Effect of Cu- and Zn-Doped Bioactive Glasses on the In Vitro Bioactivity, Mechanical and Degradation Behavior of Biodegradable PDLLA Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of PDLLA/Metal-Doped Bioactive Glass Porous Scaffolds
2.3. Characterization
2.3.1. Scaffold Porosity
2.3.2. Mechanical Evaluation
2.3.3. In Vitro Bioactivity Evaluation in Simulated Body Fluid (SBF)
2.3.4. Degradation Studies in Phosphate Buffer Solution (PBS)
3. Results and Discussion
3.1. Porosity and Pore Morphology
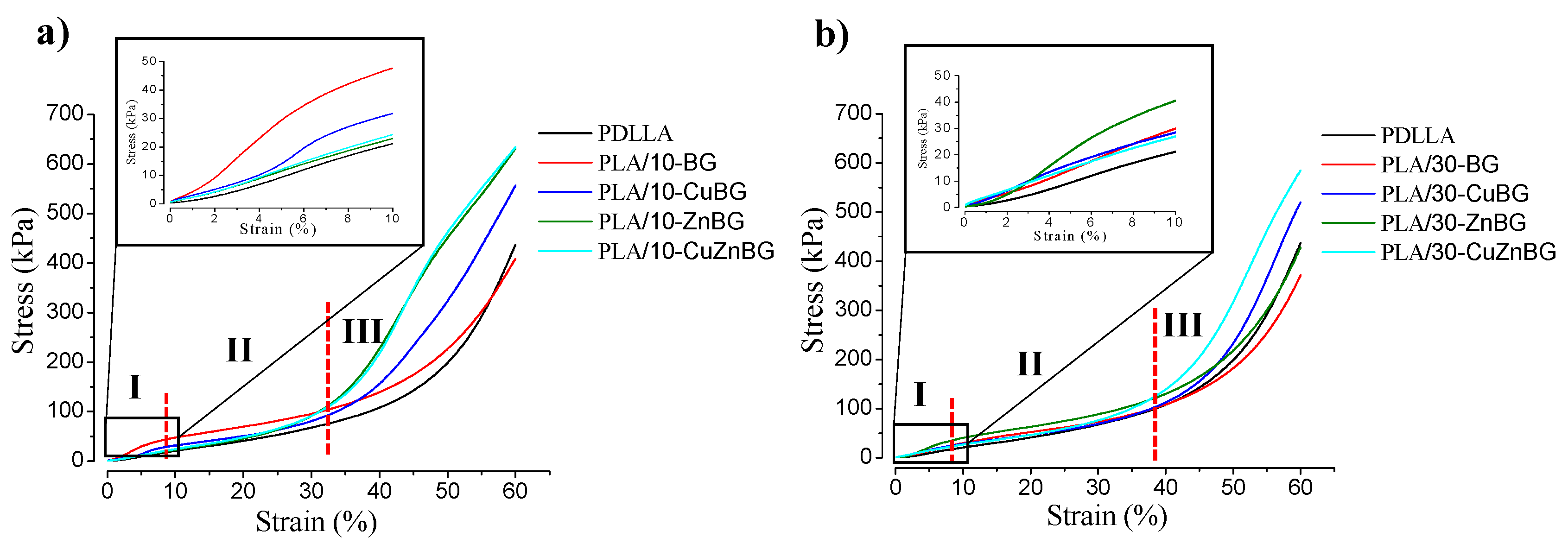
3.2. Mechanical Properties
- (I)
- Associated with the elastic deformation of the dense material forming the pore walls. This elastic deformation is reversible meaning that the polymer chains return to their equilibrium position when the stress is retired. After around 5–8% of strain, this elastic zone gradually disappears without a defined yield point because of the high porosity of these scaffolds. This transition indicates a second zone
- (II)
- Characterized by a change in the stress variation with the deformation due to the bending of the pore walls and their plastic deformation [46]. Ending the plastic deformation, the pore structure collapses starting a third zone
- (III)
- ICharacterized by the densification of the specimen at around 30–40% deformation. This denser specimen can support more load, and thus the compressive strength becomes notably higher with an exponential behavior.
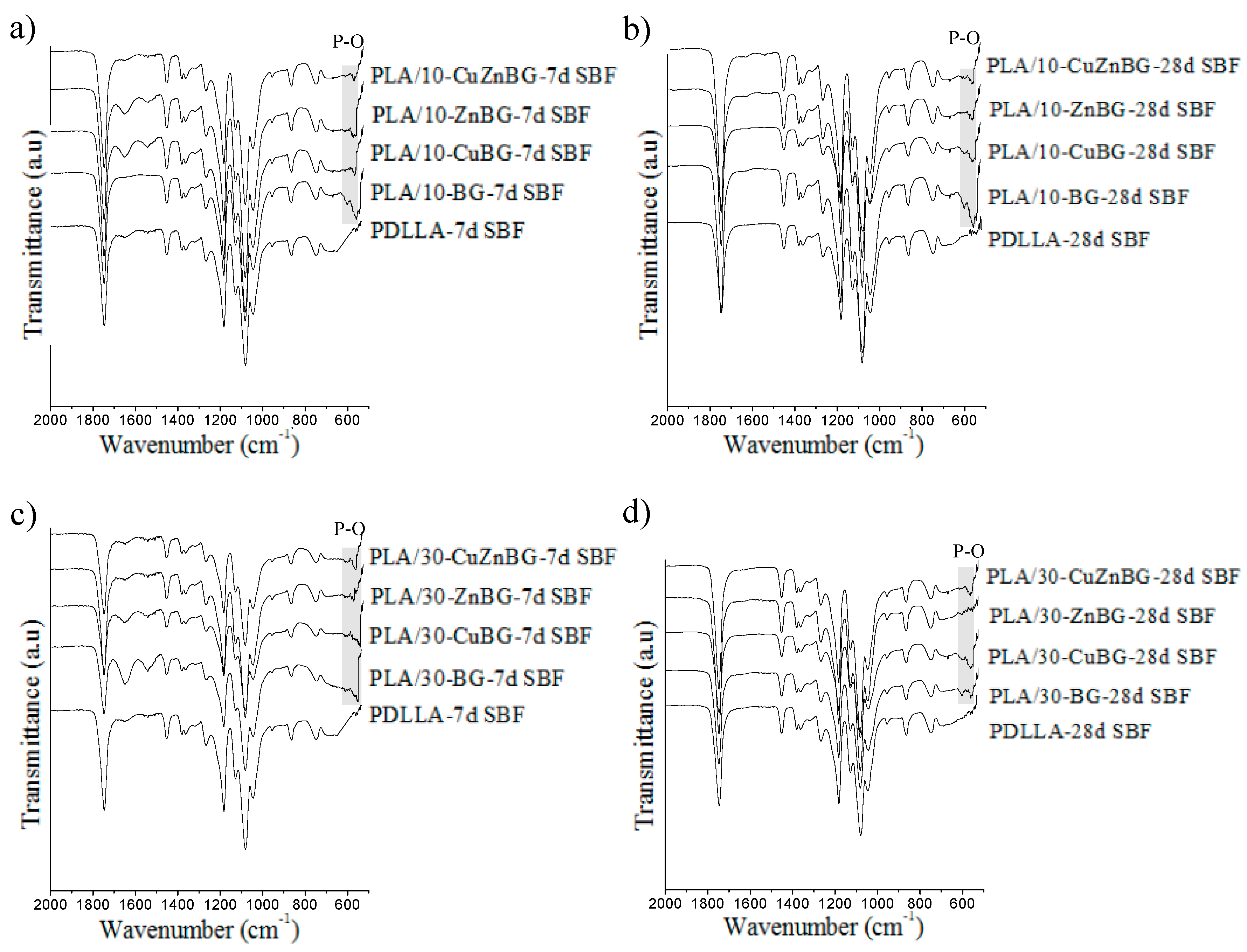
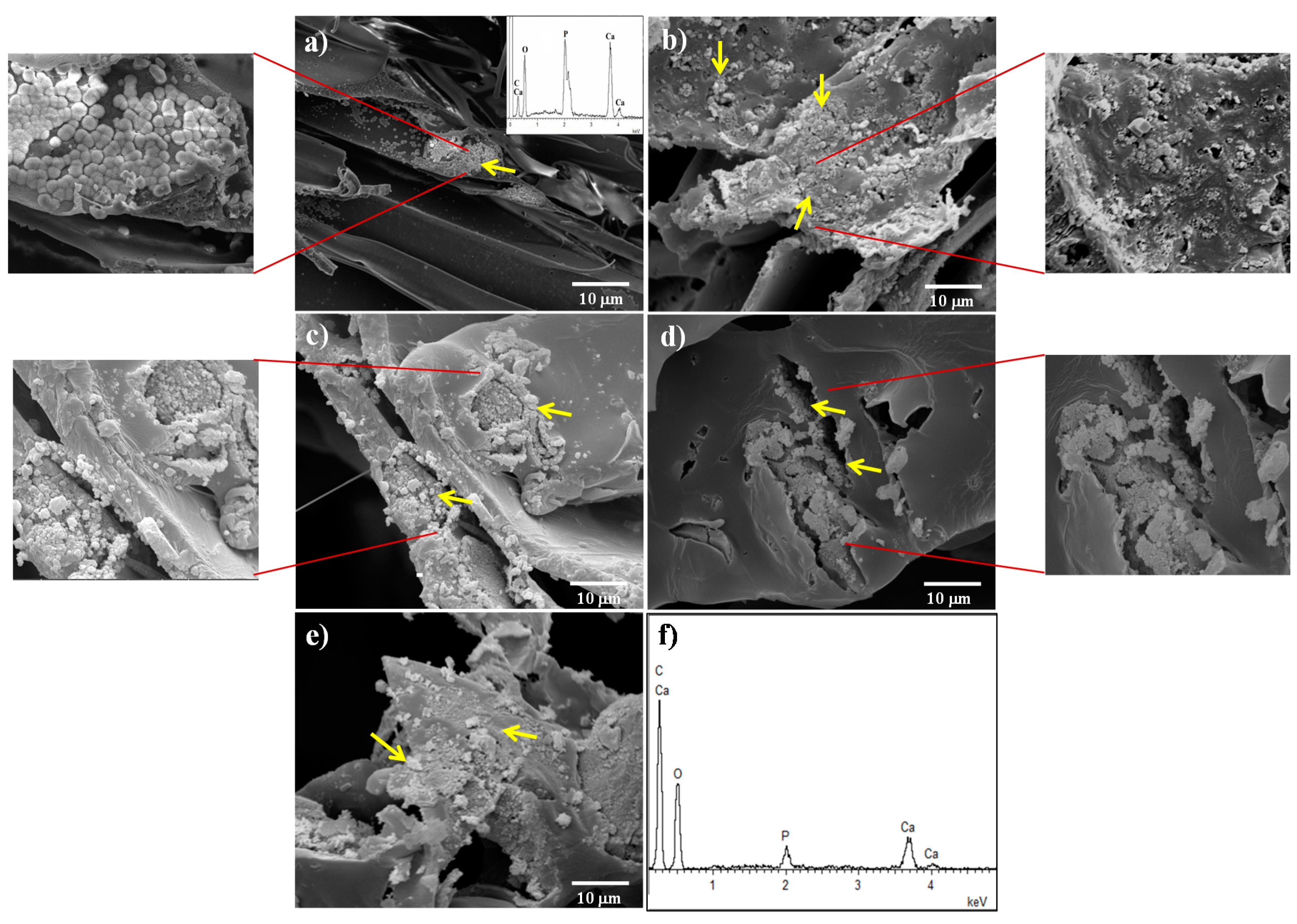
3.3. Apatite Formation in SBF
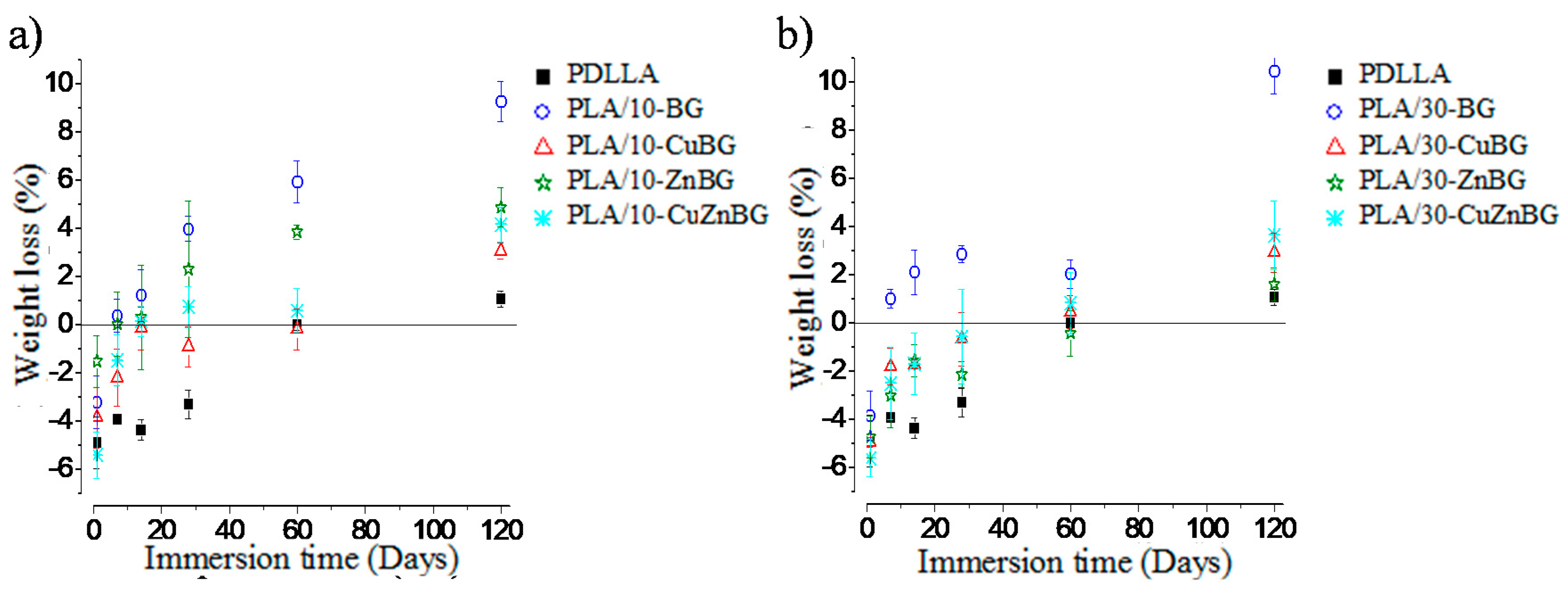
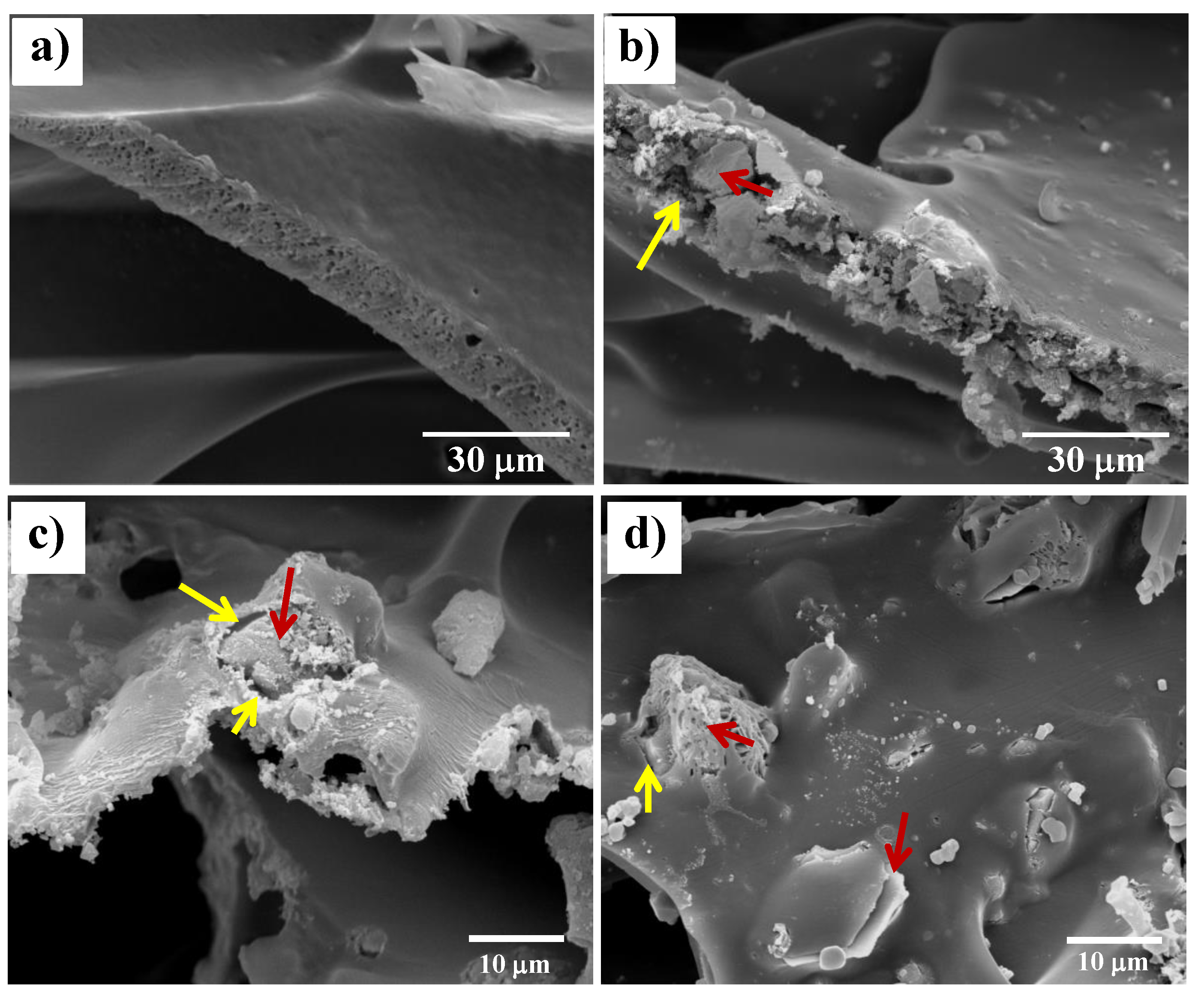
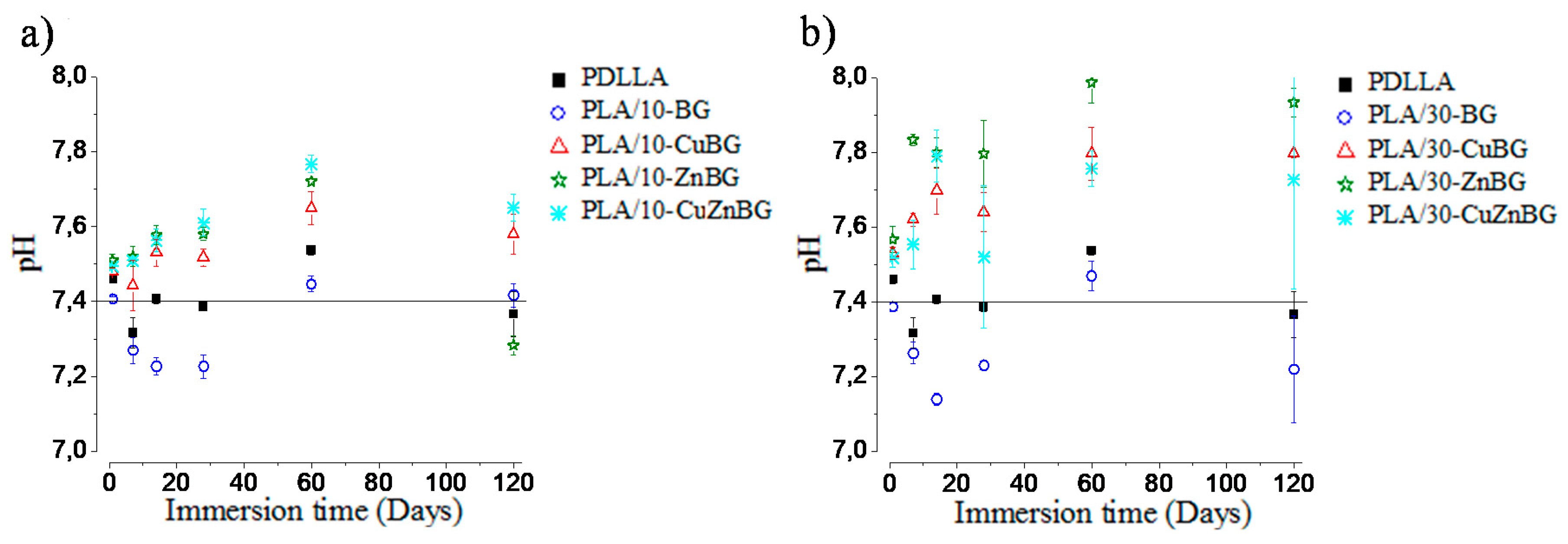
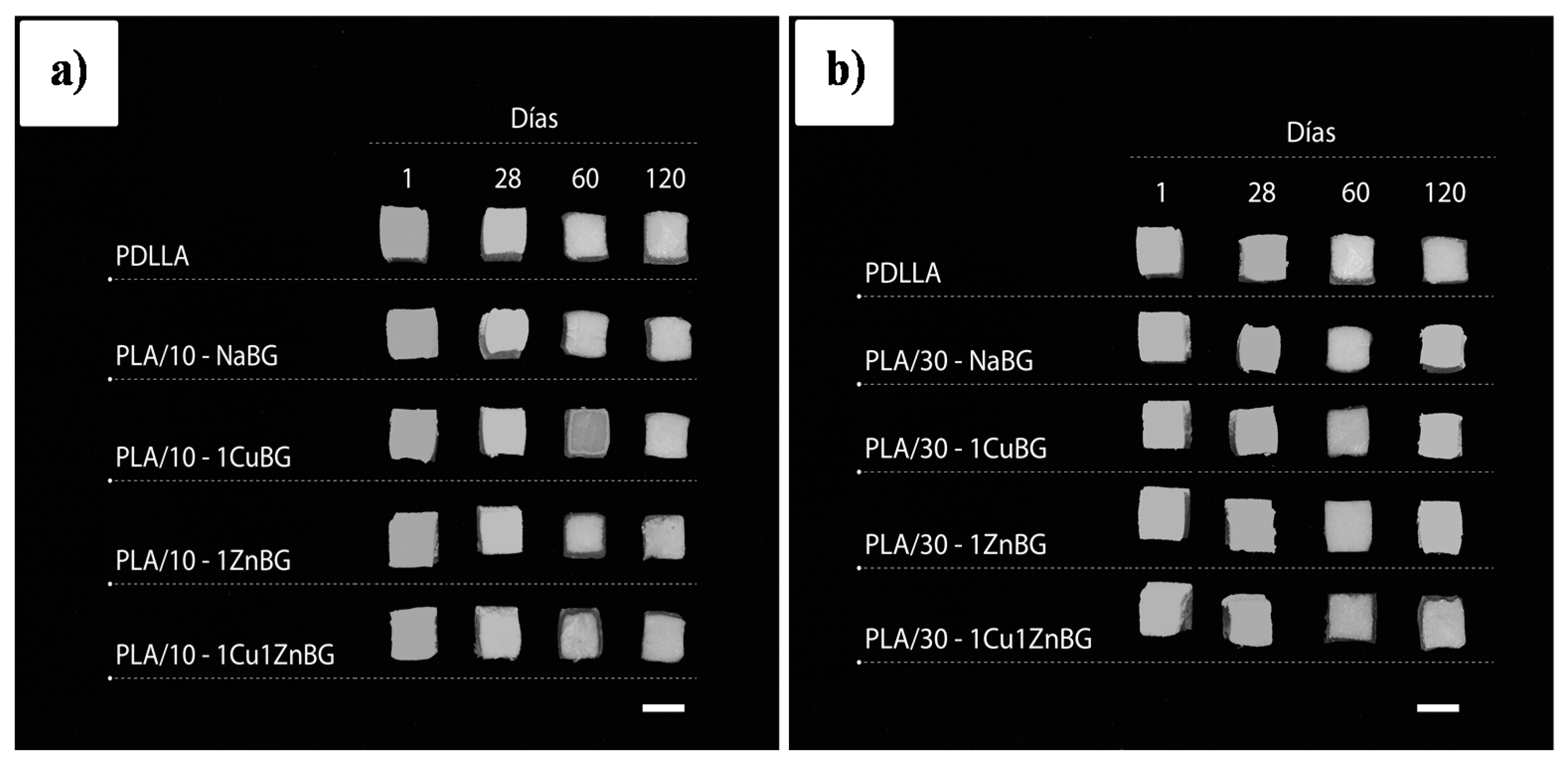
3.4. Degradation Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Mohamad, Y.D.; Bretcanu, O.; Boccaccini, A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008, 43, 4433–4442. [Google Scholar] [CrossRef]
- Wagoner, J.A.J.; Herschler, B.A. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Kroeze, R.J.; Helder, M.N.; Govaert, L.E.; Smit, T.H. Biodegradable Polymers in Bone Tissue Engineering. Materials 2009, 2, 833–856. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials 2013, 34, 8018–8029. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding Mechanisms at the Interface of Ceramic Prosthetic Materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Arcos, D.; AVallet-Regí, M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Hoppe, A.; Mouriño, V.; Boccaccini, A.R. Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomater. Sci. 2013, 1, 254. [Google Scholar] [CrossRef]
- Habibovic, P.; Barralet, J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011, 7, 3013–3026. [Google Scholar] [CrossRef]
- Schuhladen, K.; Stich, L.; Schmidt, J.; Steinkasserer, A.; Boccaccini, R.; Zinser, E. Cu, Zn doped borate bioactive glasses: Antibacterial efficacy and dose-dependent in vitro modulation of murine dendritic cells. Biomater. Sci. 2020, 21, 2143–2155. [Google Scholar] [CrossRef]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Lee, I.-H.; Kim, H.-W.; Salih, V.; Wall, I.B.; Knowles, J.C. Bone formation controlled by biologically relevant inorganic ions: Role and controlled delivery from phosphate-based glasses. Adv. Drug Deliv. Rev. 2013, 65, 405–420. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.-H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Palza, H.; Escobar, B.; Bejarano, J.; Bravo, D.; Diaz, D.M.; Perez, J. Designing antimicrobial bioactive glass materials with embedded metal ions synthesized by the sol-gel method. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3795–3801. [Google Scholar] [CrossRef]
- Bejarano, J.; Caviedes, P.; Palza, H. Sol–gel synthesis and in vitro bioactivity of copper and zinc-doped silicate bioactive glasses and glass-ceramics. Biomed. Mater. 2015, 10, 025001. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Verrier, S.; Blaker, J.J.; Maquet, V.; Hench, L.L.; Boccaccini, A.R. PDLLA/Bioglass s composites for soft-tissue and hard-tissue engineering: An in vitro cell biology assessment. Biomaterials 2004, 25, 3013–3021. [Google Scholar] [CrossRef]
- Li, S.M.; Garreau, H.; Vert, M. Structure-property relationships in the case of the degradation of massive aliphatic poly- (-hydroxy acids) in aqueous media, Poly (DL-lactic acid). J. Mater. Sci. Mater. Med. 1990, 1, 123–130. [Google Scholar] [CrossRef]
- Blaker, J.J.; Maquet, V.; Jérôme, R.; Boccaccini, A.R.; Nazhat, S.N. Mechanical properties of highly porous PDLLA/Bioglass composite foams as scaffolds for bone tissue engineering. Acta Biomater. 2005, 1, 643–652. [Google Scholar] [CrossRef]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jérôme, R. Preparation, characterization, and in vitro degradation of bioresorbable and bioactive composites based on Bioglass-filled polylactide foams. J. Biomed. Mater. Res. A 2003, 66, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jerome, R. Porous poly (a-hydroxyacid)/Bioglass composite scaffolds for bone tissue engineering. I: Preparation and in vitro characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar] [CrossRef]
- Blaker, J.J.; Nazhat, S.N.; Maquet, V.; Boccaccini, A.R. Long-term in vitro degradation of PDLLA/bioglass bone scaffolds in acellular simulated body fluid. Acta Biomater. 2011, 7, 829–840. [Google Scholar] [CrossRef]
- Tsigkou, O.; Hench, L.L.; Boccaccini, A.R.; Polak, J.M.; Stevens, M.M. Enhanced differentiation and mineralization of human fetal osteoblasts on PDLLA containing Bioglass(R) composite films in the absence of osteogenic supplements. J. Biomed. Mater. Res. Part A 2007, 80, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, J.; Detsch, R.; Boccaccini, A.R.; Palza, H. PDLLA scaffolds with Cu- and Zn-doped bioactive glasses having multifunctional properties for bone regeneration. J. Biomed. Mater. Res. Part A 2017, 105, 746–756. [Google Scholar] [CrossRef]
- Sultan, S.; Mathew, A.P. 3D printed scaffolds with gradient porosity based on a cellulose nanocrystal hydrogel. Nanoscale 2018, 10, 4421–4431. [Google Scholar] [CrossRef]
- ISO. ISO 844-Rigid Cellular Plastics-Determination of Compression Properties; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- ASTM D. 1621, Standard Test Method for Compressive Properties of Rigid Cellular Plastics; American Society for Testing and Materials: New York, NY, USA, 2010. [Google Scholar]
- Ashby, M.; Shercliff, H.; Cebon, D. Materials: Engineering, Science, Processing and Design; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; ISBN 9780750683913. [Google Scholar]
- Boccaccini, A.R.; Maquet, V. Bioresorbable and bioactive polymer/Bioglass® composites with tailored pore structure for tissue engineering applications. Compos. Sci. Technol. 2003, 63, 2417–2429. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Widdows, K.L.; Erol, M.M.; Burch, C.W.; Sanz-Herrera, J.A.; Ochoa, I.; Stämpfli, R.; Roqan, I.S.; Gabe, S.; Ansari, T.; et al. The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials 2011, 32, 4096–4108. [Google Scholar] [CrossRef]
- Houmard, M.; Qiang, F.; Saiz, E.; Tomsia, A.P. Sol-gel method to fabricate CaP scaffolds by robocasting for tissue engineering. J. Mater. Sci. Mater. Med. 2013, 23, 921–930. [Google Scholar] [CrossRef]
- Henno, S.; Lambotte, J.C.; Glez, D.; Guigand, M.; Lancien, G.; Cathelineau, G. Characterisation and quantification of angiogenesis in β-tricalcium phosphate implants by immunohistochemistry and transmission electron microscopy. Biomaterials 2003, 24, 3173–3181. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, C.; Thouas, G.A. Progress and challenges in biomaterials used for bone tissue engineering: Bioactive glasses and elastomeric composites. Prog. Biomater. 2012, 1, 2. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Kaplan, D.L. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin. Cell Dev. Biol. 2009, 20, 646–655. [Google Scholar] [CrossRef]
- Blaker, J.J.; Gough, J.E.; Maquet, V.; Notingher, I.; Boccaccini, A.R. In vitro evaluation of novel bioactive composites based on Bioglass-filled polylactide foams for bone tissue engineering scaffolds. J. Biomed. Mater. Res. A 2003, 67, 1401–1411. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 1999, 44, 446–455. [Google Scholar] [CrossRef]
- Conoscenti, G.; Carfì Pavia, F.; Ciraldo, F.E.; Liverani, L.; Brucato, V.; La Carrubba, V.; Boccaccini, A.R. In vitro degradation and bioactivity of composite poly-l-lactic (PLLA)/bioactive glass (BG) scaffolds: Comparison of 45S5 and 1393BG compositions. J. Mater. Sci. 2018, 53, 2362–2374. [Google Scholar] [CrossRef]
- Fabbri, P.; Cannillo, V.; Sola, A.; Dorigato, A.; Chiellini, F. Highly porous polycaprolactone-45S5 Bioglass® scaffolds for bone tissue engineering. Compos. Sci. Technol. 2010, 70, 1869–1878. [Google Scholar] [CrossRef]
- Torres, F.; Nazhat, S.; Sheikhmdfadzullah, S.; Maquet, V.; Boccaccini, A. Mechanical properties and bioactivity of porous PLGA/TiO2 nanoparticle-filled composites for tissue engineering scaffolds. Compos. Sci. Technol. 2007, 67, 1139–1147. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1245–1256. [Google Scholar] [CrossRef]
- Charles-Harris, M.; del Valle, S.; Hentges, E.; Bleuet, P.; Lacroix, D.; Planell, J.A. Mechanical and structural characterisation of completely degradable polylactic acid/calcium phosphate glass scaffolds. Biomaterials 2007, 28, 4429–4438. [Google Scholar] [CrossRef]
- Leal, A.I.; Caridade, S.G.; Ma, J.; Yu, N.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Asymmetric PDLLA membranes containing Bioglass® for guided tissue regeneration: Characterization and In Vitro biological behavior. Dent. Mater. 2013, 29, 427–436. [Google Scholar] [CrossRef]
- Avashia, Y.J.; Sastry, A.; Fan, K.L.; Mir, H.S.; Thaller, S.R. Materials used for reconstruction after orbital floor fracture. J. Craniofac. Surg. 2012, 23, 1991–1997. [Google Scholar] [CrossRef]
- Baino, F. Biomaterials and implants for orbital floor repair. Acta Biomater. 2011, 7, 3248–3266. [Google Scholar] [CrossRef]
- Deng, C.; Weng, J.; Lu, X.; Zhou, S.B.; Wan, J.X.; Qu, S.X.; Feng, B.; Li, X.H. Preparation and in vitro bioactivity of poly(d,l-lactide) composite containing hydroxyapatite nanocrystals. Mater. Sci. Eng. C 2008, 28, 1304–1310. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Biomimetic polymer/apatite composite scaffolds for mineralized tissue engineering. Macromol. Biosci. 2004, 4, 100–111. [Google Scholar] [CrossRef]
- Lusvardi, G.; Malavasi, G.; Menabue, L.; Menziani, M.C. Synthesis, Characterization, and Molecular Dynamics Simulation of Na2O-CaO-SiO2-ZnO Glasses. J. Phys. Chem. B 2002, 106, 9753–9760. [Google Scholar] [CrossRef]
- Courthéoux, L.; Lao, J.; Nedelec, J.; Jallot, E. Controlled Bioactivity in Zn-doped sol-gel derived SiO2 -CaO bioactive glasses. J. Phys. Chem. C 2008, 112, 13663–13667. [Google Scholar] [CrossRef]
- Linati, L.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Mustarelli, P.; Segre, U. Qualitative and quantitative structure-property relationships analysis of multicomponent potential bioglasses. J. Phys. Chem. B 2005, 109, 4989–4998. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, H.; Gorman, B.P.; Mueller, D.W.; Reidy, R.F. Behavior of copper ions in silica xerogels. J. Non. Cryst. Solids 2004, 341, 157–161. [Google Scholar] [CrossRef]
- Schreiber, H.D.; Kochanowski, B.K.; Schreiber, C.W.; Morgan, A.B.; Coolbaugh, M.T.; Dunlap, T.G. Compositional dependence of redox equilibria in sodium silicate glasses. J. Non. Cryst. Solids 1994, 177, 340–346. [Google Scholar] [CrossRef]
- Hoppe, A.; Meszaros, R.; Stähli, C.; Romeis, S.; Schmidt, J.; Peukert, W.; Marelli, B.; Nazhat, S.N.; Wondraczek, L.; Lao, J.; et al. In vitro reactivity of Cu doped 45S5 Bioglass® derived scaffolds for bone tissue engineering. J. Mater. Chem. B 2013, 1, 5659. [Google Scholar] [CrossRef]
- Láctico, L.Á.; Motta, A.C. Síntese e Caracterização do Copolímero Poli (L-co-D,L Ácido Láctico). Polimeros 2007, 17, 123–129. [Google Scholar]
- Coleman, N.J.; Bellantone, M.; Nicholson, J.W.; Mendham, A.P. Textural and structural properties of bioactive glasses in the system CaO–SiO2. Ceram. Silik. 2007, 51, 1–8. [Google Scholar]
- Srivastava, A.K.; Pyare, R. Characterization of CuO substituted 45S5 Bioactive Glasses and Glass-Ceramics. Int. J. Sci. Technol. Res. 2012, 1, 28–41. [Google Scholar] [CrossRef]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol-gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Strobel, L.A.; Kneser, U.; Boccaccini, A.R. Zinc-containing bioactive glasses for bone regeneration, dental and orthopedic applications. Biomed. Glasses 2015, 1, 51–69. [Google Scholar] [CrossRef]
- Nasrazadani, S.; Hassani, S. Modern Analytical Techniques in Failure Analysis of Aerospace, Chemical, and Oil and Gas Industries; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081001264. [Google Scholar]
- Bernstein, M.; Gotman, I.; Makarov, C.; Phadke, A.; Radin, S.; Ducheyne, P.; Gutmanas, E.Y. Low temperature fabrication of β-TCP-PCL nanocomposites for bone implants. Adv. Eng. Mater. 2010, 12, 341–347. [Google Scholar] [CrossRef]
- Lei, Y.; Rai, B.; Ho, K.H.; Teoh, S.H. In vitro degradation of novel bioactive polycaprolactone—20% tricalcium phosphate composite scaffolds for bone engineering. Mater. Sci. Eng. C 2007, 27, 293–298. [Google Scholar] [CrossRef]
- Koort, J.K.; Makinen, T.J.; Suokas, E.; Veiranto, M.; Jalava, J.; Tormala, P.; Aro, H.T. Sustained release of ciprofloxacin from an osteoconductive poly(DL)-lactide implant. Acta Orthop. 2008, 79, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Jung, S.; Day, D.; Neidig, K.; Dusevich, V.; Eick, D.; Bonewald, L. Evaluation of bone regeneration, angiogenesis, and hydroxyapatite conversion in critical-sized rat calvarial defects implanted with bioactive glass scaffolds. J. Biomed. Mater. Res. A 2012, 100, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Koyama, Y.; Yamada, T.; Imamura, Y.; Okada, T.; Shirahama, N.; Akita, K.; Takakuda, K.; Tanaka, J. Development of guided bone regeneration membrane composed of beta-tricalcium phosphate and poly (L-lactide-co-glycolide-co-epsilon-caprolactone) composites. Biomaterials 2004, 25, 5979–5986. [Google Scholar] [CrossRef]
- Albrecht, T.W.J.; Addai-Mensah, J.; Fornasiero, D. Effect of pH, Concentration and Temperature on Copper and Zinc Hydroxide Formation/Precipitation in Solution. In Proceedings of the Chemeca 2011: Engineering a Better World, Sydney, NSW, Australia, 18–21 September 2011; pp. 1–10. [Google Scholar]
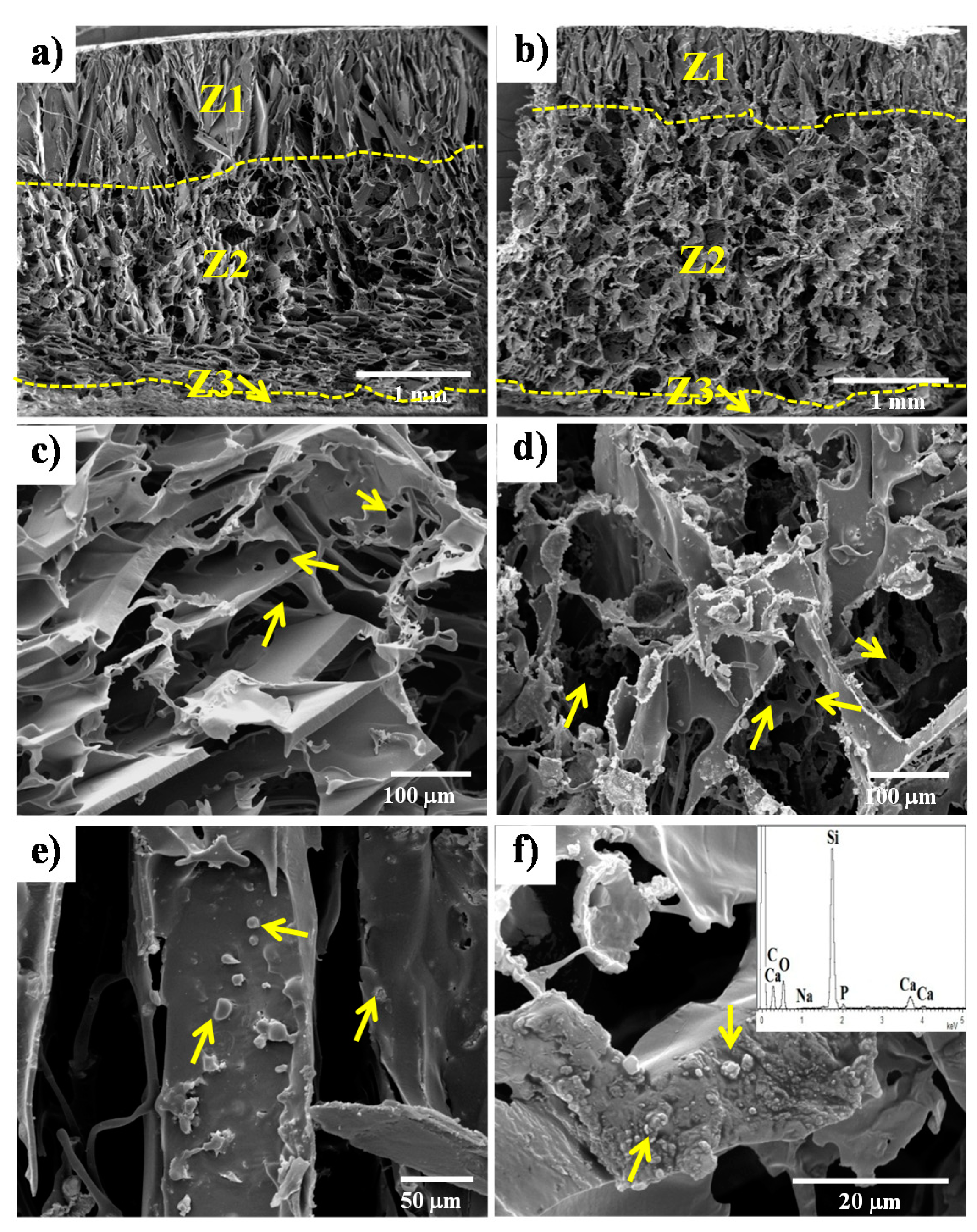
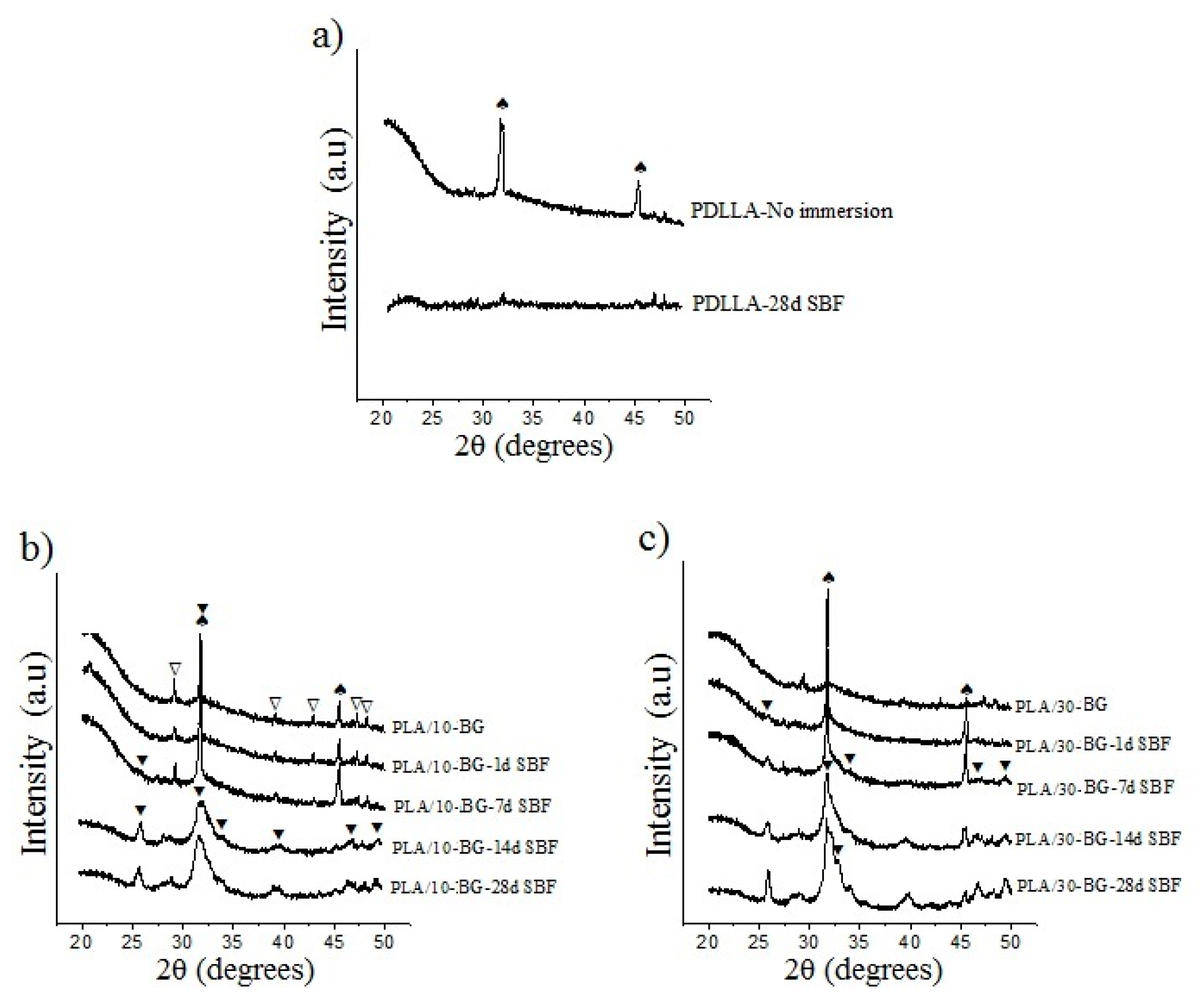
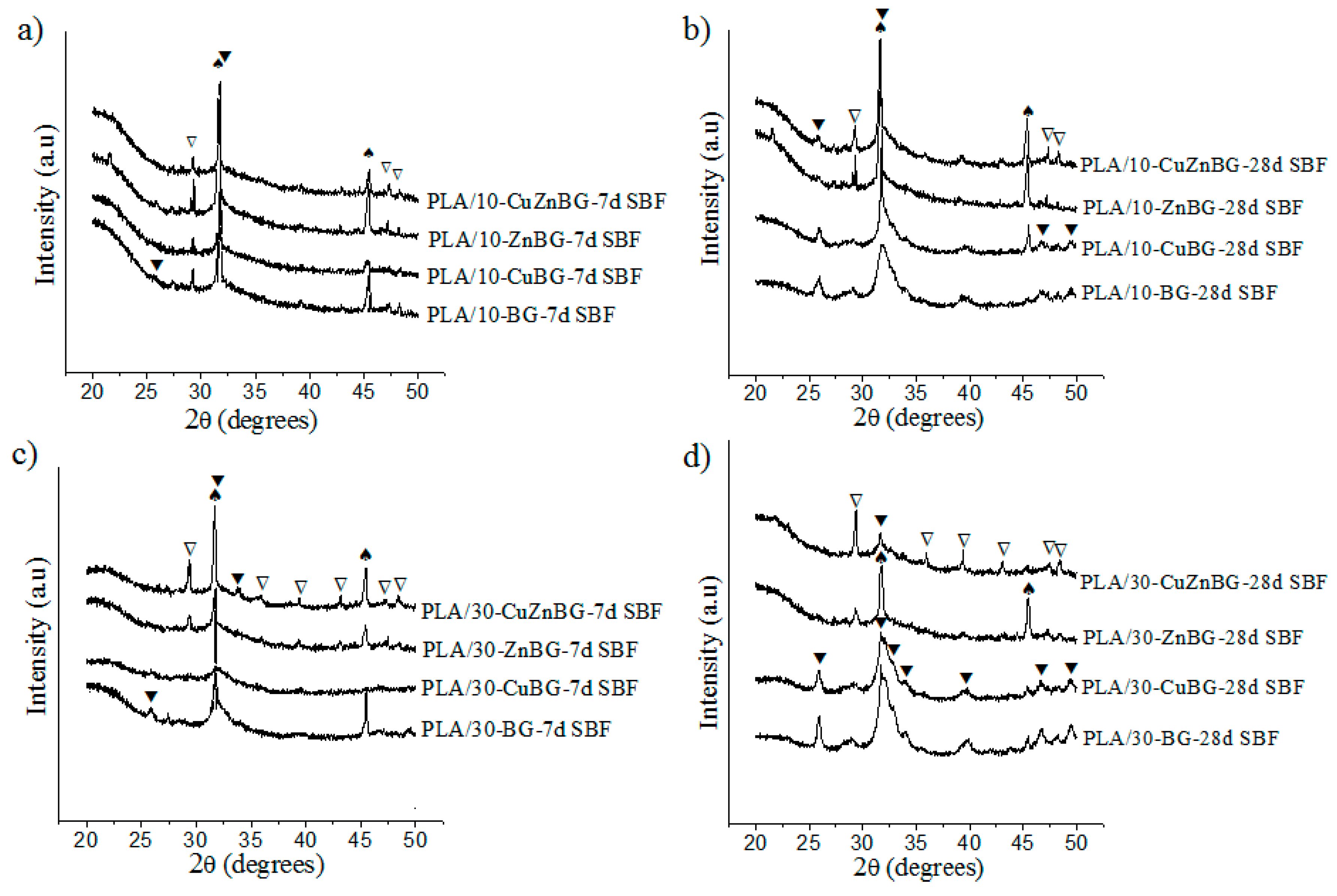
| Scaffold Sample | ρ (g/cm3) | ρ0 (g/cm3) | p (%) |
|---|---|---|---|
| Neat PDLLA | 0.083 | 1.26 | 93.4 |
| PLA/10-BG | 0.086 | 1.31 | 93.5 |
| PLA/30-BG | 0.090 | 1.42 | 93.7 |
| PLA/10-CuBG | 0.091 | 1.33 | 93.2 |
| PLA/30-CuBG | 0.102 | 1.49 | 93.2 |
| PLA/10-ZnBG | 0.095 | 1.33 | 92.8 |
| PLA/30-ZnBG | 0.106 | 1.49 | 92.9 |
| PLA/10-CuZnBG | 0.099 | 1.33 | 92.5 |
| PLA/30-CuZnBG | 0.112 | 1.48 | 92.4 |
| Scaffold | Compressive Strength at 10% Deformation (kPa) | Young’s Modulus (kPa) |
|---|---|---|
| PDLLA | 30 ± 3 | 2.3 ± 0.3 |
| PLA/10-BG | 48 ± 7 | 5.3 ± 0.3 |
| PLA/30-BG | 29 ± 4 | 3.1 ± 0.3 |
| PLA/10-CuBG | 32 ± 4 | 4 ± 1 |
| PLA/30-CuBG | 29 ± 3 | 3.5 ± 0.4 |
| PLA/10-ZnBG | 24 ± 3 | 2.8 ± 0.6 |
| PLA/30-ZnBG | 41 ± 6 | 5 ± 1 |
| PLA/10-CuZnBG | 26 ± 3 | 2.8 ± 0.6 |
| PLA/30-CuZnBG | 27 ± 3 | 2.8 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejarano, J.; Boccaccini, A.R.; Covarrubias, C.; Palza, H. Effect of Cu- and Zn-Doped Bioactive Glasses on the In Vitro Bioactivity, Mechanical and Degradation Behavior of Biodegradable PDLLA Scaffolds. Materials 2020, 13, 2908. https://doi.org/10.3390/ma13132908
Bejarano J, Boccaccini AR, Covarrubias C, Palza H. Effect of Cu- and Zn-Doped Bioactive Glasses on the In Vitro Bioactivity, Mechanical and Degradation Behavior of Biodegradable PDLLA Scaffolds. Materials. 2020; 13(13):2908. https://doi.org/10.3390/ma13132908
Chicago/Turabian StyleBejarano, Julian, Aldo R. Boccaccini, Cristian Covarrubias, and Humberto Palza. 2020. "Effect of Cu- and Zn-Doped Bioactive Glasses on the In Vitro Bioactivity, Mechanical and Degradation Behavior of Biodegradable PDLLA Scaffolds" Materials 13, no. 13: 2908. https://doi.org/10.3390/ma13132908
APA StyleBejarano, J., Boccaccini, A. R., Covarrubias, C., & Palza, H. (2020). Effect of Cu- and Zn-Doped Bioactive Glasses on the In Vitro Bioactivity, Mechanical and Degradation Behavior of Biodegradable PDLLA Scaffolds. Materials, 13(13), 2908. https://doi.org/10.3390/ma13132908






