Preparation and Characterization of TPP-Chitosan Crosslinked Scaffolds for Tissue Engineering
Abstract
1. Introduction
2. Result and Discussions
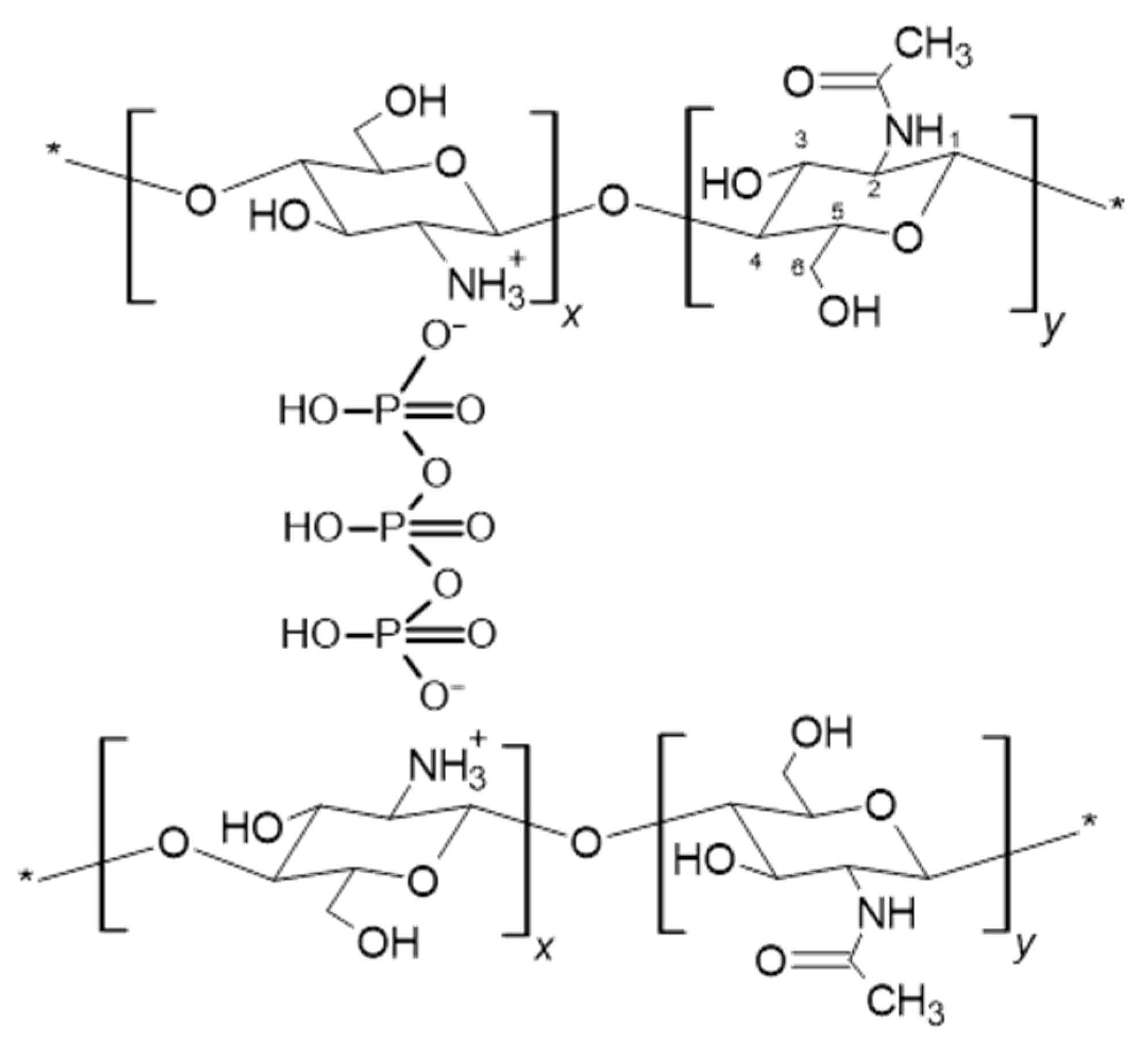
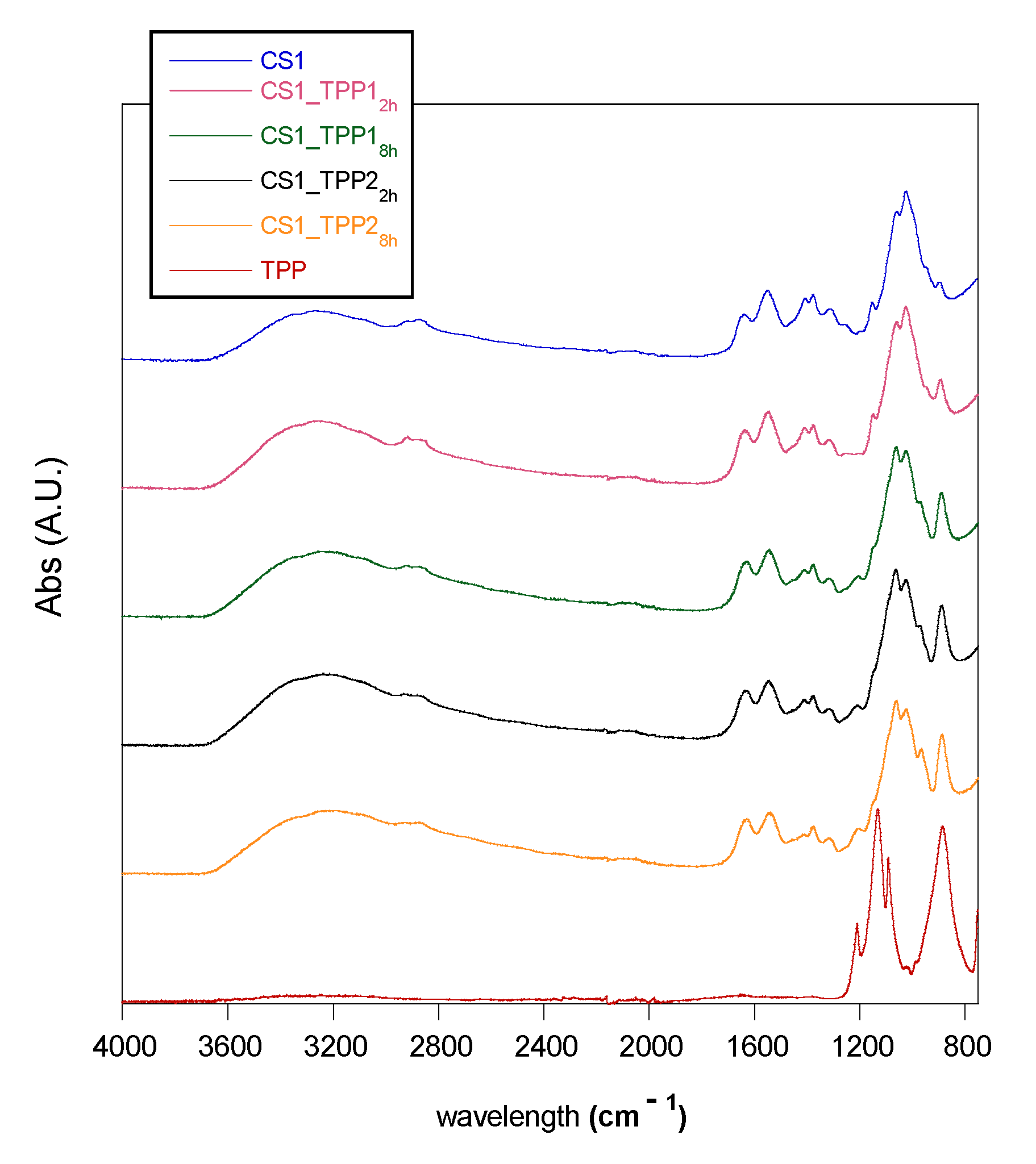
2.1. Infrared Spectroscopy Analysis
2.2. Thermogravimetric Analysis
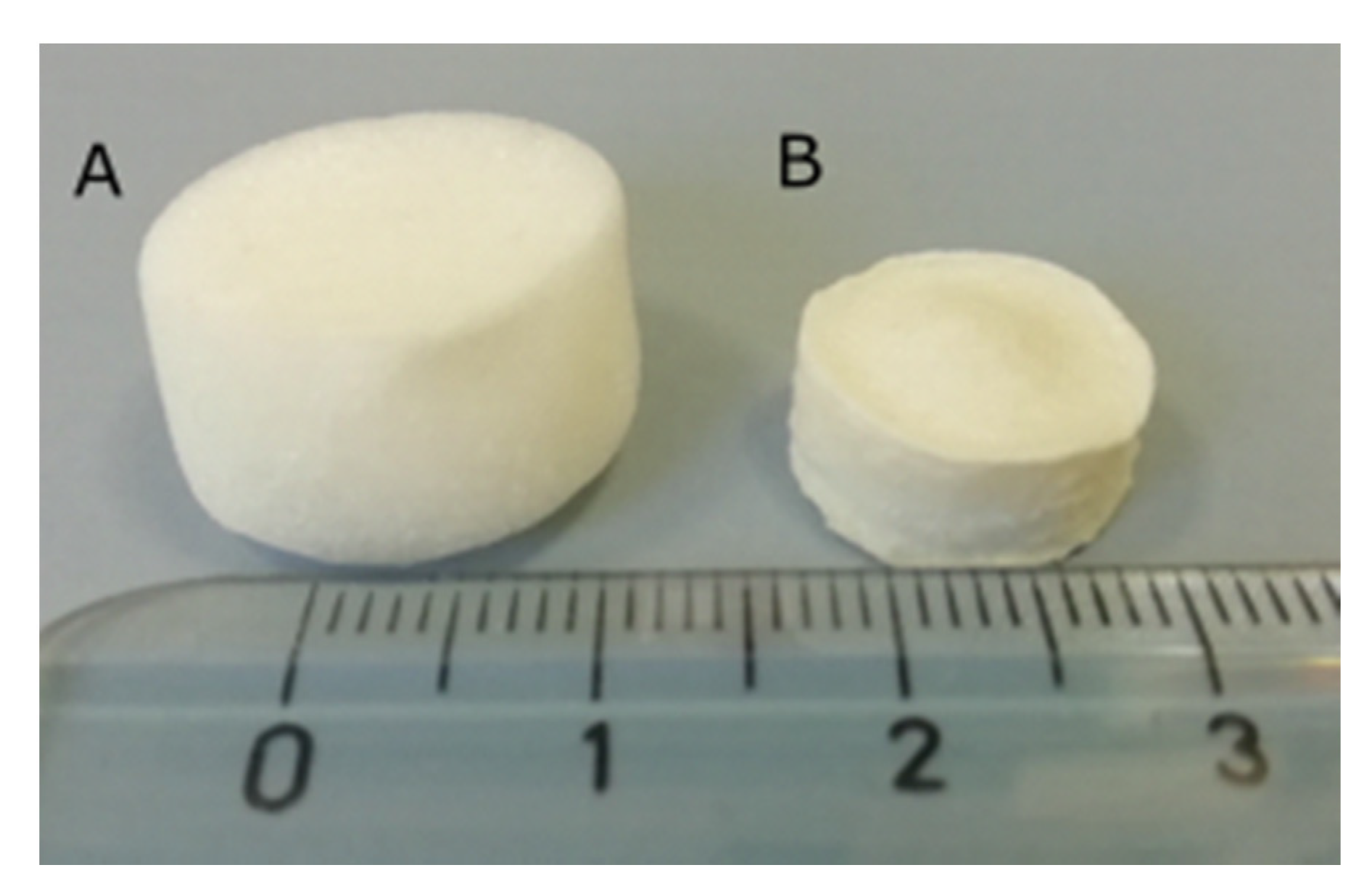
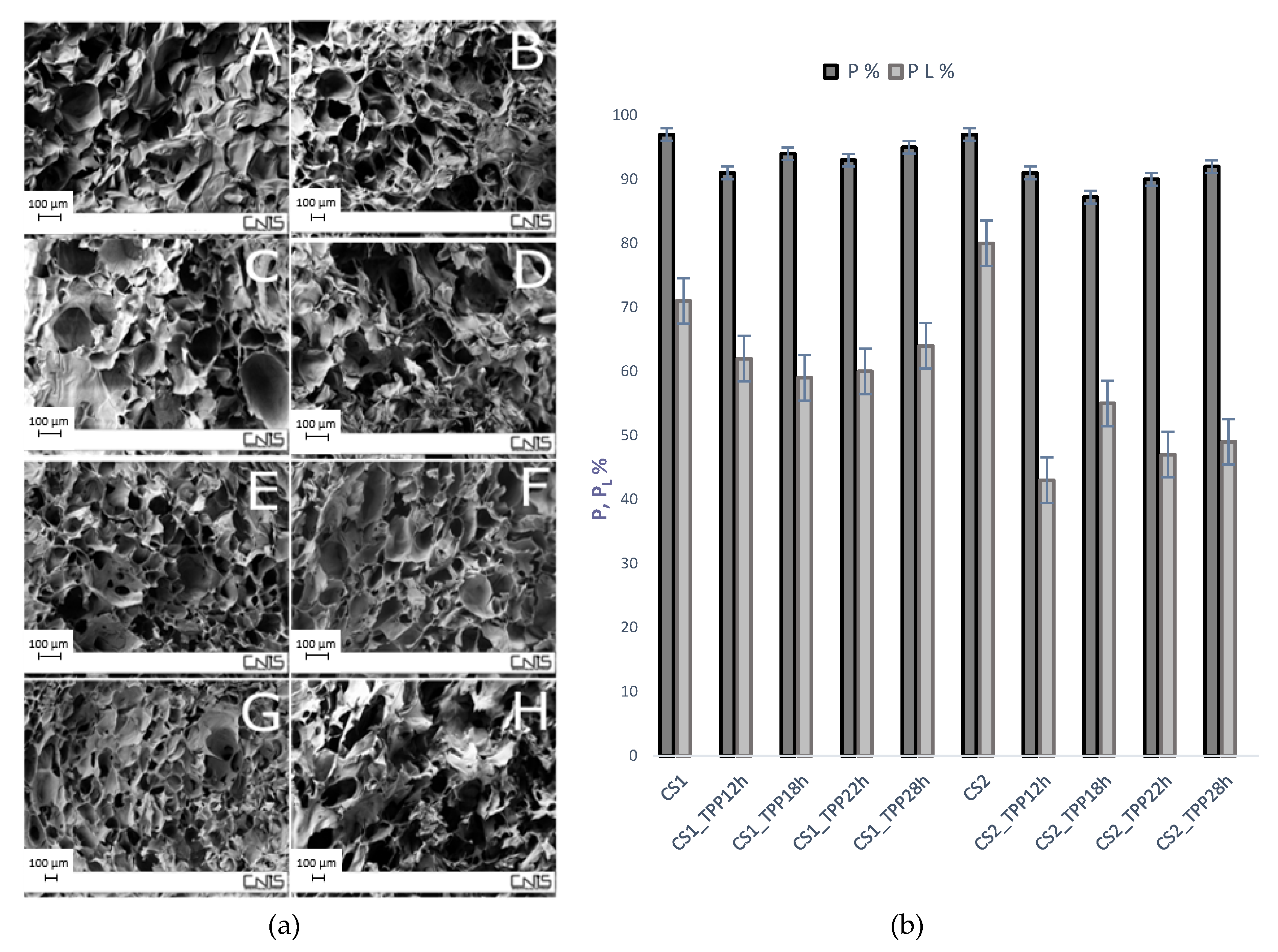
2.3. Morphological Properties and Porosity Tests
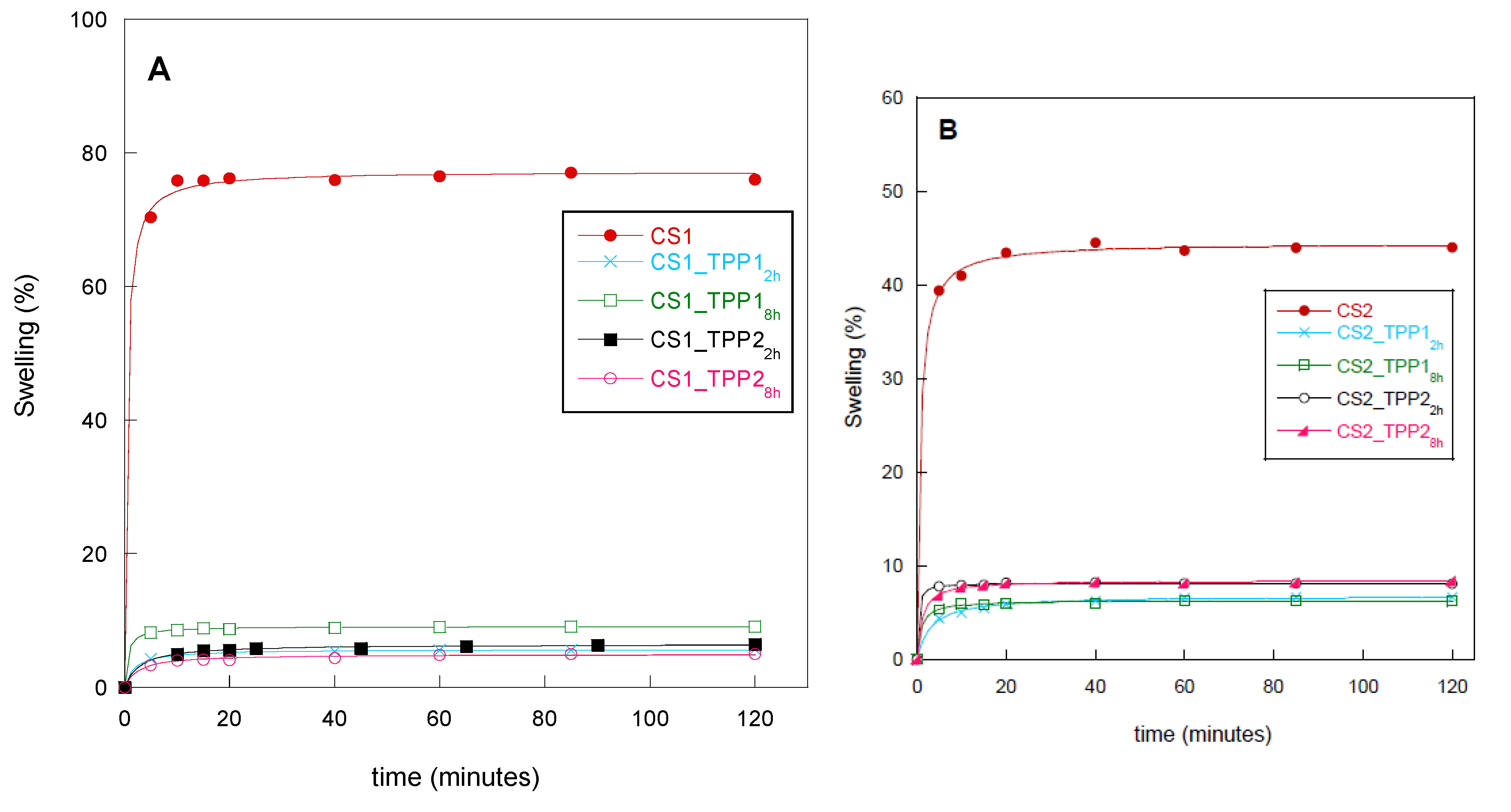
2.4. Water-Uptake Kinetics
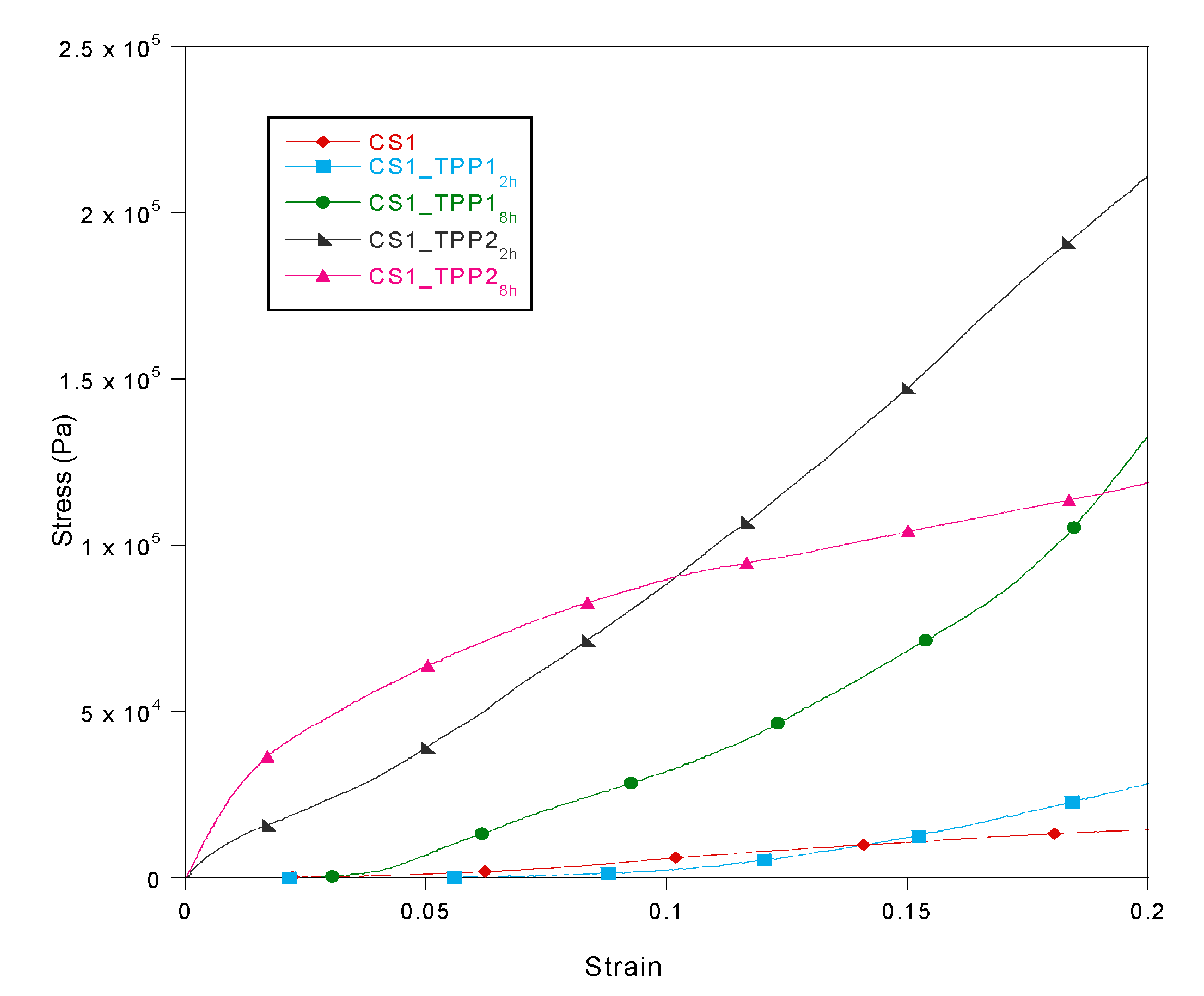
2.5. Mechanical Properties
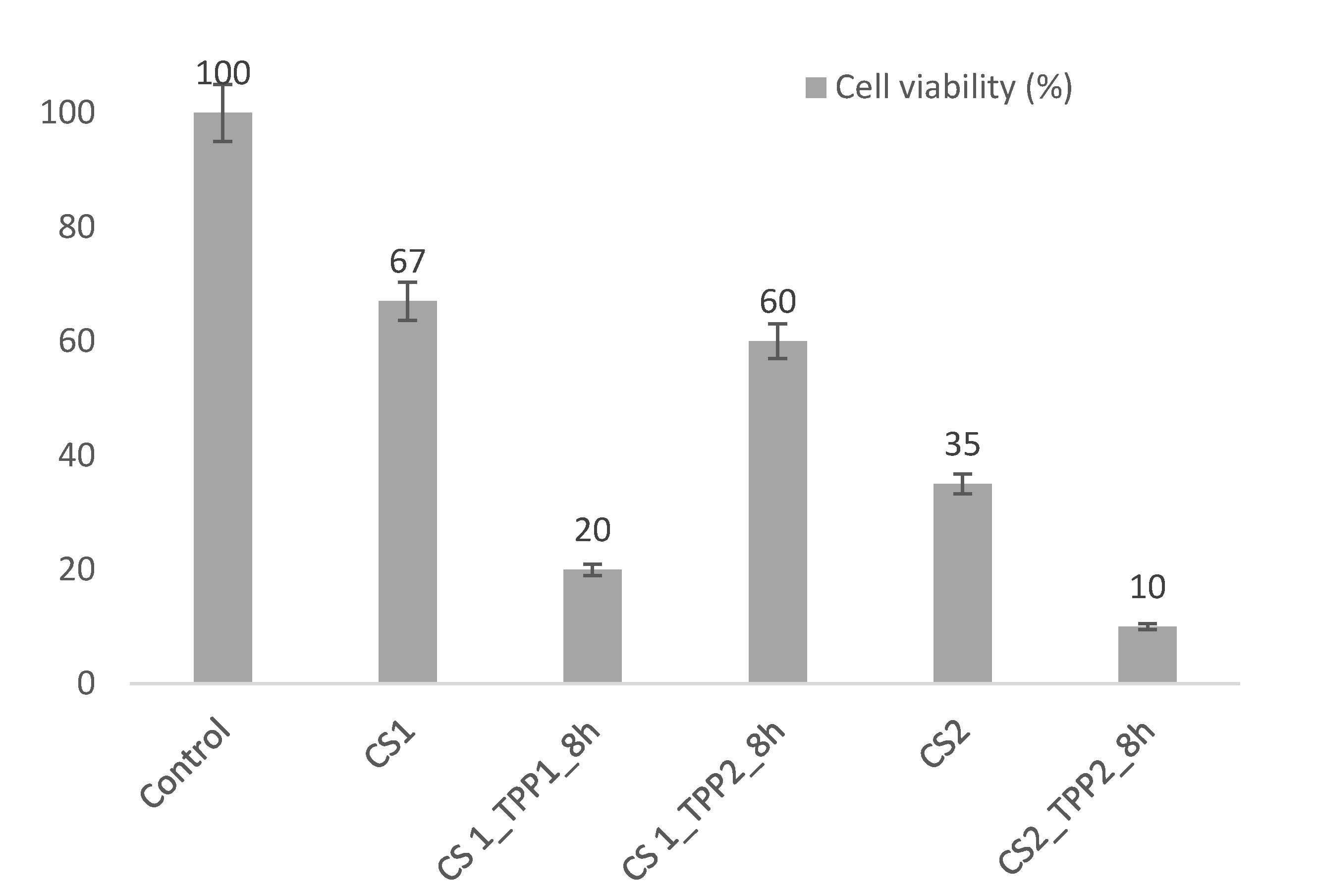
2.6. Cell Viability Tests
3. Materials and Methods
3.1. Viscosity and 1H-NMR Measurements
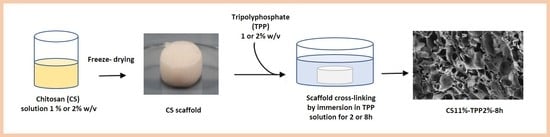
3.2. Preparation of CS_TPP Scaffolds
3.3. Infrared Spectroscopy (FTIR)
3.4. SEM Observation
3.5. Determination of the Scaffold Porosity and Interconnection Degree
3.6. Water-Uptake Kinetics of Scaffolds
3.7. Thermal and Mechanical Characterization
3.8. Cell Viability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Intern. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babushka, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials and scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mat. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef]
- Francolini, I.; Perugini, E.; Silvestro, I.; Lopreiato, M.; Scotto d’Abusco, A.; Valentini, F.; Placidi, E.; Arciprete, F.; Martinelli, A.; Piozzi, A. Graphene oxide oxygen content affects physical and biological properties of scaffolds based on chitosan/graphene oxide conjugates. Materials 2019, 12, 1142. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural polymers: Polysaccharides and their derivatives for biomedical applications. Nat. Synth. Biomed. Polym. 2014, 67–89. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Rizal, S.; Fazita, M.R.N.; Haafiz, M.K.M.; Nurmiati, Z.; Paridah, M.T. Enhancement of basic properties of polysaccharide-based composites with organic and inorganic fillers: A review. J. Appl. Polym. Sci. 2019, 136, 47251. [Google Scholar] [CrossRef]
- Silvestro, I.; Lopreiato, M.; Scotto d’Abusco, A.; Lisio, V.D.; Martinelli, A.; Piozzi, A.; Francolini, I. Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings. Intern. J. Mol. Sci. 2020, 21, 2070. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Misra, R.D.K. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Delivery Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Crucitti, V.C.; Migneco, L.M.; Piozzi, A.; Taresco, V.; Garnett, M.; Argent, R.H.; Francolini, I. Intermolecular interaction and solid state characterization of abietic acid/chitosan solid dispersions possessing antimicrobial and antioxidant properties. Eur. J. Pharm. Biopharm. 2018, 125, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Migneco, L.M.; Martinelli, A.; Pietrelli, L.; Piozzi, A.; Francolini, I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 2018, 179, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, T.; Filipkowska, U.; Szymczy, K.P.; Rodziewicz, J.; Mielcarek, A. Effect of ionic and covalent crosslinking on properties of chitosan beads and sorption effectiveness of Reactive Black 5 dye. React. Func. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J. Chitosan-based membranes with different ionic crosslinking density for pharmaceutical and industrial applications. Carbohydr. Polym. 2016, 153, 501–511. [Google Scholar] [CrossRef]
- Liao, C.-T.; Ho, M.-H. The fabrication of biomimetic chitosan scaffolds by using SBF treatment with different crosslinking agents. Membranes 2011, 1, 3–12. [Google Scholar] [CrossRef]
- Tomaz, A.F.; de Carvalho, S.M.S.; Barbosa, R.C.; Silva, L.; Suédina, M.; Gutierrez, M.A.S.; de Lima, B.; Gilson, A.; Fook, L.; Vinícius, M. Ionically Cross-linked Chitosan/Tripolyphosphate Microparticles for the Controlled Delivery of Pyrimethamine. Ibnosina J. Med. Biomed. Sci. 2011, 3. [Google Scholar] [CrossRef]
- Mi, F.-L.; Sung, H.-W.; Shyu, S.-S.; Su, C.-C.; Peng, C.-K. Synthesis and characterization of biodegradable TPP/genipin co-crosslinked chitosan gel beads. Polymer 2003, 44, 6521–6530. [Google Scholar] [CrossRef]
- Sandhya, J.; Veeralakshmi, S.; Kalaiselvam, S. Tripolyphosphate crosslinked Triticum aestivum (wheatgrass) functionalized antimicrobial chitosan: Ameliorating effect on physicochemical, mechanical, invitro cytocompatibility and cell migration properties. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Galdames, S.E.M.; Perez, A.G.M.; Ito, T.H.; Luzo, Â.C.M.; Santana, M.H.A. In vitro performance of injectable chitosan-tripolyphosphate scaffolds combined with platelet-rich plasma. Tissue Eng. Regen. Med. 2016, 13, 21–30. [Google Scholar] [CrossRef]
- Uswatta, S.P.; Okeke, I.U.; Jayasuriya, A.C. Injectable porous nano-hydroxyapatite/chitosan/tripolyphosphate scaffolds with improve compressive strength for bone regeneration. Mat. Sci. Eng. 2016, 69, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-H.; Wu, S.-J.; Wu, J.-Y.; Peng, C.-K.; Mi, F.-L. Tripolyphosphate cross-linked macromolecular composites for the growth of shape-and size-controlled apatites. Molecules 2013, 18, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Adhikari, B.; Dhara, S. Development of chitosan–tripolyphosphate fibers through pH dependent ionotropic gelation. Carbohydr. Res. 2011, 346, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Borgogna, M.; Travan, A.; Marsich, E.; Paoletti, S.; Asaro, F.; Grassi, M.; Donati, I. Polysaccharide-based networks from homogeneous chitosan-tripolyphosphate hydrogels: Synthesis and characterization. Biomacromolecules 2014, 15, 3396–3405. [Google Scholar] [CrossRef]
- Izzo, D.; Palazzo, B.; Scalera, F.; Gullotta, F.; lapesa, V.; Scialla, S.; Sannino, A.; Gervaso, F. Chitosan scaffolds for cartilage regeneration: Influence of different ionic crosslinkers on biomaterial properties. Intern. J. Polym. Mater. 2019, 68, 936–945. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1 H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J.; Charlet, G. Intrinsic Viscosity–Molecular Weight Relationship for Chitosan. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2591–2598. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Pokharkar, V.B. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: A technical note. AAPS PharmSciTech 2006, 7, E138–E143. [Google Scholar] [CrossRef]
- Chuc-Gamboa, M.G.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Escobar-García, D.M.; Pozos-Guillén, A.; del Barrio, J.S.R. The Effect of PEGDE Concentration and Temperature on Physicochemical and Biological Properties of Chitosan. Polymers 2019, 11, 1830. [Google Scholar] [CrossRef]
- Tomaz, A.F.; de Carvalho, S.M.S.; Barbosa, R.C.; Silva, L.; Suédina, M.; Gutierrez, M.A.S.; de Lima, B.; Gilson, A.; Fook, L.; Vinícius, M. Ionically Crosslinked Chitosan Membranes Used as Drug Carriers for Cancer Therapy Application. Materials 2018, 11, 2051. [Google Scholar] [CrossRef]
- Loutfy, S.A.; El-Din, H.M.A.; Elberry, M.H.; Allam, N.G.; Hasanin, M.T.M.; Abdellah, A.M. Synthesis, characterization and cytotoxic evaluation of chitosan nanoparticles: In vitro liver cancer model. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035008. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Dev. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone mechanical properties in healthy and diseased states. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, S.E.; Kwon, I.C.; Ahn, H.J.; Cho, H.; Lee, S.-H.; Kim, H.J.; Seong, S.C.; Lee, M.C. Effects of a chitosan scaffold containing TGF-β1 encapsulated chitosan microspheres on in vitro chondrocyte culture. Artif. Organs 2004, 28, 829–839. [Google Scholar] [CrossRef]
- Pati, F.; Kalita, H.; Adhikari, B.; Dhara, S. Osteoblastic cellular responses on ionically crosslinked chitosan-tripolyphosphate fibrous 3-D mesh scaffolds. J. Biomed. Mater. Res. A 2013, 101, 2526–2537. [Google Scholar] [CrossRef]
- Goh, C.Y.; Lim, S.S.; Tshai, K.Y.; Azab, A.W.Z.Z.E.; Loh, H.-S. Fabrication and in vitro biocompatibility of sodium tripolyphosphate-crosslinked chitosan–hydroxyapatite scaffolds for bone regeneration. J. Mater. Sci. 2019, 54, 3403–3420. [Google Scholar] [CrossRef]
- Han, J.; Zhou, Z.; Yin, R.; Yang, D.; Nie, J. Alginate–chitosan/hydroxyapatite polyelectrolyte complex porous scaffolds: Preparation and characterization. Intern. J. Biol. Macromol. 2010, 46, 199–205. [Google Scholar] [CrossRef]

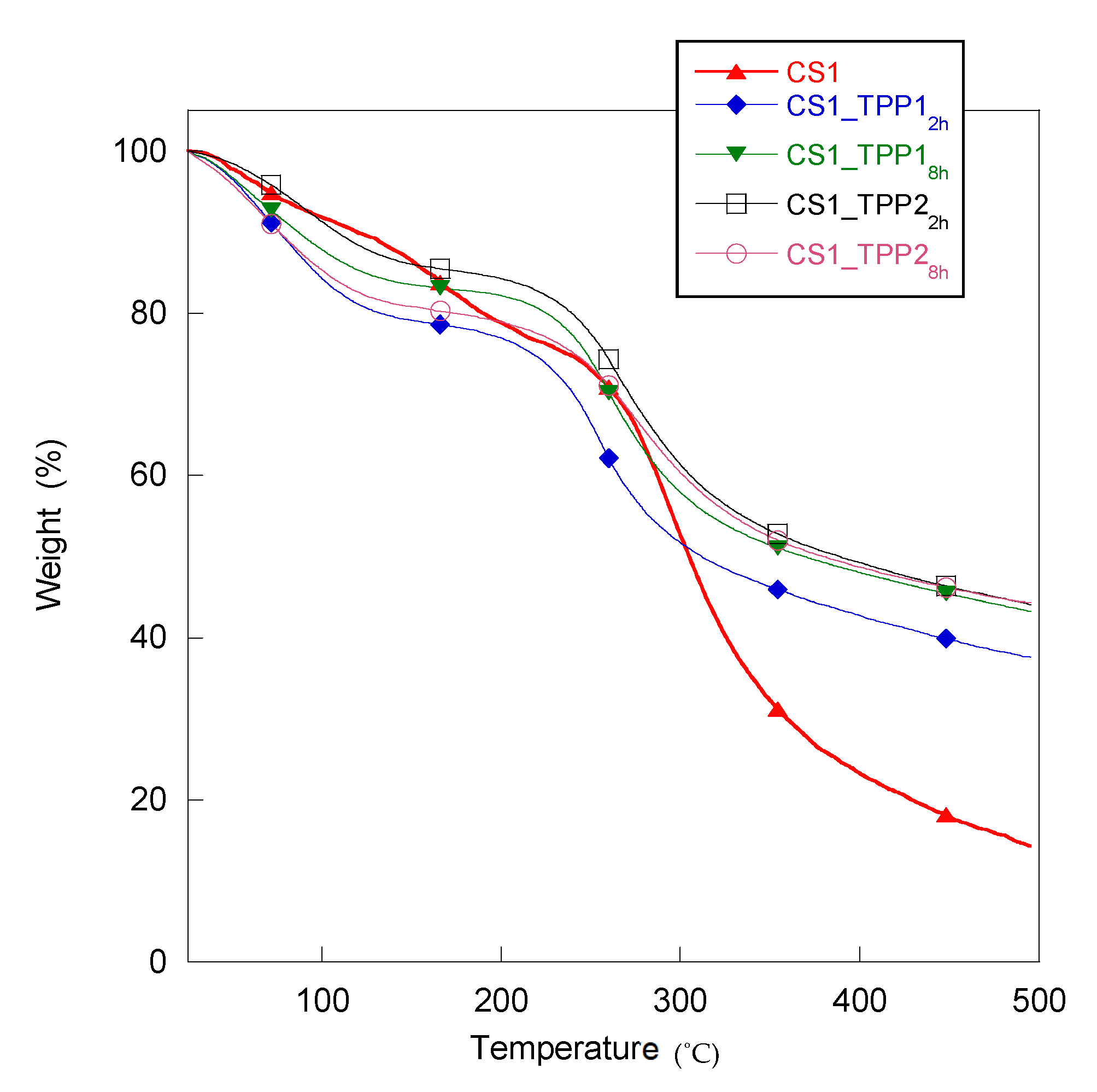
| Sample | CS Concentration (w/v %) | TPP Concentration (w/v %) | Cross-Linking Time (h) | Td (°C) | CM (MPa) | Pore Size (µm) | Pore Regularity |
|---|---|---|---|---|---|---|---|
| CS1 | 1 | - | - | 285 | 0.06 ± 0.01 | 80–180 | Good |
| CS2 | 2 | - | - | 290 | 0.2 ± 0.05 | 130–250 | Good |
| CS1_TPP12h | 1 | 1 | 2 | 255 | 0.2 ± 0.05 | 50–200 | Low |
| CS1_TPP18h | 1 | 1 | 8 | 262 | 0.5 ± 0.05 | 50–180 | Low |
| CS1_TPP22h | 1 | 2 | 2 | 270 | 0.6 ± 0.05 | 80–100 | Good |
| CS1_TPP28h | 1 | 2 | 8 | 277 | 1.2 ± 0.05 | 60–100 | Good |
| CS2_TPP12h | 2 | 1 | 2 | 262 | 0.3 ± 0.05 | 70–100 | Good |
| CS2_TPP18h | 2 | 1 | 8 | 247 | 0.9 ± 0.05 | 90–110 | Good |
| CS2_TPP22h | 2 | 2 | 2 | 282 | 1.8 ± 0.05 | 100–120 | Good |
| CS2_TPP28h | 2 | 2 | 8 | 273 | 4.7 ± 0.05 | 200–300 | Low |
| Sample | ANH2/AC = O | AP-O-P/AC = O |
|---|---|---|
| CS1 | 1.96 | 0.58 |
| CS2 | 2.02 | 0.58 |
| CS1_TPP12h | 1.29 | 0.89 |
| CS1_TPP18h | 1.10 | 1.03 |
| CS1_TPP22h | 1.15 | 1.19 |
| CS1_TPP28h | 0.94 | 1.50 |
| CS2_TPP12h | 1.31 | 0.67 |
| CS2_TPP18h | 1.28 | 1.04 |
| CS2_TPP22h | 1.29 | 1.30 |
| CS2_TPP28h | 1.00 | 1.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestro, I.; Francolini, I.; Di Lisio, V.; Martinelli, A.; Pietrelli, L.; Scotto d’Abusco, A.; Scoppio, A.; Piozzi, A. Preparation and Characterization of TPP-Chitosan Crosslinked Scaffolds for Tissue Engineering. Materials 2020, 13, 3577. https://doi.org/10.3390/ma13163577
Silvestro I, Francolini I, Di Lisio V, Martinelli A, Pietrelli L, Scotto d’Abusco A, Scoppio A, Piozzi A. Preparation and Characterization of TPP-Chitosan Crosslinked Scaffolds for Tissue Engineering. Materials. 2020; 13(16):3577. https://doi.org/10.3390/ma13163577
Chicago/Turabian StyleSilvestro, Ilaria, Iolanda Francolini, Valerio Di Lisio, Andrea Martinelli, Loris Pietrelli, Anna Scotto d’Abusco, Andromeda Scoppio, and Antonella Piozzi. 2020. "Preparation and Characterization of TPP-Chitosan Crosslinked Scaffolds for Tissue Engineering" Materials 13, no. 16: 3577. https://doi.org/10.3390/ma13163577
APA StyleSilvestro, I., Francolini, I., Di Lisio, V., Martinelli, A., Pietrelli, L., Scotto d’Abusco, A., Scoppio, A., & Piozzi, A. (2020). Preparation and Characterization of TPP-Chitosan Crosslinked Scaffolds for Tissue Engineering. Materials, 13(16), 3577. https://doi.org/10.3390/ma13163577