschi, S.; Conti, G.N.; Bányász, I.; Watterich, A.; Khanh, N.Q.; Fried, M.; Pászti, F.; Brenci, M.; Pelli, S.; Righini, G.C. Ion beam irradiated channel waveguides in Er3+ -doped tellurite glass. Appl. Phys. Lett. 2007, 90, 121136. [Google Scholar] [CrossRef]
- Gupta, P.; Jain, H.; Williams, D.B.; Kanert, O.; Kuechler, R. Structural evolution of LaBGeO5 transparent ferroelectric nano-composites. Proc. J. Non Cryst. Solids 2004, 349, 291–298. [Google Scholar] [CrossRef]
- Takahashi, Y.; Saitoh, K.; Benino, Y.; Fujiwara, T.; Komatsu, T. Formation of langasite-type crystals in corresponding glasses and their second-order optical nonlinearities. J. Ceram. Soc. Jpn. 2004, 112, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Jain, H.; Williams, D.B.; Toulouse, J.; Veltchev, I. Creation of tailored features by laser heating of Nd0.2La0.8BGeO5 glass. Opt. Mater. 2006, 29, 355–359. [Google Scholar] [CrossRef]
- Gupta, P.; Jain, H.; Williams, D.B.; Honma, T.; Benino, Y.; Komatsu, T. Creation of ferroelectric, single-crystal architecture in Sm0.5La0.5BGeO5 Glass. J. Am. Ceram. Soc. 2007, 91, 110–114. [Google Scholar] [CrossRef]
- Stone, A.; Sakakura, M.; Shimotsuma, Y.; Stone, G.; Gupta, P.; Miura, K.; Hirao, K.; Dierolf, V.; Jain, H. Directionally controlled 3D ferroelectric single crystal growth in LaBGeO_5 glass by femtosecond laser irradiation. Opt. Express 2009, 17, 23284. [Google Scholar] [CrossRef]
- Stone, A.; Jain, H.; Dierolf, V.; Sakakura, M.; Shimotsuma, Y.; Miura, K.; Hirao, K. Multilayer aberration correction for depth-independent three-dimensional crystal growth in glass by femtosecond laser heating. J. Opt. Soc. Am. B 2013, 30, 1234. [Google Scholar] [CrossRef] [Green Version]
- Lipatiev, A.S.; Lipateva, T.O.; Lotarev, S.V.; Okhrimchuk, A.G.; Larkin, A.S.; Presnyakov, M.Y.; Sigaev, V.N. Direct laser writing of LaBGeO5 crystal-in-glass waveguide enabling frequency conversion. Cryst. Growth Des. 2017, 17, 4670–4675. [Google Scholar] [CrossRef]
- Knorr, B.; Veenhuizen, K.; Stone, A.; Jain, H.; Dierolf, V. Optical properties and structure of Er:LaBGeO_5 laser-induced crystals-in-glass. Opt. Mater. Express 2017, 7, 4095. [Google Scholar] [CrossRef]
- McAnany, S.D.; Veenhuizen, K.; Nolan, D.A.; Aitken, B.G.; Dierolf, V.; Jain, H. Challenges of Laser-Induced Single-Crystal Growth in Glass: Incongruent Matrix Composition and Laser Scanning Rate. Cryst. Growth Des. 2019, 19, 4489–4497. [Google Scholar] [CrossRef]
- Shi, J.; Feng, X. Laser-induced nonlinear crystalline waveguide on glass fiber format and diode-pumped second harmonic generation. Opt. Fiber Technol. 2018, 41, 118–124. [Google Scholar] [CrossRef]
- Madhar, N.A.; Varma, K.B.R. Spinodal decomposition in tellurite-based glasses induced by excimer laser irradiation. J. Am. Ceram. Soc. 2009, 92, 2609–2615. [Google Scholar] [CrossRef]
- Cvecek, K.; Miyamoto, I.; Schmidt, M. Gas bubble formation in fused silica generated by ultra-short laser pulses. Opt. Express 2014, 22, 15877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uebbing, J.J.; Hengstler, S.; Schroeder, D.; Venkatesh, S.; Haven, R. Heat and fluid flow in an optical switch bubble. J. Microelectromech. Syst. 2006, 15, 1528–1539. [Google Scholar] [CrossRef]
- Wang, J.-C.; Guo, Q.-B.; Liu, X.-F.; Dai, Y.; Wang, Z.-Y.; Qiu, J.-R. Bubble Generation in Germanate Glass Induced by Femtosecond Laser*. Chin. Phys. Lett. 2016, 33, 036101. [Google Scholar] [CrossRef]
- Kishi, T.; Kumagai, T.; Yano, T.; Shibata, S. On-chip fabrication of air-bubble-containing Nd3+-doped tellurite glass microsphere for laser emission. AIP Adv. 2012, 2, 042169. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, K.; Masuno, A.; Ueda, M.; Inoue, H.; Yamamoto, H.; Kawashima, T. Low phonon energies and wideband optical windows of La2O3-Ga2O3 glasses prepared using an aerodynamic levitation technique. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Vangheluwe, M.; Petit, Y.; Marquestaut, N.; Corcoran, A.; Fargin, E.; Vallée, R.; Cardinal, T.; Canioni, L. Nanoparticle generation inside Ag-doped LBG glass by femtosecond laser irradiation. Opt. Mater. Express 2016, 6, 743. [Google Scholar] [CrossRef]

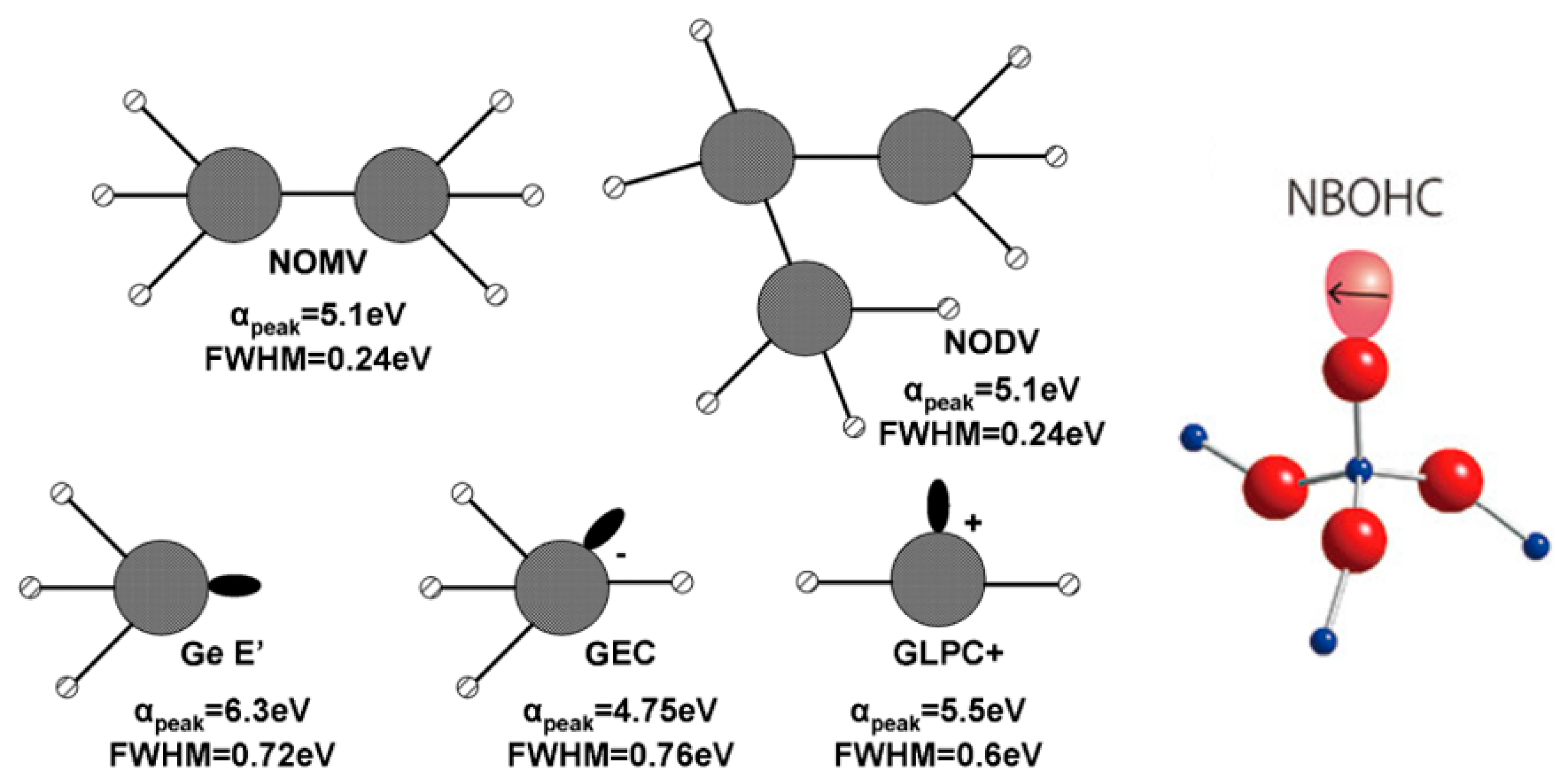
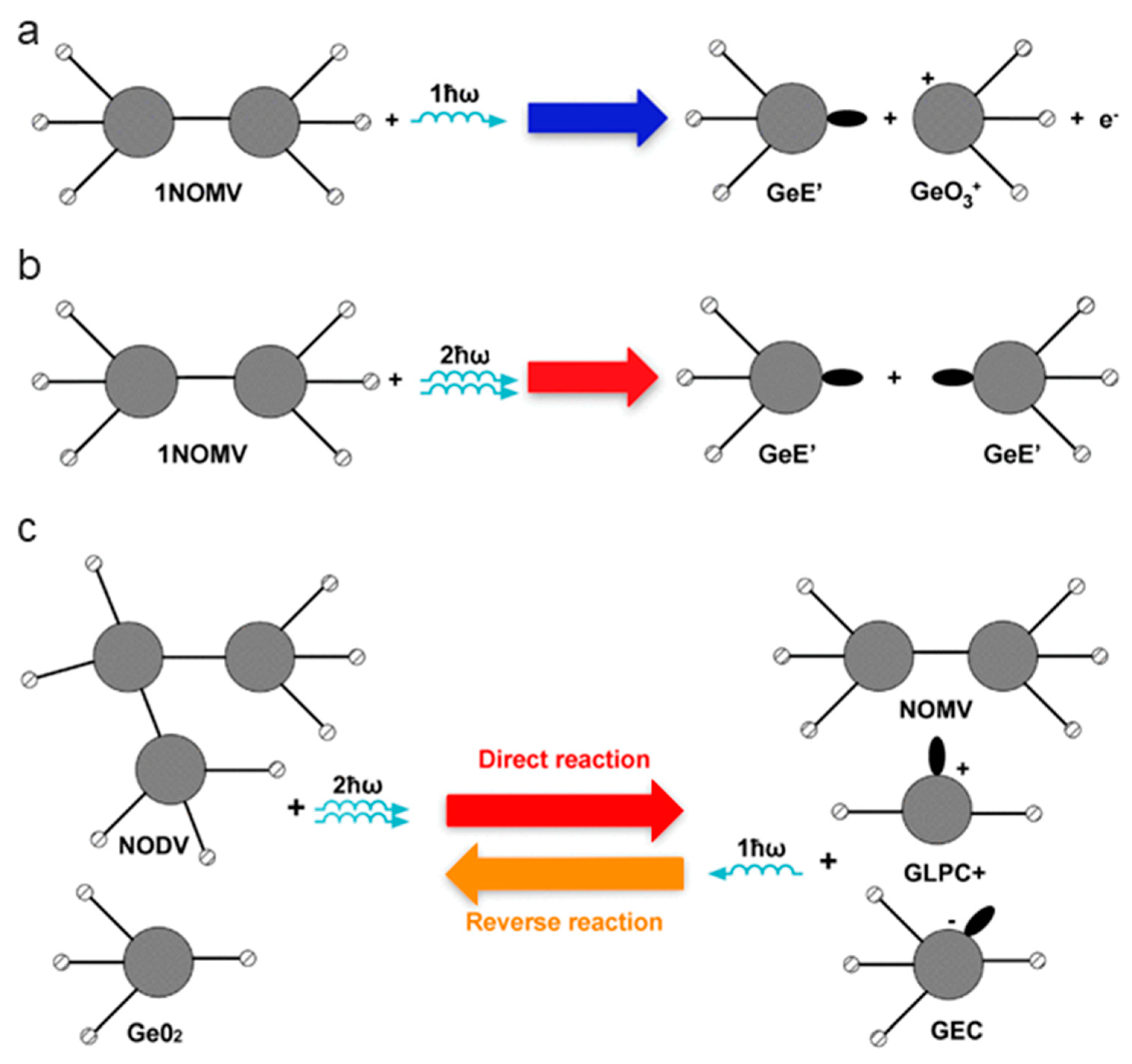
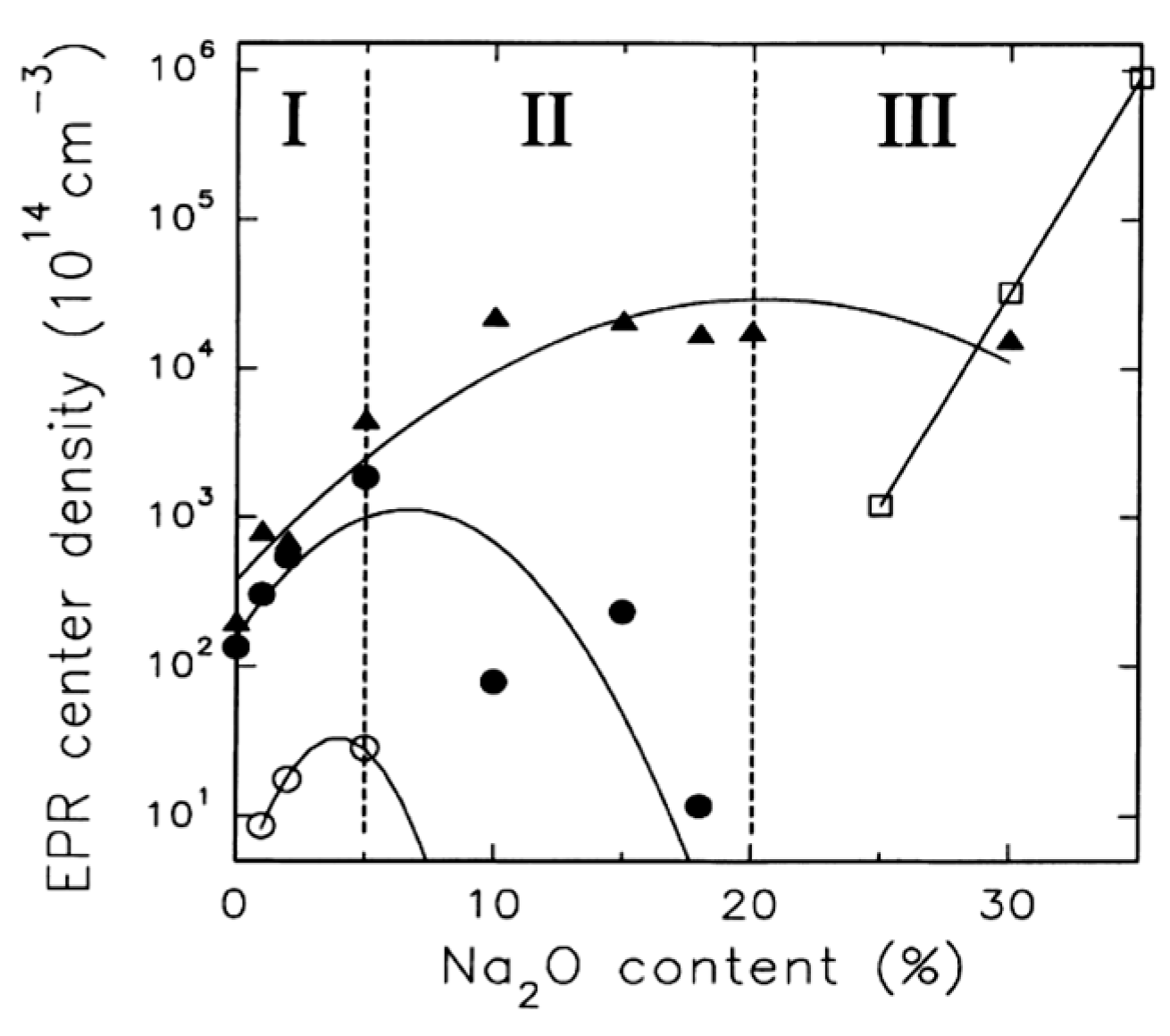
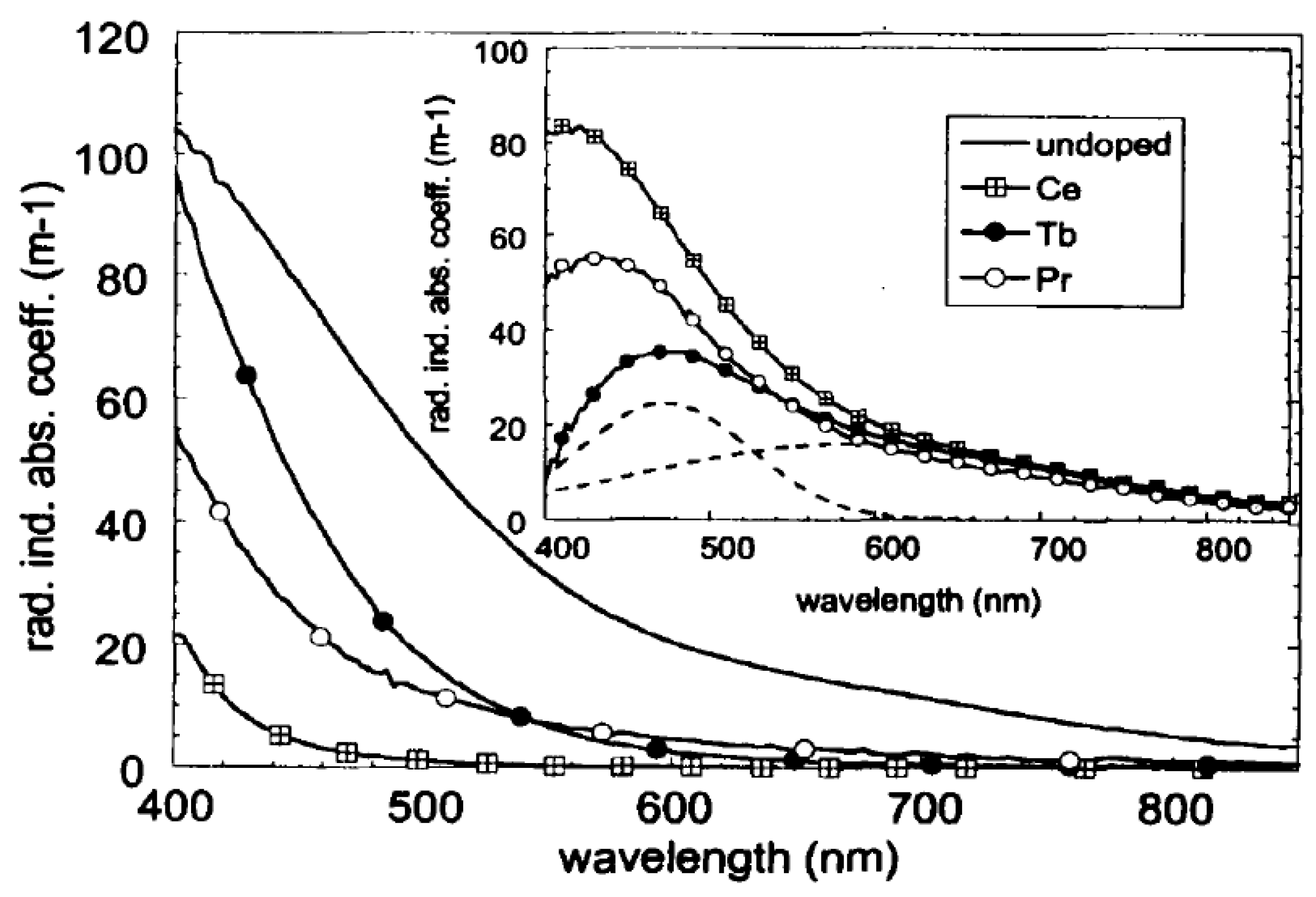
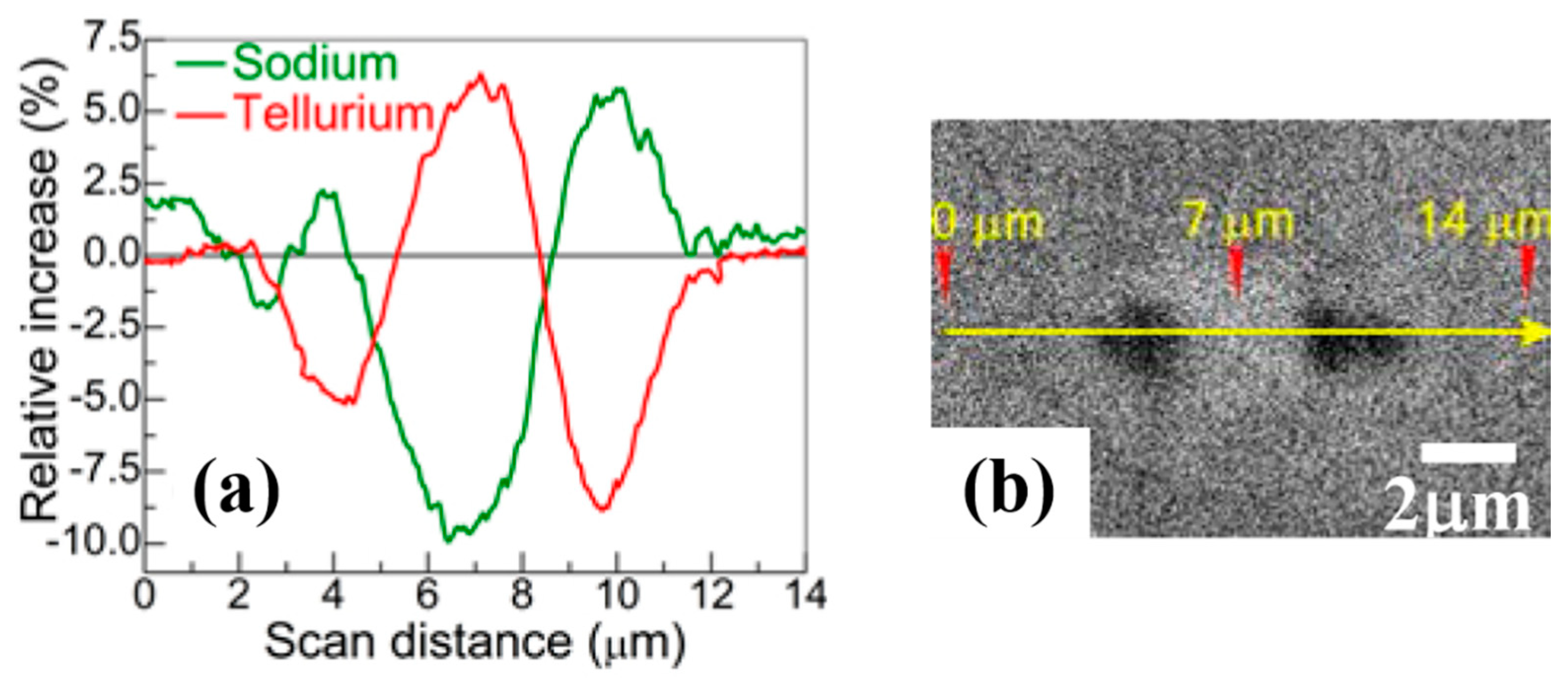
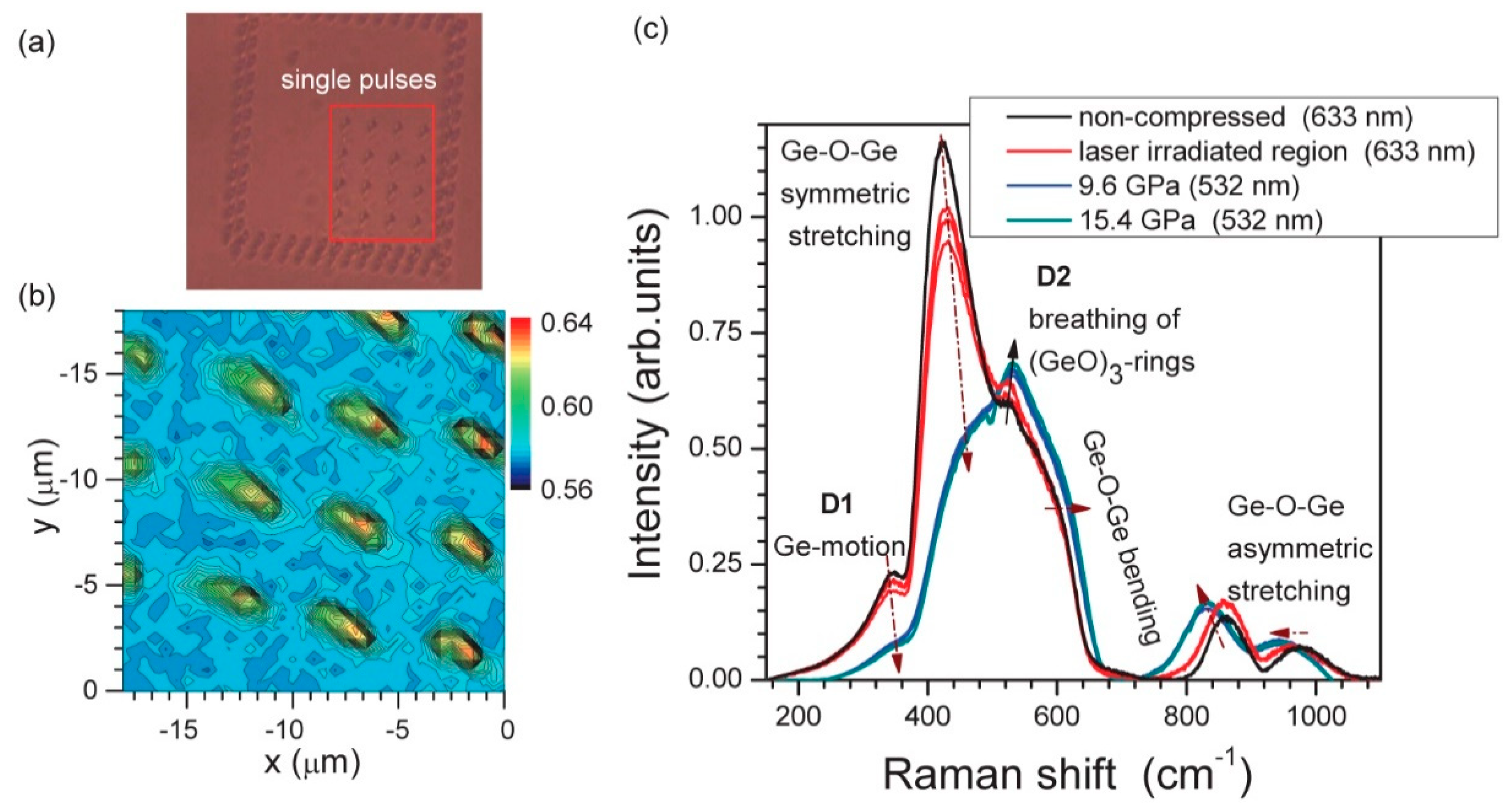
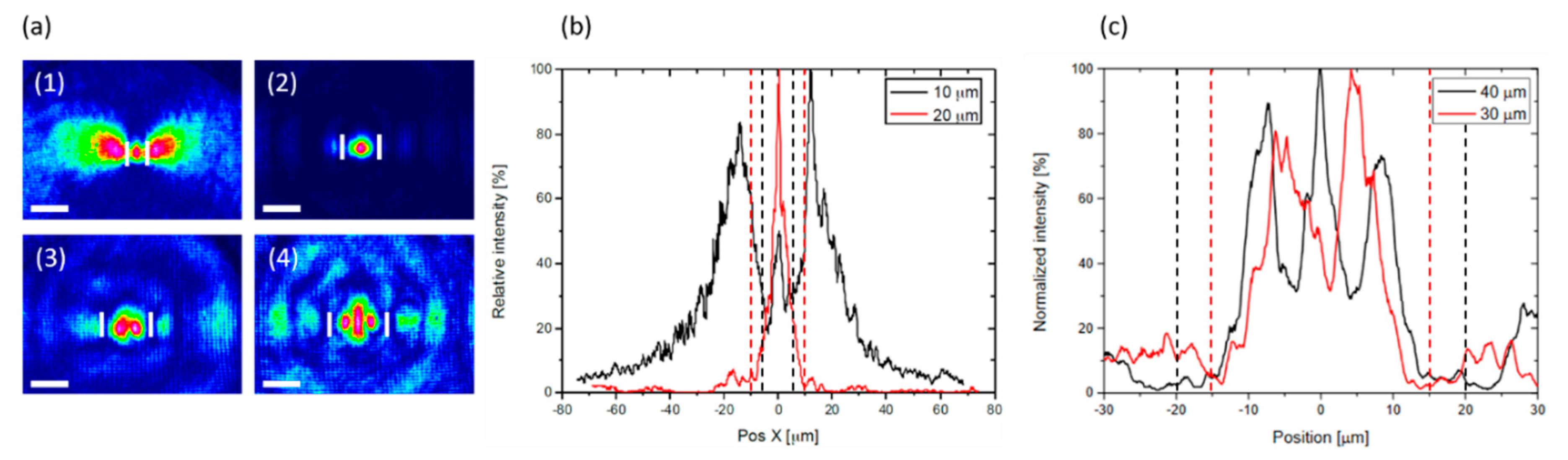
| Defect | EPR g-Value |
|---|---|
| Te-NBOHC | 1.9960 [62] |
| TeEC/VO | 1.9705 [66] |
| Modifier related trap | 2.0010 [63] |
| TeOHC | 2.0747 [63] |
| Defect | EPR g-Value | Abs. Wavelength nm [eV] | PL Wavelength nm [eV] |
|---|---|---|---|
| (GLPC)+ | 1.9866 [70] | 225 [5.5] [69] | 400 [3.1] [71] 1 |
| GEC | 1.9933 [70] | 261 [4.75] [69] and 315 [3.9] [72] | - |
| Ge E′ | 2.0011 [73] | 197 [6.3] [68] | 590 [2.1] [61] 2 |
| Ge-NBOHC | 2.0076 [74] | 375 [3.3] [72] | 590 [2.1] [61] 2 650 [1.9] [71] 1 |
| GeO3+ | 2.008 [73] | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hongisto, M.; Veber, A.; Petit, Y.; Cardinal, T.; Danto, S.; Jubera, V.; Petit, L. Radiation-Induced Defects and Effects in Germanate and Tellurite Glasses. Materials 2020, 13, 3846. https://doi.org/10.3390/ma13173846
Hongisto M, Veber A, Petit Y, Cardinal T, Danto S, Jubera V, Petit L. Radiation-Induced Defects and Effects in Germanate and Tellurite Glasses. Materials. 2020; 13(17):3846. https://doi.org/10.3390/ma13173846
Chicago/Turabian StyleHongisto, Mikko, Alexander Veber, Yannick Petit, Thierry Cardinal, Sylvain Danto, Veronique Jubera, and Laeticia Petit. 2020. "Radiation-Induced Defects and Effects in Germanate and Tellurite Glasses" Materials 13, no. 17: 3846. https://doi.org/10.3390/ma13173846
APA StyleHongisto, M., Veber, A., Petit, Y., Cardinal, T., Danto, S., Jubera, V., & Petit, L. (2020). Radiation-Induced Defects and Effects in Germanate and Tellurite Glasses. Materials, 13(17), 3846. https://doi.org/10.3390/ma13173846








