Microstructures and Mechanical Properties of Al-2Fe-xCo Ternary Alloys with High Thermal Conductivity
Abstract
1. Introduction
2. Materials and Methods
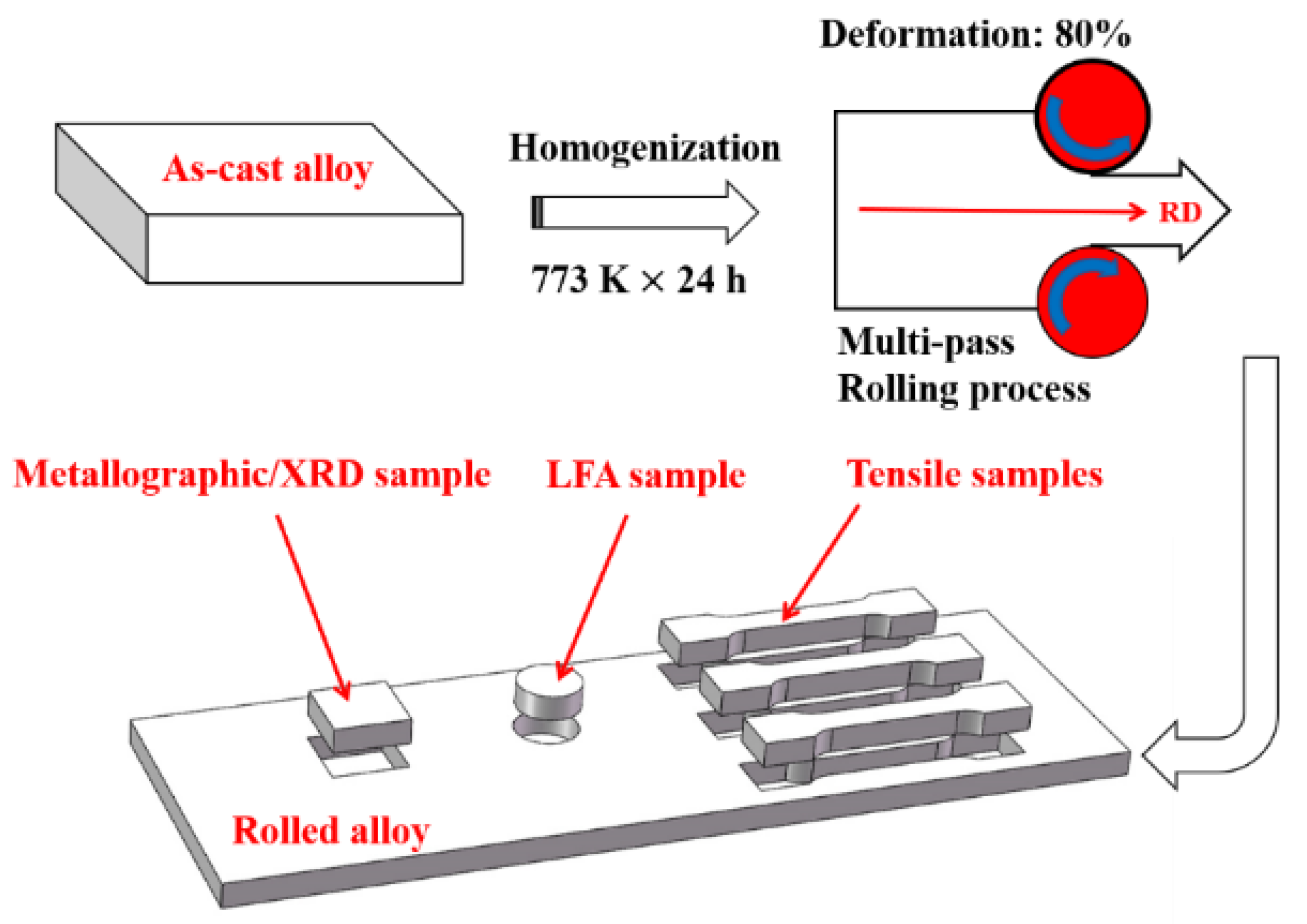
2.1. Preparation of Samples
2.2. Measurements
3. Results and Discussion
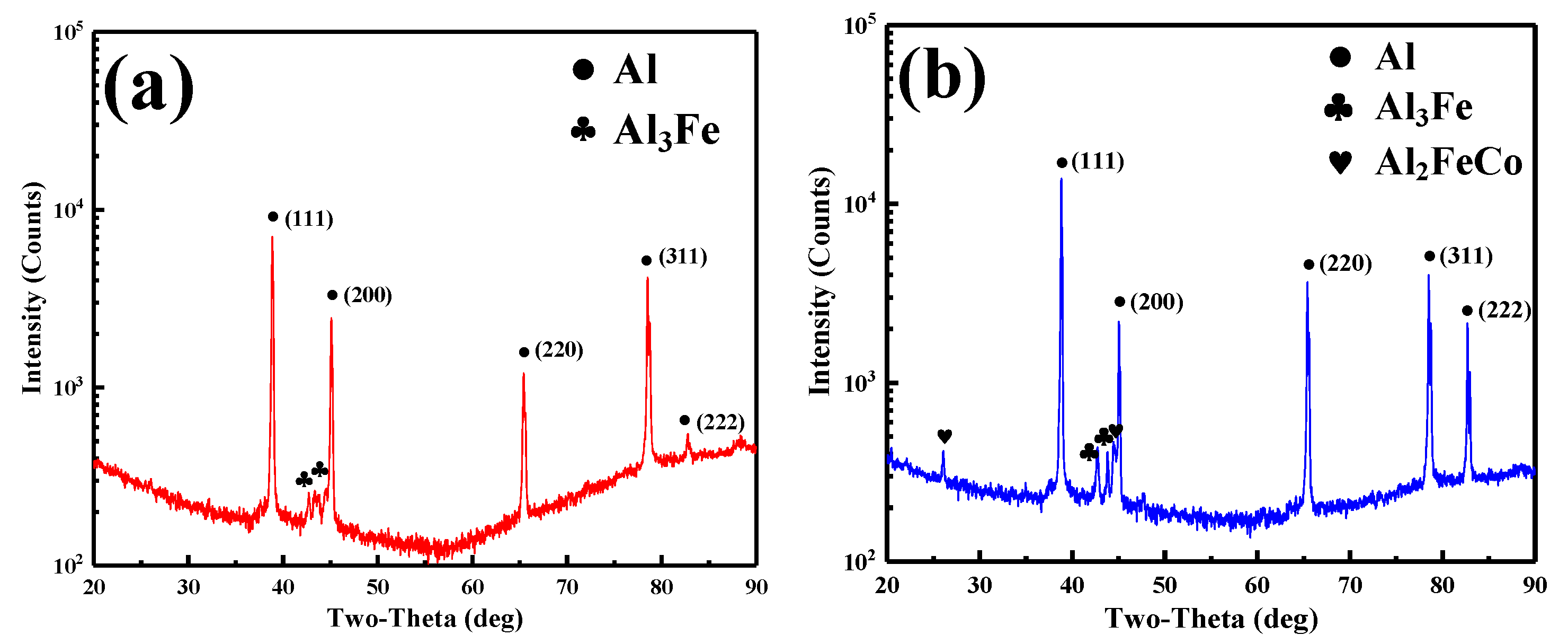
3.1. XRD Results
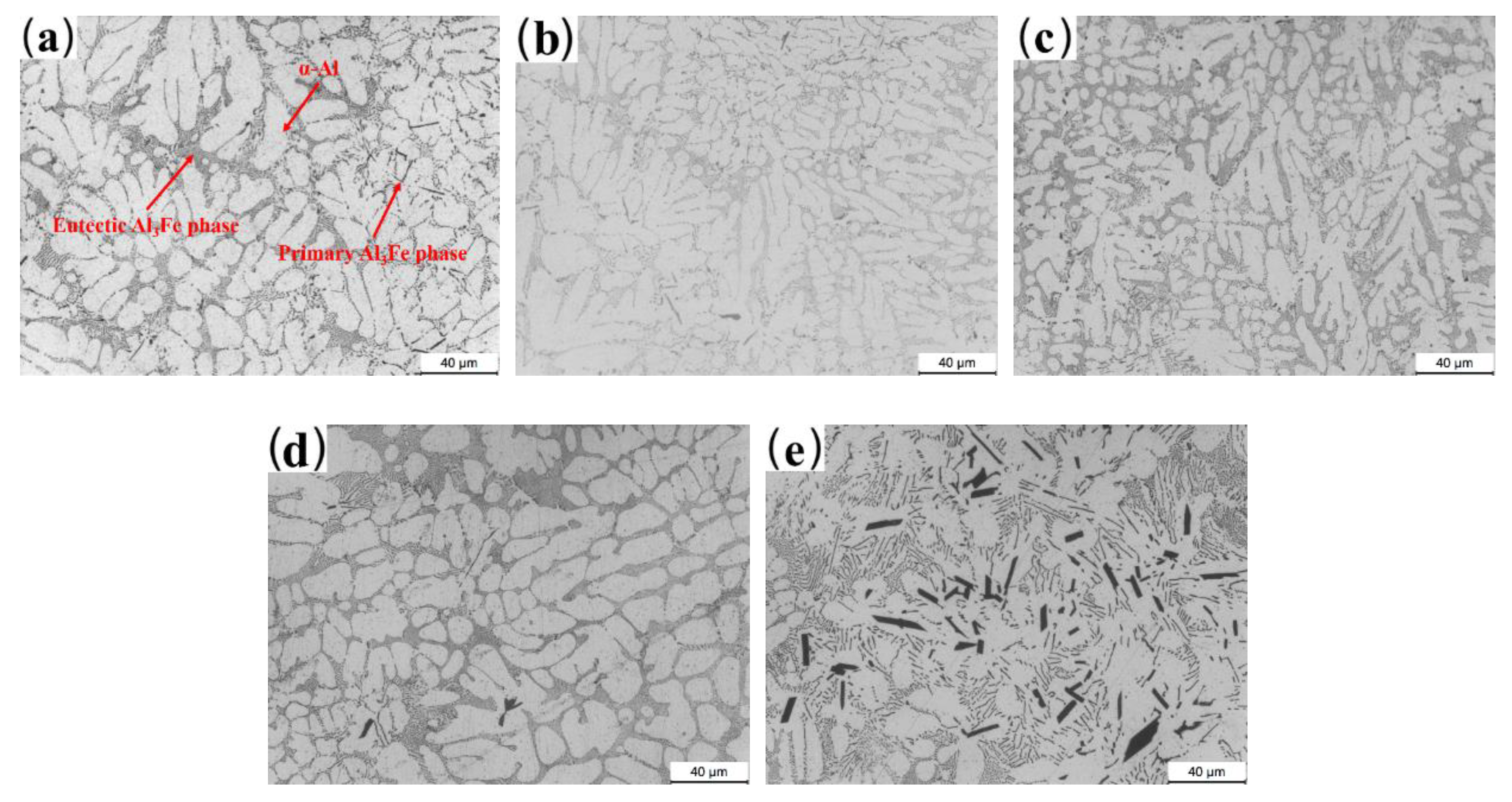
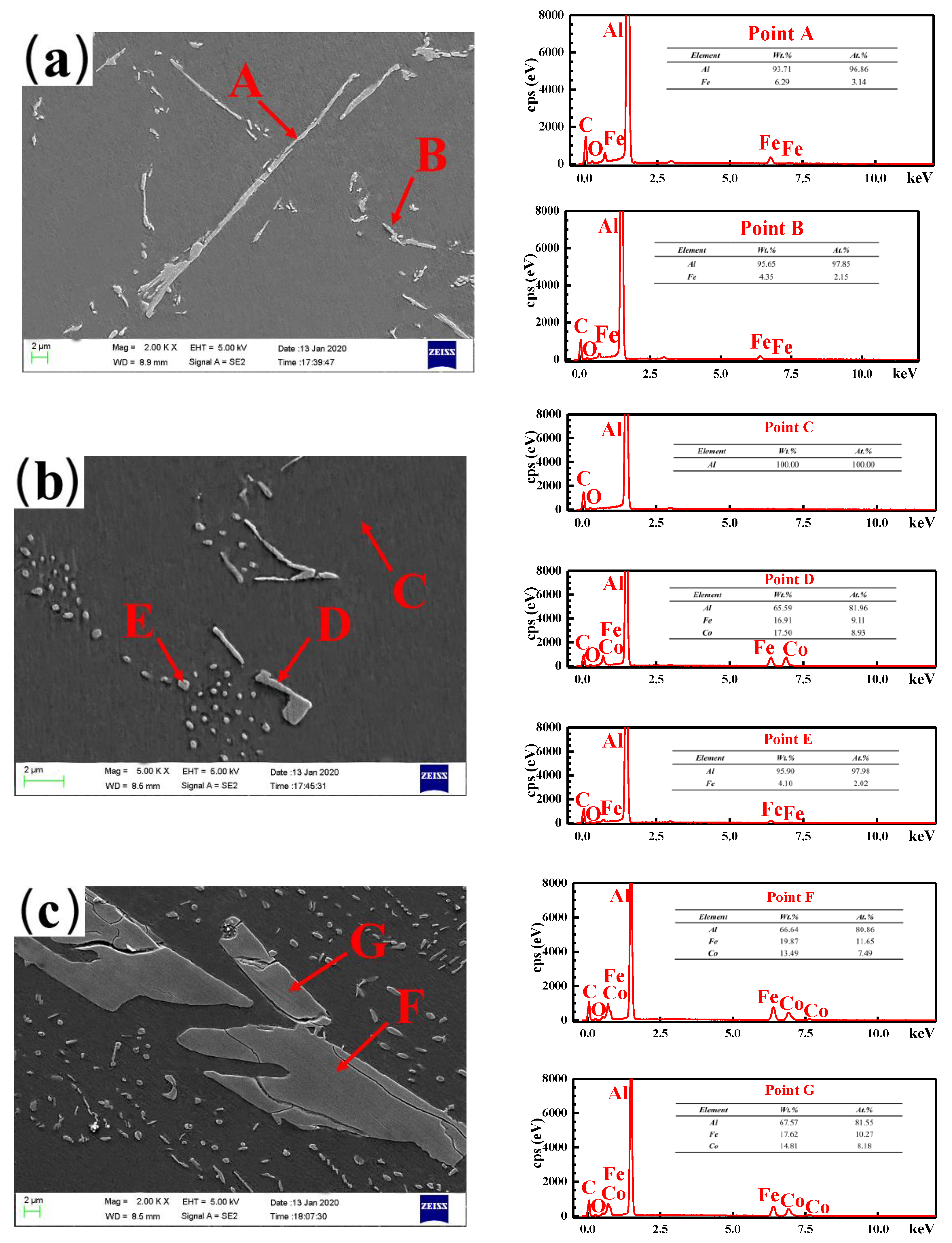
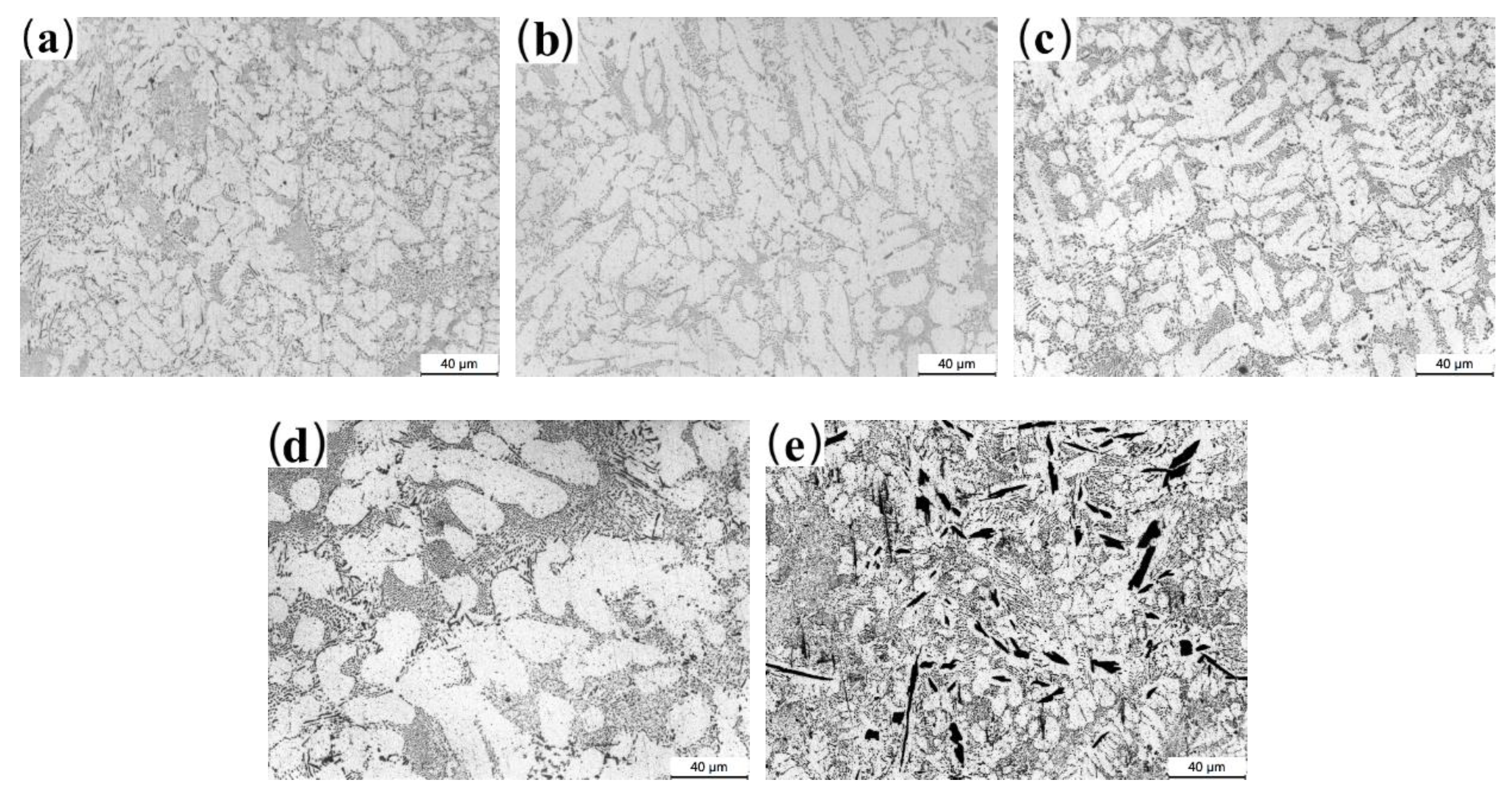
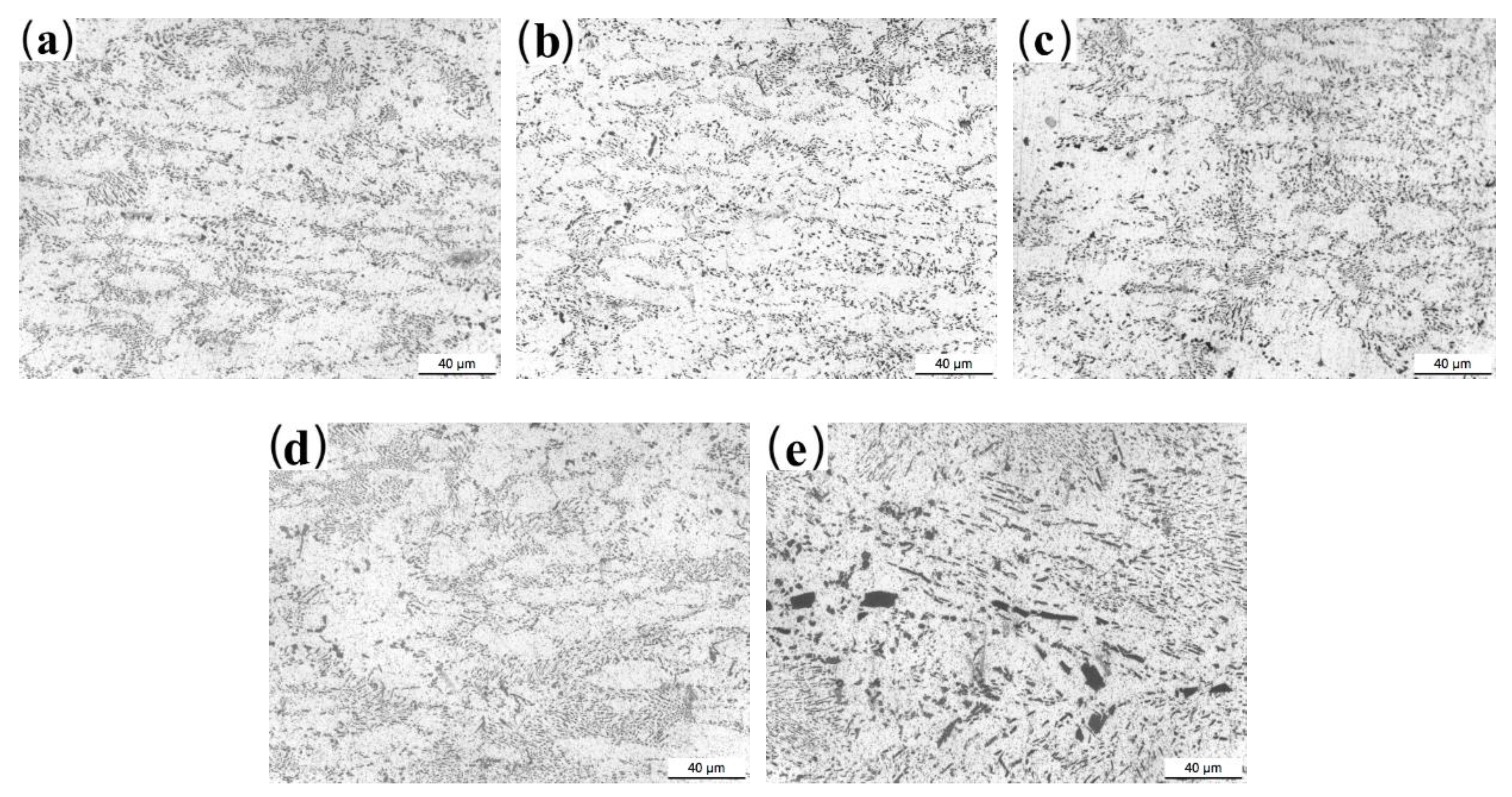
3.2. Microstructure Characterization
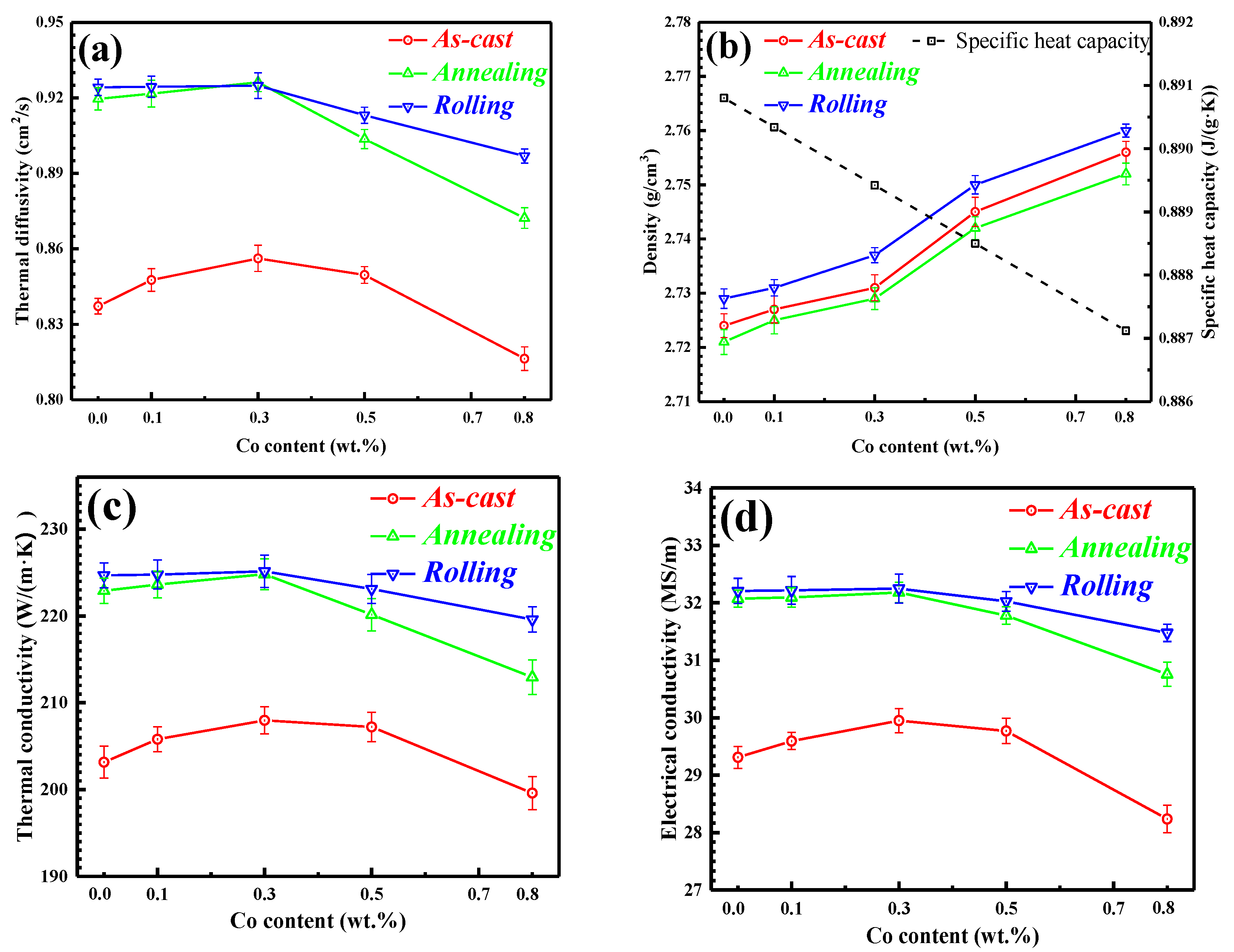
3.3. Conductivity Performance of Al-2Fe-xCo Ternary Alloys
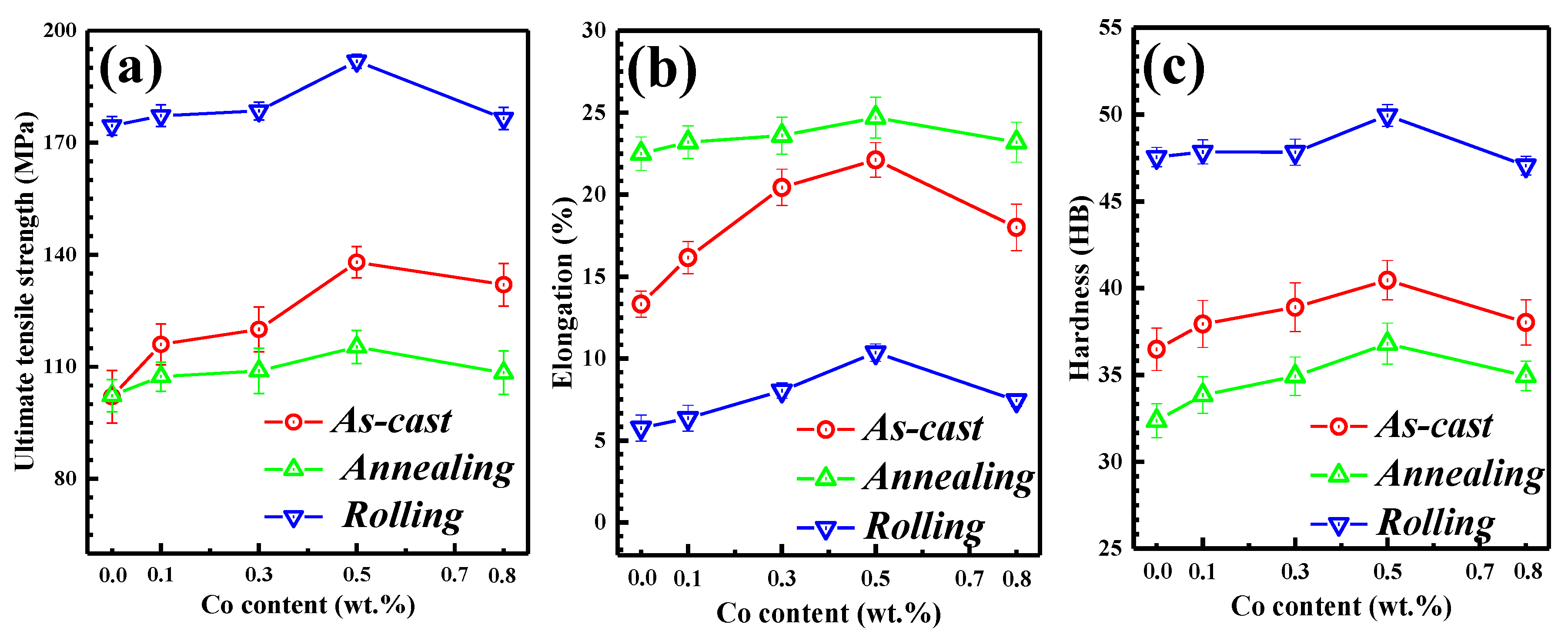
3.4. Mechanical Properties of Al-2Fe-xCo Ternary Alloys
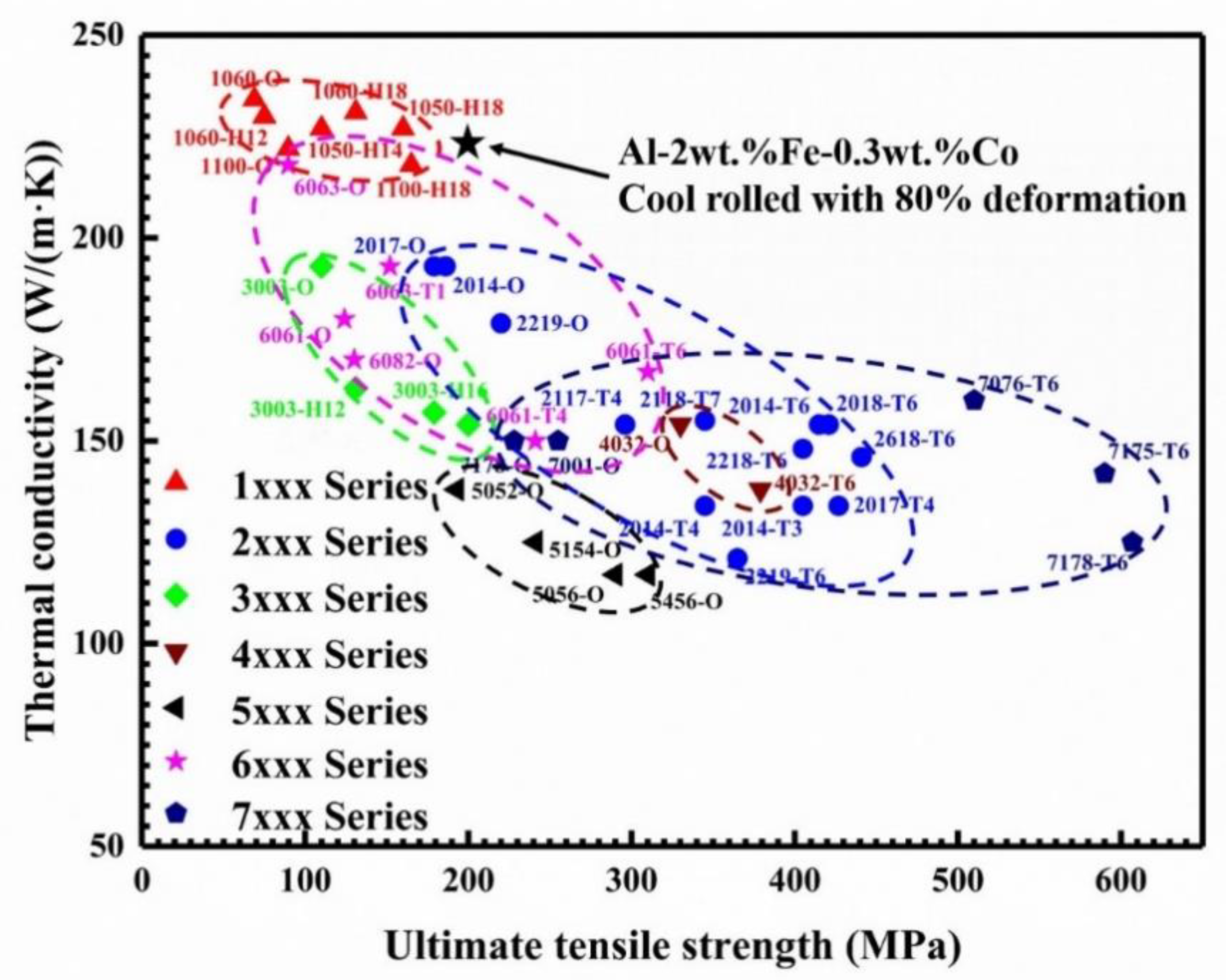
3.5. The Correlation between Thermal Conductivity and Mechanical Properties
4. Conclusions
- (1)
- The addition amount of Co in the range from 0 to 0.3% can transform the morphology of primary Al3Fe phases from long needles to fine particles. The thermal conductivity of the Al-2Fe matrix would slightly increase from 203 W/(m·K) to 208 W/(m·K).
- (2)
- Because of the elimination of lattice defects and spheroidization of Al3Fe phases, the thermal conductivity of annealed Al-2Fe-xCo alloys is higher than that of as-cast alloys. After cool rolling with 80% deformation, the thermal conductivity of alloys slightly increases due to the breaking down of Al2FeCo phases.
- (3)
- Linear fitting was conducted to match this relationship for Al-2Fe-xCo ternary alloys in different states. The best fitting effect was obtained when the intercept was 6.96. In other words, the Lorentz number (L) is equal to 2.34 × 10−8 V−2K−2 for the Al-2Fe-xCo alloys.
- (4)
- The UTS and EL of the Al-2Fe-0.5Co alloy were close to 140 MPa and 22.0%, respectively, i.e., about 35% and 69% higher than those of the matrix Al-2Fe alloy. The improvement of mechanical properties was attributed to the refinement of α-Al grains and second phase strengthening.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, N.N.; Ban, C.Y.; Wang, H.F.; Cui, J.Z. Optimized Combination of Strength and Electrical Conductivity of Al-Mg-Si Alloy Processed by ECAP with Two-Step Temperature. Materials 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, D.; Luo, A.A.; Tao, Y.; Zeng, X. Effect of solute atoms and second phases on the thermal conductivity of Mg-RE alloys: A quantitative study. J. Alloys Compd. 2018, 747, 431–437. [Google Scholar] [CrossRef]
- Shi, Z.M.; Gao, K.; Shi, Y.T.; Wang, Y. Microstructure and mechanical properties of rare-earth-modified Al−1Fe binary alloys. Mater. Sci. Eng. A-Struct 2015, 632, 62–71. [Google Scholar] [CrossRef]
- Qi, M.; Kang, Y.; Li, J.; Shang, B. Improvement in mechanical, thermal conductivity and corrosion performances of a new high-thermally conductive Al-Si-Fe alloy through a novel R-HPDC process. J. Mater. Process. Tech. 2020, 279, 116586. [Google Scholar] [CrossRef]
- Kim, C.W.; Cho, J.I.; Choi, S.W.; Kim, Y.C. The effect of alloying elements on thermal conductivity of aluminum alloys in high pressure die casting. Adv. Mater. Res. 2013, 813, 175–178. [Google Scholar]
- Zhong, L.; Peng, J.; Sun, Y.; Wang, Y.; Pan, F. Microstructure and thermal conductivity of as-cast and as-extruded binary Mg–Mn alloys. Mater. Sci. Technol. 2016, 33, 1–6. [Google Scholar] [CrossRef]
- Chen, J.K.; Hung, H.Y.; Wang, C.F.; Tang, N.K. Thermal and electrical conductivity in Al–Si/Cu/Fe/Mg binary and ternary Al alloys. J. Mater. Sci 2015, 50, 5630–5639. [Google Scholar] [CrossRef]
- Konovalov, S.V.; Danilov, V.I.; Zuev, L.B.; Filip’Ev, R.A.; Gromov, V.E. On the influence of the electrical potential on the creep rate of aluminum. Phys. Solid State 2007, 49, 1457–1459. [Google Scholar] [CrossRef]
- Ivanov, Y.F.; Alsaraeva, K.V.; Gromov, V.E.; Popova, N.A.; Konovalov, S.V. Fatigue life of silumin treated with a high-intensity pulsed electron beam. J. Surf. Investig. X-Ray 2015, 9, 1056–1059. [Google Scholar] [CrossRef]
- Chen, J.K.; Hung, H.Y.; Wang, C.F.; Tang, N.K. Effects of casting and heat treatment processes on the thermal conductivity of an Al-Si-Cu-Fe-Zn alloy. Int. J. Heat Mass Tran. 2017, 105, 189–195. [Google Scholar] [CrossRef]
- Kim, Y.M.; Choi, S.W.; Hong, S.K. The behavior of thermal diffusivity change according to the heat treatment in Al-Si binary system. J. Alloys Compd. 2016, 687, 54–58. [Google Scholar] [CrossRef]
- Okamoto, H. Phase Diagrams for Binary Alloy, 2nd ed.; ASM International: Cleveland, OH, USA, 2010; p. 44. [Google Scholar]
- Mbuya, T.; Odera, B.; Ng’ang’a, S. Influence of iron on castability and properties of aluminium silicon alloys: Literature review. Int. J. Cast Met. Res. 2003, 16, 451–465. [Google Scholar] [CrossRef]
- Hu, Z. Microstructure and mechanical properties of Al-Fe-V-Si aluminum alloy produced by electron beam melting. Mater. Sci. Eng. A-Struct 2016, 659, 207–214. [Google Scholar]
- Mulazimoglu, M.H.; Drew, R.A.; Gruzleski, J.E. The electrical conductivity of cast Al−Si alloys in the range 2 to 12.6 wt pct silicon. Metall. Trans. A 1989, 20, 383–389. [Google Scholar] [CrossRef]
- Luo, S.; Shi, Z.; Li, N.; Lin, Y.; Liang, Y.; Zeng, Y. Crystallization inhibition and microstructure refinement of Al-5Fe alloys by addition of rare earth elements. J. Alloys Compd. 2019, 789, 90–99. [Google Scholar] [CrossRef]
- Sasaki, T.; Ohkubo, T.; Hono, K. Microstructure and mechanical properties of bulk nanocrystalline Al–Fe alloy processed by mechanical alloying and spark plasma sintering. Acta Mater. 2009, 57, 3529–3538. [Google Scholar] [CrossRef]
- Krasnowski, M.; Kulik, T. Nanocrystalline and amorphous Al-Fe alloys containing 60–85% of Al synthesised by mechanical alloying and phase transformations induced by heating of milling products. Mater. Chem. Phys. 2009, 116, 631–637. [Google Scholar] [CrossRef]
- Ban, C.Y.; Zhang, X.; Qian, P.; Han, Y.; Cui, J.Z. Study on the solidification structures of Al-Fe-Si alloy under DC and AC magnetic fields. Adv. Mat. Res. 2011, 189, 4477–4482. [Google Scholar]
- Nayak, S.; Chang, H.; Kim, D.H.; Pabi, S.; Murty, B. Formation of metastable phases and nanocomposite structures in rapidly solidified Al-Fe alloys. Mater. Sci. Eng. A 2011, 528, 5967–5973. [Google Scholar] [CrossRef]
- Vourlias, G.; Pistofidis, N.; Pavlidou, E.; Stergioudis, G. Reinforcement of Al-Fe-Ni alloys with the in situ formation of composite materials. J. Alloys Compd. 2009, 483, 178–181. [Google Scholar] [CrossRef]
- Wang, J.Y. The Effect of Grain Refiner on Hypereutectic Al-Fe Alloys and the Study of Refining Mechanism; Shenyang University of Technology: Shenyang, China, 2007. [Google Scholar]
- Wang, Z.J. Grain Refinement Behavior of Al-10% Mg Master Alloy on Al-5% Fe Alloy. Foundry Technol. 2009, 9, 77–79. [Google Scholar]
- Kaufman, M.J. The effects of Mn additions on the microstructure and mechanical properties of Al-Si-Cu casting alloys. Mater. Sci. Eng. A-Struct 2008, 488, 496–504. [Google Scholar]
- Sun, C.M.; Shi, Z.M.; Li, Z.F. Improvement of morphology of Fe-rich phase in commercial pure aluminum by Ce-rich rare earth modification. J. Chin. Soc. Rare Earths 2007, 25, 318–322. [Google Scholar]
- Sha, M.; Wu, S.; Wan, L. Combined effects of cobalt addition and ultrasonic vibration on microstructure and mechanical properties of hypereutectic Al–Si alloys with 0.7% Fe. Mater. Sci. Eng. A-Struct 2012, 554, 142–148. [Google Scholar] [CrossRef]
- Sha, M.; Wu, S.; Wang, X.; Wan, L.; An, P. Effects of cobalt content on microstructure and mechanical properties of hypereutectic Al–Si alloys. Mater. Sci. Eng. A 2012, 535, 258–263. [Google Scholar] [CrossRef]
- Rudajevová, A.; Buch, F.V.; Mordike, B.L. Thermal diffusivity and thermal conductivity of MgSc alloys. J. Alloys Compd. 1999, 292, 27–30. [Google Scholar] [CrossRef]
- Pan, H.; Pan, F.; Yang, R.; Peng, J.; Zhao, C.; She, J.; Gao, Z.; Tang, A. Thermal and electrical conductivity of binary magnesium alloys. J. Mater. Sci. 2014, 49, 3107–3124. [Google Scholar] [CrossRef]
- Hanawalt, J.; Rinn, H.; Frevel, L.J.I. Chemical analysis by X-ray diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Ellner, M. Polymorphic phase transformation of Fe4Al13 causing multiple twinning with decagonal pseudo-symmetry. Acta Crystallogr. B Struct. Sci. 1995, B51, 31–36. [Google Scholar] [CrossRef]
- Többens, D.; Stuesser, N.; Knorr, K.; Mayer, H.M.; Lampert, G. Calculated from ICSD using POWD-12++. Mater. Sci. Forum 2001, 378, 288. [Google Scholar]
- L’Heureux, I. Oscillatory zoning in crystal growth: A constitutional undercooling mechanism. Phys. Rev. E 1993, 48, 4460. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Mangelinck-Noël, N.; Bergman, C.; Billia, B. Determination of the average nucleation undercooling of primary Al-phase on refining particles from Al–5.0wt% Ti–1.0wt% B in Al-based alloys using DSC. J. Alloys Compd. 2009, 477, 622–627. [Google Scholar] [CrossRef]
- Li, R.D.; Ma, J.C.; Zhou, Z.P.; Yu, H.S. Effect of Rare Earth on Microstructure of Eutectic A1-2% Fe Alloy. J. Rare Earth 2004, 22, 722–724. [Google Scholar]
- Nagaumi, H. Effects of Mg contents on thermal properties of Al-Mg alloys. Keikinzoku 2000, 50, 49–53. [Google Scholar]
- Stadler, F.; Antrekowitsch, H.; Fragner, W.; Kaufmann, H.; Uggowitzer, P.J. The effect of nickel on the thermal conductivity of Al-Si cast alloys. ICAA13 Pittsburgh 2016, 137–142. [Google Scholar]
- Cong, Z.; Yong, D.; Liu, S.; Liu, S.; Jie, W.; Sundman, B. Microstructure and Thermal Conductivity of the As-Cast and Annealed Al-Cu-Mg-Si Alloys in the Temperature Range from 25℃ to 400℃. Int. J. Thermophys. 2015, 36, 2869–2880. [Google Scholar]
- Tie, D.; Guan, R.-g.; Guo, N.; Zhao, Z.; Su, N.; Li, J.; Zhang, Y. Effects of different heat treatment on microstructure, mechanical and conductive properties of continuous Rheo-extruded Al-0.9 Si-0.6 Mg (wt%) alloy. Metals 2015, 5, 648–655. [Google Scholar] [CrossRef]
- Cui, X.; Cui, H.; Wu, Y.; Liu, X. The improvement of electrical conductivity of hypoeutectic Al-Si alloys achieved by composite melt treatment. J. Alloys Compd. 2019, 788, 1322–1328. [Google Scholar] [CrossRef]
- Zhao, Q.; Cui, X.; Qian, Z.; Liu, X. The synergistic effect of Al–B–C master alloy to improve conductivity and strength of 1070 alloy. J. Alloys Compd. 2015, 639, 478–482. [Google Scholar] [CrossRef]
- Ying, T.; Zheng, M.Y.; Li, Z.T.; Qiao, X.G.; Xu, S.W. Thermal conductivity of as-cast and as-extruded binary Mg–Zn alloys. J. Alloys Compd. 2015, 621, 250–255. [Google Scholar] [CrossRef]
- Zhao, X. Thermodynamics and Kinetics of Materials; Zhejiang University Press: Hangzhou, China, 2016. [Google Scholar]
- Tan, R. Preparation and Study of Properties of Moderate Strength and High Conductivity Aluminum Alloy Wire; Zhengzhou University: Zhengzhou, China, 2017. [Google Scholar]
- Yuan, Z.; Wang, S. Plastic Deformation and Rolling Principles; Metallurgical Industry Press: Beijing, China, 2008. [Google Scholar]
- Chester, G.; Thellung, A. The law of Wiedemann and Franz. Proc. Phys. Soc. 1961, 77, 1005. [Google Scholar] [CrossRef]
- JE, H. Aluminium: Properties and Physical Metallurgy; ASM International: Mater. Park, OH, USA, 1984. [Google Scholar]
- Wilson, R.B.; Apgar, B.A.; Martin, L.W.; Cahill, D.G. Thermoreflectance of metal transducers for optical pump-probe studies of thermal properties. Opt. Express 2012, 20, 28829–28838. [Google Scholar] [CrossRef] [PubMed]
- Inogamov, N.A.; Petrov, Y.V. Thermal conductivity of metals with hot electrons. J. Exp. Theor. Phys. 2010, 110, 446–468. [Google Scholar] [CrossRef]
- Matweb. Available online: https://www.matweb.com/ (accessed on 10 March 2020).
- Salazar-Guapuriche, M.A.; Zhao, Y.Y.; Pitman, A.; Greene, A. Correlation of strength with hardness and electrical conductivity for aluminium alloy 7010. Mater. Sci. Forum. 2006, 519–521, 853–858. [Google Scholar]
- Yang, Y.; Zhang, L.; Zhong, J. Approach to thermal conductivity improvement of 6063 aluminium alloy. Met. Funct. Mater. 2004, 11, 23–25. [Google Scholar]
- Zhang, J.; Gao, A. Effect of heat treatment on thermal conductivity of 6063 aluminum alloy. In Proceedings of the Second International Conference on Mechanic Automation and Control Engineering, Hohhot, China, 15–17 July 2011; pp. 6449–6451. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Huang, Y.; Li, C.; Huang, Z.; Du, J. Microstructures and Mechanical Properties of Al-2Fe-xCo Ternary Alloys with High Thermal Conductivity. Materials 2020, 13, 3728. https://doi.org/10.3390/ma13173728
Luo G, Huang Y, Li C, Huang Z, Du J. Microstructures and Mechanical Properties of Al-2Fe-xCo Ternary Alloys with High Thermal Conductivity. Materials. 2020; 13(17):3728. https://doi.org/10.3390/ma13173728
Chicago/Turabian StyleLuo, Gan, Yujian Huang, Chengbo Li, Zhenghua Huang, and Jun Du. 2020. "Microstructures and Mechanical Properties of Al-2Fe-xCo Ternary Alloys with High Thermal Conductivity" Materials 13, no. 17: 3728. https://doi.org/10.3390/ma13173728
APA StyleLuo, G., Huang, Y., Li, C., Huang, Z., & Du, J. (2020). Microstructures and Mechanical Properties of Al-2Fe-xCo Ternary Alloys with High Thermal Conductivity. Materials, 13(17), 3728. https://doi.org/10.3390/ma13173728




