What We Should Consider for Full Densification when Sintering
Abstract
1. Introduction
2. Preventing the Entrapment of Insoluble Gases
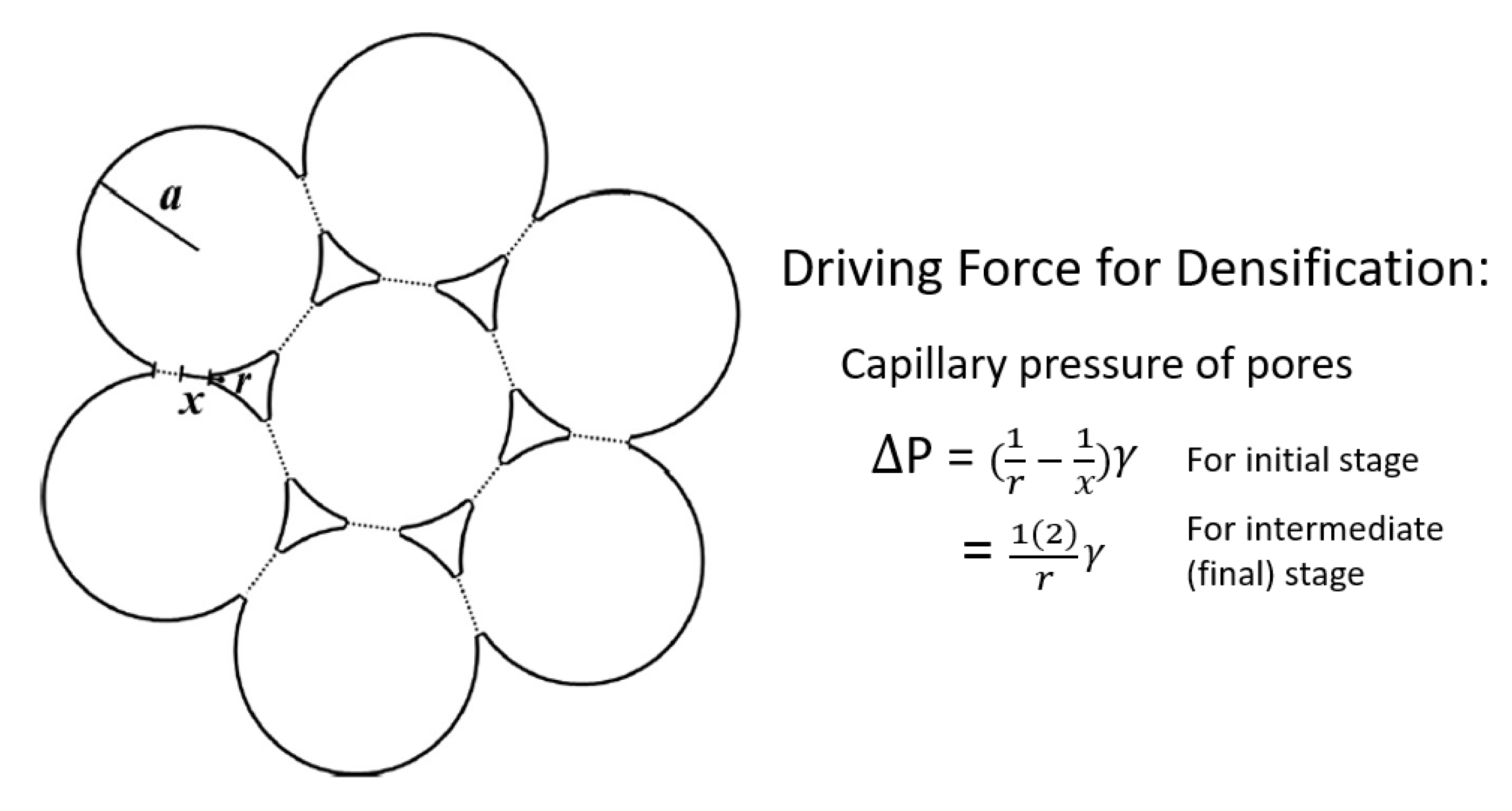
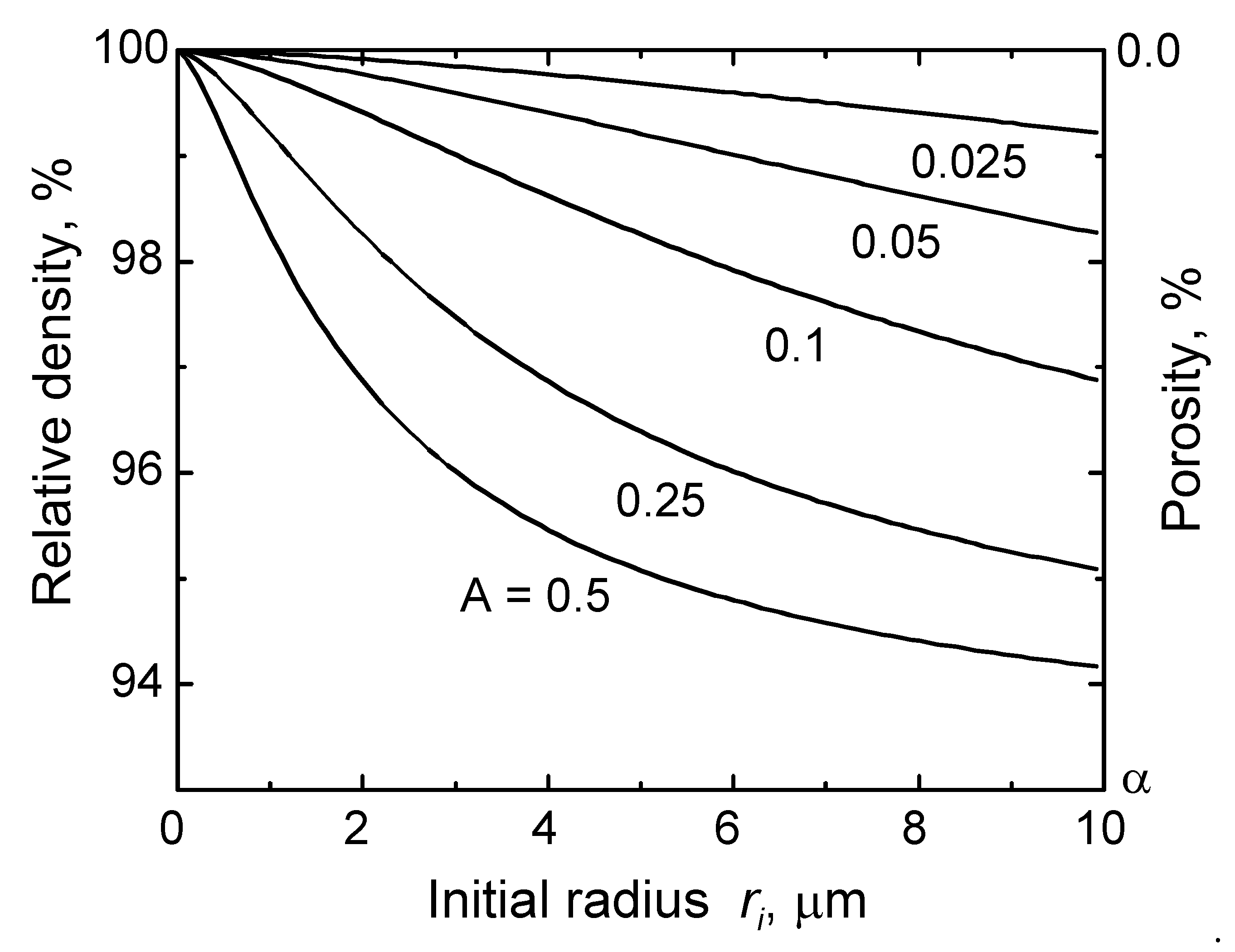
3. Pore Entrapment within Grains and Its Classical Analysis
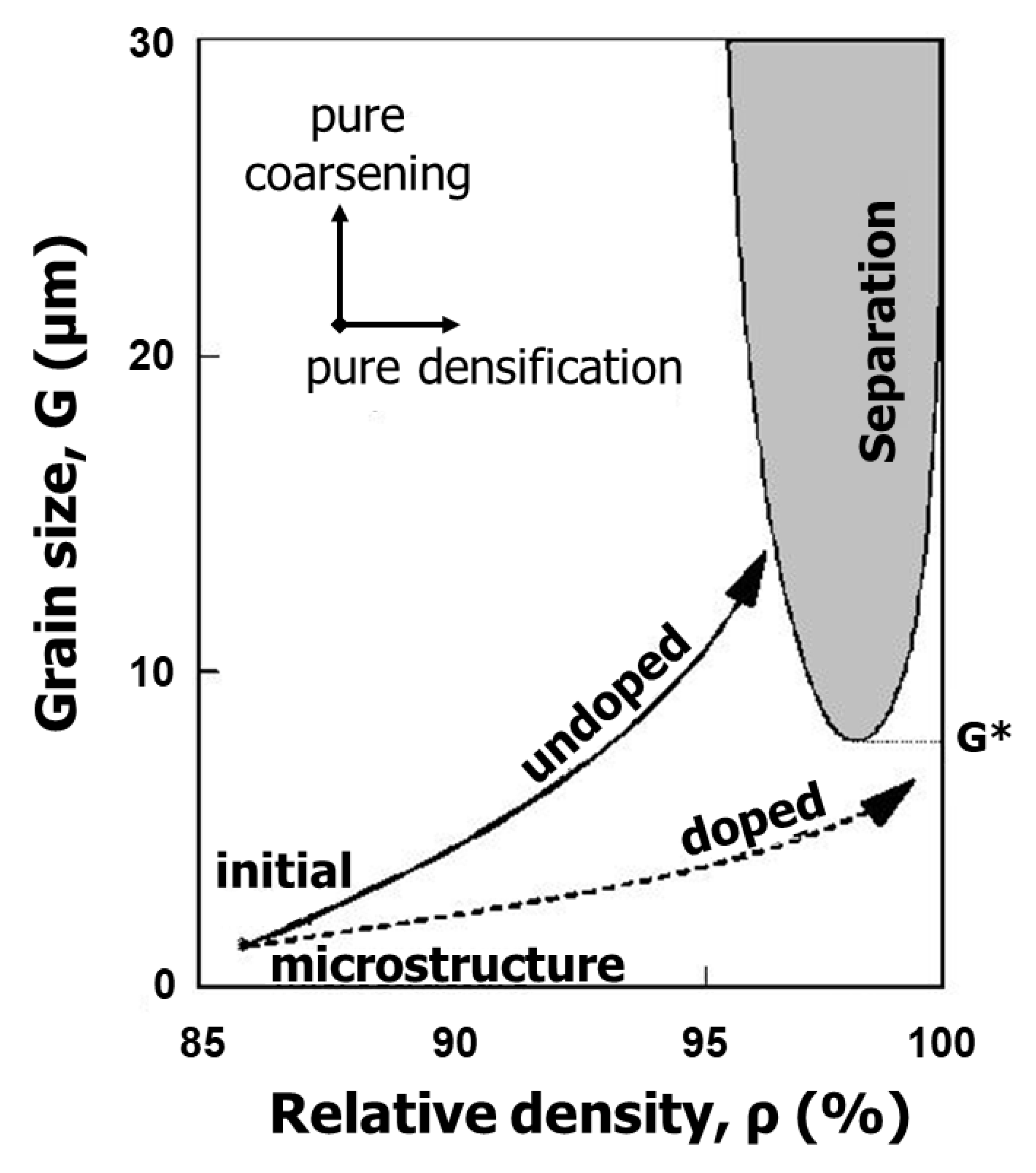
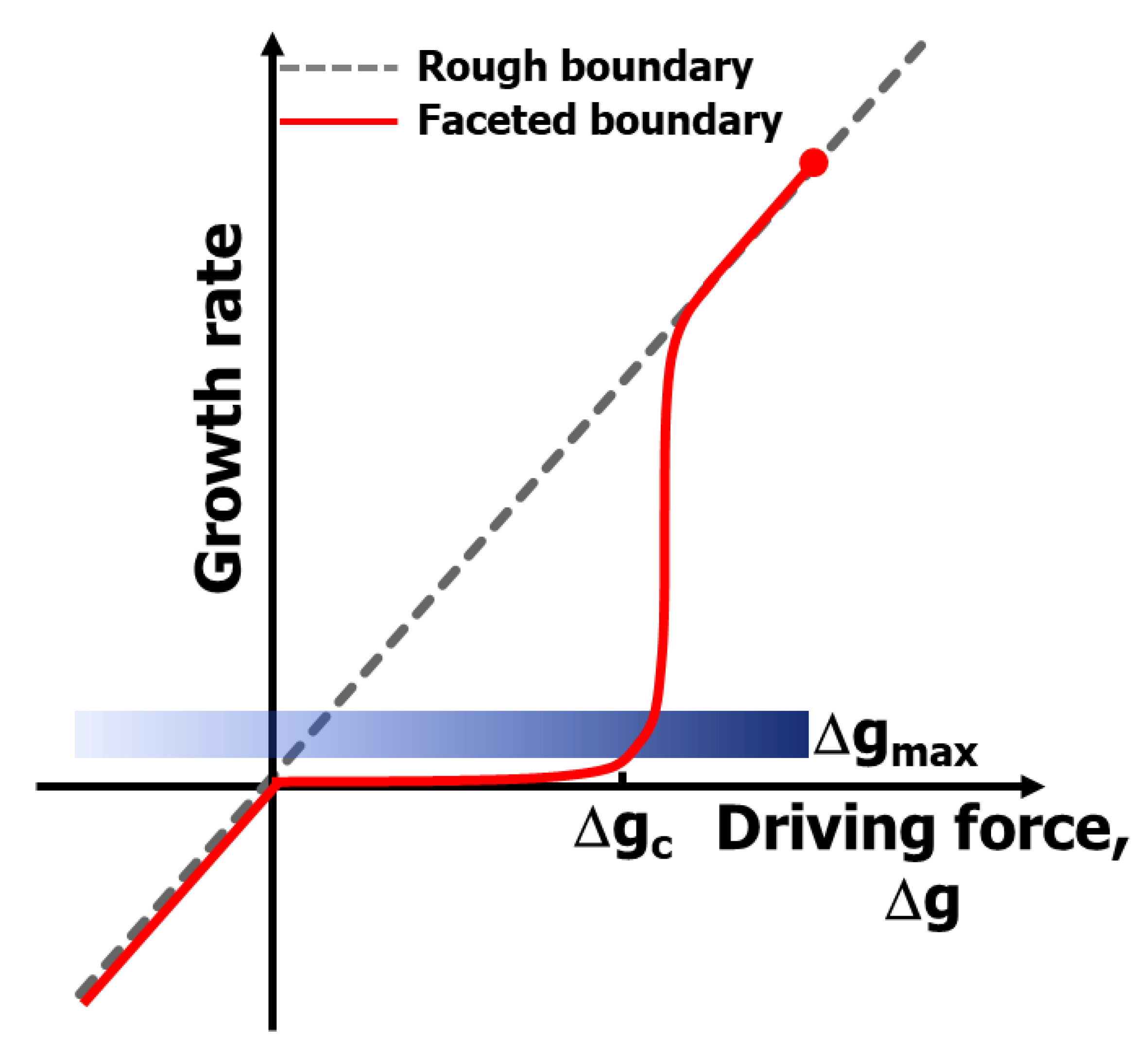
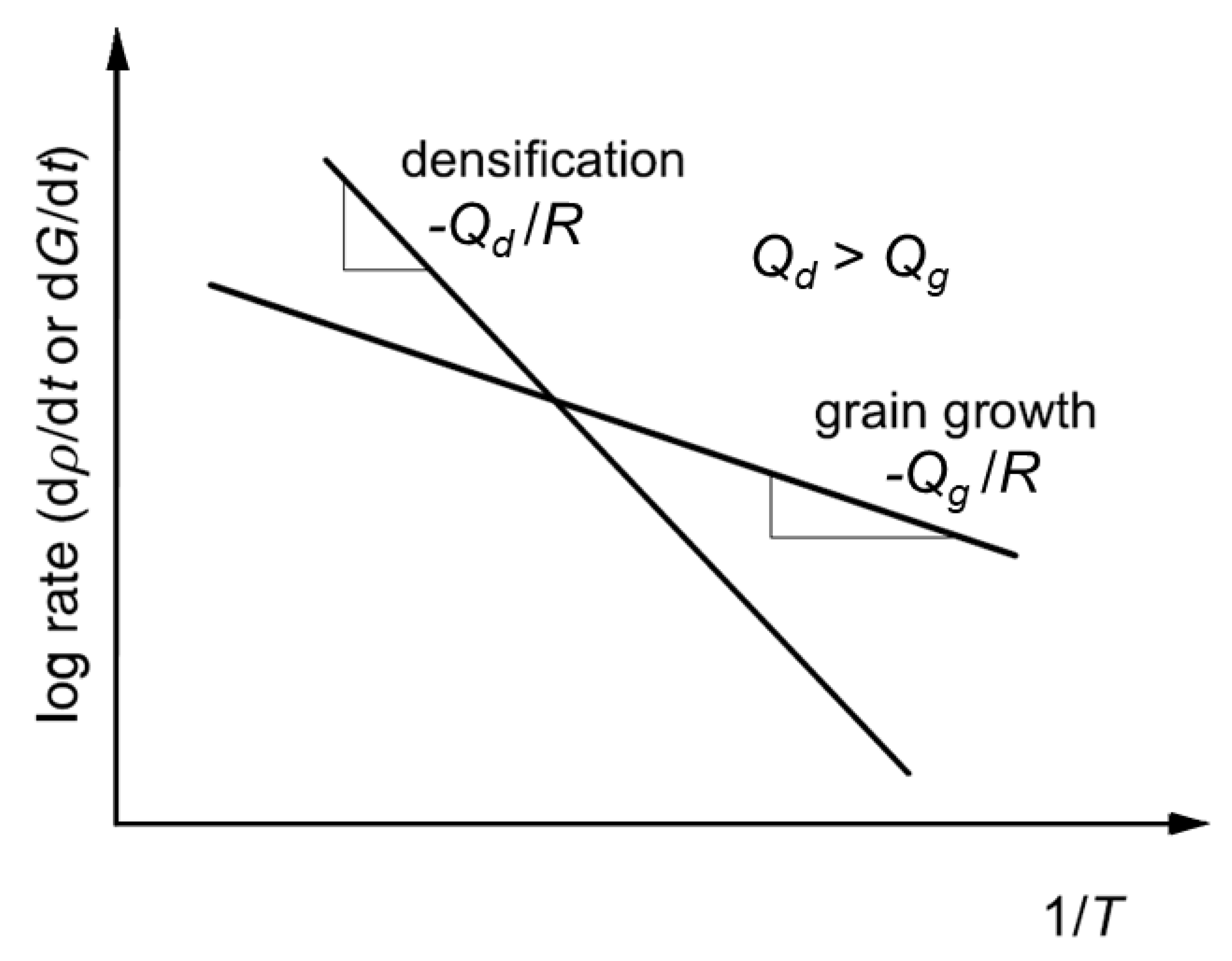
4. Suppression of Pore Entrapment: Grain Growth Control
5. Techniques for Enhancing Densification Relative to Grain Growth
5.1. Application of External Pressure
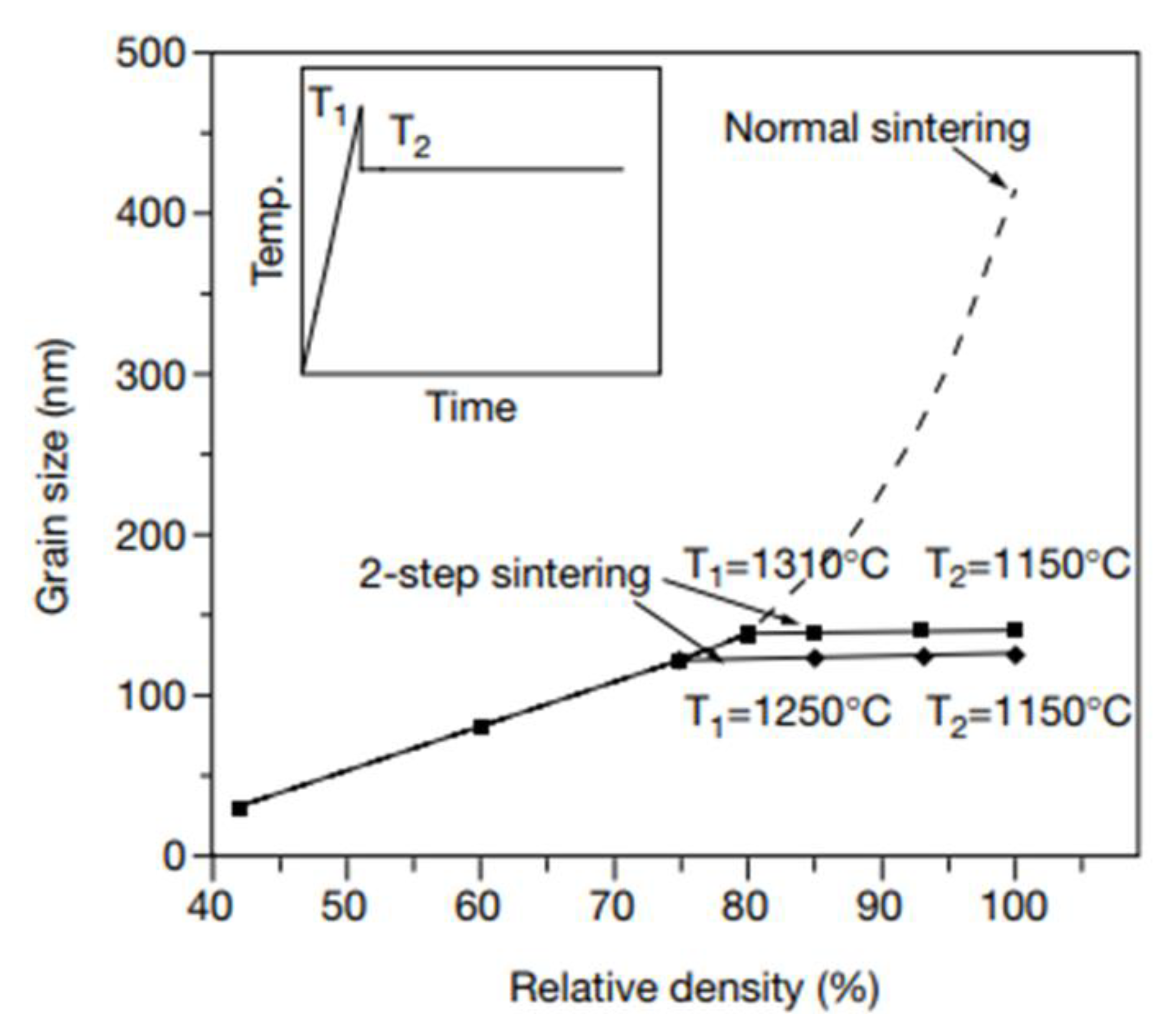
5.2. Modification of Thermal Cycle
5.3. Application of an External Field
5.4. Addition of Dopants
6. Concluding Remarks
Funding
Conflicts of Interest
References
- Kang, S.-J.L. Sintering: Densification, Grain Growth and Microstructure; Elsevier Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Bordia, R.K.; Kang, S.-J.L.; Olevsky, E. Current understanding and future research directions at the onset of the next century of sintering science and technology. J. Am. Ceram. Soc. 2017, 100, 2314–2352. [Google Scholar] [CrossRef]
- Herring, C. Effect of change of scale on sintering phenomena. J. Appl. Phys. 1950, 21, 301–303. [Google Scholar] [CrossRef]
- Liniger, E.; Raj, R. Packing and sintering of two-dimensional structures made from bimodel particle size distributions. J. Am. Ceram. Soc. 1987, 70, 843–849. [Google Scholar] [CrossRef]
- Choi, J.-P.; Lyu, H.-G.; Lee, W.-S.; Lee, J.-S. Densification and microstructural development during sintering of powder injection molded Fe micro-nanopowder. Powder Technol. 2014, 253, 596–601. [Google Scholar] [CrossRef]
- Kang, S.-J.L.; Yoon, K.J. Densification of ceramics containing entrapped gases. J. Eur. Ceram. Soc. 1989, 5, 135–139. [Google Scholar] [CrossRef]
- Paek, Y.-K.; Eun, K.Y.; Kang, S.-J.L. Effect of sintering atmosphere on densification of MgO doped Al2O3. J. Am. Ceram. Soc. 1988, 71, C380–C382. [Google Scholar] [CrossRef]
- Coble, R.L. Sintering alumina: Effect of atmospheres. J. Am. Ceram. Soc. 1962, 45, 123–127. [Google Scholar] [CrossRef]
- Yoon, B.K.; Chin, E.-Y.; Kang, S.-J.L. Dedensification during sintering of BaTiO3 caused by the decomposition of residual BaCO3. J. Am. Ceram. Soc. 2008, 91, 4121–4124. [Google Scholar] [CrossRef]
- Alexander, B.H.; Balluffi, R.W. The mechanism of sintering of copper. Acta Metall. 1957, 5, 666–677. [Google Scholar] [CrossRef]
- Kwon, S.-T.; Kim, D.-Y.; Kang, T.-K.; Yoon, D.N. Effect of sintering temperature on the densification of Al2O3. J. Am. Ceram. Soc. 1987, 70, C69–C70. [Google Scholar] [CrossRef]
- Brook, R.J. Pore-grain boundary interactions and grain growth. J. Am. Ceram. Soc. 1969, 52, 56–57. [Google Scholar] [CrossRef]
- Hsueh, C.H.; Evans, A.G.; Coble, R.L. Microstructure development during final/intermediate stage sintering I. Pore/grain boundary separation. Acta Metall. 1982, 30, 1269–1279. [Google Scholar] [CrossRef]
- Harmer, M.P. Use of solid-solution additives in ceramic processing. In Structure and Properties of MgO and Al2O3 Ceramics; Kingery, W.D., Ed.; Am. Ceram. Soc. Inc.: Columbus, OH, USA, 1985. [Google Scholar]
- Lee, B.K.; Chung, S.-Y.; Kang, S.-J.L. Grain boundary faceting and abnormal grain growth in BaTiO3. Acta Mater. 2000, 48, 1575–1580. [Google Scholar] [CrossRef]
- Kang, S.-J.L.; Lee, M.G.; An, S.M. Microstructural evolution during sintering with control of the interface structure. J. Am. Ceram. Soc. 2009, 92, 1464–1471. [Google Scholar] [CrossRef]
- Kang, S.-J.L.; Park, J.-H.; Ko, S.Y.; Lee, H.-Y. Solid-state conversion of single crystals: The principle and the state-of-the-art. J. Am. Ceram. Soc. 2015, 98, 347–360. [Google Scholar] [CrossRef]
- Kang, S.-J.L.; Ko, S.-Y.; Moon, S.Y. Mixed Control of boundary migration and the principle of microstructural evolution. J. Ceram. Soc. Japan 2016, 124, 259–267. [Google Scholar] [CrossRef]
- Park, C.W.; Yoon, D.Y. Effects of SiO2, CaO, and MgO additions on the grain growth of alumina. J. Am. Ceram. Soc. 2000, 83, 2605–2609. [Google Scholar] [CrossRef]
- An, S.M.; Kang, S.-J.L. Boundary structural transition and grain growth behavior in BaTiO3 with Nd2O3 doping and oxygen partial pressure change. Acta Mater. 2011, 59, 1964–1973. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kang, S.-J.L. Repetitive grain growth behavior with increasing temperature and grain boundary roughening in a model Ni system. Acta Mater. 2014, 69, 283–291. [Google Scholar] [CrossRef]
- Coble, R.L. Diffusion models for hot pressing with surface energy and pressure effects as driving forces. J. Appl. Phys. 1970, 41, 4798–4807. [Google Scholar] [CrossRef]
- Arzt, E.; Ashby, M.F.; Eastering, K.E. Practical applications of hot-isostatic pressing diagrams: Four case studies. Metall. Trans. A. 1983, 14, 211–221. [Google Scholar] [CrossRef]
- Mitomo, M. Pressure sintering of Si3N4. J. Mater. Sci. 1976, 11, 1103–1107. [Google Scholar] [CrossRef]
- Greskovich, C. Preparation of high density Si3N4 by a gas-pressure sintering process. J. Am. Ceram. Soc. 1981, 64, 725–730. [Google Scholar] [CrossRef]
- Harmer, M.P.; Brook, R.J. Fast firing—Microstructural benefits. J. Br. Ceram. Soc. 1981, 80, 147–148. [Google Scholar]
- Mostaghaci, H.; Brook, R.J. Production of dense and fine grain size BaTiO3 by fast firing. Trans. J. Br. Ceram. Soc. 1983, 82, 167–170. [Google Scholar]
- Chen, I.-W.; Wang, X.-H. Sintering dense nanocrystalline ceramics without final-stage grain growth. Nature 2000, 404, 168–171. [Google Scholar] [CrossRef]
- Wang, X.-H.; Deng, X.-Y.; Bai, H.-L.; Zhou, H.; Qu, W.-G.; Li, L.-T.; Chen, I.-W. Two-step sintering of ceramics with constant grain-size, II: BaTiO3 and Ni–Cu–Zn ferrite. J. Am. Ceram. Soc. 2006, 89, 438–443. [Google Scholar] [CrossRef]
- Lee, Y.-I.; Kim, Y.-W.; Mitomo, M.; Kim, D.-Y. Fabrication of dense nanostructured silicon carbide ceramics through two-step sintering. J. Am. Ceram. Soc. 2003, 86, 1803–1805. [Google Scholar] [CrossRef]
- Yang, D.-Y.; Yoon, D.Y.; Kang, S.-J.L. Suppression of abnormal grain growth in WC-Co via two-step liquid phase sintering. J. Am. Ceram. Soc. 2011, 94, 1019–1024. [Google Scholar] [CrossRef]
- Agrawal, D.K. Microwave processing of ceramics. Curr. Opin. Solid State Mater. Sci. 1998, 3, 480–486. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Olevsky, E.A.; Krikun, E.V. Microwave sintering: Fundamentals and modeling. J. Am. Ceram. Soc. 2013, 96, 1003–1020. [Google Scholar] [CrossRef]
- Tokita, M. Trends in advanced SPS spark plasma sintering systems and technology. J. Soc. Powder Technol. Jpn. 1993, 30, 790–804. [Google Scholar] [CrossRef]
- Nishimura, T.; Mitomo, M.; Hirotsuru, H.; Kawahara, M. Fabrication of silicon nitride nano-ceramics by spark plasma sintering. J. Mater. Sci. Lett. 1995, 14, 1046–1047. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Raethel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Cologna, M.; Rashkova, B.; Raj, R. Flash sintering of nanograin zirconia in <5 s at 850 °C. J. Am. Ceram. Soc. 2010, 93, 3556–3559. [Google Scholar]
- Ji, W.; Parker, B.; Zhang, J.Y.; Todd, R.I. Ultra-fast firing: Effect of heating rate on sintering of 3YSZ, with and without an electric field. J. Eur. Ceram. Soc. 2017, 37, 2547–2551. [Google Scholar] [CrossRef]
- Lee, M.-G.; Chung, S.-Y.; Kang, S.-J.L. Boundary faceting dependent densification in a BaTiO3 model system. Acta Mater. 2011, 59, 692–698. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-J.L. What We Should Consider for Full Densification when Sintering. Materials 2020, 13, 3578. https://doi.org/10.3390/ma13163578
Kang S-JL. What We Should Consider for Full Densification when Sintering. Materials. 2020; 13(16):3578. https://doi.org/10.3390/ma13163578
Chicago/Turabian StyleKang, Suk-Joong L. 2020. "What We Should Consider for Full Densification when Sintering" Materials 13, no. 16: 3578. https://doi.org/10.3390/ma13163578
APA StyleKang, S.-J. L. (2020). What We Should Consider for Full Densification when Sintering. Materials, 13(16), 3578. https://doi.org/10.3390/ma13163578



