On the Microstructure and Properties of Nb-12Ti-18Si-6Ta-5Al-5Cr-2.5W-1Hf (at.%) Silicide-Based Alloys with Ge and Sn Additions
Abstract
1. Introduction
2. Alloy Design and Selection
2.1. Rationale and Objectives
2.2. Alloy Design
2.2.1. Constraints of Alloy Design
- (a)
- (b)
- contain Al and Cr (because of objectives (i), (ii) and (iii)),
- (c)
- have density lower than state of the art Ni-based superalloys (ρ ≈ 9 g/cm3 for 3rd generation, ρ ≈ 8.64 to 8.95 g/cm3 for 2nd generation [3,29]), lower than the density of single phase bcc solid solution RCCAs with Al, Nb and Ta additions (ρ ≈ 6.85 to 9.08 g/cm3 [2]), and multiphase bcc solid solution + intermetallic(s) (Laves, M5Si3) RCCAs with Al, Nb and Ta or Cr, Nb and Ta additions (ρ ≈ 7.14 to 8.58 g/cm3 [2]). To our knowledge there is no data about the density of RCCAs with simultaneous Al, Cr, Nb and Ta additions, and there is no data about RCCAs with Ge and Sn addition [2],
- (d)
- (e)
- (f)
- have Al/Cr = 1 and Sn/Ge = 1 based on the results reported in [13] and
- (g)
2.2.2. Alloy Selection
3. Experimental
4. Results
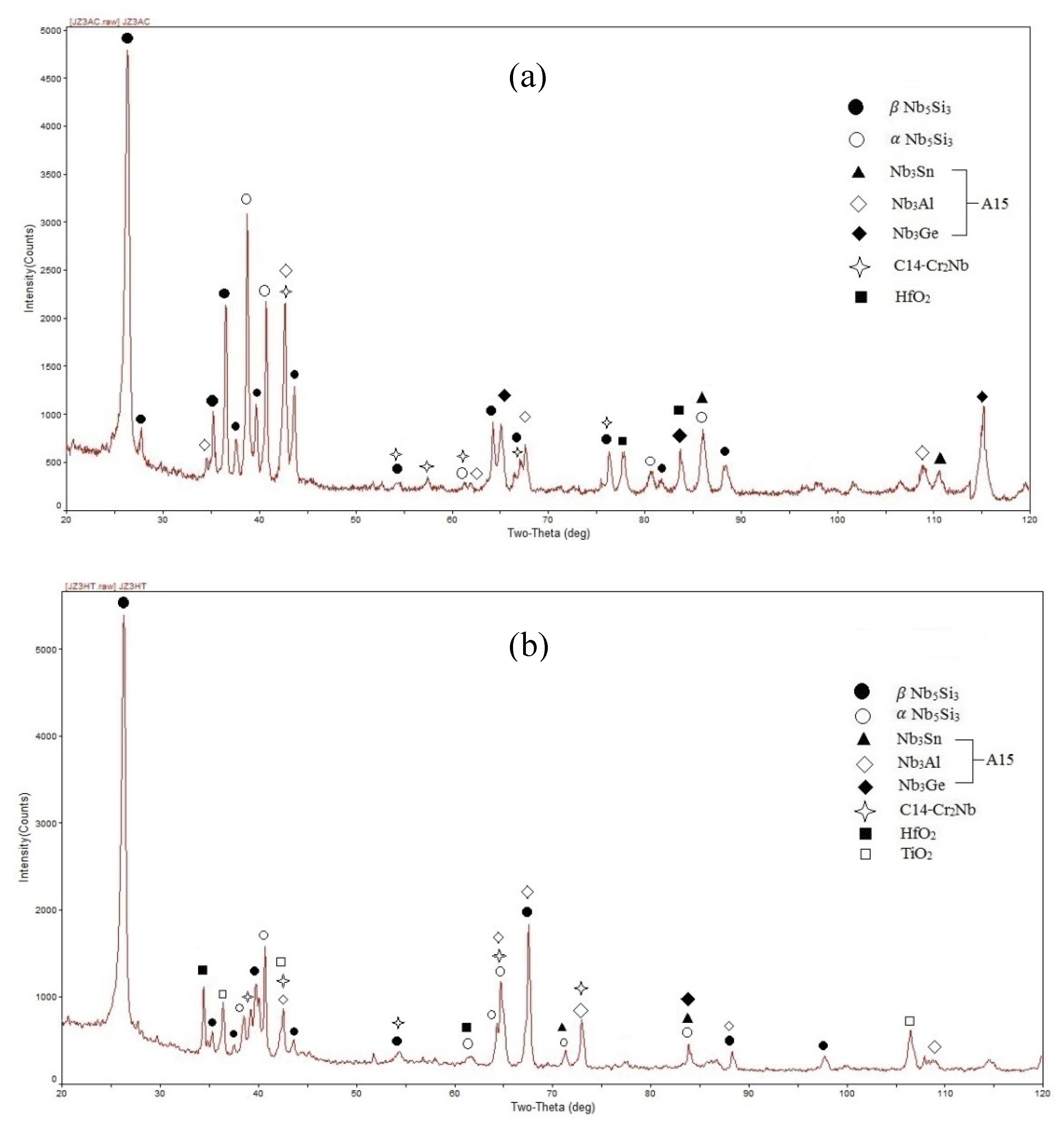
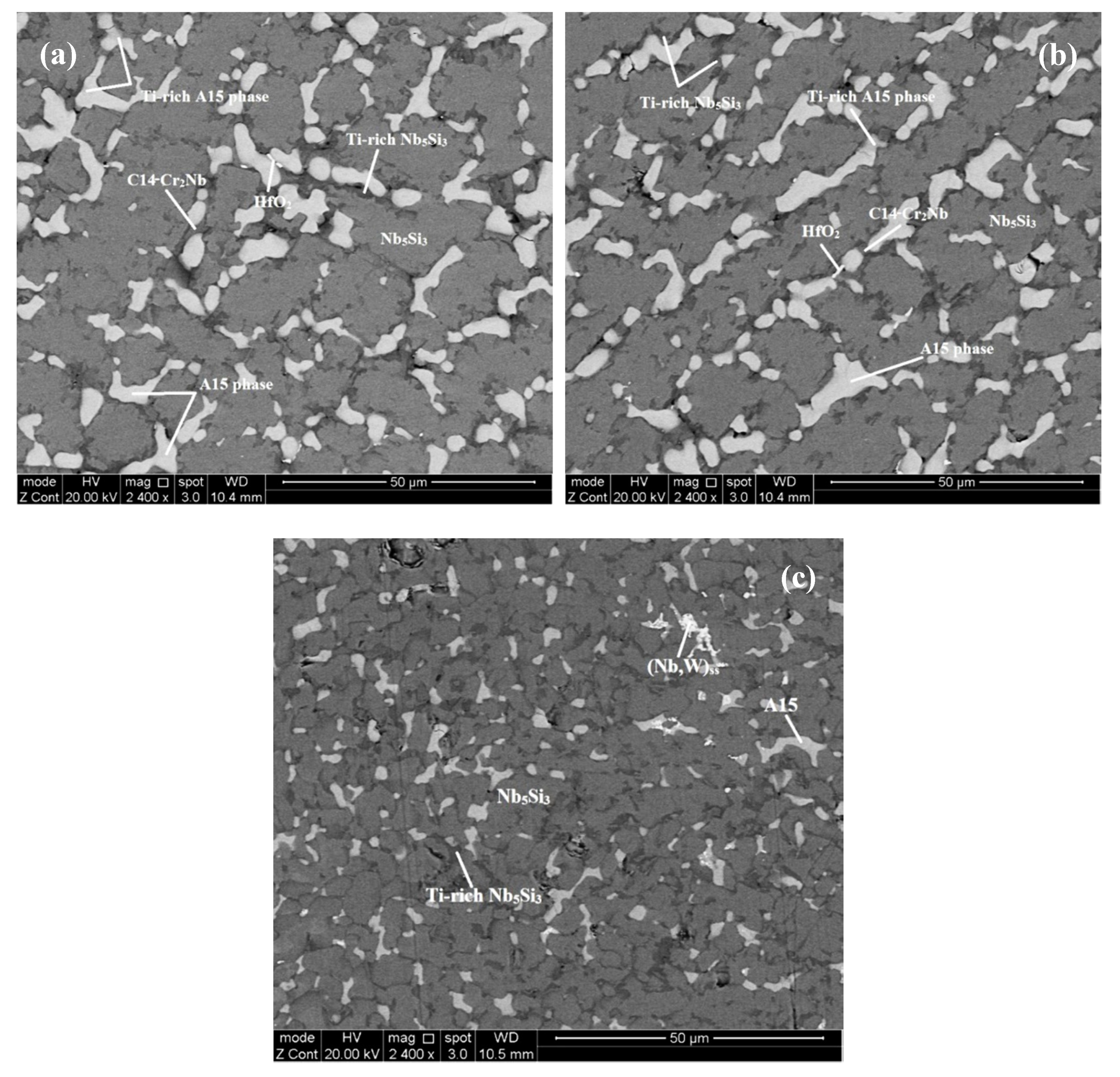
4.1. Microstructures
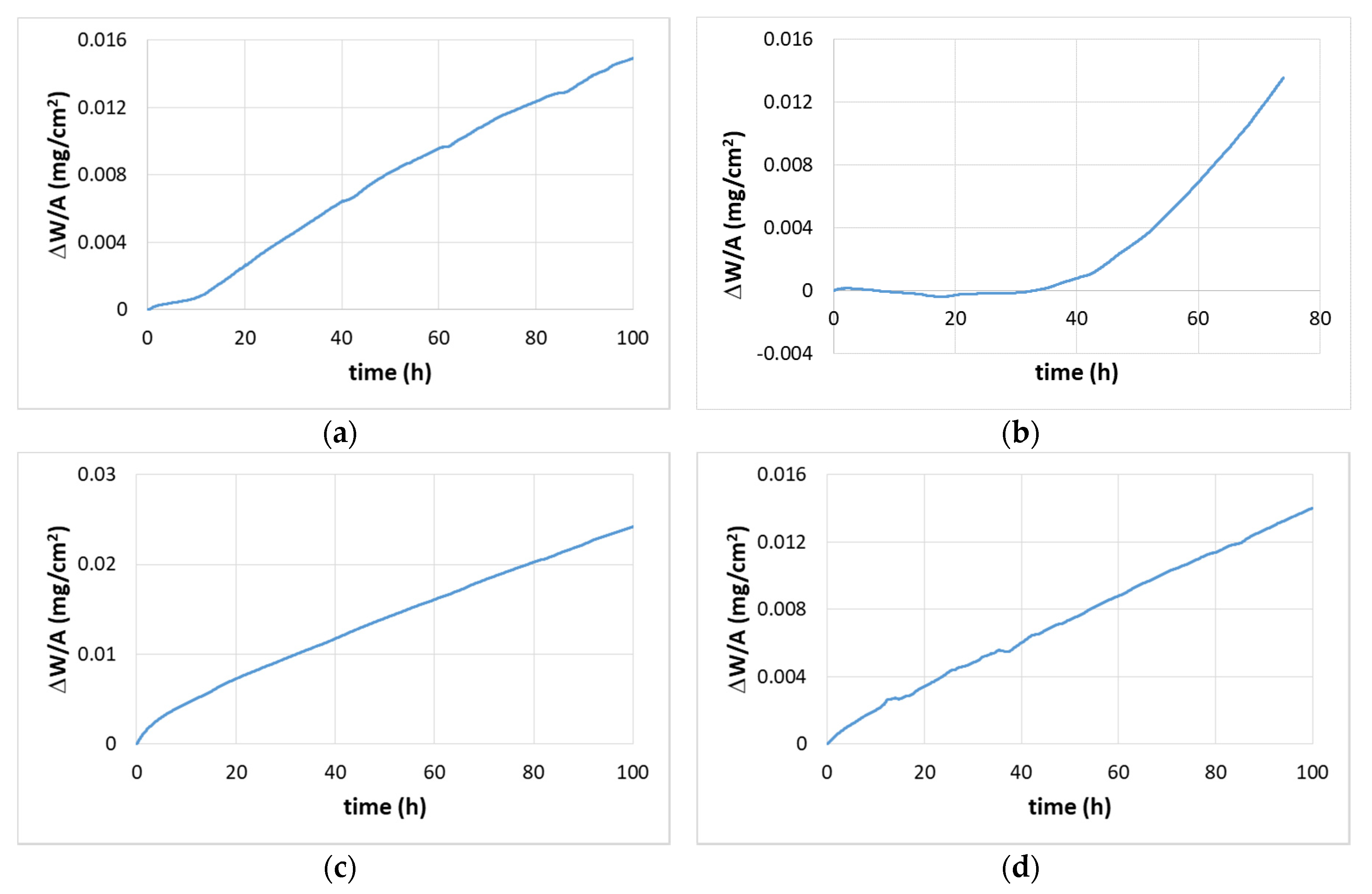
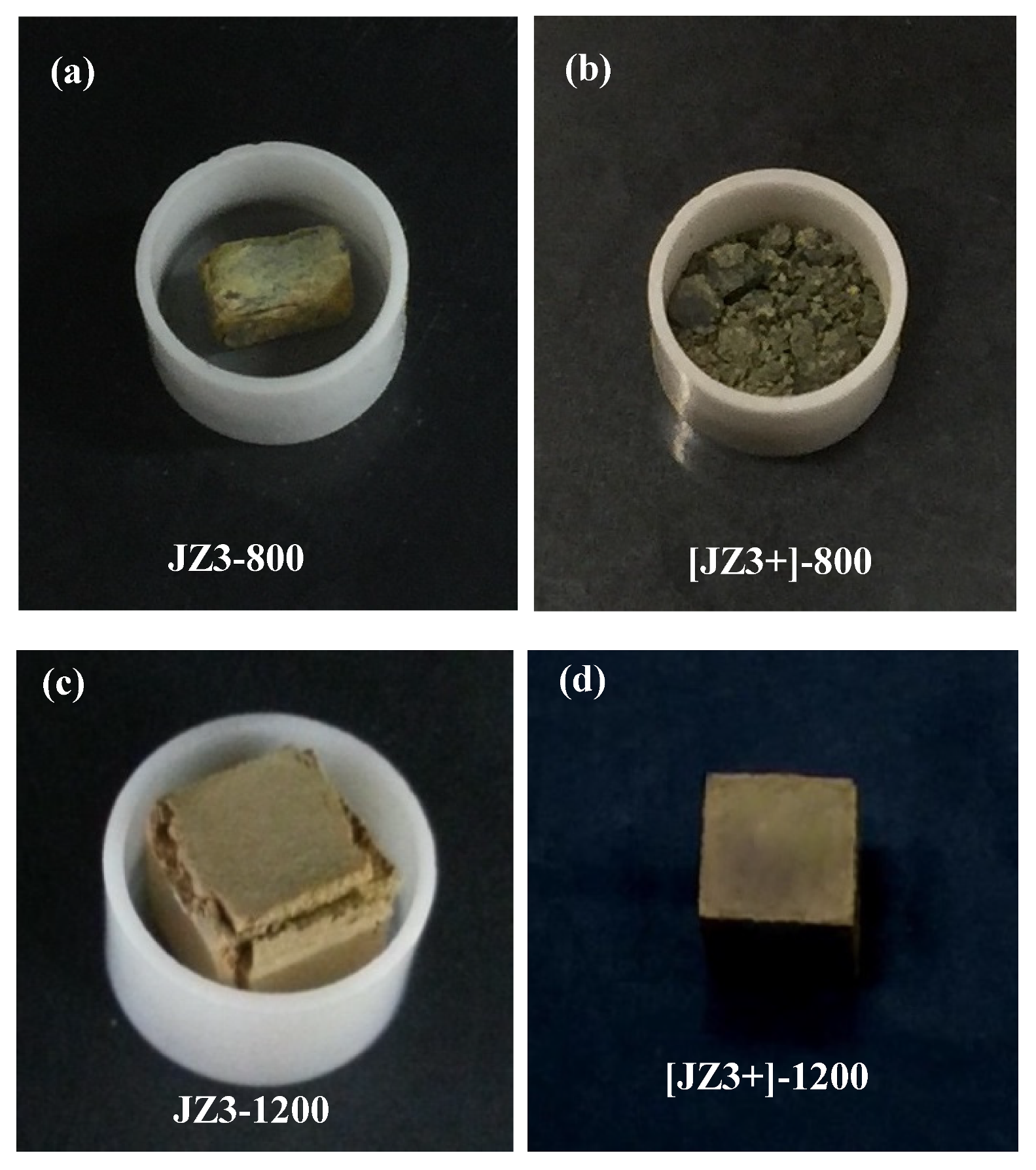
4.2. Oxidation
4.2.1. Oxidation at 800 °C
4.2.2. Oxidation at 1200 °C
5. Discussion
5.1. Density
Comparison with RCCAs
5.2. Macrosegregation
5.3. Microstructures
5.4. Oxidation
5.4.1. Oxidation at 800 °C
5.4.2. Oxidation at 1200 °C
5.4.3. Comparison with RCCAs
6. Comparisons of Experimental Data with NICE
6.1. Macrosegregation of Si
6.2. Volume Fraction of Solid Solution
6.3. Chemical Composition of Solid Solution
The Parameters VEC, δ and Δχ of the Solid Solution
6.4. Composition of Nb5Si3
6.5. Weight Change in Isothermal Oxidation
6.6. Calculated Creep Rate for Creep Goal
7. Summary and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | as cast |
| BSE | back scattered electron image |
| CCA | complex concentrated alloy |
| DBTT | ductile to brittle transition temperature |
| DSC | differential scanning calorimetry |
| EDS | energy dispersive X-ray spectrometry |
| HEA | high entropy alloy |
| HT | heat treated |
| HV | Hardness Vickers |
| MACSi | macrosegregation of Si |
| MASC | metal and silicide composite |
| M5Si3 | 5-3 silicide of element M, where M is TM or RM |
| Nbss | niobium solid solution |
| NICE | Niobium Intermetallic Composite Elaboration |
| RM | refractory metal |
| RCCA | refractory complex concentrated alloy |
| RHEA | refractory high entropy alloy |
| RMIC | refractory metal intermetallic composite |
| SEM | scanning electron microscope |
| TG | thermogravimetric analysis |
| TM | transition metal |
| VEC | valence electron concentration |
| XRD | X-ray diffraction |
| Δ | parameter related to atomic size |
| ΔW/A | weight change per unit area |
| Δχ | parameter related to electronegativity |
| Έ | creep rate (s−1) |
| ρ | density |
| <Si> | Al+Ge+Si+Sn |
| Tm | melting point |
Appendix A
Appendix A.1. Definition of the Parameters δ, Δχ and VEC
Appendix A.2. Alloys and Their Nominal Compositions (at.%)
| JZ1 = Nb-12Ti-18Si-6Ta-2.5W-1Hf-2Sn-2Ge |
| JZ2 = Nb-12Ti-18Si-6Ta-2.5W-1Hf-5Sn-5Ge |
| KZ4 = Nb-24Ti-18Si-5Cr |
| KZ5 = Nb-24Ti-18Si-5Al-5Cr |
| KZ6 = Nb-24Ti-18Si-6Ta-5Al-5Cr |
| KZ7 = Nb-24Ti-18Si-5Al |
| KZ8 = Nb-24Ti-18Si-8Cr-4Al |
| MASC =Nb-25Ti-16Si-8Hf-2Al-2Cr |
| OHS1 = Nb-24Ti-18Si-5Al-5Cr-5Ge-5Sn |
| ZF5 = Nb-24Ti-18Si-5Al-5Ge |
| ZX8= Nb-24Ti-18Si-5Al-5Cr-5Sn |
References
- Balsone, S.J.; Bewlay, B.P.; Jackson, M.R.; Subramanian, P.R.; Zhao, J.-C.; Chatterjee, A.; Heffernan, T.M. Materials beyond superalloy-exploiting high temperature composites. In Structural Intermetallics 2001; Hemker, K.J., Dimiduk, B.M., Clemens, H., Darolia, R., Inui, M., Larsen, J.M., Sikka, V.K., Thomas, M., Whittenberger, J.D., Eds.; TMS (The Minerals, Metals & Materials Society): Warrendale, PA, USA, 2001; pp. 99–108. ISBN 0-87339-511-5. [Google Scholar]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Jackson, M.R.; Gigliotti, M.F.X. Niobium Silicide High Temperature in Situ Composites. In Intermetallic Compounds: Principles and Practice; Westbrook, J.H., Fleischer, R.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2002; Volume 3. [Google Scholar]
- Tsakiropoulos, P. On Nb silicide based alloys: Alloy design and selection. Materials 2018, 11, 844. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Alloys for application at ultra-high temperatures: Nb-silicide in situ composites-Challenges, breakthroughs and opportunities. Prog. Mater. Sci. 2020, 100714. [Google Scholar] [CrossRef]
- Jackson, M.R.; Bewlay, B.P.; Briant, C.L. Creep Resistant Nb-Silicide Based Two Phase Composites. U.S. Patent 6,447,623 B1, 10 September 2002. [Google Scholar]
- Tsakiropoulos, P. Alloys. U.S. Patent 10,227,680, 9 October 2019. [Google Scholar]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J.-C. Niobium Silicide Based Composites Resistant to Low Temperature Pesting. U.S. Patent 6,419,765, 16 July 2002. [Google Scholar]
- Menon, E.S.K.; Mendiratta, M.G.D.; Dimiduk, M. Oxidation behavior of complex niobium based alloys. In Niobium Science & Technology: Proceedings of the International Symposium Niobium 2001, held in Orlando, Florida, USA, 2–5 December 2001; Niobium 2001 Limited: Bridgeville, PA, USA, 2002; pp. 121–145. ISBN 0971206805. [Google Scholar]
- Xu, Z.; Utton, C.; Tsakiropoulos, P. A study of the effect of 5 at.% Sn on the microstructure and isothermal oxidation at 800 and 1200 °C of Nb-24Ti-18Si based alloys with Al and/or Cr additions. Materials 2020, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tsakiropoulos, P. The effect of Ge addition on the oxidation of Nb-24Ti-18Si silicide based alloys. Materials 2019, 12, 3120. [Google Scholar] [CrossRef] [PubMed]
- Knittel, S.; Mathieu, S.; Portebois, L.; Vilasi, M. Effect of tin addition on Nb-Si based in situ composites. Part II: Oxidation behaviour. Intermetallics 2014, 47, 43–52. [Google Scholar] [CrossRef]
- Hernandez-Negrete, O.; Tsakiropoulos, P. On the microstructure and isothermal oxidation at 800 and 1200 °C of the Nb-24Ti-18Si-5Al-5Cr-5Ge-5Sn (at.%) silicide based alloy. Materials 2020, 13, 722. [Google Scholar] [CrossRef]
- Zhao, J.; Utton, C.; Tsakiropoulos, P. On the microstructure and properties of Nb-12Ti-18Si-6Ta-2.5W-1Hf (at.%) silicide based alloys with Ge and Sn additions. Materials 2020, 13, 1778. [Google Scholar] [CrossRef]
- Schneibel, J.H. Beyond Nickel-Base Superalloys. In Processing and Fabrication of Advanced Materials XIII; Stallion Press: Hawthorne, CA, USA, 2005; Volume 2. [Google Scholar]
- Bewlay, B.P.; Jackson, M.R.; Zhao, J.-C.; Subramanian, P.R.; Mendiratta, M.G.; Lewandowski, J.J. Ultrahigh temperature Nb-silicide based composites. MRS Bull. 2003, 28, 646–653. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Sevast’yanov, D.V.; Simonenko, N.P. Promising ultra-high-temperature ceramic materials for aerospace applications. Russ. J. Inorg. Chem. 2013, 58, 1669–1693. [Google Scholar] [CrossRef]
- Tang, S.; Hu, C. Design, preparation and properties of carbon fiber reinforced ultra-high temperature ceramic composites for aerospace applications: A review. J. Mater. Sci. Technol. 2017, 33, 117–130. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Nb silicide based alloys; Part II. J. Alloy. Compd. 2018, 748, 569–576. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Whiting, P.W.; Davis, A.W.; Briant, C.L. Creep Mechanisms in Niobium-Silicide Based in-Situ Composites. Mat. Res. Soc. Symp. Proc. 1999, 552, KK6.11.1–KK6.11.5. [Google Scholar] [CrossRef]
- Prokoshkin, D.A.; Vasileva, E.V. Alloys of Niobium; Samarin, A.M., Ed.; Israel Program for Scientific Translations: Jerusalem, Israel, 1965. [Google Scholar]
- Kim, J.-H.; Tabaru, T.; Sakamoto, M.; Hanada, S. Mechanical properties and fracture behaviour of an NbSS/Nb5Si3 in-situ composite modified by Mo and Hf alloying. Mater. Sci. Eng. 2004, A372, 137–144. [Google Scholar] [CrossRef]
- Guo, H.; Guo, X. Microstructure evolution and room temperature fracture toughness of an integrally directionally solidified Nb-Ti-Si based ultrahigh temperature alloys. Scripta Mater. 2011, 64, 637–640. [Google Scholar] [CrossRef]
- Xu, Z.; Utton, C.; Tsakiropoulos, P. A study of the effect of 2 at.% Sn on the microstructure and isothermal oxidation at 80 and 1200 °C of Nb-24Ti-18Si based alloys with Al and/or Cr additions. Materials 2018, 11, 1826. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P.; Shao, G. A thermo-gravimetric and microstructural study of the oxidation of Nbss/Nb5Si3 based in situ composites with Sn addition. Intermetallics 2007, 15, 270–281. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the Nb silicide based alloys: Part I-The bcc Nb solid solution. J. Alloy. Compd. 2017, 708, 961–971. [Google Scholar] [CrossRef]
- Grammenos, I.; Tsakiropoulos, P. Study of the role of Hf, Mo and W additions in the microstructure of Nb-20Si silicide based alloy. Intermetallics 2011, 19, 1612–1621. [Google Scholar] [CrossRef]
- McCaughey, C.; Tsakiropoulos, P. Type of primary Nb5Si3 and precipitation of Nbss in αNb5Si3 in a Nb-8.3Ti-21.1Si-5.4Mo-4W-0.7Hf (at.%) near eutectic Nb-silicide based alloy. Materials 2018, 11, 967. [Google Scholar] [CrossRef]
- MacKay, R.A.; Gabb, T.P.; Smialek, J.L.; Nathal, M.V. Alloy Design Challenge: Development of Low Density Superalloys for Turbine Blade Applications; NASA/TM-2009-215819; Technical Report; NASA Glenn Research Center: Cleveland, OH, USA, 2009. [Google Scholar]
- Begley, R.T.; Bechtold, J.H. Effect of alloying on the mechanical properties of Niobium. J. Less Common Met. 1961, 3, 1–12. [Google Scholar] [CrossRef]
- Menon, E.S.K.; Mendiratta, M.G.; Dimiduk, D.M. High temperature oxidation mechanisms in Nb-silicide bearing multicomponent alloys. In Structural Intermetallics 2001; Hemker, K.J., Dimiduk, D.M.H., Darolia, C.R., Inui, H.D., Larsen, J.M., Sikka, V.K., Thomas, M., Whittenberger, J.D., Eds.; TMS: Warrendale, PA, USA, 2001; pp. 591–600. [Google Scholar]
- Tsakiropoulos, P. On the alloying and properties of tetragonal Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Fujikara, M.; Kasama, A.; Tanaka, R.; Hanada, S. Effect of alloy chemistry on the high temperature strengths and room temperature fracture toughness of advanced Nb-based alloys. Mater. Trans. 2004, 45, 493–501. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the macrosegregation of silicon in niobium silicide based alloys. Intermetallics 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Li, Z.; Tsakiropoulos, P. On the microstructure and hardness of the Nb-24Ti-18Si-5Al-5Cr-5Ge and Nb-24Ti-18Si-5Al-5Cr-5Ge-5Hf (at.%) silicide based alloys. Materials 2019, 12, 2655. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Ta and Cr additions in the microstructure of Nb-Ti-Si-Al in situ composites. Intermetallics 2006, 14, 639–659. [Google Scholar] [CrossRef]
- Li, Z.; Tsakiropoulos, P. The microstructures of Nb-18Si-5Al-5Ge and Nb-24Ti-18Si-5Al-5Ge in situ composites. J. Alloy. Compd. 2013, 550, 553–560. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Cr and Al additions in the microstructure of Nb-Ti-Si in situ composites. Intermetallics 2005, 13, 1079–1095. [Google Scholar] [CrossRef]
- Okamoto, H. Phase Diagrams for Binary Alloys: Desk Handbook; ASM International: Metals Park, OH, USA, 2000. [Google Scholar]
- Grammenos, I. Ultra-High Temperature Nb-Silicide Based Alloys. Ph.D. Thesis, University of Surrey, Guildford, UK, 2008. [Google Scholar]
- Begley, R.T. Columbium alloy development at Westinghouse. In Evolution of Refractory Metals and Alloys; E Dalder, N.C., Grobstein, T., Chen, C.S., Eds.; TMS: Warrendale, PA, USA, 1994; pp. 29–48. [Google Scholar]
- Schlesinger, M.E.; Okamoto, H.; Gokhale, A.B.; Abbaschian, R. The Nb-Si (Niobium-Silicon) System. J. Phase Equilibria 1993, 14, 502–509. [Google Scholar] [CrossRef]
- Toffolon, C.; Servant, C.; Gachon, J.C.; Sundman, B. Reassessment of the Nb-Sn System. J. Phase Equilibria 2002, 23, 134–139. [Google Scholar] [CrossRef]
- Massalski, T.B.; Subramanian, P.R.; Okamoto, H.; Kacprzak, L. (Eds.) Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990; Volume 3. [Google Scholar]
- Tsakiropoulos, P. Alloying and properties of C14-NbCr2 and A15-Nb3X (X = Al,Ge,Si,Sn) in Nb-silicide based alloys. Materials 2018, 11, 11395. [Google Scholar] [CrossRef]
- Sarath, E.; Menon, K. Phase transformations and oxidation resistance of Nb-Ti-Si based alloys. In Niobium for High Temperature Applications; Kim, Y.-W., Corneiro, T., Eds.; TMS: Warrendale, PA, USA, 2004; pp. 63–74. [Google Scholar]
- Nelson, J.; Ghadyani, M.; Utton, C.; Tsakiropoulos, P. A study of the effects of Al, Cr, Hf and Ti additions on the microstructure and oxidation of Nb-24Ti-18Si silicide based alloys. Materials 2018, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.; Wood, D. Pest degradation in beryllides, silicides, aluminides, and related compounds. J. Nucl. Mater. 1964, 12, 208–215. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Effect of Al, Cr and Ta additions on the oxidation behaviour of Nb–Ti–Si in situ composites at 800 °C. Mater. Sci. Eng. A 2006, 416, 269–280. [Google Scholar] [CrossRef]
- Vellios, N. Design of Nb-Silicide Based Alloys for Aero-Engines. Ph.D. Thesis, University of Surrey, Guildford, UK, 2007. [Google Scholar]
- Bewlay, B.P.; Cretegny, L.; Jackson, M.R.; Subramanian, P.R. Niobium Silicide Based Composites and Related Articles. U.S. Patent 2006/0147335 A1, 6 July 2006. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. Study of the effect of Cr and Ti additions in the microstructure of Nb-18Si-5Ge based in situ composites. Intermetallics 2012, 26, 18–25. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Lipsitt, H.A.; Reeder, W.J.; Jackson, M.R.; Sutliff, J.A. Toughening mechanisms in directionally solidified Nb-Nb3Si-Nb3Si5 in-situ composites. In Processing and Fabrication of Advanced Materials III; Ravi, V.A., Srivatsan, T.S., Moore, J.J., Eds.; The Minerals Metals and Materials Society: Pittsburgh, PA, USA, 1994; pp. 547–565. [Google Scholar]
- Sun, Z.; Guo, X.; Zhang, C. Thermodynamic modeling of the Nb-rich corner in the Nb–Si–Sn system. CALPHAD Comput. Coupling Phase Diagr. Thermochem. 2012, 36, 82–88. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. Ab initio study of ternary W5Si3 type TM5Sn2X compounds (TM = Nb, Ti, X = Al, Si). Materials 2019, 12, 3217. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Zherebtsov, S.; Salischev, G.; Stepanov, N. Oxidation behaviour of refractory AlNbTiVZr0.25 high entropy alloys. Materials 2018, 11, 2526. [Google Scholar] [CrossRef]





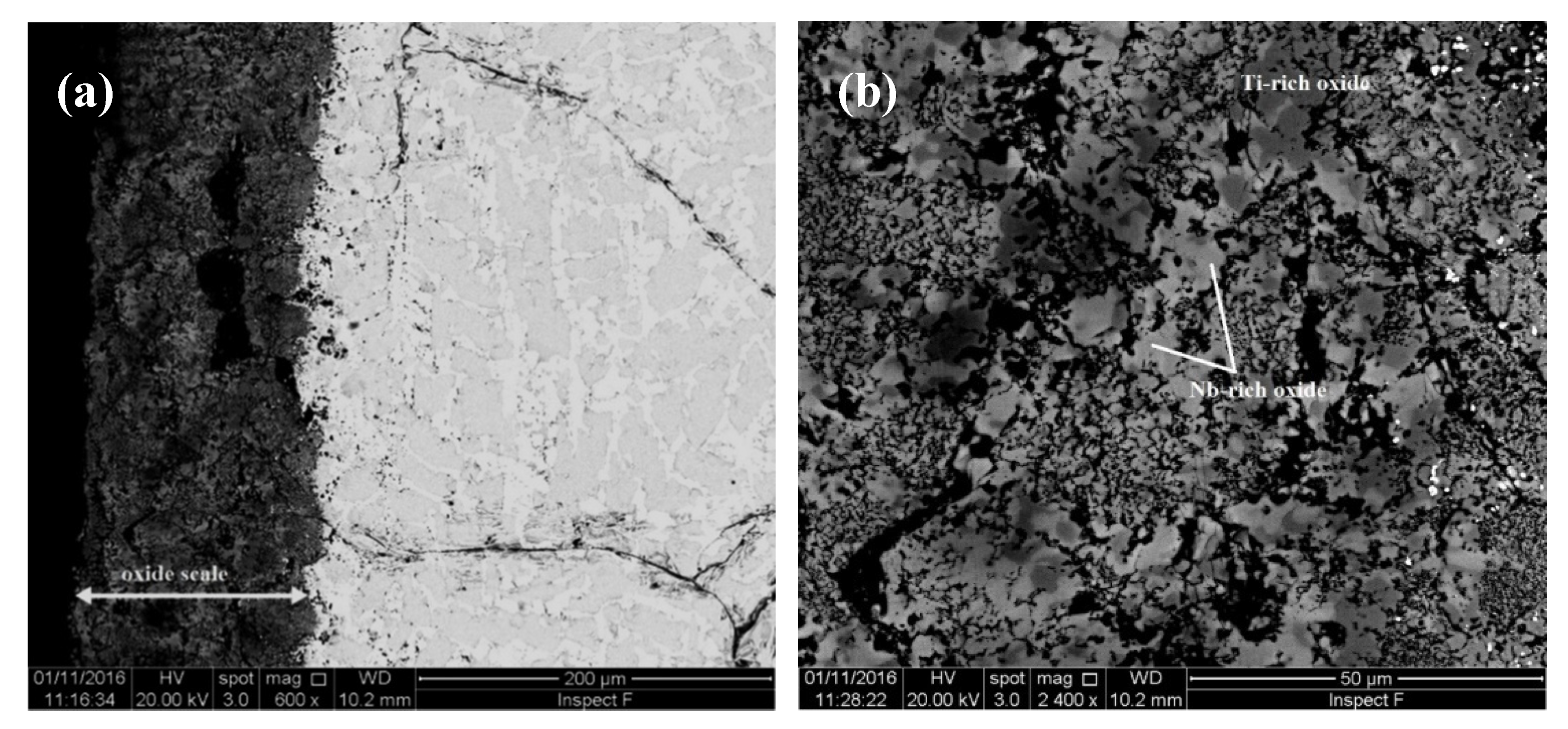
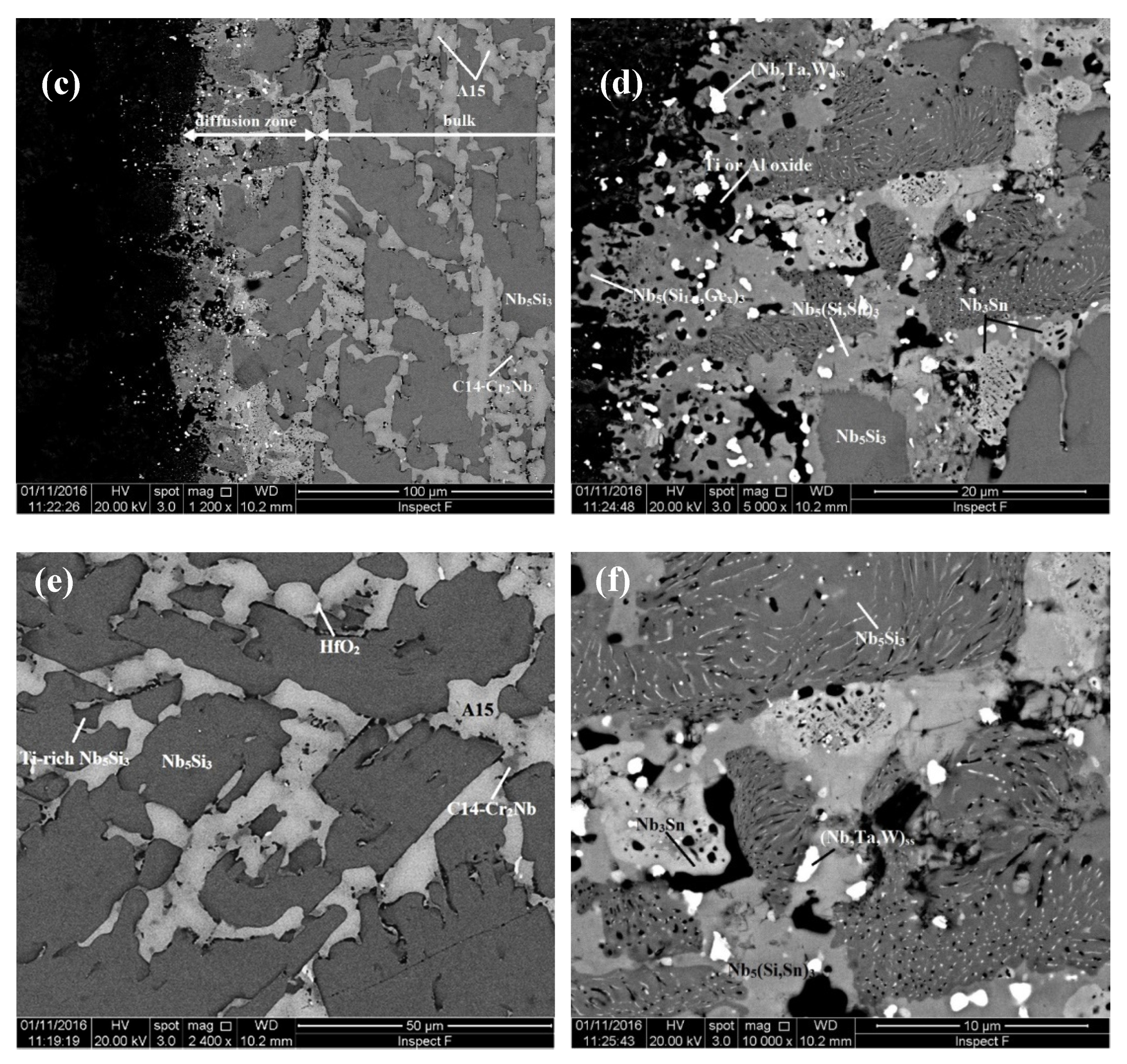
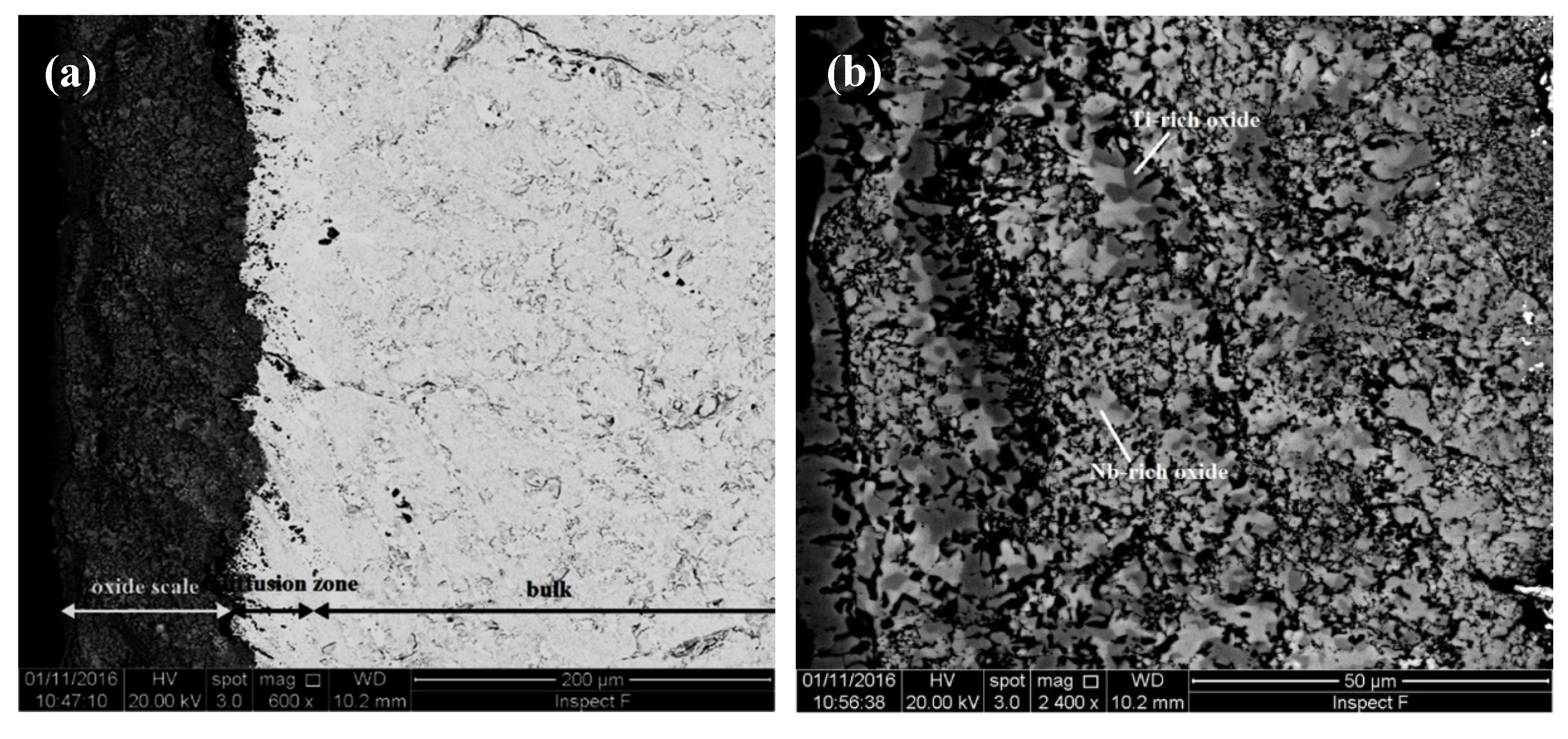
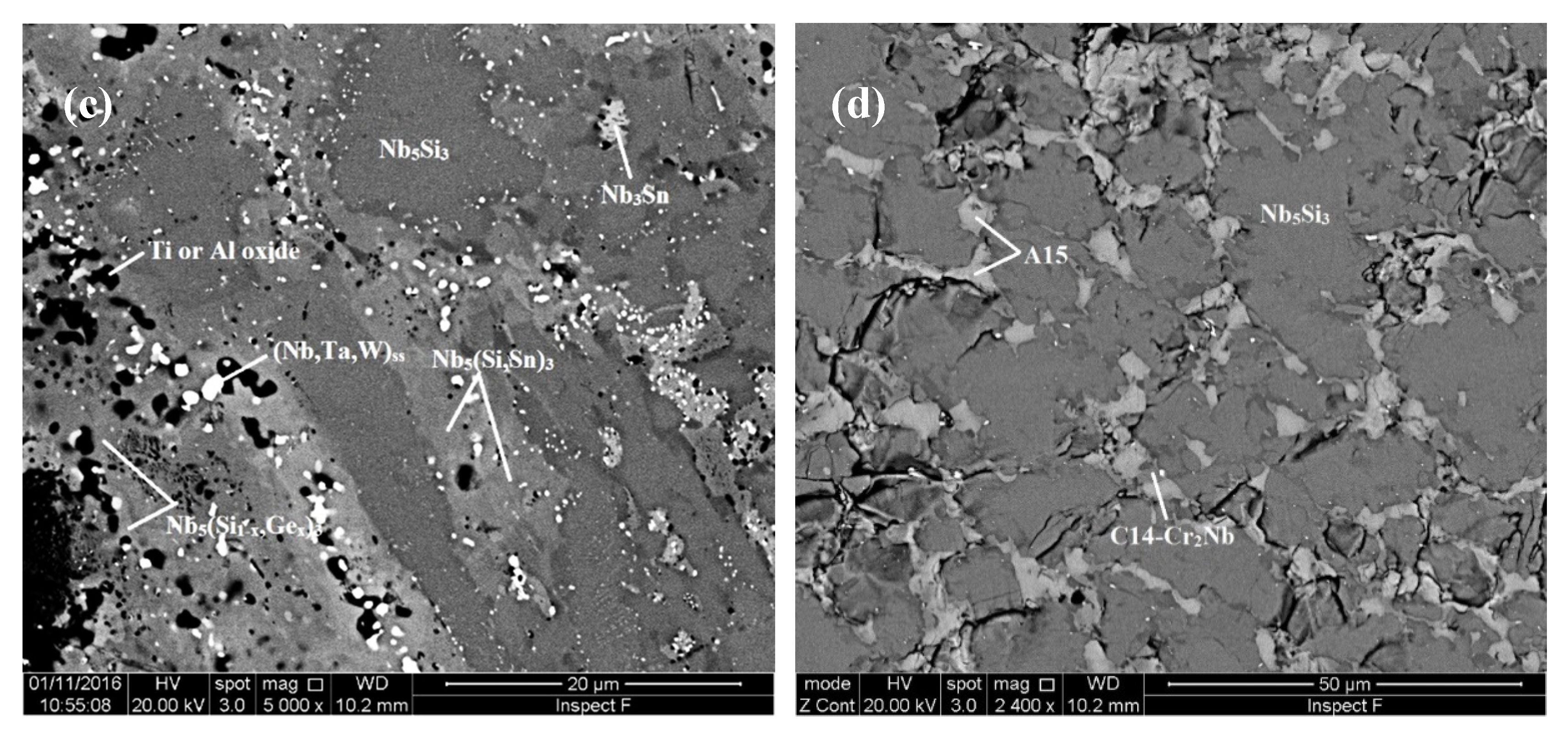
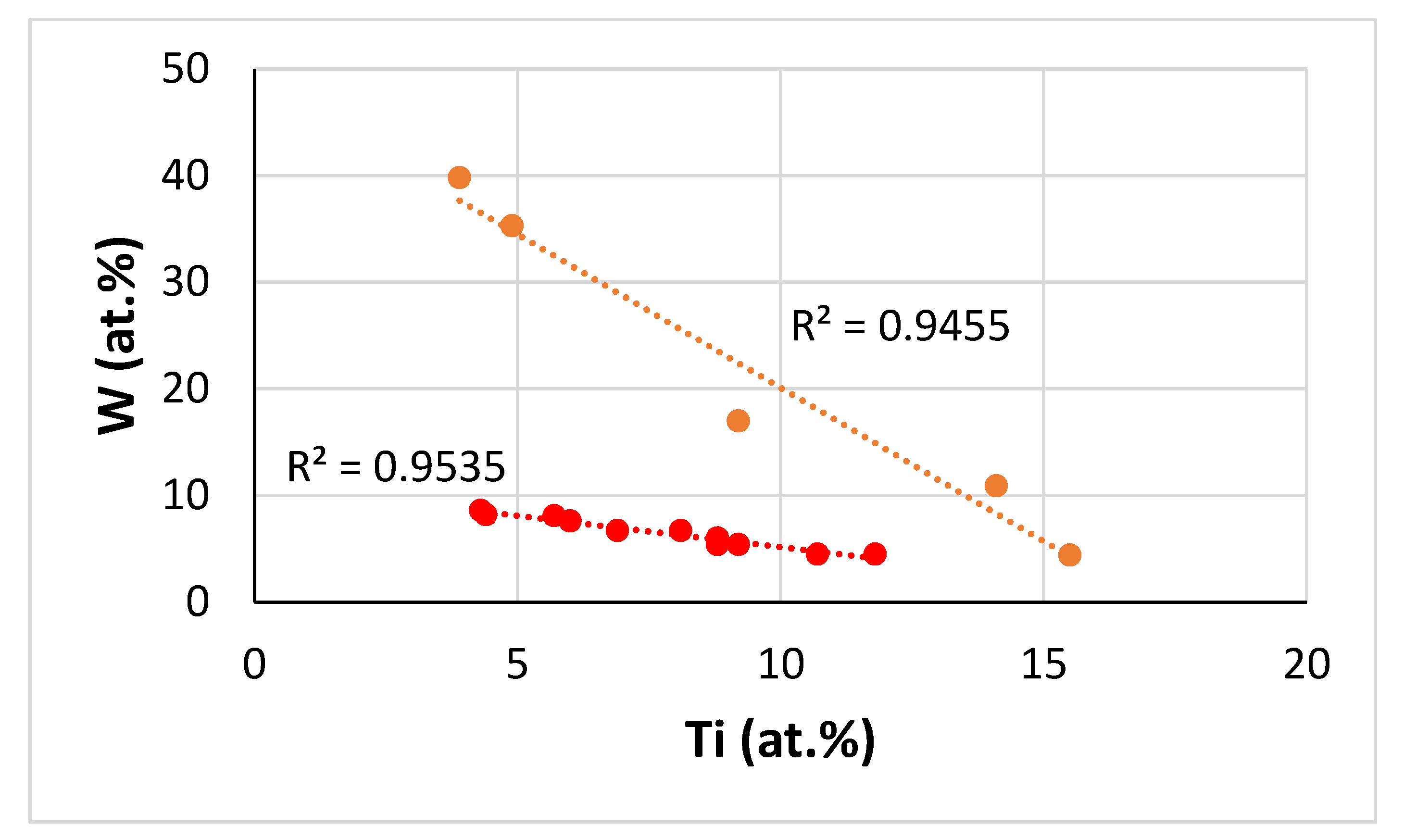
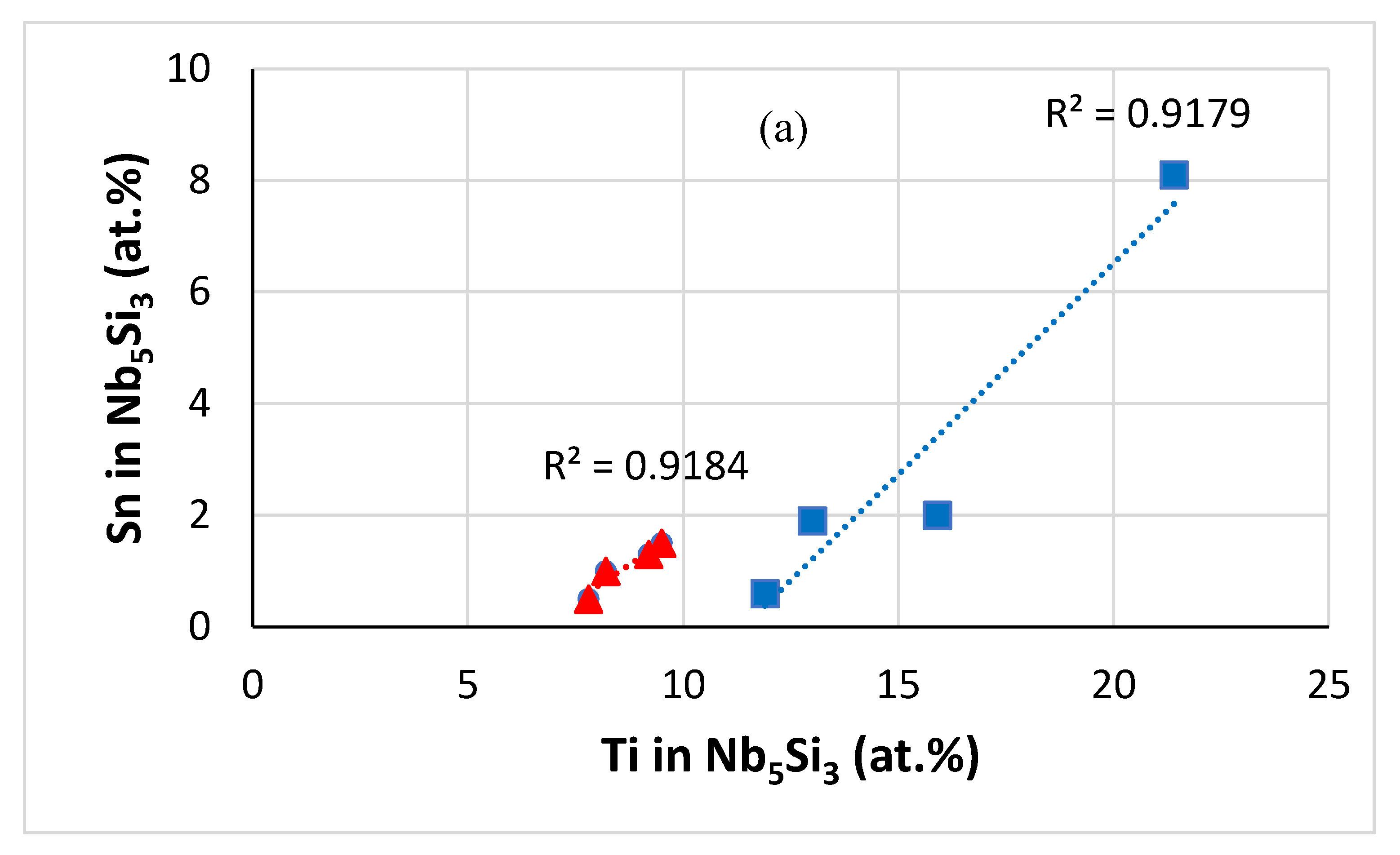
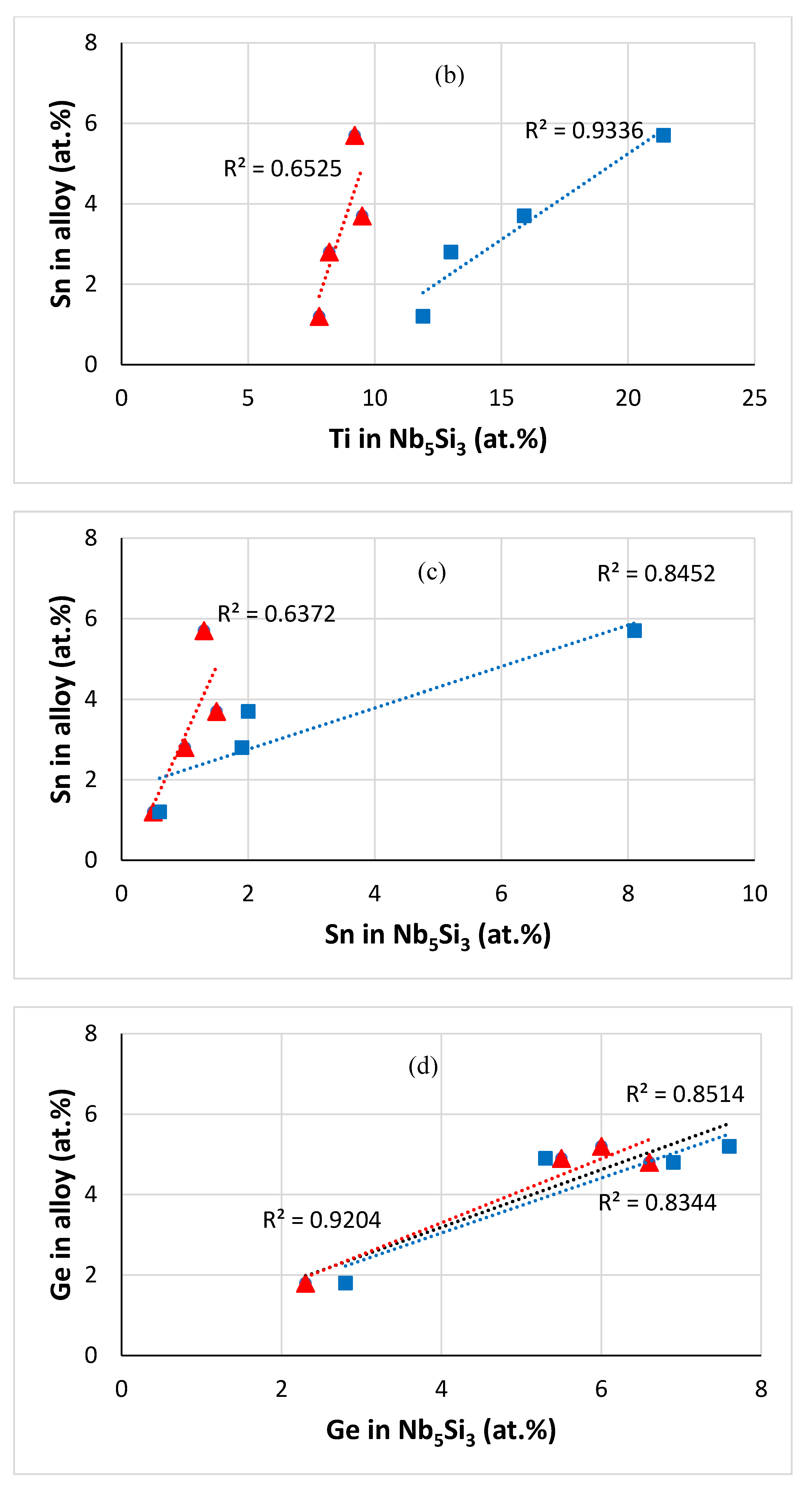
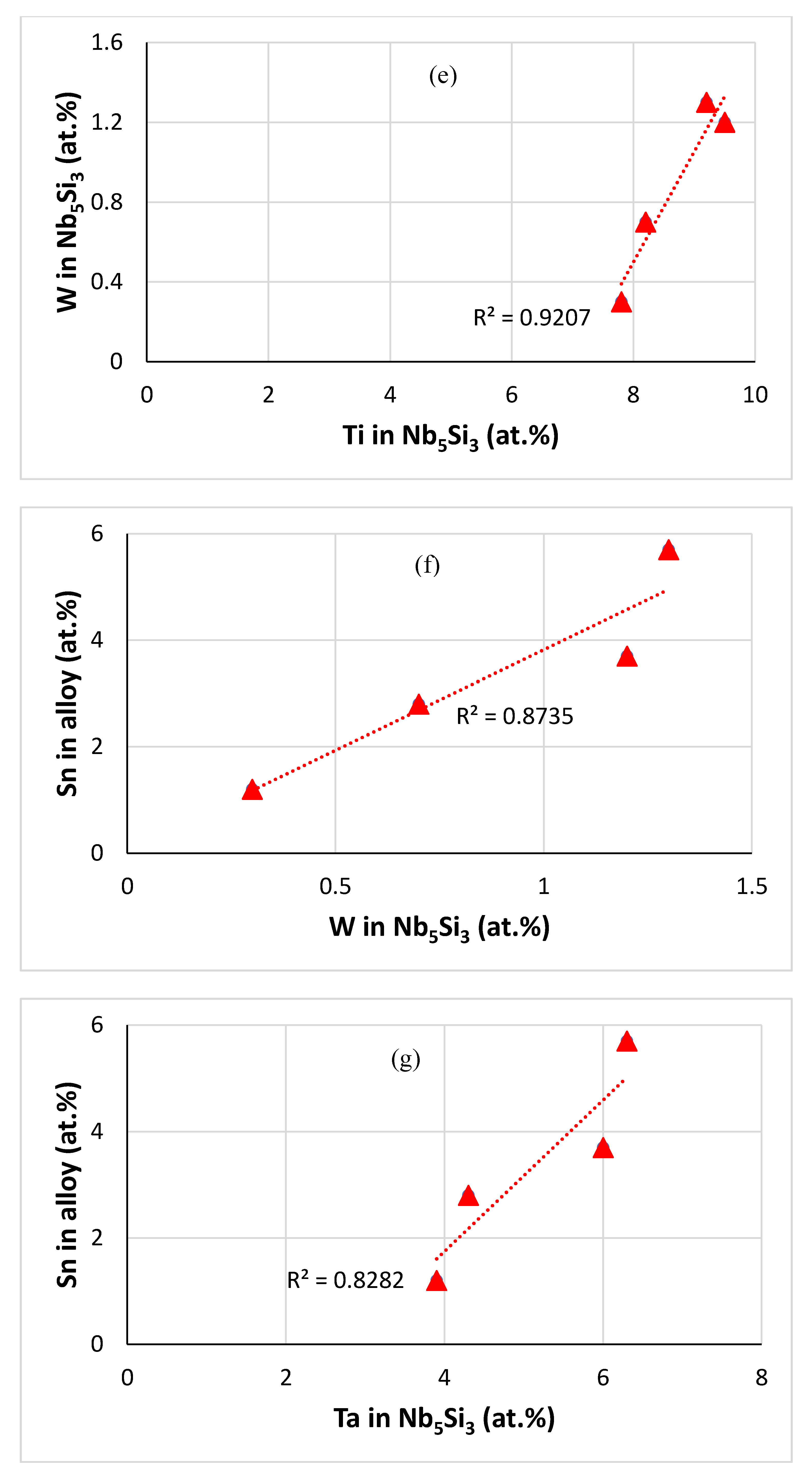
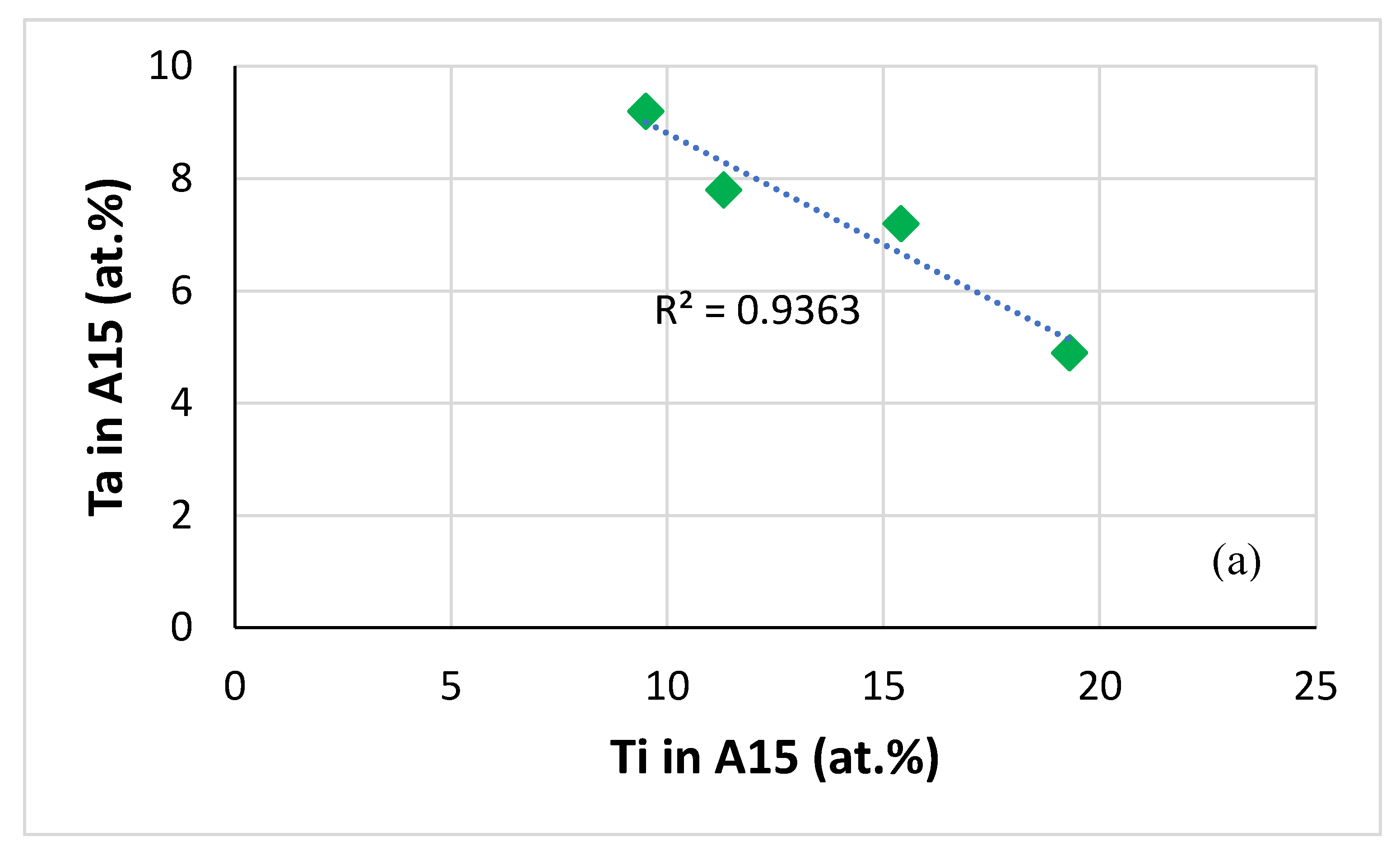

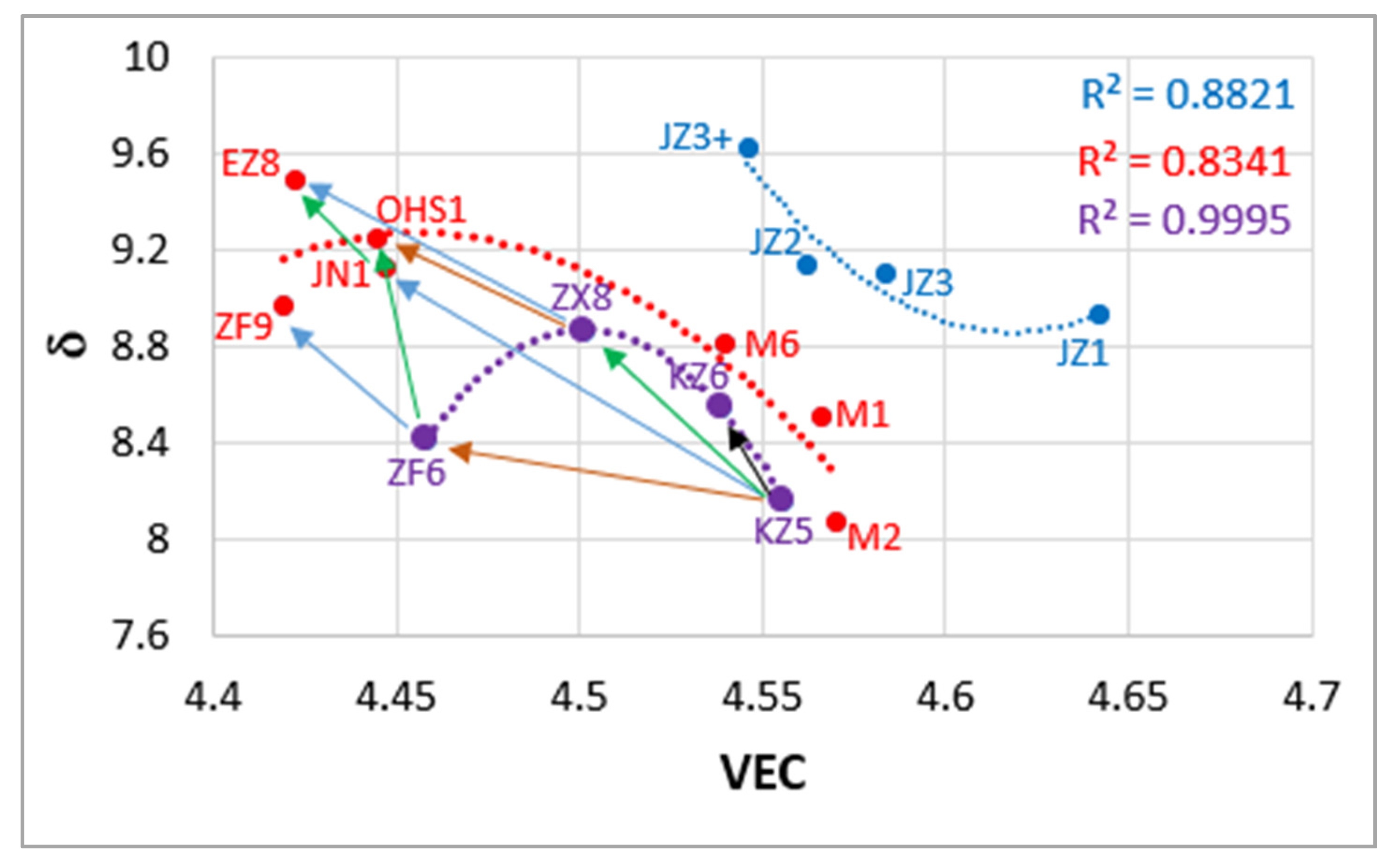
| Alloy | Density (g/cm3) | Nbss (%) | A15-Nb3X (%) |
|---|---|---|---|
| JZ3-AC | 7.94 ± 0.01 7.93 − 7.96 | - | 26.8 ± 2.3 24.2 − 28.8 |
| JZ3-HT a | - | 4.7 ± 0.7 4.1 − 5.5 | 31.2 ± 3.4 27.5 − 34.2 |
| [JZ3+]-AC | 7.54 ± 0.01 7.53 − 7.55 | - | 13.3 ± 0.3 12.9 − 13.5 |
| [JZ3+]-HT a | - | 1.2 ± 0.2 1.1 − 1.4 | 13.9 ± 0.9 12.9 − 14.6 |
| Alloy | |
|---|---|
| JZ3 | JZ3+ |
| As Cast | |
| Nb-12.4Ti-17.7Si-6Ta-2.7W-3.7Sn-4.8Ge-1Hf-4.7Al-5.2Cr | Nb-12.4Ti-19.7Si-5.7Ta-2.3W-5.7Sn-4.9Ge-0.8Hf-4.6Al-5.2Cr |
| Nbss | (Nb,W)ss |
| Nb5Si3, Ti-rich Nb5Si3 | Nb5Si3, Ti-rich Nb5Si3 |
| A15-Nb3X, Ti-rich A15, Cr-rich A15 | A15-Nb3X, Ti-rich A15 |
| C14-NbCr2 Laves | C14-NbCr2 Laves |
| HfO2 | HfO2 |
| Heat Treated | |
| Nb-12.8Ti-18.3Si-5.7Ta-2.5W-3.4Sn-5.2Ge-0.8Hf-4.8Al-4.8Cr | Nb-12.3Ti-20.7Si-5.7Ta-2W-4.8Sn-5.1Ge-0.8Hf-4.6Al-4.7Cr |
| Nbss | (Nb,W)ss |
| Nb5Si3, Ti-rich Nb5Si3 | Nb5Si3, Ti-rich Nb5Si3 |
| A15-Nb3X | A15-Nb3X |
| C14-NbCr2 Laves | C14-NbCr2 Laves |
| HfO2 | HfO2 |
| Alloy | 800 °C | 1200 °C | |||
|---|---|---|---|---|---|
| Weight Gain (mg/cm2) | Rate Constant | Weight Gain (mg/cm2) | Rate Constant | ||
| kl (g cm−2 s−1) | kl (g cm−2 s−1) | kp (g2 cm−4 s−1) | |||
| JZ3 | 14.9 (100 h) | 4.4 × 10−8 (0–100 h) | 24.2 (100 h) | 6 × 10−8 (9–100 h) | 5.9 × 10−10 (0–9 h) |
| JZ3+ | 13.9 (74 h) | 6.5 × 10−9 (0–40 h) 1.2 × 10−7 (40–74 h) | 14 (100 h) | 5.5 × 10−10 (0–100 h) 4.7 × 10−11 (0–14 h) 5.8 × 10−10 (6–100 h) | |
| Alloy | ΔHm (kJ/mol) | Tm (K) | ΔHm/Tm(J/molK) | ΔHmsd/ΔHmsp | Tmsd (K) | Tmsp (K) | Tmsd/Tmsp | [ΔHm/Tm] × [ΔHmsd/ΔHmsp]−1 | MACSi (at.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OHS1 | 27.7 | 2090 | 13.25 |  | 1.37 |  | 1653 |  | 437 |  | 3.8 |  | 9.67 |  | 6.8 |  |
| JZ3 | 29.1 | 2242 | 12.98 | 1.6 | 1823 | 419 | 4.35 | 8.11 | 4 | |||||||
| KZ6 | 28.6 | 2257 | 12.67 | 1.86 | 1894 | 363 | 5.23 | 6.81 | 2.5 | |||||||
| KZ5 | 27.5 | 2239 | 12.28 | 2.05 | 1909 | 330 | 5.78 | 5.99 | 1.3 | |||||||
| Alloy | ΔHm (kJ/mol) | Tm (K) | ΔHm/Tm (J/molK) | ΔHmsd/ΔHmsp | Tmsd (K) | Tmsp (K) | Tmsd/Tmsp | [ΔHm/Tm] × [ΔHmsd/ΔHmsp]−1 | MACSi (at.%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JZ3 | 29.1 | 2242 | 12.98 | 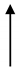 | 1.6 | 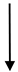 | 1823 | 419 |  | 4.35 | 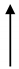 | 8.11 | 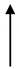 | 4 |  |
| ZF5 (Al) | 28 | 2202 | 12.72 | 1.72 | 1820 | 382 | 4.76 | 7.39 | 2.9 | ||||||
| KZ7 (Al) | 27.7 | 2272 | 12.19 | 2.15 | 1948 | 324 | 5.78 | 6 | 2.3 | ||||||
| Alloy | ΔHm (kJ/mol) | Tm (K) | ΔHm/Tm (J/molK) | ΔHmsd/ΔHmsp | Tmsd (K) | Tmsp (K) | Tmsd/Tmsp | [ΔHm/Tm] × [ΔHmsd/ΔHmsp]−1 | MACSi (at.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OHS1 | 27.7 | 2090 |  | 13.25 |  | 1.37 |  | 1653 |  | 437 | 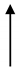 | 3.8 | 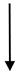 | 9.67 | 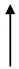 | 6.8 | 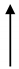 |
| JZ3 | 29.1 | 2242 | 13 | 1.6 | 1823 | 419 | 4.35 | 8.11 | 4 | ||||||||
| KZ4 (Cr) | 28.2 | 2335 | 12.1 | 2.44 | 2060 | 275 | 7.5 | 4.96 | 1.9 | ||||||||
| Phase | Alloy | |||
|---|---|---|---|---|
| OHS1 | JZ2 | JZ3 | JZ3+ | |
| Nbss | √ | - | - | - |
| (Nb,W)ss | - | √ W/Ta = 0.7 | √ W/Ta = 3.15 | √ W/Ta = 3.64 |
| A15 | √ | √ <Si> = 18.2 | √ <Si> = 25.8 | √ <Si> * |
| (Ti,Nb)6Sn5 | √ | - | - | - |
| Nb5Sn2Si | √ Nb/Ti = 1.95 <Si> = 37.6 | - | - | - |
| NbGe2 | - | √ | - | - |
| Nb5(Si,Ge)3 | √ <Si> = 37.9 | √ RM/(Ti+Hf) = 38 <Si> = 37.7 | √ RM/(Ti+Hf) = 30 <Si> = 37.8 | √ RM/(Ti+Hf) = 33 <Si> = 38.5 |
| W-rich Nb5(Si,Ge)3 | - | √ RM/(Ti+Hf) = 51 <Si> = 35.2 | - | - |
| Nb5Si3 | √ | √ RM/(Ti+Hf) = 6.2 <Si> = 37.9 | √ RM/(Ti+Hf) = 4.8 <Si> = 37.3 | √ RM/(Ti+Hf) = 2.9 <Si> = 39.2 |
| Ti rich Nb5Si3 | - | √ RM/(Ti+Hf) = 3. <Si> = 34.9 | - | - |
| Nb5(Si,Sn)3 | - | - | √ RM/(Ti+Hf) = 117 <Si> = 38.6 | √ RM/(Ti+Hf) = 6.5 <Si> = 38.8 |
| Sn rich layer | - | √ | - | - |
| Ge rich layer | - | √ | - | - |
| Alloy | Nbsscal | Nbssexp | (ΔW/A)cal | (ΔW/A)exp | (ΔW/A)cal | (ΔW/A)exp | MACSical | MACSiexp |
|---|---|---|---|---|---|---|---|---|
| As cast | 800 °C | 1200 °C | As cast | |||||
| JZ1 | 44.1 | 46.5 | 17 | 33.6 | 77 | 91 | 4.8 | 5.6 |
| JZ2 | 34.8 | 34.5 | 12 | 28.9 | 54 | 72 | 5.2 | 4.9 |
| JZ3 | 9.9 | 4.7 | 9.9 | 14.9 | 42 | 24.2 | 4.6 | 4 |
| JZ3+ | 7.4 | 1.2 | 7.4 | 13.9 | 15 | 14 | 3.5 | 3.1 |
| Alloy | VEC | Δχ | δ | Sd/Sp | Nb/(Ti+Hf) |
|---|---|---|---|---|---|
| JZ1 | 4.642 | 0.1745 | 8.93 | 2.92 | 4.41 |
| JZ2 | 4.562 | 0.1859 | 9.14 | 2.39 | 3.95 |
| JZ3 | 4.584 | 0.1942 | 9.1 | 2.24 | 3.11 |
| JZ3+ | 4.546 | 0.1932 | 9.63 | 1.89 | 2.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Utton, C.; Tsakiropoulos, P. On the Microstructure and Properties of Nb-12Ti-18Si-6Ta-5Al-5Cr-2.5W-1Hf (at.%) Silicide-Based Alloys with Ge and Sn Additions. Materials 2020, 13, 3719. https://doi.org/10.3390/ma13173719
Zhao J, Utton C, Tsakiropoulos P. On the Microstructure and Properties of Nb-12Ti-18Si-6Ta-5Al-5Cr-2.5W-1Hf (at.%) Silicide-Based Alloys with Ge and Sn Additions. Materials. 2020; 13(17):3719. https://doi.org/10.3390/ma13173719
Chicago/Turabian StyleZhao, Jiang, Claire Utton, and Panos Tsakiropoulos. 2020. "On the Microstructure and Properties of Nb-12Ti-18Si-6Ta-5Al-5Cr-2.5W-1Hf (at.%) Silicide-Based Alloys with Ge and Sn Additions" Materials 13, no. 17: 3719. https://doi.org/10.3390/ma13173719
APA StyleZhao, J., Utton, C., & Tsakiropoulos, P. (2020). On the Microstructure and Properties of Nb-12Ti-18Si-6Ta-5Al-5Cr-2.5W-1Hf (at.%) Silicide-Based Alloys with Ge and Sn Additions. Materials, 13(17), 3719. https://doi.org/10.3390/ma13173719






