Preparation and Photocatalytic Properties of Metal-Doped ZnO Nanofilms Grown on Graphene-Coated Flexible Substrates
Abstract
1. Introduction
2. Experimental Methods
2.1. Synthesis of M-ZnO Nanofilms on GPET Substrates
2.2. Structural Characteristics
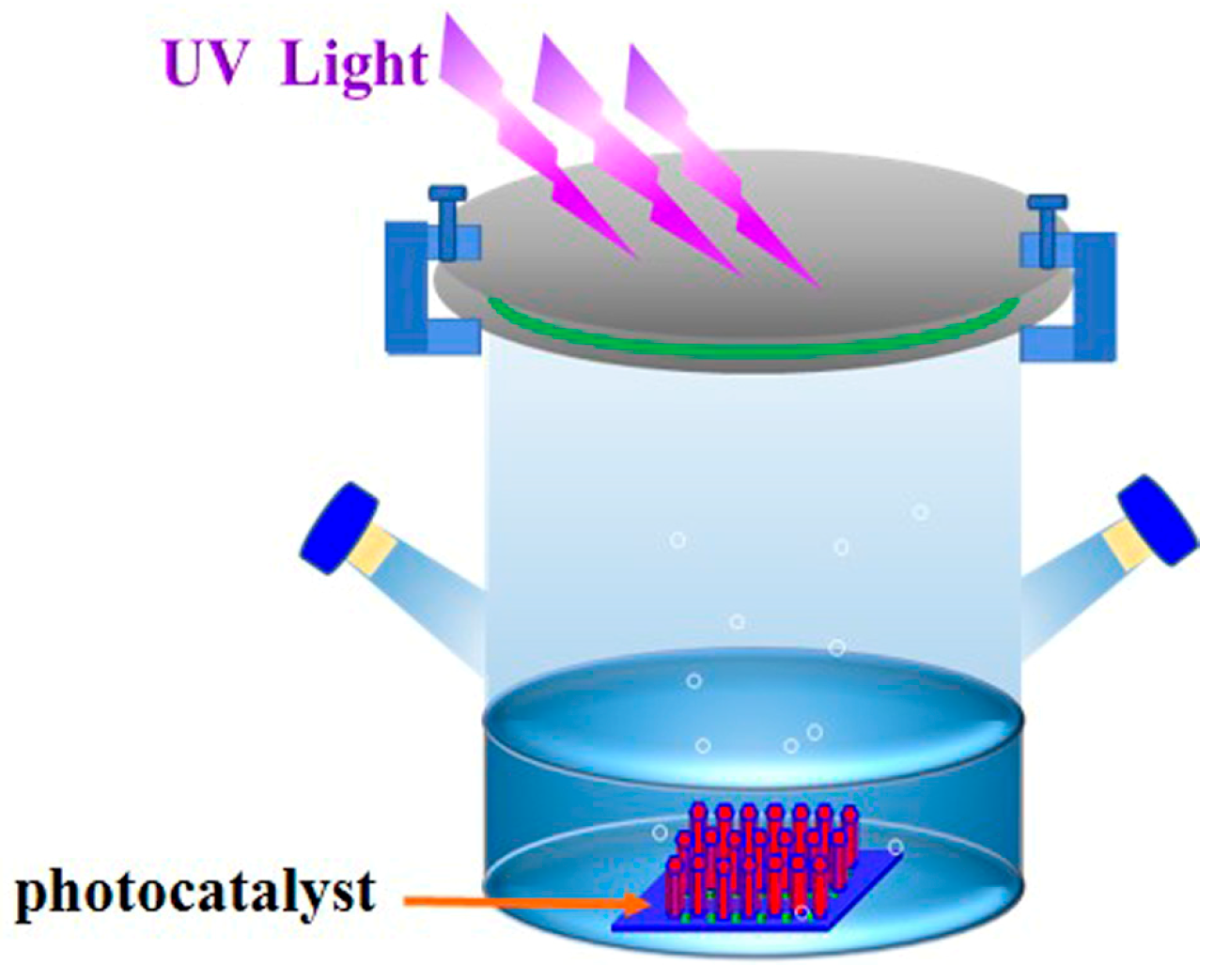
2.3. Photocatalytic Measurement
3. Results and Discussion
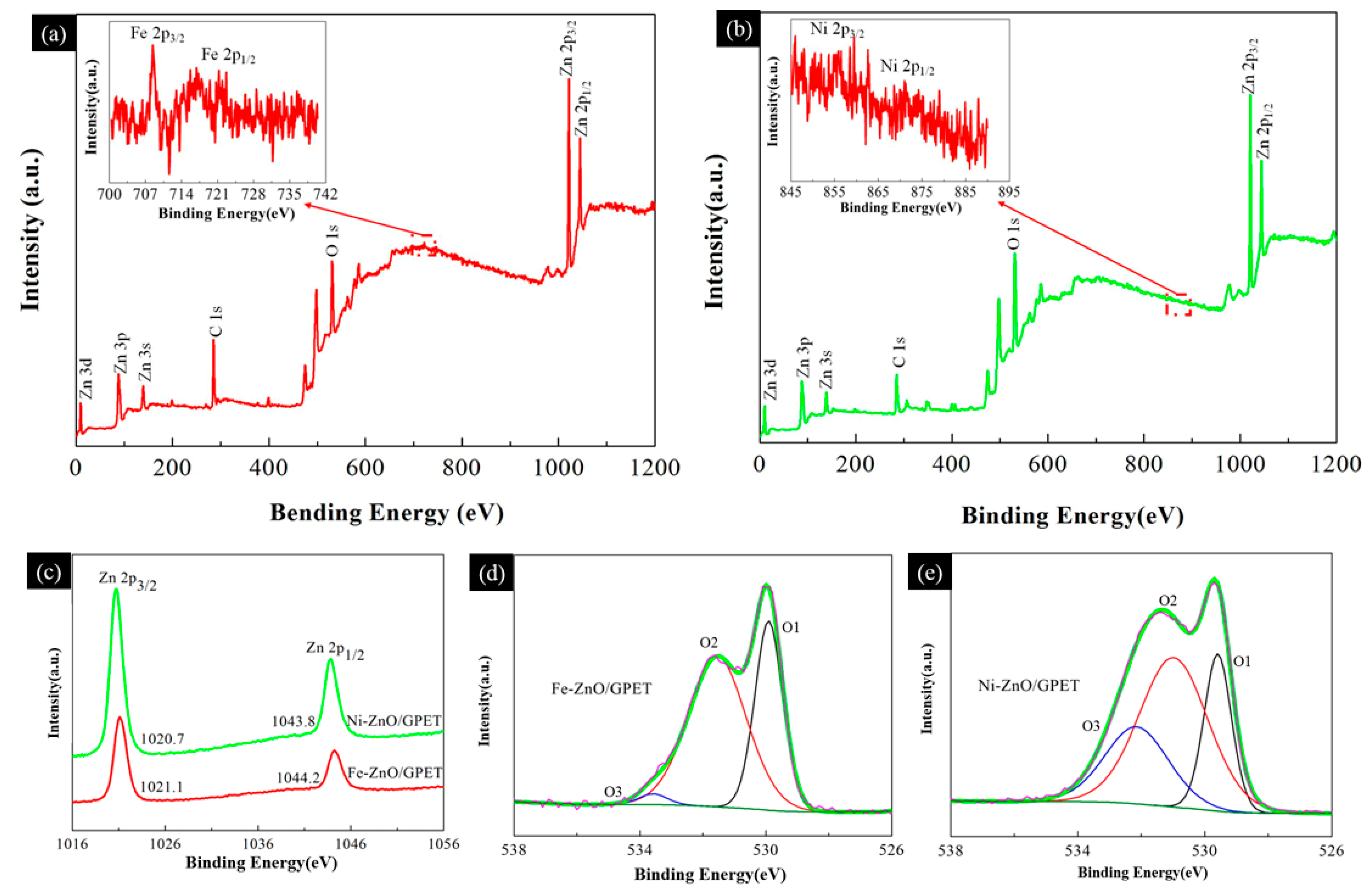
3.1. Morphology and Structure of M-ZnO/GPET Composites
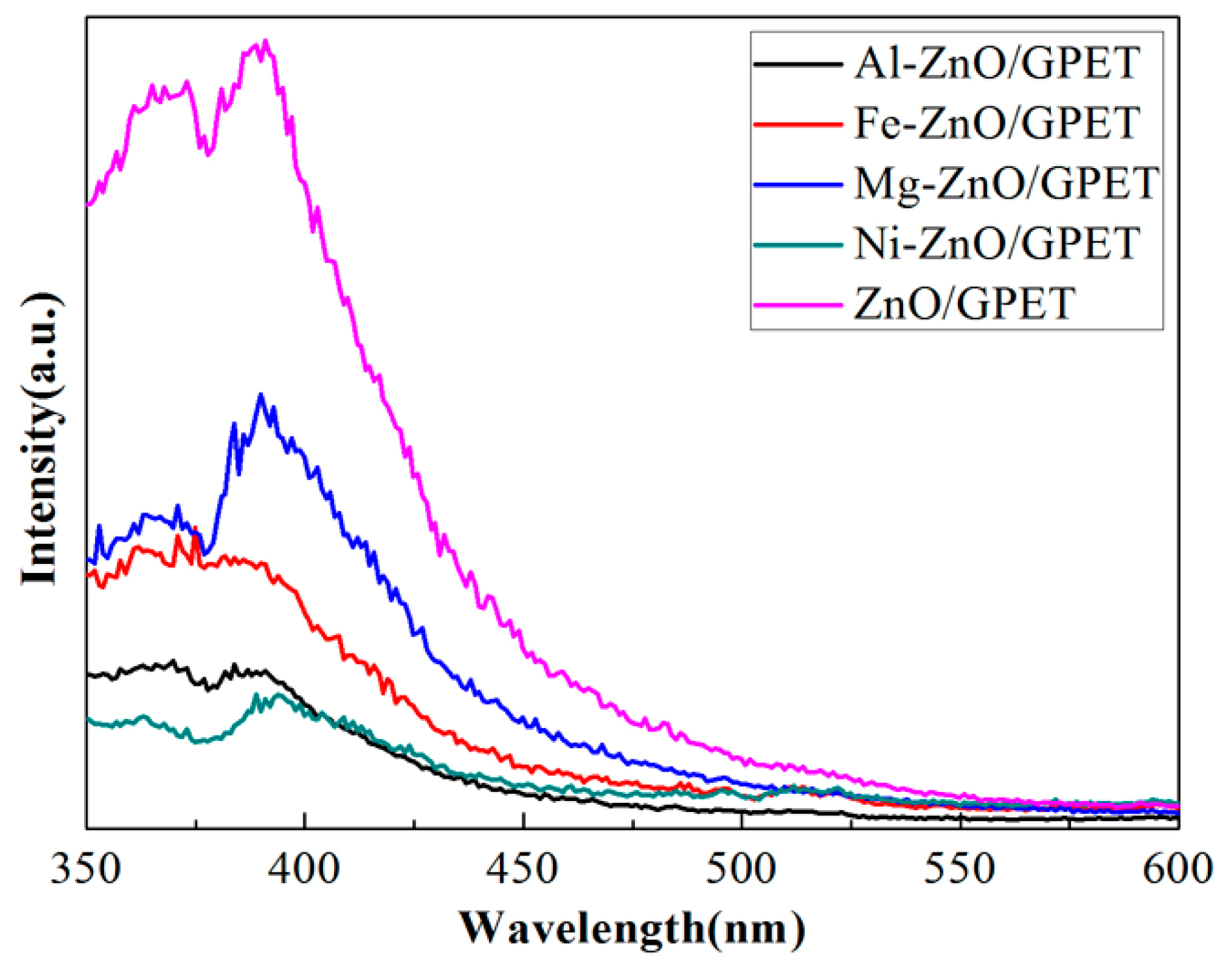
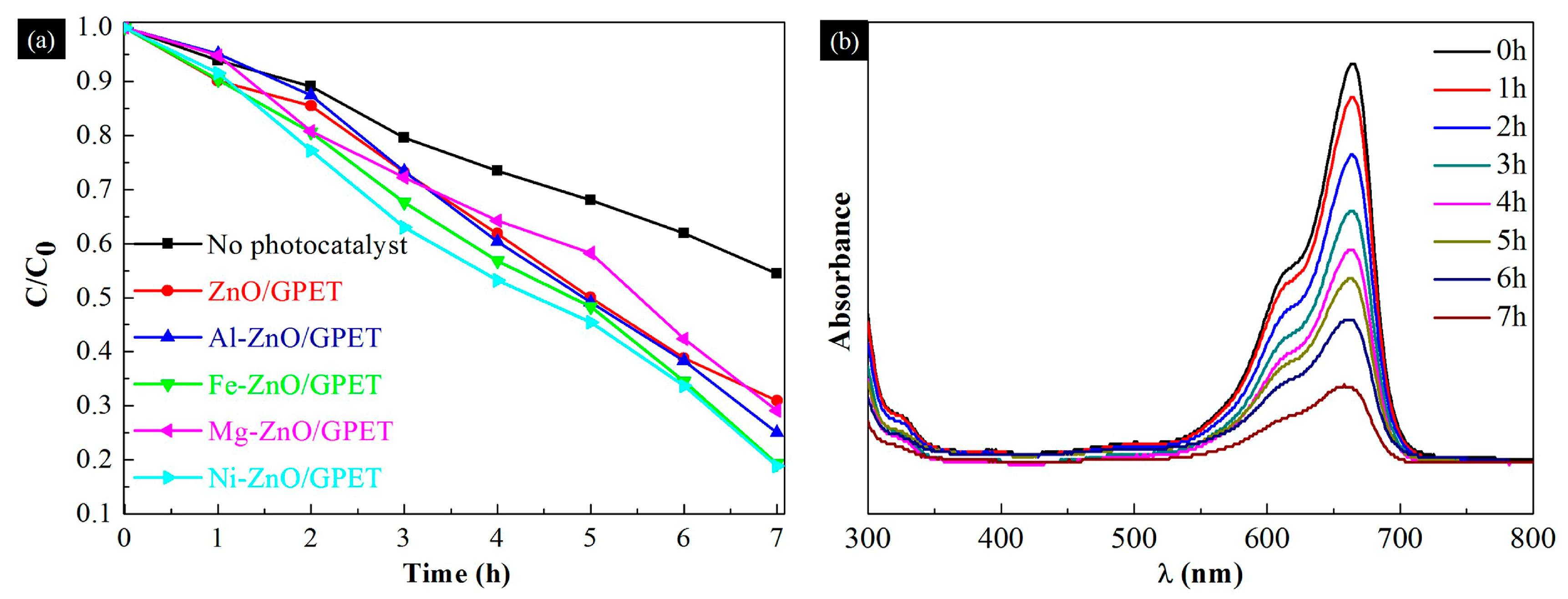
3.2. Photocatalytic Performance
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications-a review. J. Chem. Technol. Biot. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Lee, C. Sonochemical synthesis of Ce-doped TiO2 nanostructure: A visible-light-driven photocatalyst for degradation of toluene and O-xylene. Materials 2019, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Yurddaskal, M.; Yldrm, S.; Dikici, T.; Yurddaskal, M.; Elik, E. Effects of Zn-doping on the photocatalytic activity and microstructures of nanocrystalline SnO2 powders. Chem. J. Turk. Chem. Soc. 2017, 5, 9–14. [Google Scholar] [CrossRef]
- Wang, K.; Xu, J.M.; Wang, X.T. The effects of zno morphology on photocatalytic efficiency of zno/rgo nanocomposites. Appl. Surf. Sci. 2016, 360, 270–275. [Google Scholar]
- Klingshirn, C. The luminescence of ZnO under high one-and two-quantum excitation. Phys. Status Solidi 1975, 71, 547–556. [Google Scholar] [CrossRef]
- Awasthi, G.P.; Adhikari, S.P.; Ko, S.; kim, H.J.; Park, C.H.; kim, C.S. Facile synthesis of ZnO flowers modified graphene like MoS2 sheets for enhanced visible-light-driven photocatalytic activity and antibacterial properties. J. Alloy Compod. 2016, 682, 208–215. [Google Scholar] [CrossRef]
- Zheng, D.; Gong, P.; Chen, Y.Z.; Wang, C.; Peng, D.L. Preparation of multi-branched Au-ZnO hybrid nanocrystals on graphene for enhanced photocatalytic performance. Mater. Lett. 2015, 161, 379–383. [Google Scholar] [CrossRef]
- Zhuang, H.F.; Lin, C.J.; Lai, Y.K.; Sun, L.; Jing, L.I. Some critical structure factors of titanium oxide nanotube array in its photocatalytic activity. Environ. Sci. Technol. 2007, 41, 4735–4740. [Google Scholar] [CrossRef]
- Ameur, S.B.; Barhoumi, A.; Duponchel, B.; Hadjltaief, H.B.; Leroy, G.; Amlouk, M.; Guermazi, H. Physical investigations and photocatalytic activities on ZnO and SnO2 thin films deposited on flexible polymer substrate. Vacuum 2018, 155, 546–552. [Google Scholar] [CrossRef]
- Sanchez, L.; Guz, L.; García, P.; Rodriguez, J.; Ponce, S.; Goyanes, S.; Marchi, M.C.; Candal, R. Synthesis and characterization of ZnO nanorod films on PET for photocatalytic disinfection of water. J. Adv. Oxid. Technol. 2015, 18, 246–252. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Ai, T.T.; Li, W.H.; Jing, R.; Fei, Y.H.; Feng, X.M. Photoelectric and electrochemical performance of Al-doped ZnO thin films hydrothermally grown on graphene-coated polyethylene terephthalate bilayer flexible substrates. J. Phys. Chem. C 2017, 121, 28148–28157. [Google Scholar] [CrossRef]
- Lai, C.; Wang, M.; Zheng, G.M.; Liu, Y.G.; Zhang, C.; Wang, R.Z.; Xu, P.; Cheng, M.; Huang, C.; Wu, H.P.; et al. Synthesis of surface molecular imprinted TiO2/graphene photocatalyst and its highly efficient photocatalytic degradation of target pollutant under visible light irradiation. Appl. Surf. Sci. 2016, 390, 368–376. [Google Scholar] [CrossRef]
- Xu, J.; Cui, Y.; Han, Y.; Hao, M.; Zhang, X. ZnO-graphene composites with high photocatalytic activities under visible light. RSC Adv. 2016, 6, 96778–96784. [Google Scholar] [CrossRef]
- Thangavel, S.K.; Krishnamoorthy, V.; Krishnaswamy, N.; Raju, S.J.; Kim, G.J. Venugopal, Graphdiyne-ZnO nanohybrids as an advanced photocatalytic material. J. Phys. Chem. C 2015, 119, 22057–22065. [Google Scholar] [CrossRef]
- Türkyılmaz, Ş.Ş.; Güy, N.; Özacar, M. Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals. J. Photochem. Photobiol. A 2017, 341, 39–50. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Visible light photocatalytic activity of Fe3+-doped ZnO nanoparticle prepared via sol-gel technique. Chemosphere 2013, 91, 1604–1611. [Google Scholar] [CrossRef]
- Yousefi, R.; Beheshtian, J.; Seyed-Talebi, S.M.; Azimi, H.R.; Jamali-Sheini, F. Experimental and theoretical study of enhanced photocatalytic activity of Mg-doped ZnO NPs and ZnO/rGO nanocomposites. Chem. Asian J. 2018, 13, 194–203. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, C.G.; Wu, A.Q.; Tan, T.X.; Sun, L.; Wang, L.; Fu, H.G. A facile one-pot route for the controllable growth of small sized and well-dispersed ZnO particles on GO-derived grapheme. J. Mater. Chem. 2012, 22, 11778–11784. [Google Scholar] [CrossRef]
- Meng, Q.N.; Wang, K.; Tang, Y.F.; Zhao, K.; Zhang, G.J.; Zhao, L. One-pot synthesis of Fe2O3, loaded SiO2, hollow particles as effective visible light photo-Fenton catalyst. J. Alloy. Compd. 2017, 722, 8–16. [Google Scholar] [CrossRef]
- Huang, Z.J.; Wu, P.X.; Zhang, X.; Wang, X.R.; Zhu, N.W.; Wu, J.H.; Li, P. Intercalation of Fe (III) complexes into layered double hydroxides: Synthesis and structural preservation. Appl. Clay Sci. 2012, 65, 87–94. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.K.; Wang, J.Q. Photo-Fenton degradation of rhodamine B using Fe2O3-Kaolin as heterogeneous catalyst: Characterization, process optimization and mechanism. J. Colloid Interface Sci. 2014, 433, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rambu, A.P.; Ursu, L.; Iftimie, N.; Nica, V.; Dobromir, M.; Lacomi, F. Study on Ni-doped ZnO films as gas sensors. Appl. Surf. Sci. 2013, 280, 598–604. [Google Scholar] [CrossRef]
- Singh, V.P.; Rath, C. Passivation of native defects of ZnO by doping Mg detected through various spectroscopic techniques. RSC Adv. 2015, 5, 44390–44397. [Google Scholar] [CrossRef]
- Delarosa, E.; Sepulveda-Guzman, S.; Reeja-Jayan, B.; Torres, A.; Salas, P.; Elizondo, N.; Yacaman, M.J. Controlling the growth and luminescence properties of well-faceted ZnO nanorods. J. Phys. Chem. C 2007, 111, 8489–8495. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloy. Compd. 2017, 698, 555–564. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, X.R.; Duan, L.B.; Liu, R.D.; Wu, H.G.; Hou, T.; Jiang, X.W.; Gao, H.D. Influence of interface combination of RGO-photosensitized SnO2@ RGO core-shell structures on their photocatalytic performance. App. Surf. Sci. 2017, 391, 627–634. [Google Scholar] [CrossRef]
- Xie, H.; Gu, Y.; Mu, H. Photocatalytic performance of 3D Ni/graphene/ZnO composites fabricated by hydrothermal processing. J. Nanosci. Nanotechnol. 2018, 18, 4822–4833. [Google Scholar] [CrossRef]
- Wang, W.; Ai, T.; Yu, Q. Electrical and photocatalytic properties of boron-doped ZnO nanostructure grown on PET–ITO flexible substrates by hydrothermal method. Sci. Rep. 2017, 7, 42615. [Google Scholar] [CrossRef]
- Heinonen, S.; Kannisto, M.; Nikkanen, J.P.; Huttuncn-Saarivirta, E.; Karp, M.; Levanen, E. Photocatalytic and antibacterial properties of ZnO films with different surface topographies on stainless steel substrate. Thin Solid Film. 2016, 616, 842–849. [Google Scholar] [CrossRef]
- Kim, H.J.; Joshi, M.K.; Pant, H.R.; Kim, J.H.; Kim, C.S. One-pot hydrothermal synthesis of multifunctional Ag/ZnO/fly ash nanocomposite. Colloids Surf. A 2015, 469, 256–262. [Google Scholar] [CrossRef]
- Wei, A.; Xiong, L.; Sun, L.; Liu, Y.J.; Li, W.W. CuO nanoparticle modified ZnO nanorods with improved photocatalytic activity. Chin. Phys. Lett. 2013, 30, 046202. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, P.; Ren, S.; Jiang, J.; Yu, Q.; Jiang, L.; Zhang, Y. Preparation and Photocatalytic Properties of Metal-Doped ZnO Nanofilms Grown on Graphene-Coated Flexible Substrates. Materials 2020, 13, 3589. https://doi.org/10.3390/ma13163589
Rong P, Ren S, Jiang J, Yu Q, Jiang L, Zhang Y. Preparation and Photocatalytic Properties of Metal-Doped ZnO Nanofilms Grown on Graphene-Coated Flexible Substrates. Materials. 2020; 13(16):3589. https://doi.org/10.3390/ma13163589
Chicago/Turabian StyleRong, Ping, Shuai Ren, Jianchao Jiang, Qi Yu, Liyun Jiang, and Yonghong Zhang. 2020. "Preparation and Photocatalytic Properties of Metal-Doped ZnO Nanofilms Grown on Graphene-Coated Flexible Substrates" Materials 13, no. 16: 3589. https://doi.org/10.3390/ma13163589
APA StyleRong, P., Ren, S., Jiang, J., Yu, Q., Jiang, L., & Zhang, Y. (2020). Preparation and Photocatalytic Properties of Metal-Doped ZnO Nanofilms Grown on Graphene-Coated Flexible Substrates. Materials, 13(16), 3589. https://doi.org/10.3390/ma13163589




