Influence of Wooden Sawdust Treatments on Cu(II) and Zn(II) Removal from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Sorbent Preparation
2.2. Synthetic Solutions
2.3. Sorption Experiments
2.4. Adsorption Models
2.4.1. Langmuir Model
2.4.2. Freundlich Model
3. Results and Discussion
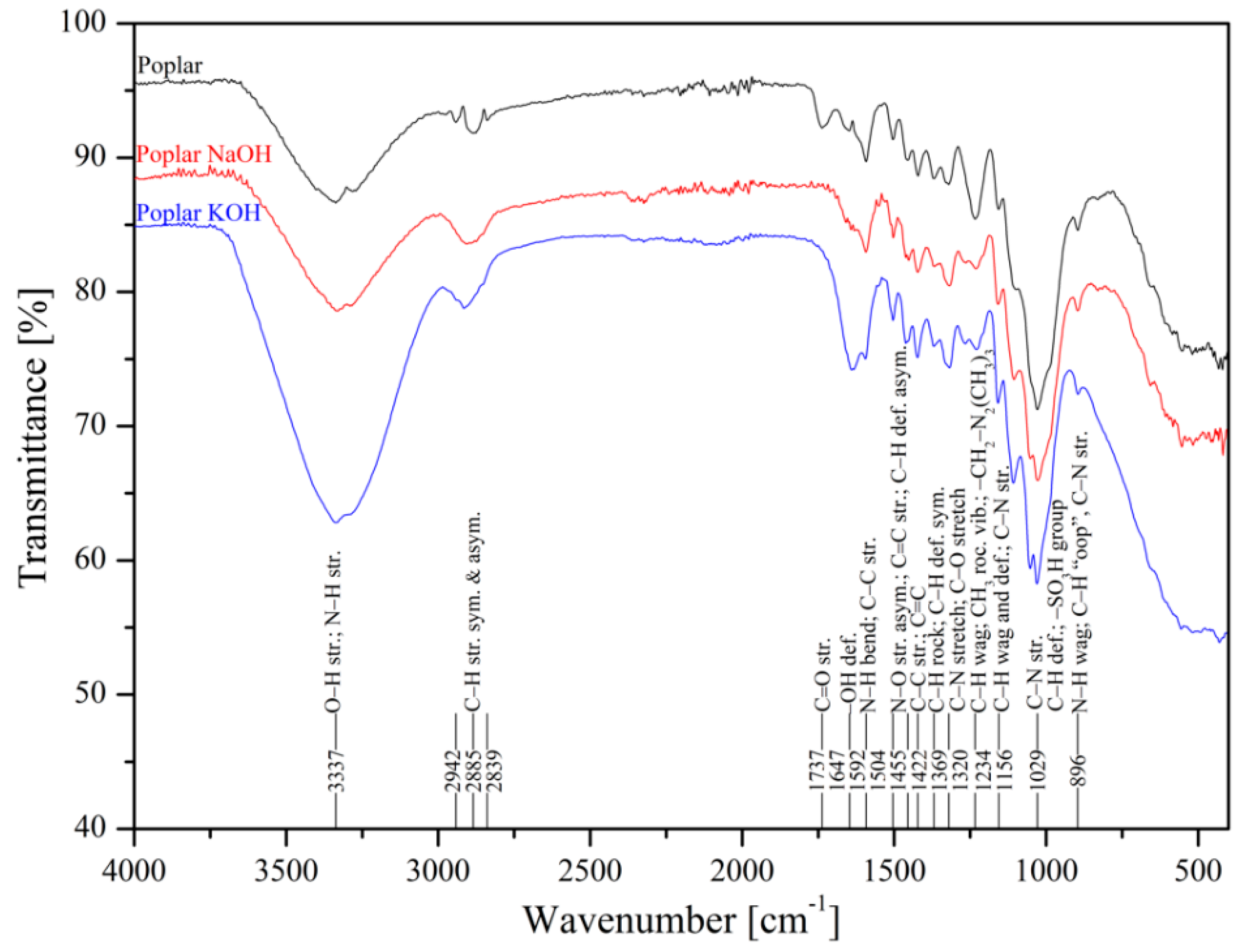
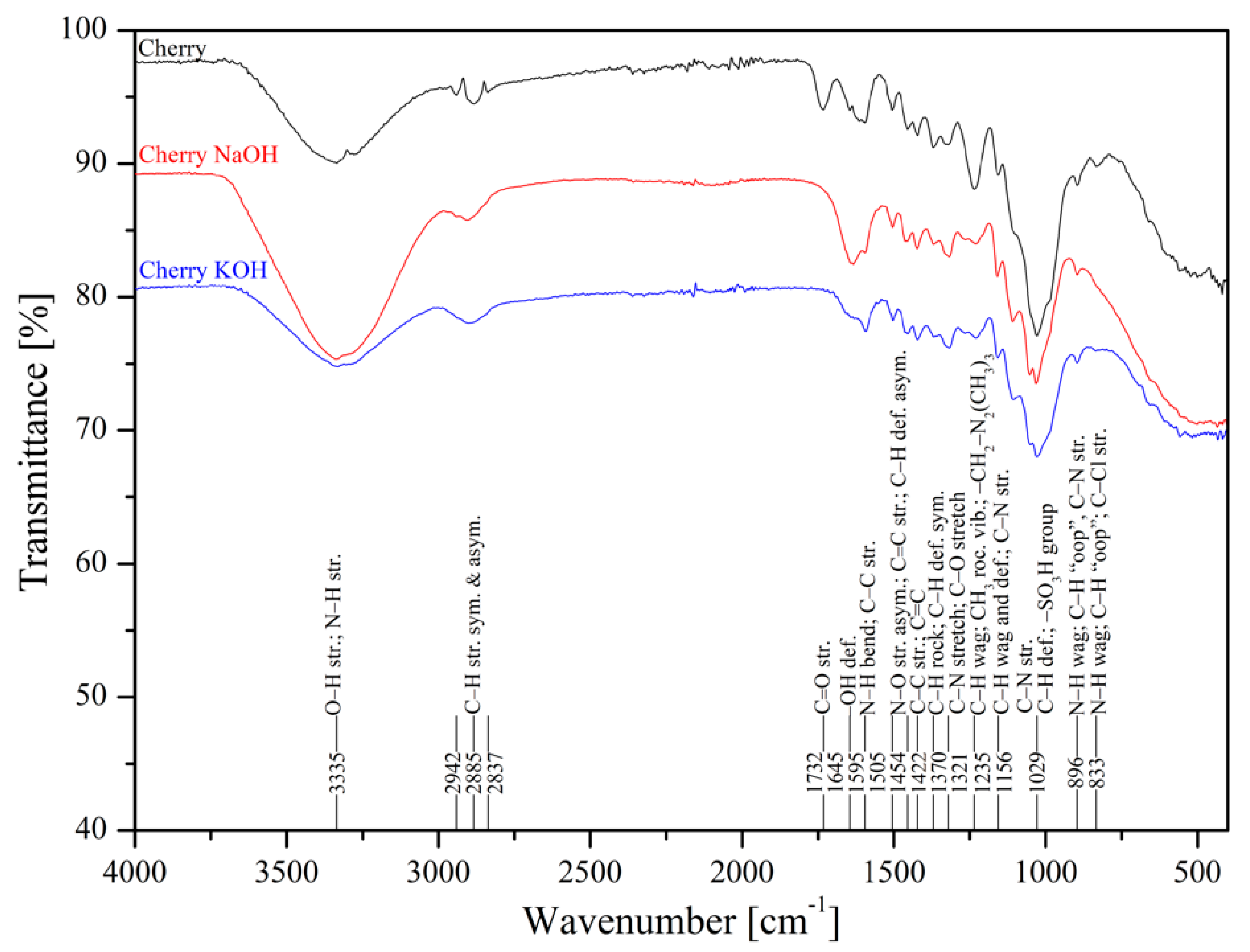
3.1. FTIR Spectra
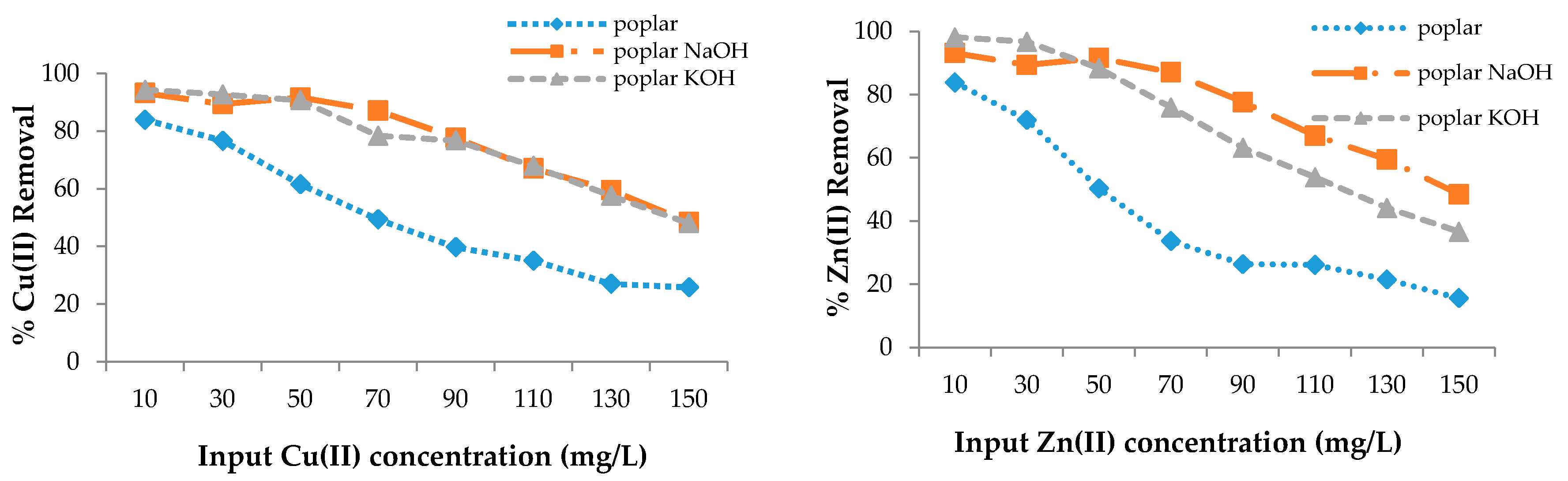
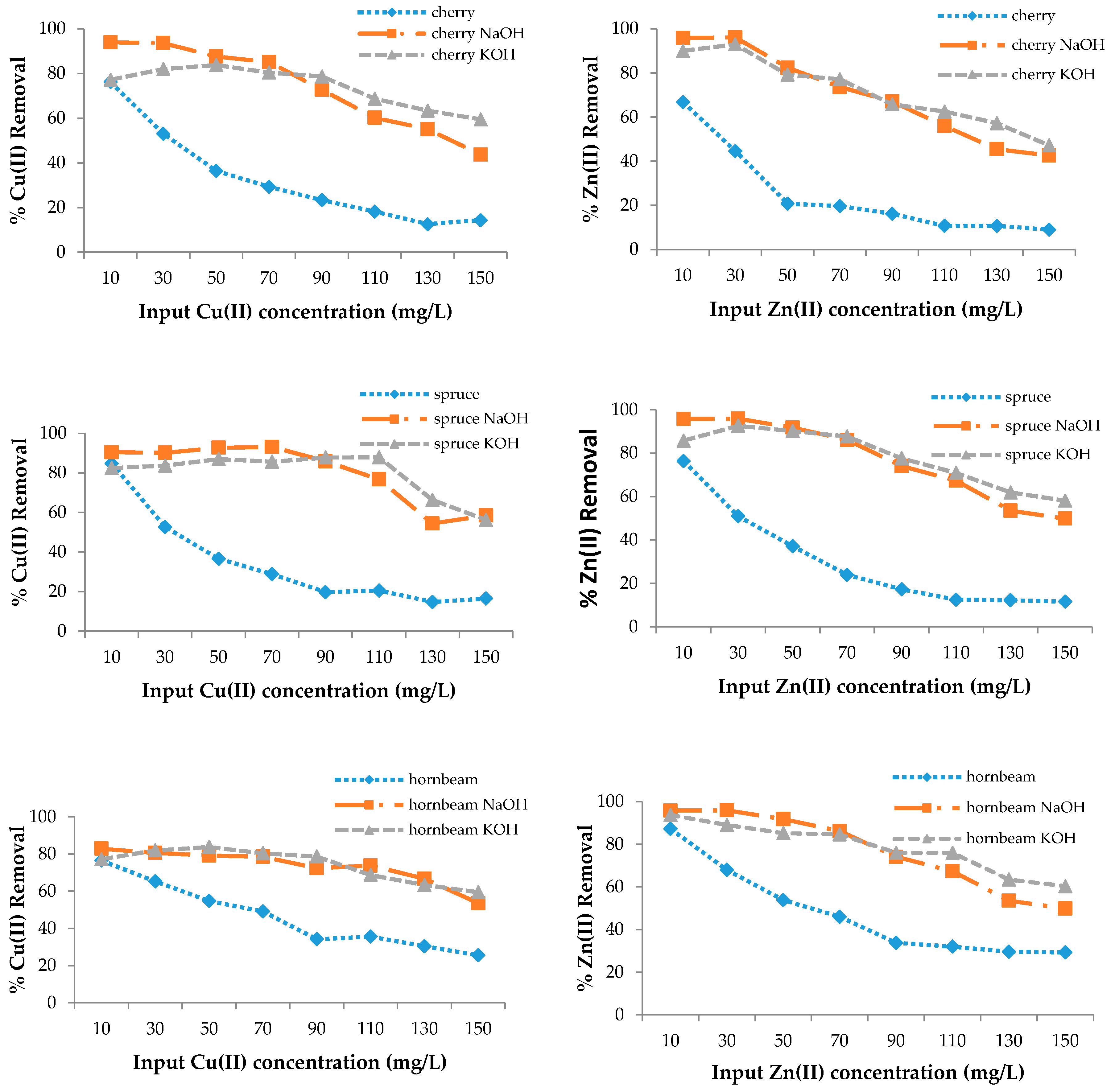
3.2. Removal Efficiency
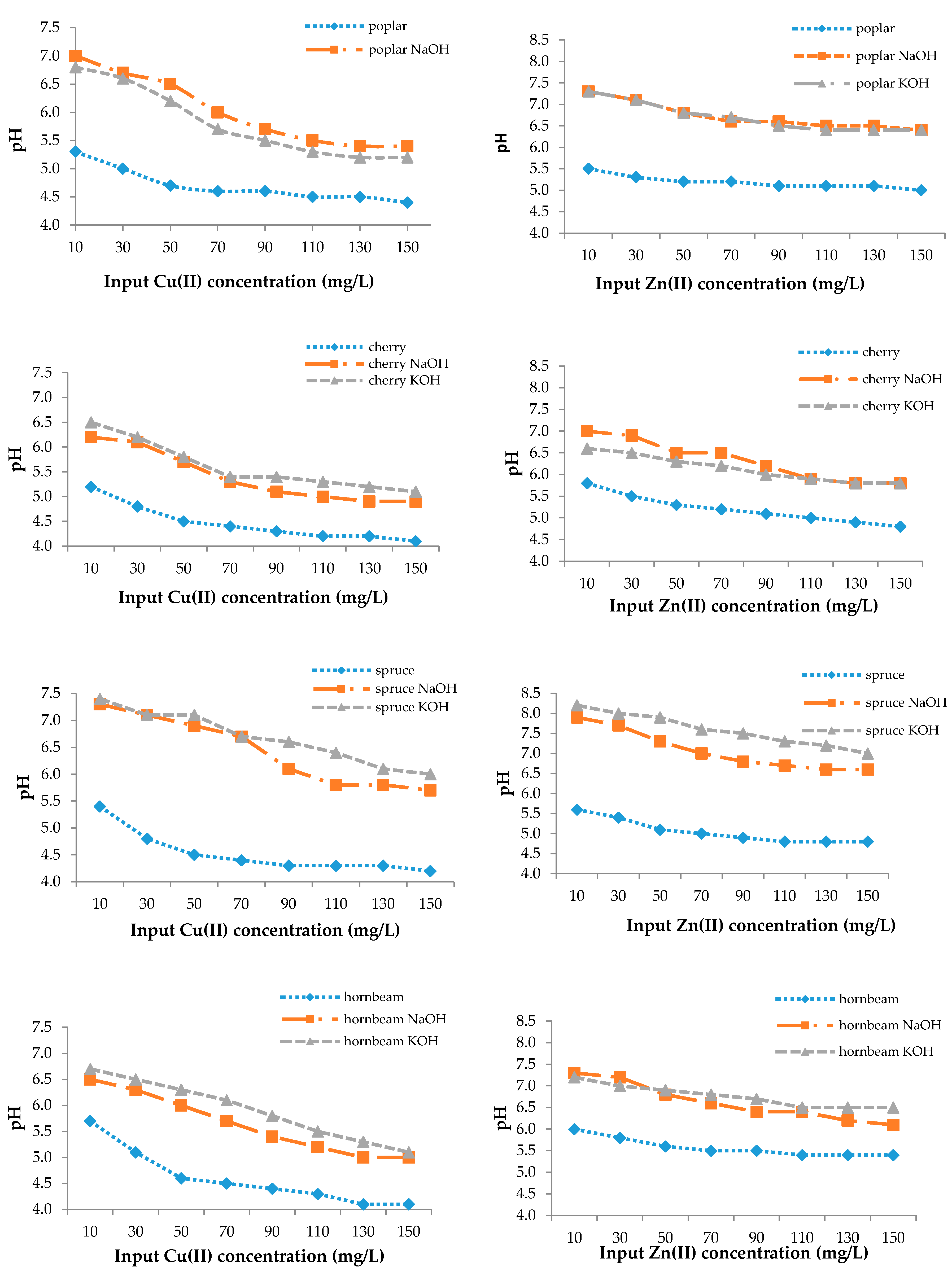
3.3. Effect of pH
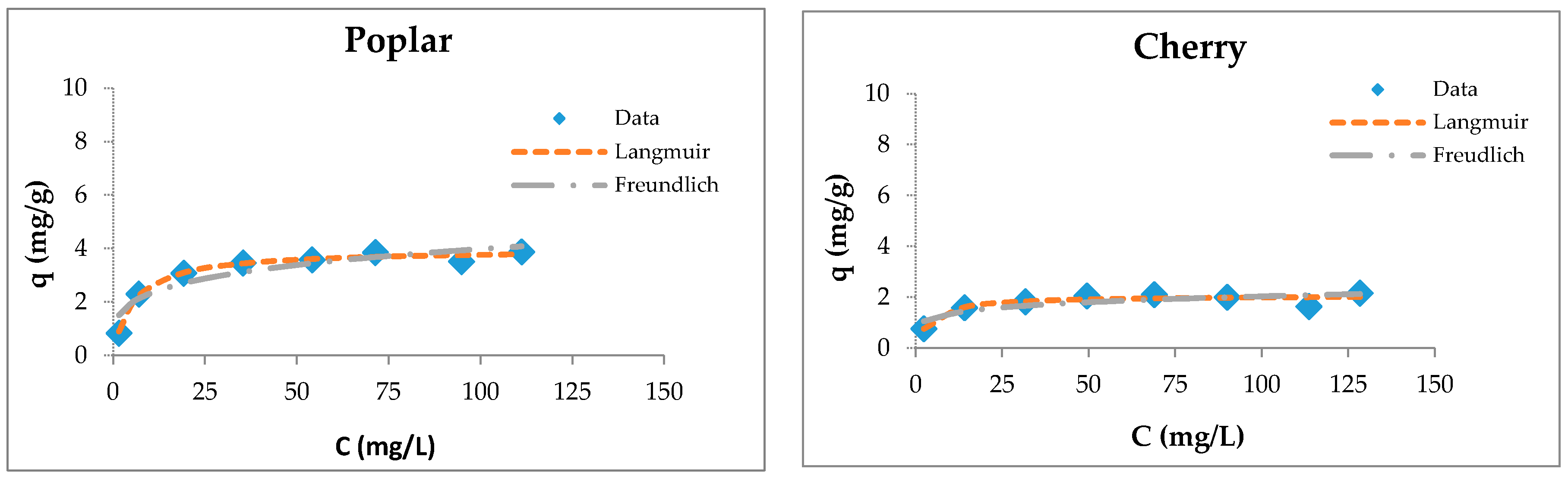
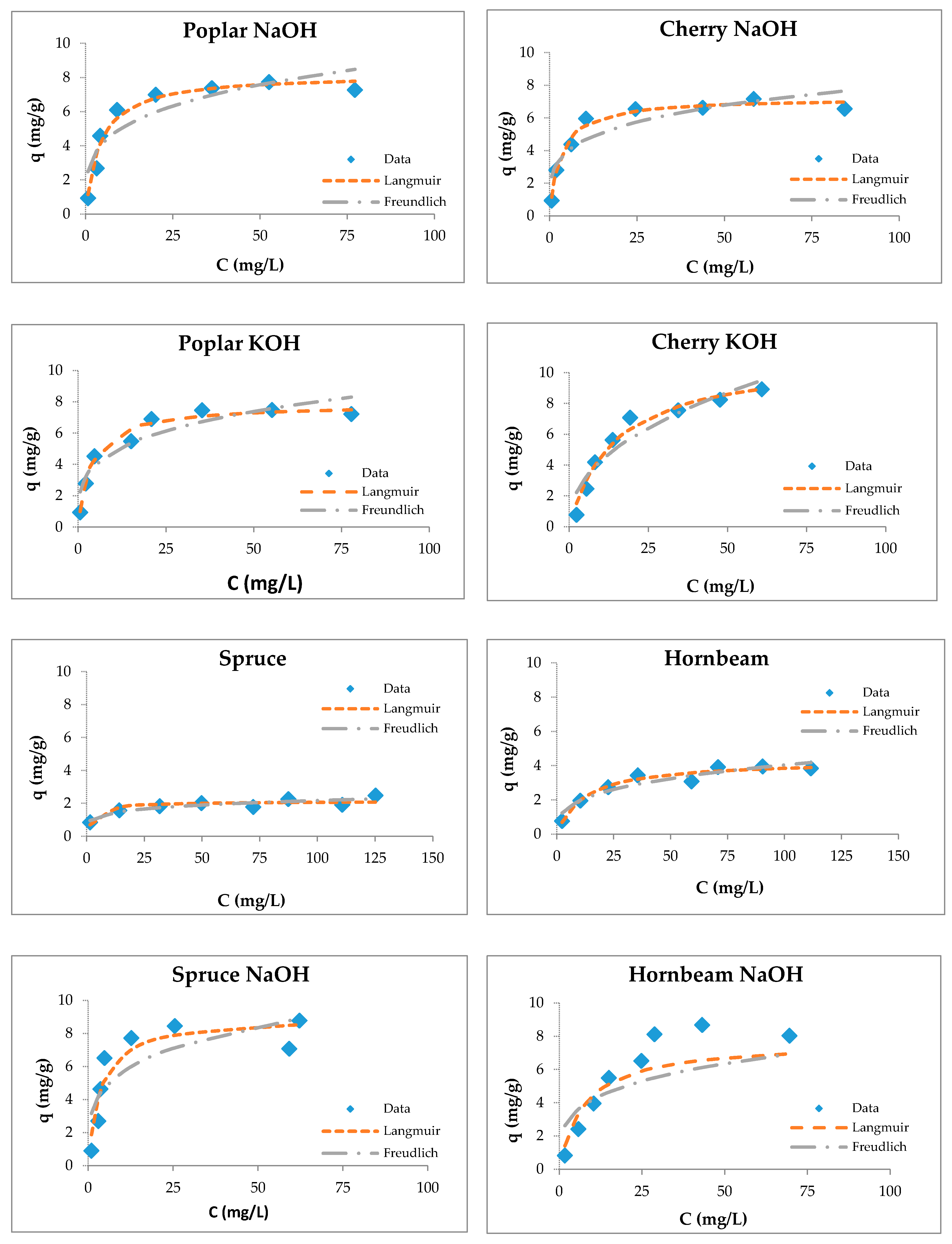
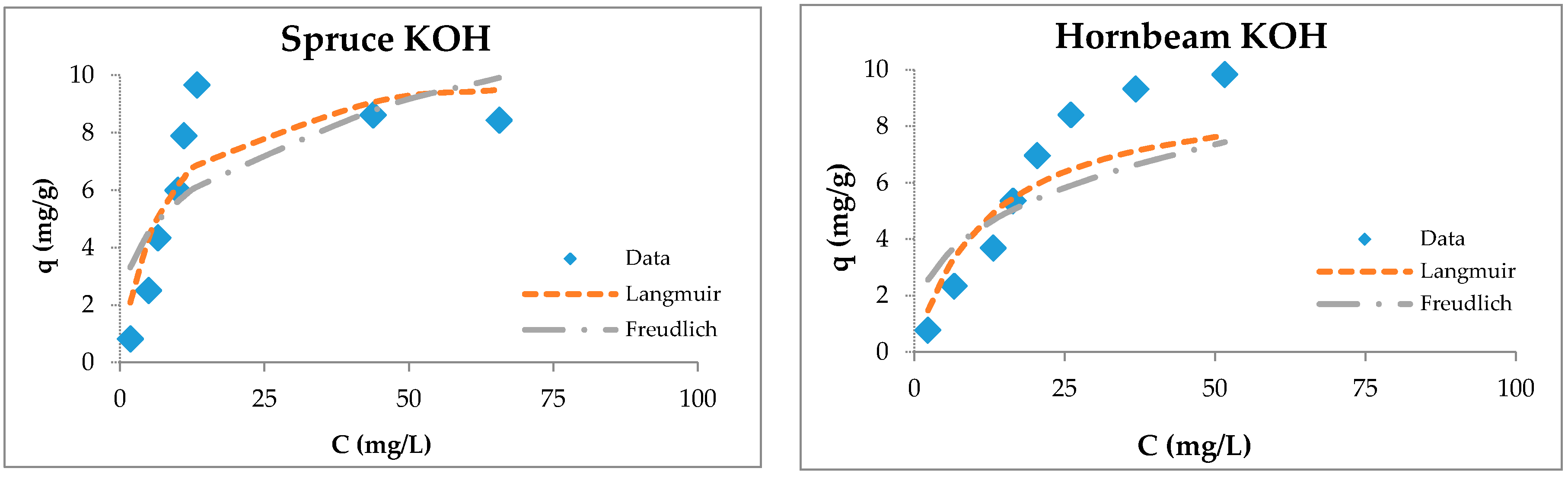
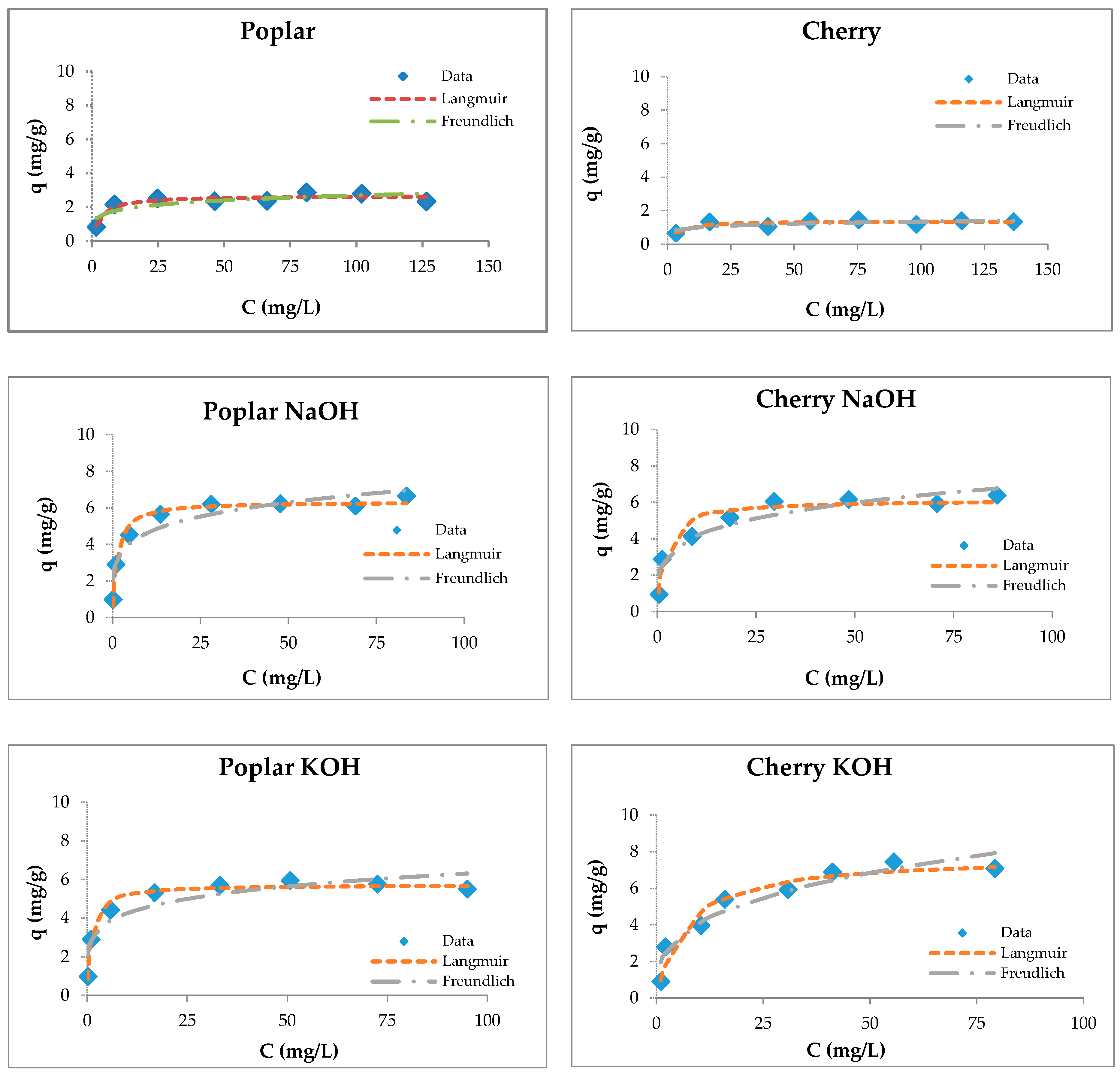
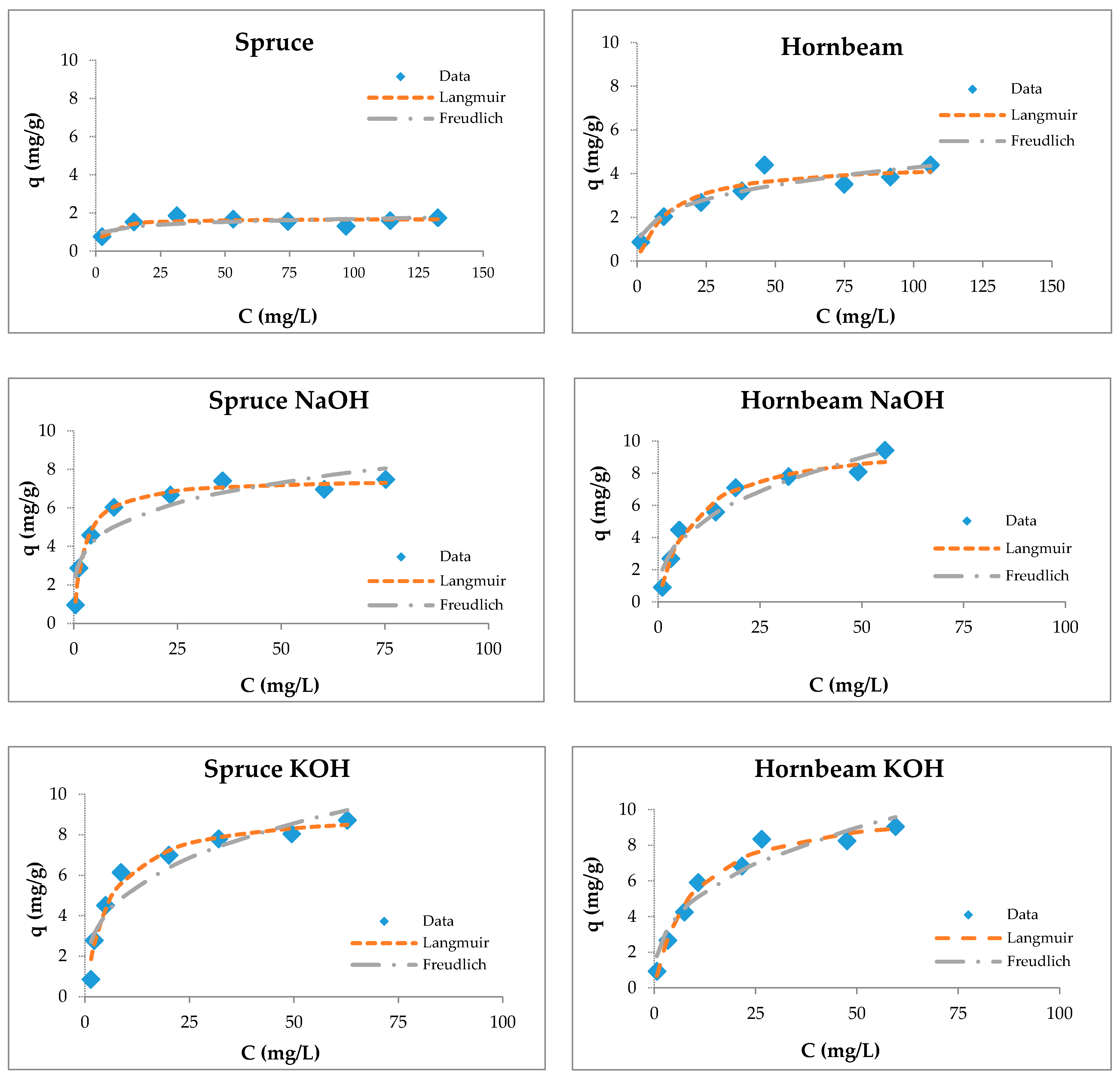
3.4. Sorption Studies
3.5. Maximum Sorption Capacities of Natural Sorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- El Hajam, M.; Kandri, N.I.; Plavan, G.-I.; Harrath, A.H.; Mansour, L.; Boufahja, F.; Zerouale, A. Pb2+ ions adsorption onto raw and chemically activated Dibetou sawdust: Application of experimental designs. J. King Saud Univ. Sci. 2020, 32, 2176–2189. [Google Scholar] [CrossRef]
- Slesarova, A.; Kusnierova, M.; Luptakova, A.; Zeman, J. An Overview of Occurrence and Evolution of Acid Mine Drainage in The Slovak Republic. In Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy, Amherst, MA, USA, 16–19 October 2007. [Google Scholar]
- Demcak, S.; Balintova, M.; Holub, M. Monitoring of the abandoned mine Smolnik (Slovakia) influence on the aquatic environment. IOP Conf. Ser. Earth Environ. Sci. 2020, 444, 12010. [Google Scholar] [CrossRef]
- Balintova, M.; Petrilakova, A.; Singovszka, E. Study of metal ion sorption from acidic solutions. Theor. Found. Chem. Eng. 2012, 46, 727–731. [Google Scholar] [CrossRef]
- Balintova, M.; Petrilakova, A.; Singovszka, E. Study of metals distribution between water and sediment in the Smolnik Creek (Slovakia) contaminated by acid mine drainage. Chem. Eng. Trans. 2012, 28, 73–78. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Sulaiman, O.; Ibrahim, M.H.; Chii, Y.Y.; Siddique, B.M. Removal of Cu(II) and Pb(II) ions from aqueous solutions by adsorption on sawdust of Meranti wood. Desalination 2009, 247, 636–646. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.-M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Chapter 1. Contamination of heavy metals in aquatic media: Transport, toxicity and technologies for remediation. In Heavy Metals in Water; Sharma, S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 1–24. ISBN 978-1-84973-885-9. [Google Scholar]
- Larous, S.; Meniai, A.-H. Removal of copper (II) from aqueous solution by agricultural by-products-sawdust. Energy Proc. 2012, 18, 915–923. [Google Scholar] [CrossRef]
- Gakwisiri, C.; Raut, N.; Al-Saadi, A.; Al-Aisri, S.; Al-Ajmi, A. A Critical Review of Removal of Zinc from Wastewater. In Proceedings of the World Congress on Engineering, London, UK, 4–6 July 2012. [Google Scholar]
- Zwain, H.M.; Vakili, M.; Dahlan, I. Waste material adsorbents for zinc removal from wastewater: A comprehensive review. Int. J. Chem. Eng. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Osredkar, J. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clin. Toxicol. 2011, 3, 1–18. [Google Scholar] [CrossRef]
- Smieja-Król, B.; Janeczek, J.; Bauerek, A.; Thorseth, I.H. The role of authigenic sulfides in immobilization of potentially toxic metals in the Bagno Bory wetland, southern Poland. Environ. Sci. Pollut. Res. 2015, 22, 15495–15505. [Google Scholar] [CrossRef]
- Dore, E.; Fancello, D.; Rigonat, N.; Medas, D.; Cidu, R.; Da Pelo, S.; Frau, F.; Lattanzi, P.; Marras, P.A.; Meneghini, C.; et al. Natural attenuation can lead to environmental resilience in mine environment. Appl. Geochem. 2020, 117, 104597. [Google Scholar] [CrossRef]
- Shikazono, N.; Zakir, H.M.; Sudo, Y. Zinc contamination in river water and sediments at Taisyu Zn–Pb mine area, Tsushima Island, Japan. J. Geochem. Explor. 2008, 98, 80–88. [Google Scholar] [CrossRef]
- Luptakova, A.; Balintova, M.; Jencarova, J.; Macingova, E.; Prascakova, M. Metals recovery from acid mine damage. Nova Biotechnol. 2010, 22, 1111–1118. [Google Scholar]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. Chembioeng. Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Blais, J.F.; Djedidi, Z.; Cheikh, R.B.; Tyagi, R.D.; Mercier, G. Metals Precipitation from effluents: Review. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 135–149. [Google Scholar] [CrossRef]
- Chang, L.; Cao, Y.; Fan, G.; Li, C.; Peng, W. A review of the applications of ion floatation: Wastewater treatment, mineral beneficiation and hydrometallurgy. RSC Adv. 2019, 9, 20226–20239. [Google Scholar] [CrossRef]
- Kyzas, G.; Matis, K. Flotation in water and wastewater treatment. Processes 2018, 6, 116. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Yaser, A.Z.; Cassey, T.L.; Hairul, M.A.; Shazwan, A.S. current review on the coagulation/flocculation of lignin containing wastewater. Int. J. Waste Res. 2014, 4, 153–159. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater Treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Wan Ikhsan, S.N.; Yusof, N.; Aziz, F.; Nurasyikin, M. A review of oilfield wastewater treatment using membrane filtration over conventional technology. Malays. J. Anal. Sci. 2017, 21, 643–658. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis applications in wastewater treatment for environmental protection and resources recovery: A systematic review on progress and perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef]
- Gunatilake, S. Methods of removing heavy metals from industrial wastewater. Methods 2015, 1, 14. [Google Scholar]
- Asadi, F.; Shariatmadari, H.; Mirghaffari, N. Modification of rice hull and sawdust sorptive characteristics for remove heavy metals from synthetic solutions and wastewater. J. Hazard. Mater. 2008, 154, 451–458. [Google Scholar] [CrossRef]
- Meena, A.K.; Kadirvelu, K.; Mishra, G.K.; Rajagopal, C.; Nagar, P.N. Adsorptive removal of heavy metals from aqueous solution by treated sawdust (Acacia arabica). J. Hazard. Mater. 2008, 150, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Demcak, S.; Balintova, M.; Hurakova, M.; Frontasyeva, M.V.; Zinicovscaia, I.; Yushin, N. Utilization of poplar wood sawdust for heavy metals removal from model solutions. Nova Biotechnol. Chim. 2017, 16, 26–31. [Google Scholar] [CrossRef]
- Nacu, G.; Bulgariu, D.; Cristina Popescu, M.; Harja, M.; Toader Juravle, D.; Bulgariu, L. Removal of Zn(II) ions from aqueous media on thermal activated sawdust. Desalin. Water Treat. 2016, 57, 21904–21915. [Google Scholar] [CrossRef]
- Božić, D.; Stanković, V.; Gorgievski, M.; Bogdanović, G.; Kovačević, R. Adsorption of heavy metal ions by sawdust of deciduous trees. J. Hazard. Mater. 2009, 171, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Tang, J.; Gong, Y.; Zhang, H. Characterization of potassium hydroxide (KOH) modified hydrochrochars from different feedstock for enhanced removal of heavy metals from water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. [Google Scholar] [CrossRef]
- Şen, A.; Pereira, H.; Olivella, M.A.; Villaescusa, I. Heavy metals removal in aqueous environments using bark as a biosorbent. Int. J. Environ. Sci. Technol. 2015, 12, 391–404. [Google Scholar] [CrossRef]
- Low, K.S.; Lee, C.K.; Liew, S.C. Sorption of cadmium and lead from aqueous solutions by spent grain. Process Biochem. 2000, 36, 59–64. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer’eb, M. Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Hussein, B.I. Removal of copper ions from waste water by adsorption with modified and unmodified sunflower stalks. J. Eng. 2010, 16, 12. [Google Scholar]
- Qin, F.; Wen, B.; Shan, X.-Q.; Xie, Y.-N.; Liu, T.; Zhang, S.-Z.; Khan, S.U. Mechanisms of competitive adsorption of Pb, Cu, and Cd on peat. Environ. Pollut. 2006, 144, 669–680. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, J.; Zhang, W.; Wang, M.; Zhou, J. Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J. Hazard. Mater. 2007, 141, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, S.S.; Goyal, D. Removal of heavy metals by waste tea leaves from aqueous solution. Eng. Life Sci. 2005, 5, 158–162. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Delacroix, A. Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J. Hazard. Mater. 2003, 105, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, Y.; Shukla, A.; Shukla, S.S.; Dorris, K.L. The removal of heavy metal from aqueous solutions by sawdust adsorption—Removal of copper. J. Hazard. Mater. 2000, 80, 33–42. [Google Scholar] [CrossRef]
- Shukla, A.; Zhang, Y.-H.; Dubey, P.; Margrave, J.L.; Shukla, S.S. The role of sawdust in the removal of unwanted materials from water. J. Hazard. Mater. 2002, 95, 137–152. [Google Scholar] [CrossRef]
- Shukla, S.; Pai, R.S. Removal of Pb(II) from solution using cellulose-containing materials. J. Chem. Technol. Biotechnol. 2005, 80, 176–183. [Google Scholar] [CrossRef]
- Demcak, S.; Balintova, M.; Demcakova, M.; Csach, K.; Zinicovscaia, I.; Yushin, N.; Frontasyeva, M. Effect of alkaline treatment of wooden sawdust for the removal of heavy metals from aquatic environments. DWT 2019, 155, 207–215. [Google Scholar] [CrossRef]
- Memon, S.Q.; Memon, N.; Shah, S.W.; Khuhawar, M.Y.; Bhanger, M.I. Sawdust—A green and economical sorbent for the removal of cadmium (II) ions. J. Hazard. Mater. 2007, 139, 116–121. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Duca, G.; Cepoi, L.; Chiriac, T.; Rudi, L.; Mitina, T.; Frontasyeva, M.V.; Pavlov, S.; Gundorina, S.F. Biotechnology of metal removal from industrial wastewater: Zinc case study: Biotechnology of zinc removal from wastewater. Clean Soil Air Water 2015, 43, 112–117. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Šćiban, M.; Klašnja, M.; Škrbić, B. Modified softwood sawdust as adsorbent of heavy metal ions from water. J. Hazard. Mater. 2006, 136, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Memon, S.Q.; Memon, N.; Solangi, A.R.; Memon, J.-R. Sawdust: A green and economical sorbent for thallium removal. Chem. Eng. J. 2008, 140, 235–240. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Yeddou, A.R. Potential of sawdust materials for the removal of dyes and heavy metals: Examination of isotherms and kinetics. Desalin. Water Treat. 2016, 57, 24019–24034. [Google Scholar] [CrossRef]
- Pearce, J.M. Digital designs and scientific hardware. In Open-Source Lab; Elsevier: Amsterdam, The Netherlands, 2014; pp. 165–252. ISBN 978-0-12-410462-4. [Google Scholar]
- Nakano, S. Studies on 2, 2′-Biquinoline derivatives. VI. Yakugaku Zasshi 1962, 82, 486–491. [Google Scholar] [CrossRef]
- Colorimeter DR 890 Manual; Hach Company: Loveland, CT, USA, 2013.
- Federal Register, 45 (105) 36166 (29 May 1980). Available online: https://www.loc.gov/item/fr045105 (accessed on 25 May 2020).
- Larous, S.; Meniai, A.-H.; Lehocine, M.B. Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination 2005, 185, 483–490. [Google Scholar] [CrossRef]
- Semerjian, L. Removal of heavy metals (Cu, Pb) from aqueous solutions using pine (Pinus halepensis) sawdust: Equilibrium, kinetic, and thermodynamic studies. Environ. Technol. Innov. 2018, 12, 91–103. [Google Scholar] [CrossRef]
- Šćiban, M.; Radetić, B.; Kevrešan, Ž.; Klašnja, M. Adsorption of heavy metals from electroplating wastewater by wood sawdust. Bioresour. Technol. 2007, 98, 402–409. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.; Heller, W. The Adsorption of cis- and trans-Azobenzene. J. Am. Chem. Soc. 1939, 61, 2228–2230. [Google Scholar] [CrossRef]
- Bodirlau, R.; Teaca, C. Fourier transform infrared spectroscopy and thermal analysis of lignocellulose fillers treated with organic anhydrides. Rom. J. Phys. 2009, 54, 93–104. [Google Scholar]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties characterization of chemically modified hemp hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Dong, S.-J.; Ma, H.-H.; Zhang, B.-X.; Wang, Y.-F.; Hu, X.-M. Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Ind. Crop. Prod. 2015, 76, 688–696. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- El-Saied, F.A.; Abo-Elenan, S.A.; El-Shinawy, F.H. Removal of lead and copper ions from polluted aqueous solutions using nano-sawdust particles. Int. J. Waste Resour. 2017, 7. [Google Scholar] [CrossRef]
- Šćiban, M.; Klašnja, M. Wood sawdust and wood originate materials as adsorbents for heavy metal ions. Holz Roh. Werkst. 2004, 62, 69–73. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Removal of copper(II) from aqueous solution by pine and base modified pine cone powder as biosorbent. J. Hazard. Mater. 2009, 168, 909–917. [Google Scholar] [CrossRef]
- Keränen, A.; Leiviskä, T.; Zinicovscaia, I.; Frontasyeva, M.V.; Hormi, O.; Tanskanen, J. Quaternized pine sawdust in the treatment of mining wastewater. Environ. Technol. 2016, 37, 1390–1397. [Google Scholar] [CrossRef]
- Ouafi, R.; Rais, Z.; Taleb, M.; Benabbou, M.; Asri, M. Sawdust in the treatment of heavy metals-contaminated wastewater. In Sawdust: Properties, Potential Uses and Hazards; Nova Science Publishers: New York, NY, USA, 2017; pp. 145–182. [Google Scholar]
- Sciban, M.; Klasnja, M.; Skrbic, B. Modified hardwood sawdust as adsorbent of heavy metal ions from water. Wood Sci. Technol. 2006, 40, 217–227. [Google Scholar] [CrossRef]
- Özacar, M.; Şengil, İ.A. Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour. Technol. 2005, 96, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Islam, M.R. Effects of pH on isotherms modeling for Cu(II) ions adsorption using maple wood sawdust. Chem. Eng. J. 2009, 149, 273–280. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Atif, M.; Prasad, B.; Mishra, I.M. Batch adsorption of zinc on tea factory waste. Desalination 2009, 244, 66–71. [Google Scholar] [CrossRef]
- Boonamnuayvitaya, V.; Chaiya, C.; Tanthapanichakoon, W.; Jarudilokkul, S. Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay. Sep. Purif. Technol. 2004, 35, 11–22. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Szafran, R.G.; Modelski, S. Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 2011, 265, 126–134. [Google Scholar] [CrossRef]
- Thirumavalavan, M.; Lai, Y.-L.; Lin, L.-C.; Lee, J.-F. Cellulose-based native and surface modified fruit peels for the adsorption of heavy metal ions from aqueous solution: Langmuir adsorption isotherms. J. Chem. Eng. Data 2010, 55, 1186–1192. [Google Scholar] [CrossRef]
- Božić, D.; Gorgievski, M.; Stanković, V.; Štrbac, N.; Šerbula, S.; Petrović, N. Adsorption of heavy metal ions by beech sawdust—Kinetics, mechanism and equilibrium of the process. Ecol. Eng. 2013, 58, 202–206. [Google Scholar] [CrossRef]
- Shukla, S.R.; Pai, R.S. Adsorption of Cu(II), Ni(II) and Zn(II) on dye loaded groundnut shells and sawdust. Sep. Purif. Technol. 2005, 43, 1–8. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of copper (II), chromium (III), nickel (II) and lead (II) ions from aqueous solutions by meranti sawdust. J. Hazard. Mater. 2009, 170, 969–977. [Google Scholar] [CrossRef]
| Techniques | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Chemical precipitation | Simple procedure, inexpensive, useful for high concentrations of metals | Production of sludge, ineffective at low concentration of contaminants | [18,19] |
| Flotation | Selective metal ion recovery, low sludge generation, high separation efficiency | High cost | [20,21] |
| Ion exchange | No change in pH of wastewater, process reliability, stability, and chemical safety | High membrane cost, requirement of resin fouling, resin regeneration | [22,23] |
| Coagulation and flocculation | Settling and dewatering of sludge | High cost, large consumption of chemicals | [24,25] |
| Membrane filtration | Low production of solid waste, low consumption of chemicals, high efficiency | Low flow rate, high cost | [26,27] |
| Electrodialysis | Relatively low energy consumption, suitable for non-ionized from ionized components | Organic matter and colloids are not removed, elaborate controls are required, selection materials of construction membranes is important | [28,29] |
| Adsorption | Low formation of chemical and biological sludge, high efficiency, relatively low cost, regeneration of sorbents, and possibility of metal recovery | High cost of engineering sorbent | [7,30] |
| Wooden Sawdust | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (1/mg) | R2 | KF (1/g) | 1/n | R2 | |
| Poplar | 3.97 ± 0.26 | 0.19 | 0.99 | 1.35 | 0.23 | 0.87 |
| Poplar NaOH | 8.20 ± 1.43 | 0.24 | 0.96 | 2.75 | 0.26 | 0.82 |
| Poplar KOH | 7.86 ± 1.19 | 0.26 | 0.98 | 2.61 | 0.27 | 0.88 |
| Cherry | 2.08 ± 0.06 | 0.25 | 0.87 | 0.92 | 0.17 | 0.71 |
| Cherry NaOH | 7.38 ± 0.88 | 0.31 | 0.98 | 2.70 | 0.23 | 0.83 |
| Cherry KOH | 11.06 ± 1.93 | 0.07 | 0.97 | 1.54 | 0.44 | 0.90 |
| Spruce | 2.13 ± 0.05 | 0.32 | 0.79 | 0.88 | 0.20 | 0.84 |
| Spruce NaOH | 9.02 ± 1.73 | 0.28 | 0.87 | 3.21 | 0.25 | 0.68 |
| Spruce KOH | 10.52 ± 1.74 | 0.14 | 0.77 | 2.80 | 0.30 | 0.58 |
| Hornbeam | 4.32 ± 0.41 | 0.08 | 0.96 | 0.94 | 0.32 | 0.90 |
| Hornbeam NaOH | 7.71 ± 0.93 | 0.13 | 0.80 | 2.28 | 0.26 | 0.63 |
| Hornbeam KOH | 9.49 ± 1.04 | 0.08 | 0.78 | 1.95 | 0.34 | 0.65 |
| Wooden Sawdust | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (1/mg) | R2 | KF (1/g) | 1/n | R2 | |
| Poplar | 2.69 ± 0.08 | 0.35 | 0.89 | 1.27 | 0.16 | 0.69 |
| Poplar NaOH | 6.34 ± 0.47 | 0.82 | 0.978 | 3.00 | 0.19 | 0.90 |
| Poplar KOH | 5.73 ± 0.31 | 0.97 | 0.98 | 2.85 | 0.17 | 0.86 |
| Cherry | 1.38 ± 0.03 | 0.33 | 0.71 | 0.72 | 0.14 | 0.57 |
| Cherry NaOH | 6.15 ± 0.38 | 0.50 | 0.94 | 2.46 | 0.23 | 0.91 |
| Cherry KOH | 7.79 ± 0.96 | 0.14 | 0.95 | 1.96 | 0.32 | 0.93 |
| Spruce | 1.71 ± 0.04 | 0.35 | 0.71 | 0.86 | 0.15 | 0.78 |
| Spruce NaOH | 7.53 ± 0.98 | 0.42 | 0.99 | 2.97 | 0.23 | 0.87 |
| Spruce KOH | 9.26 ± 1.68 | 0.18 | 0.97 | 2.42 | 0.32 | 0.88 |
| Hornbeam | 4.54 ± 0.41 | 0.09 | 0.88 | 1.07 | 0.30 | 0.87 |
| Hornbeam NaOH | 10.02 ± 1.83 | 0.12 | 0.97 | 2.02 | 0.38 | 0.94 |
| Hornbeam KOH | 10.31 ± 1.71 | 0.11 | 0.98 | 2.13 | 0.37 | 0.93 |
| Serial Number | Low-Cost Sorbent | qmax (mg/g) | Heavy Metals | Optimal pH | Reference |
|---|---|---|---|---|---|
| 1 | Tea Waste | 8.9 | Zn | 4.2 | [80] |
| 2 | Coffee residues | 13.4 31.2 | Zn Cu | - | [81] |
| 3 | Peanut shells | 25.4 | Cu | 5.0 | [82] |
| 4 | Banana peel | 21.9 52.4 | Zn Cu | 4.0–6.0 | [83] |
| 5 | Lemon peel | 27.9 70.9 | Zn Cu | 4.0–6.0 | [83] |
| 6 | Orange peel | 27.1 63.3 | Zn Cu | 4.0–6.0 | [83] |
| 7 | Peat (Danish) | 34.1 | Cu | 4.0 | [43] |
| 8 | Peat (Heilongjiang) | 25.4 | Cu | 4.0 | [43] |
| 9 | Poplar sawdust (raw) | 0.74 0.86 | Zn Cu | 5.8 5.2 | [55] |
| 10 | Beech sawdust | 2.0 4.5 | Zn Cu | 4.8–5.3 | [84] |
| 11 | Teakwood sawdust | 4.9 11.0 | Zn Cu | 5.88 5.24 | [85] |
| 12 | Meranti sawdust | 32.1 | Cu | 6.0 | [86] |
| 13 | Cherry sawdust (raw) | 1.46 2.16 | Zn Cu | 5.1 4.1 | Present study |
| 14 | Poplar sawdust (raw) | 2.88 3.88 | Zn Cu | 5.1 4.4 | Present study |
| 15 | Hornbeam sawdust (raw) | 4.4 3.96 | Zn Cu | 5.4 4.1 | Present study |
| 16 | Spruce sawdust (raw) | 2.01 2.48 | Zn Cu | 5.6 4.2 | Present study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacova, Z.; Demcak, S.; Balintova, M.; Pla, C.; Zinicovscaia, I. Influence of Wooden Sawdust Treatments on Cu(II) and Zn(II) Removal from Water. Materials 2020, 13, 3575. https://doi.org/10.3390/ma13163575
Kovacova Z, Demcak S, Balintova M, Pla C, Zinicovscaia I. Influence of Wooden Sawdust Treatments on Cu(II) and Zn(II) Removal from Water. Materials. 2020; 13(16):3575. https://doi.org/10.3390/ma13163575
Chicago/Turabian StyleKovacova, Zdenka, Stefan Demcak, Magdalena Balintova, Cocencepcion Pla, and Inga Zinicovscaia. 2020. "Influence of Wooden Sawdust Treatments on Cu(II) and Zn(II) Removal from Water" Materials 13, no. 16: 3575. https://doi.org/10.3390/ma13163575
APA StyleKovacova, Z., Demcak, S., Balintova, M., Pla, C., & Zinicovscaia, I. (2020). Influence of Wooden Sawdust Treatments on Cu(II) and Zn(II) Removal from Water. Materials, 13(16), 3575. https://doi.org/10.3390/ma13163575







