Magnetically Functionalized Moss Biomass as Biosorbent for Efficient Co2+ Ions and Thioflavin T Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Preparation
2.2. Magnetic Biosorbent Preparation
2.3. Adsorption of Co2+ Ions and Thioflavin T
2.4. Magnetic Biosorbent Reusability and Stability
2.5. SEM-EDX, FTIR, and XRD Analyses
2.6. Radiometric Analysis
3. Results and Discussion
3.1. Magnetic Biosorbent Characterization
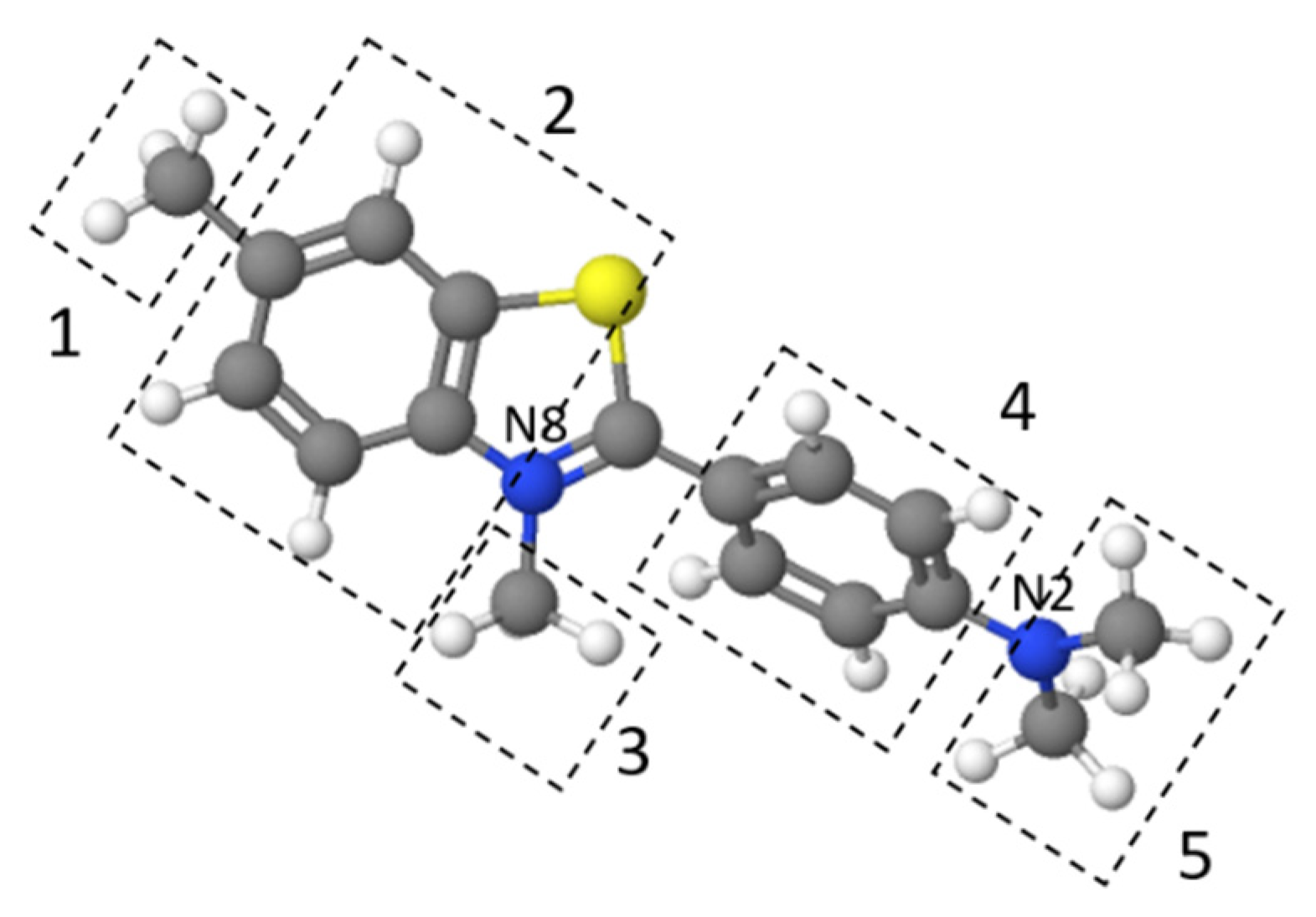
3.2. Thioflavin T and Co2+ Ions Adsorption
3.3. Magnetic Biosorbent Reusability and Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shah, A.I.; Dar, M.U.D.; Bhat, R.A.; Singh, J.; Singh, K.; Bhat, S.A. Prospectives and challenges of wastewater treatment technologies to combat contaminants of emerging concerns. Ecol. Eng. 2020, 152, 105882. [Google Scholar] [CrossRef]
- Mullerova, S.; Baldikova, E.; Prochazkova, J.; Pospiskova, K.; Safarik, I. Magnetically modified macroalgae Cymopolia barbata biomass as an adsorbent for safranin O removal. Mater. Chem. Phys. 2019, 225, 174–180. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Escudero, L.B.; Quintas, P.Y.; Wuilloud, R.G.; Dotto, G.L. Recent advances on elemental biosorption. Environ. Chem. Lett. 2019, 17, 409–427. [Google Scholar] [CrossRef]
- De Freitas, G.R.; Da Silva, M.G.C.; Vieira, M.G.A. Biosorption technology for removal of toxic metals: A review of commercial biosorbents and patents. Environ. Sci. Pollut. Res. 2019, 26, 19097–19118. [Google Scholar] [CrossRef]
- Mahamadi, C. Will nano-biosorbents break the Achilles’ heel of biosorption technology? Environ. Chem. Lett. 2019, 17, 1753–1768. [Google Scholar] [CrossRef]
- Gan, P.P.; Li, S.F.Y. Biosorption of elements. In Elements Recovery and Sustainability; Hunt, A., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 80–113. [Google Scholar]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef]
- Hassan, M.; Naidu, R.; Du, J.; Liu, Y.; Qi, F. Critical review of magnetic biosorbents: Their preparation, application, and regeneration for wastewater treatment. Sci. Total Environ. 2020, 702, 134893. [Google Scholar] [CrossRef]
- Sharma, R.; Sarswat, A.; Pittman, C.U.; Mohan, D. Cadmium and lead remediation using magnetic and non-magnetic sustainable biosorbents derived from Bauhinia purpurea pods. RSC Adv. 2017, 7, 8606–8624. [Google Scholar] [CrossRef]
- Daneshfozoun, S.; Abdullah, M.; Abdullah, B. Preparation and characterization of magnetic biosorbent based on oil palm empty fruit bunch fibers, cellulose and Ceiba pentandra for heavy metal ions removal. Ind. Crop. Prod. 2017, 105, 93–103. [Google Scholar] [CrossRef]
- Trakal, L.; Veselská, V.; Šafařík, I.; Vítková, M.; Číhalová, S.; Komárek, M. Lead and cadmium sorption mechanisms on magnetically modified biochars. Bioresour. Technol. 2016, 203, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Adeogun, A.I.; Akande, J.A.; Idowu, M.A.; Kareem, S.O. Magnetic tuned sorghum husk biosorbent for effective removal of cationic dyes from aqueous solution: Isotherm, kinetics, thermodynamics and optimization studies. Appl. Water Sci. 2019, 9, 160. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Shudayfat, A.M.; Fasfous, I.I. Preparation of magnetic biosorbents based on cypress wood that was pretreated by heating or TiO2 deposition. Ind. Crop. Prod. 2019, 129, 105–113. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Y.; Liu, Y.; Liang, Y.; Zhang, K.; Wang, S.; Liang, Y. Effect of competitive adsorption on zinc removal from aqueous solution and zinc smelting effluent by eucalyptus leaf-based magnetic biosorbent. J. Environ. Sci. Health Part A 2017, 16, 1–17. [Google Scholar] [CrossRef]
- Safarik, I.; Šafaříková, M. One-step magnetic modification of non-magnetic solid materials. Int. J. Mater. Res. 2014, 105, 104–107. [Google Scholar] [CrossRef]
- Maderova, Z.; Baldikova, E.; Pospiskova, K.; Safarik, I.; Safarikova, M. Removal of dyes by adsorption on magnetically modified activated sludge. Int. J. Environ. Sci. Technol. 2016, 13, 1653–1664. [Google Scholar] [CrossRef]
- Winsett, J.; Moilanen, A.; Paudel, K.; Kamali, S.; Ding, K.; Cribb, W.; Seifu, D.; Neupane, S. Quantitative determination of magnetite and maghemite in iron oxide nanoparticles using Mössbauer spectroscopy. SN Appl. Sci. 2019, 1, 1636. [Google Scholar] [CrossRef]
- Baldikova, E.; Mullerova, S.; Prochazkova, J.; Rouskova, M.; Solcova, O.; Safarik, I.; Pospiskova, K. Use of waste Japonochytrium sp. biomass after lipid extraction as an efficient adsorbent for triphenylmethane dye applied in aquaculture. Biomass Convers. Biorefinery 2019, 9, 479–488. [Google Scholar] [CrossRef]
- Su, S.; Liu, Q.; Liu, J.; Zhang, H.; Li, R.; Jing, X.; Wang, J. Functionalized Sugarcane Bagasse for U(VI) Adsorption from Acid and Alkaline Conditions. Sci. Rep. 2018, 8, 793. [Google Scholar] [CrossRef]
- Angelova, R.; Baldikova, E.; Pospiskova, K.; Maderova, Z.; Safarikova, M.; Šafarík, I. Magnetically modified Sargassum horneri biomass as an adsorbent for organic dye removal. J. Clean. Prod. 2016, 137, 189–194. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Z.; Shang, Z.; Zhao, J.-C. Classification and identification of Rhodobryum roseum Limpr. and its adulterants based on fourier-transform infrared spectroscopy (FTIR) and chemometrics. PLoS ONE 2017, 12, e0172359. [Google Scholar] [CrossRef] [PubMed]
- Remenárová, L.; Pipíška, M.; Horník, M.; Augustín, J. Sorption of cationic dyes from aqueous solutions by moss Rhytidiadelphus squarrosus: Kinetics and equilibrium studies. Nova Biotechnol. 2009, 9, 53–61. [Google Scholar]
- Marešová, J.; Pipíška, M.; Rozložník, M.; Horník, M.; Remenárová, L.; Augustín, J. Cobalt and strontium sorption by moss biosorbent: Modeling of single and binary metal systems. Desalination 2011, 266, 134–141. [Google Scholar] [CrossRef]
- Lei, T.; Li, S.-J.; Jiang, F.; Ren, Z.-X.; Wang, L.-L.; Yang, X.-J.; Tang, L.-H.; Wang, S. Adsorption of Cadmium Ions from an Aqueous Solution on a Highly Stable Dopamine-Modified Magnetic Nano-Adsorbent. Nanoscale Res. Lett. 2019, 14, 352. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, L. A general rate law equation for biosorption. Biochem. Eng. J. 2008, 38, 390–394. [Google Scholar] [CrossRef]
- Fraga, T.J.M.; da Silva, L.F.F.; Ferreira, L.E.M.d.L.; Da Silva, M.P.; Fraga, D.M.d.S.M.; de Araújo, C.M.B.; Carvalho, M.N.; Cavalcanti, J.V.F.d.L.; Ghislandi, M.G.; Sobrinho, M.A.d.M. Amino-Fe3O4-functionalized multi-layered graphene oxide as an ecofriendly and highly effective nanoscavenger of the reactive drimaren red. Environ. Sci. Pollut. Res. 2020, 27, 9718–9732. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhoub, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef]
- Pipíška, M.; Valica, M.; Partelová, D.; Horník, M.; Lesný, J.; Hostin, S. Removal of Synthetic Dyes by Dried Biomass of Freshwater Moss Vesicularia Dubyana: A Batch Biosorption Study. Environments 2018, 5, 10. [Google Scholar] [CrossRef]
- Partelová, D.; Šuňovská, A.; Marešová, J.; Horník, M.; Pipíška, M.; Hostin, S. Removal Of Contaminants From Aqueous Solutions Using Hop (Humulus lupulus L.) Agricultural By-Products. Nova Biotechnol. Chim. 2015, 14, 212–227. [Google Scholar] [CrossRef]
- Remenárová, L.; Pipíška, M.; Horník, M.; Augustín, J. Mechanistic evaluation of Co and Zn sorption processes using equilibrium modeling, FTIR and SEM-EDX analysis. Chem. Listy 2010, 104, 722–728. [Google Scholar]
- Shin, W.S. Competitive sorption of anionic and cationic dyes onto cetylpyridinium-modified montmorillonite. J. Environ. Sci. Health Part A 2008, 43, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Maurya, N.; Mittal, A.K. Biosorptive uptake of cationic dyes from aqueous phase using immobilised dead macro fungal biomass. Int. J. Environ. Technol. Manag. 2011, 14, 282–293. [Google Scholar] [CrossRef]
- Lian, G.; Zhang, X.; Zhang, S.; Liu, D.; Cui, D.; Wang, Q. Controlled fabrication of ultrathin-shell BN hollow spheres with excellent performance in hydrogen storage and wastewater treatment. Energy Environ. Sci. 2012, 5, 7072–7080. [Google Scholar] [CrossRef]
- Hashemian, S.; Saffari, H.; Ragabion, S. Adsorption of Cobalt(II) from Aqueous Solutions by Fe3O4/Bentonite Nanocomposite. Water Air Soil Pollut. 2015, 226, 2212. [Google Scholar] [CrossRef]
- Motl, A.; Šebesta, F.; Navratil, J.D.; Hlavicova, J. Sorption of cobalt on magnetite. Czechoslov. J. Phys. 2003, 53, A515–A523. [Google Scholar] [CrossRef]
- Zhong, Q.-Q.; Zhao, Y.-Q.; Shen, L.; Hao, B.; Xu, X.; Gao, B.-Y.; Shang, Y.-N.; Chu, K.-Z.; Zhang, X.-H.; Yue, Q.-Y. Single and binary competitive adsorption of cobalt and nickel onto novel magnetic composites derived from green macroalgae. Environ. Eng. Sci. 2020, 37, 188–200. [Google Scholar] [CrossRef]
- Hymavathi, D.; Prabhakar, G. Studies on the removal of Cobalt(II) from aqueous solutions by adsorption withFicus benghalensisleaf powder through response surface methodology. Chem. Eng. Commun. 2017, 204, 1401–1411. [Google Scholar] [CrossRef]
- Popper, Z.A.; Fry, S.C. Primary Cell Wall Composition of Bryophytes and Charophytes. Ann. Bot. 2003, 91, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution. Water 2020, 12, 446. [Google Scholar] [CrossRef]
- Sulatskaya, A.I.; Maskevich, A.A.; Kuznetsova, I.M.; Uversky, V.N.; Turoverov, K.K. Fluorescence Quantum Yield of Thioflavin T in Rigid Isotropic Solution and Incorporated into the Amyloid Fibrils. PLoS ONE 2010, 5, e15385. [Google Scholar] [CrossRef]
- Songsaeng, S.; Thamyongkit, P.; Poompradub, S. Natural rubber/reduced-graphene oxide composite materials: Morphological and oil adsorption properties for treatment of oil spills. J. Adv. Res. 2019, 20, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Guo, W.; Gao, F.; Wang, Y.; Gao, Y. Lead and uranium sorptive removal from aqueous solution using magnetic and nonmagnetic fast pyrolysis rice husk biochars. RSC Adv. 2018, 8, 13205–13217. [Google Scholar] [CrossRef]
- Philben, M.; Butler, S.; Billings, S.A.; Benner, R.; Edwards, K.A.; Ziegler, S.E. Biochemical and structural controls on the decomposition dynamics of boreal upland forest moss tissues. Biogeosciences 2018, 15, 6731–6746. [Google Scholar] [CrossRef]










| C0 (µmol L−1) | Qe cal (µmol g−1) | kn (min−1) (mg g−1)1−n | n | R (µmol g−1 min−1) | R2 | Qe exp (µmol g−1) | |
|---|---|---|---|---|---|---|---|
| Co2+ | 500 | 87.6 ± 2.5 | 0.0010 ± 0.0002 | 2.41 | 48.0 | 0.995 | 88.5 |
| 1000 | 131.9 ± 4.3 | 0.0013 ± 0.0003 | 2.20 | 60.1 | 0.991 | 135.8 | |
| TT | 313 | 106.7 ± 0.7 | 0.0448 ± 00235 | 1.51 | 51.7 | 0.999 | 105.0 |
| 626 | 235.8 ± 2.3 | 0.0017 ± 0.0005 | 2.08 | 146.3 | 0.998 | 228.3 |
| Sorbent | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qmax [µmol g−1] | b [L µmol−1] | R2 | K [µmol g−1 (L µmol−1)1/n] | 1/n | R2 | ||
| Magnetic biomass | Co2+ | 218 ± 14 | 0.0025 ± 0.0006 | 0.980 | 12.35 ± 5.16 | 0.35 ± 0.06 | 0.956 |
| TT | 483 ± 35 | 0.0072 ± 0.0015 | 0.961 | 39.78 ± 16.46 | 0.34 ± 0.07 | 0.858 | |
| Native biomass | Co2+ | 208 ± 3 | 0.008 ± 0.001 | 0.997 | 32.2 ± 16.5 | 0.24 ± 0.07 | 0.856 |
| TT | 395 ± 10 | 0.06 ± 0.01 | 0.906 | 99.7 ± 5.5 | 0.19 ± 0.01 | 0.983 | |
| Sorbent | Qmax Co (µmol g−1) | QmaxTT (µmol g−1) | pH | T (°C) | Reference |
|---|---|---|---|---|---|
| Vesicularia dubyana moss | - | 373 | 6.0 | 25 | Pipíška et al. [29] |
| Hop leaf biomass | - | 243 | 6.0 | 25 | Partelová et al. [30] |
| Rhytidiadelphus squarrosus | 208 | 395 | 6.0 | 25 | Remenárová et al. [23] Remenárová et al. [31] |
| Modified montmorillonite | - | 298 | 6.0 | 25 | Shin [32] |
| Fomitopsis carnea | - | 68.7 | - | 30 | Maurya and Mittal [33] |
| ultrathin-shell boron nitride hollow spheres | - | 479 | - | - | Lian et al. [34] |
| Magnetic R. squarrosus | 218 | 483 | 6.0 | 22 | Present study |
| Fe3O4/bentonite nanocomposite | 323 | - | 8.0 | 25 | Hashemian et al. [35] |
| Magnetite | 25 | - | 8.0 | - | Motl et al. [36] |
| Green microalgae magnetic composite | 2327 | - | 6.5 | 20 | Zhong et al. [37] |
| Ficus benghalensis | 97 | - | 5.0 | 25 | Hymavathi and Prabhakar [38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipíška, M.; Zarodňanská, S.; Horník, M.; Ďuriška, L.; Holub, M.; Šafařík, I. Magnetically Functionalized Moss Biomass as Biosorbent for Efficient Co2+ Ions and Thioflavin T Removal. Materials 2020, 13, 3619. https://doi.org/10.3390/ma13163619
Pipíška M, Zarodňanská S, Horník M, Ďuriška L, Holub M, Šafařík I. Magnetically Functionalized Moss Biomass as Biosorbent for Efficient Co2+ Ions and Thioflavin T Removal. Materials. 2020; 13(16):3619. https://doi.org/10.3390/ma13163619
Chicago/Turabian StylePipíška, Martin, Simona Zarodňanská, Miroslav Horník, Libor Ďuriška, Marián Holub, and Ivo Šafařík. 2020. "Magnetically Functionalized Moss Biomass as Biosorbent for Efficient Co2+ Ions and Thioflavin T Removal" Materials 13, no. 16: 3619. https://doi.org/10.3390/ma13163619
APA StylePipíška, M., Zarodňanská, S., Horník, M., Ďuriška, L., Holub, M., & Šafařík, I. (2020). Magnetically Functionalized Moss Biomass as Biosorbent for Efficient Co2+ Ions and Thioflavin T Removal. Materials, 13(16), 3619. https://doi.org/10.3390/ma13163619






