The Effects of Various Parameters of the Microwave-Assisted Solvothermal Synthesis on the Specific Surface Area and Catalytic Performance of MgF2 Nanoparticles
Abstract
1. Introduction
2. Experimental Part
2.1. MgF2 Nanoparticles—Catalyst Preparation
2.2. HF Activation and Catalytic Measurements
2.3. Characterizations
3. Results and Discussion
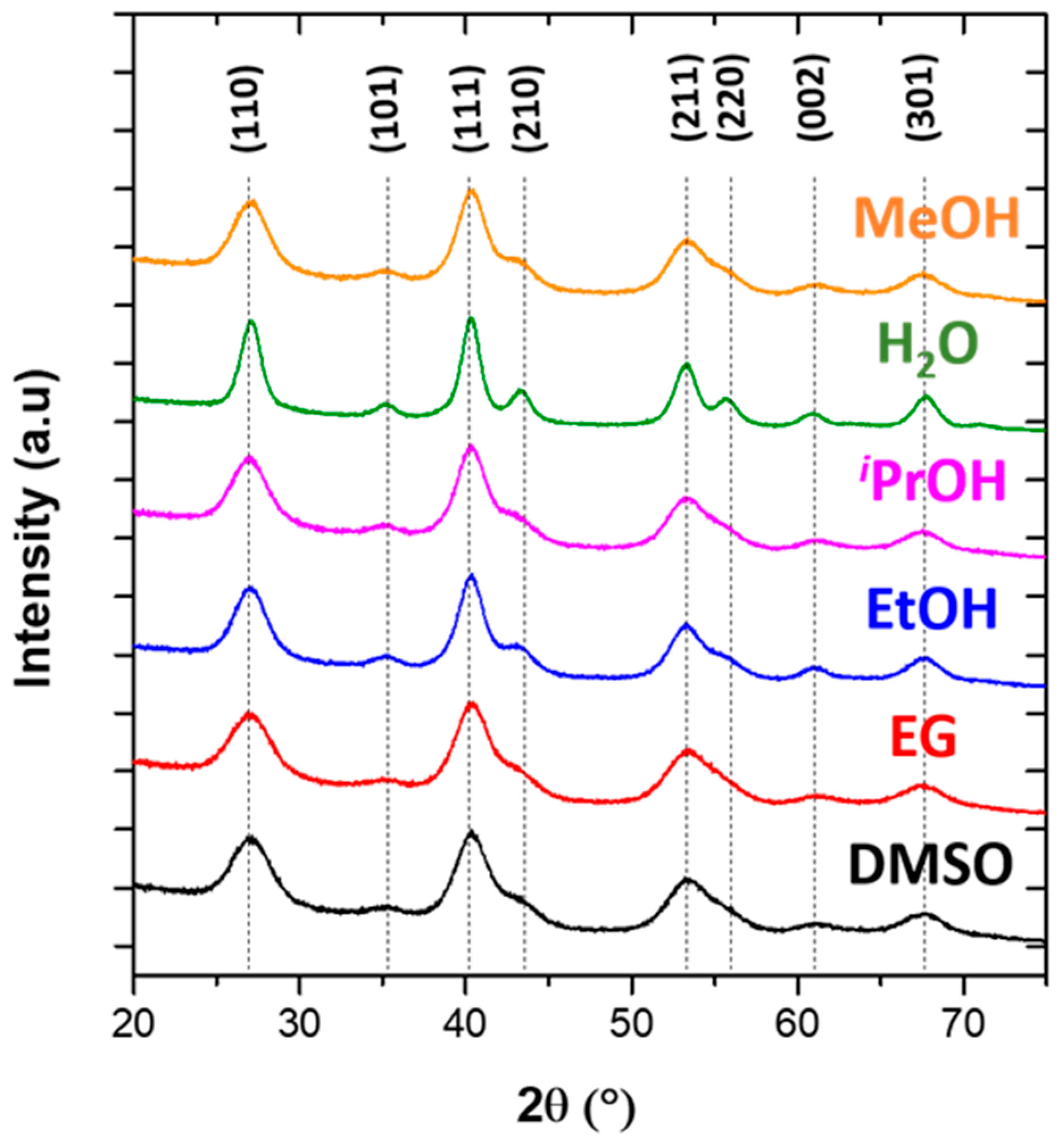
3.1. Synthesis of MgF2 Nanofluorides
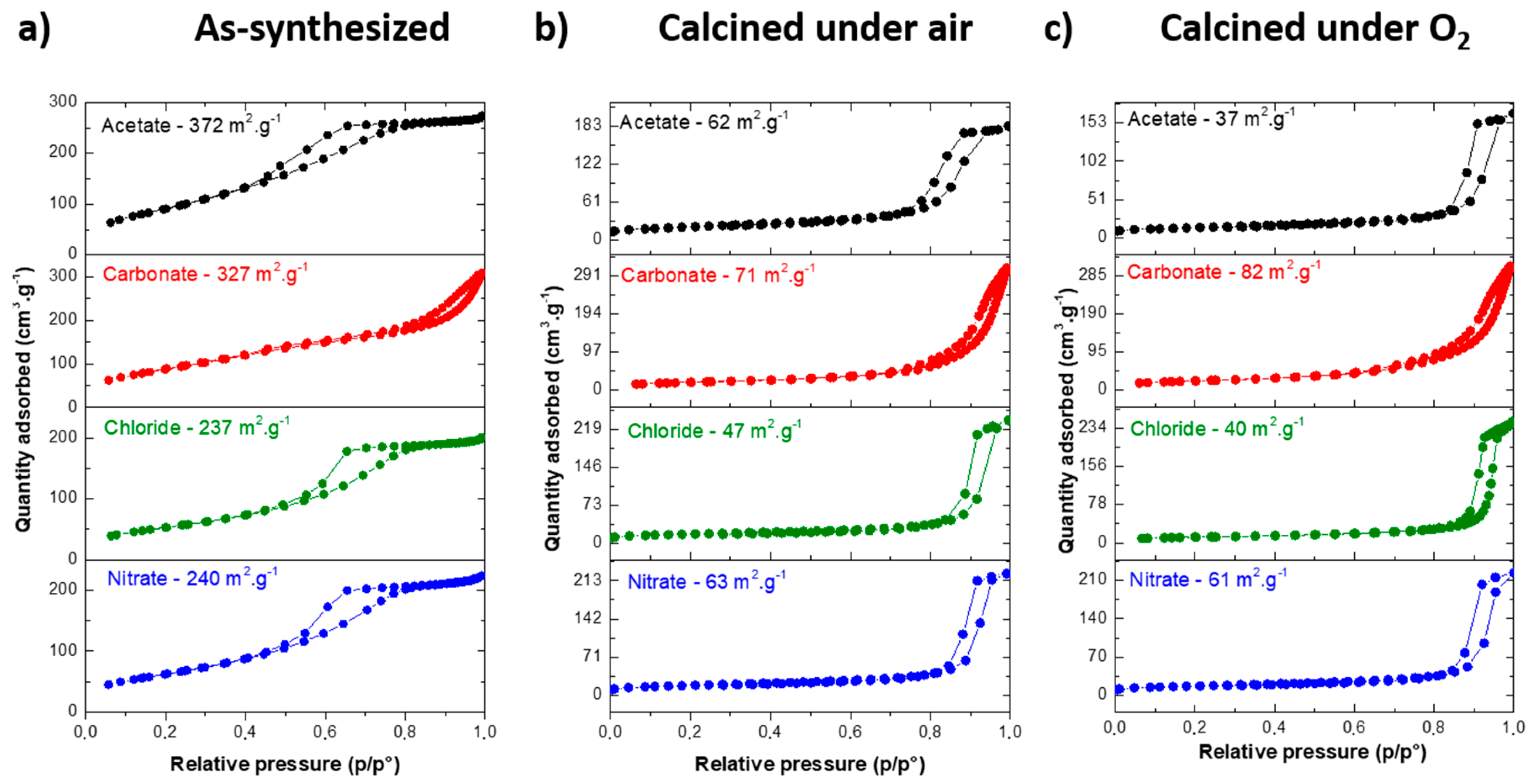
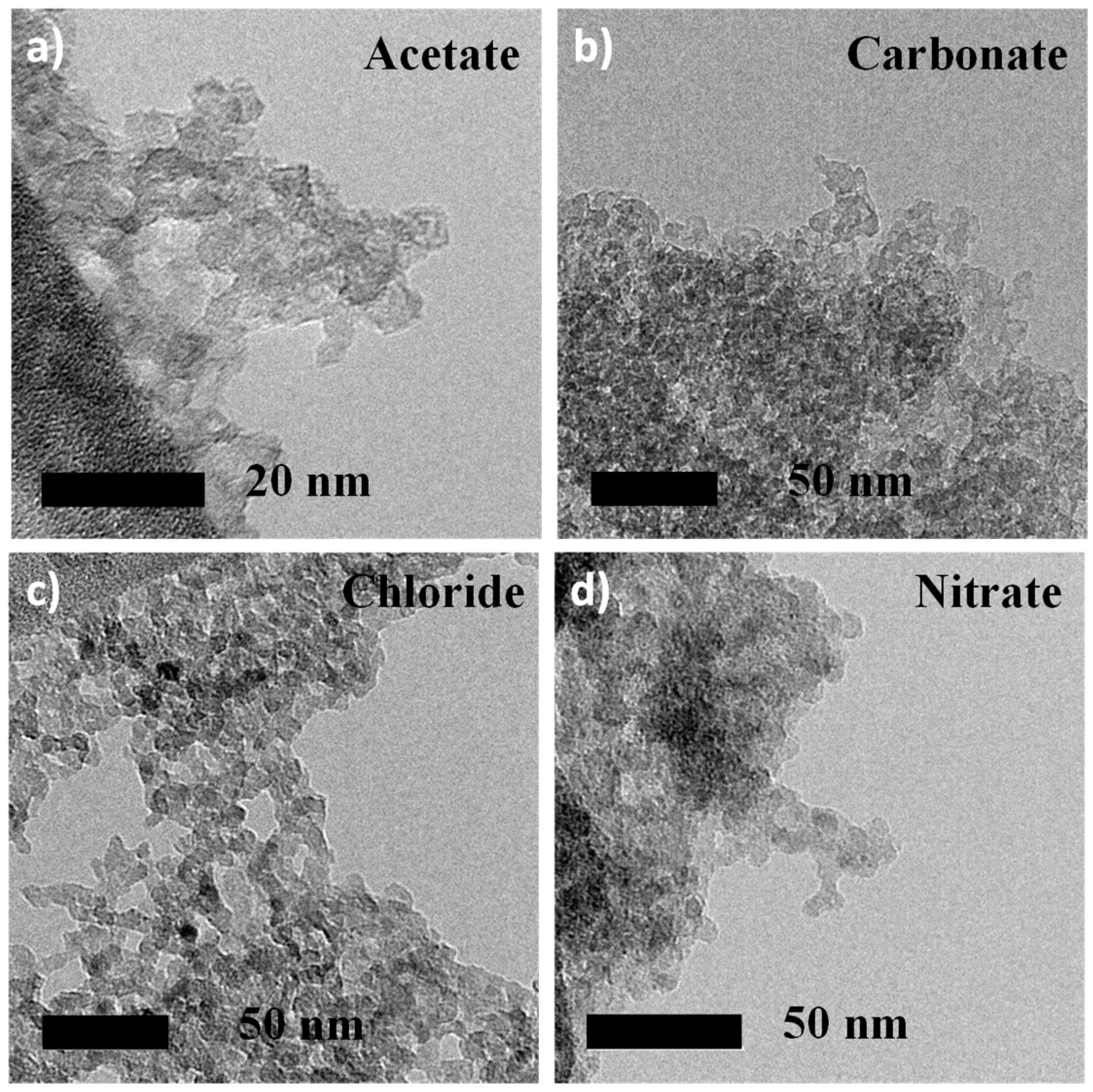
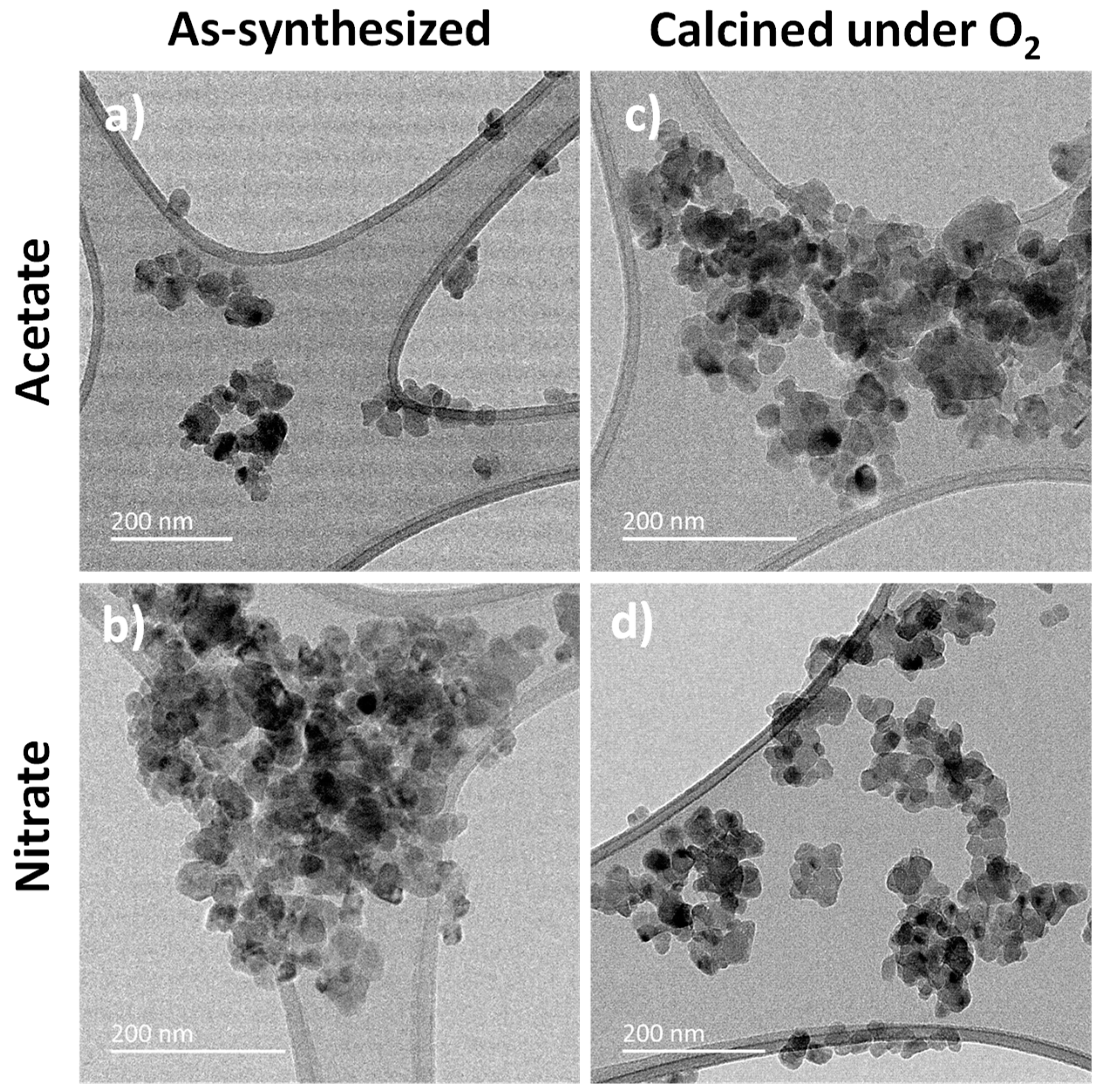
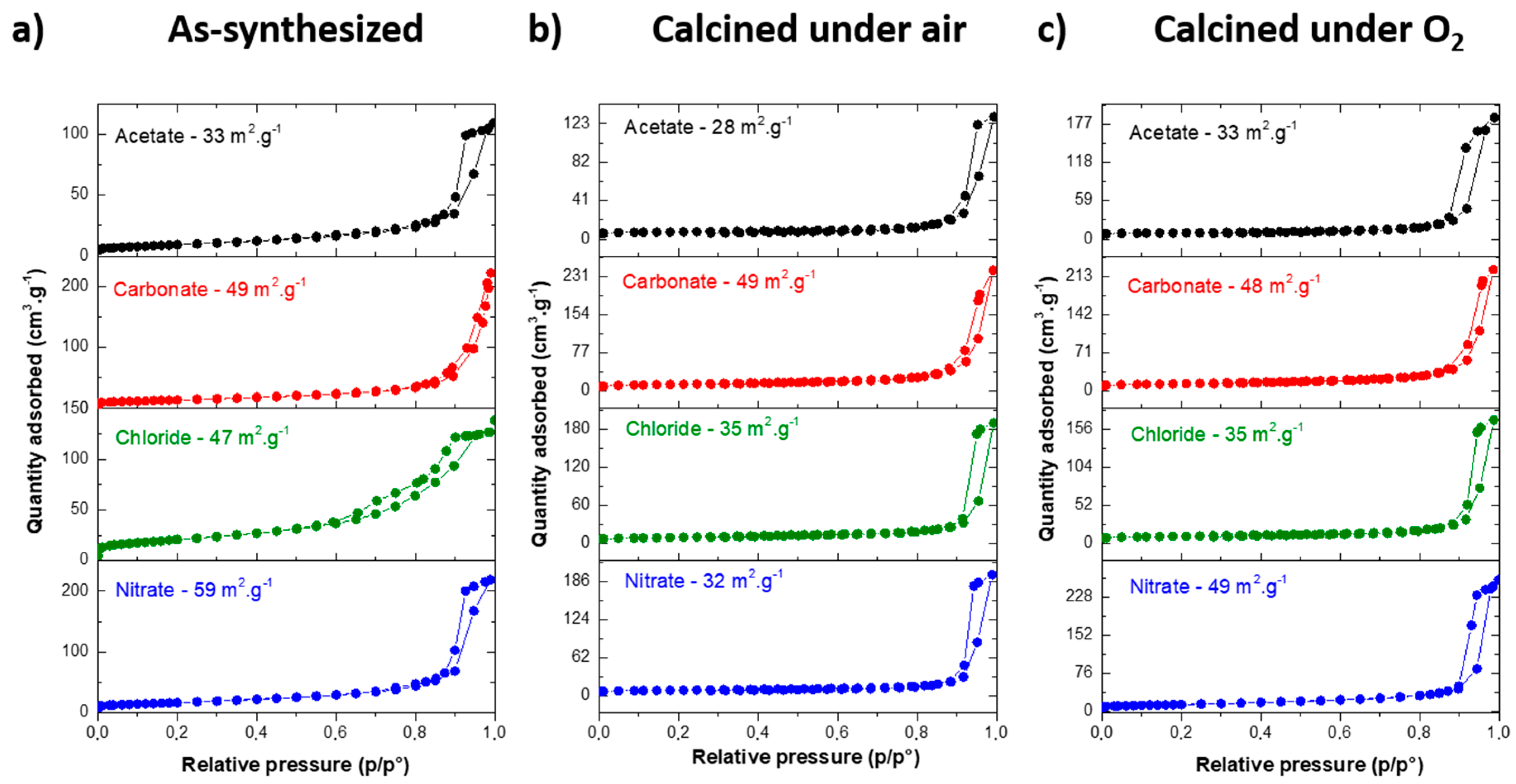
3.2. MgF2 Nanofluoride’s Characterization by XRPD, N2 Sorption, FT–IR and TEM
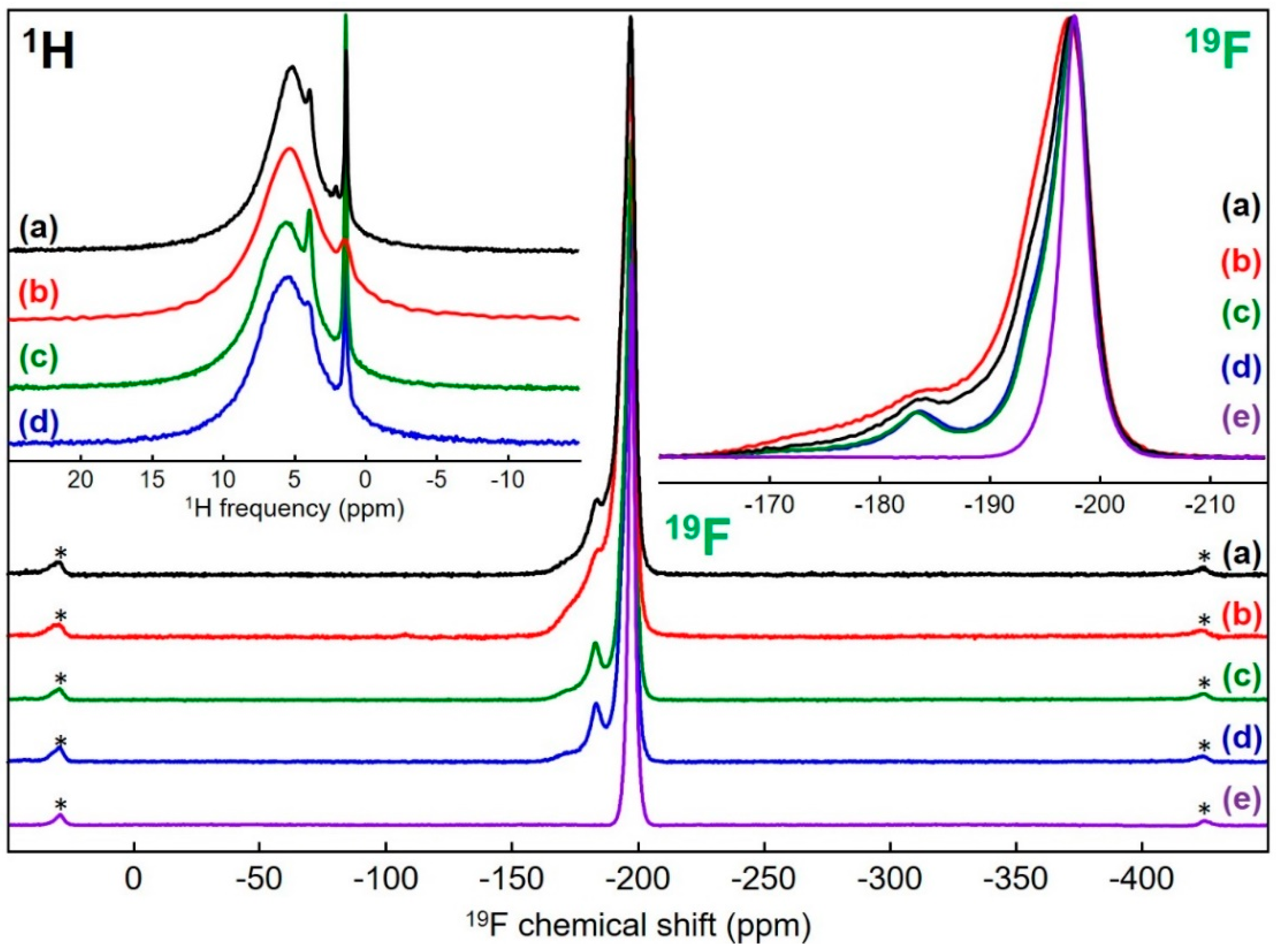
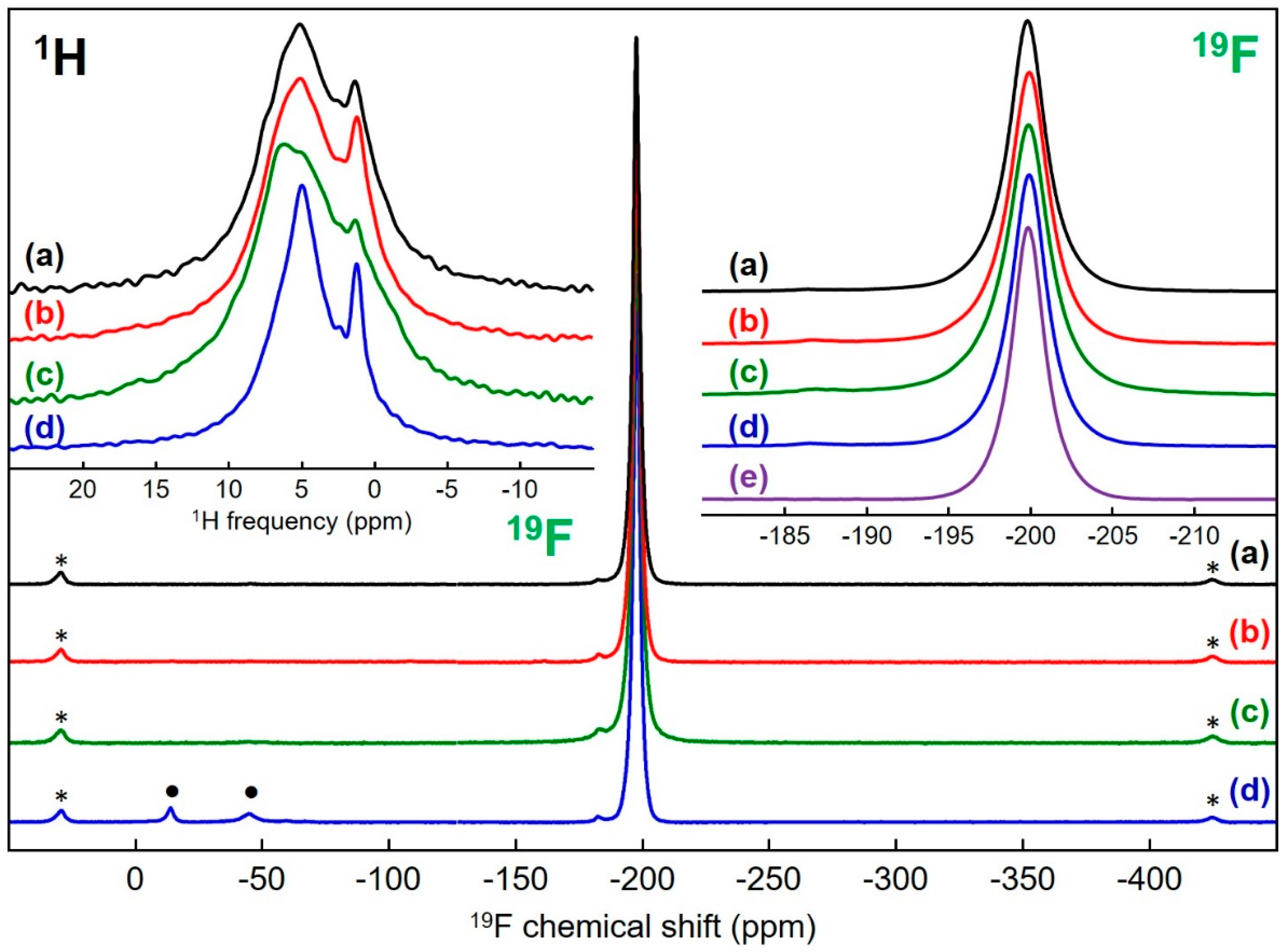
3.3. MgF2 Nanofluoride’s Characterization by 19F and 1H Solid-State MAS NMR
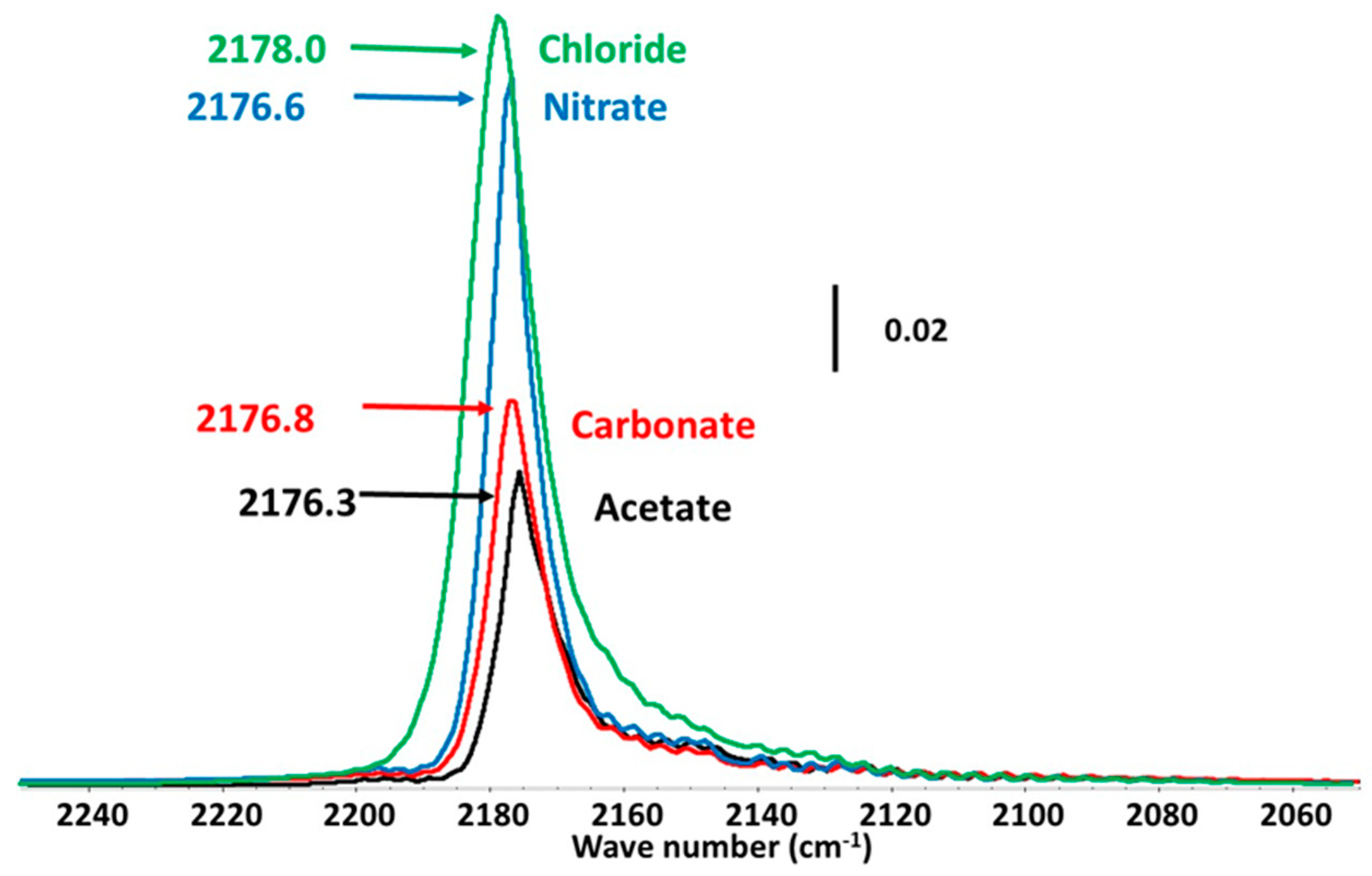
3.4. Properties of the Active Sites Characterized by Adsorption of CO as a Probe Molecule Followed by IR Spectroscopy
3.5. Catalytic Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fedorov, P.P.; Luginina, A.A.; Kuznetsov, S.V.; Osiko, V.V. Nanofluorides. J. Fluor. Chem. 2011, 132, 1012–1039. [Google Scholar] [CrossRef]
- Löbmann, P. Sol-Gel processing of MgF2 antireflective coatings. Nanomaterials 2018, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Krahl, T.; Broßke, D.; Scheurell, K.; Lintner, B.; Kemnitz, E. Novel aspects in the chemistry of the non-aqueous fluorolytic Sol–Gel synthesis of nanoscaled homodisperse MgF2 sols for antireflective coatings. J. Mater. Chem. C 2016, 4, 1454–1466. [Google Scholar] [CrossRef]
- Scheurell, K.; Noack, J.; König, R.; Hegmann, J.; Jahn, R.; Hofmann, T.; Löbmann, P.; Lintner, B.; Garcia-Juan, P.; Eicher, J.; et al. Optimisation of a Sol–Gel synthesis route for the preparation of MgF2 particles for a large scale coating process. Dalton Trans. 2015, 44, 19501. [Google Scholar] [CrossRef]
- Nakamura, F.; Kato, T.; Okada, G.; Kawano, N.; Kawaguchi, N.; Fukuda, K.; Yanagida, T. Scintillation, dosimeter and optical properties of MgF2 transparent ceramics doped with Gd3+. Mater. Res. Bull. 2018, 98, 83–88. [Google Scholar] [CrossRef]
- Kemnitz, E. Nanoscale metal fluorides: A new class of heterogeneous catalysts. Catal. Sci. Technol. 2015, 5, 786–806. [Google Scholar] [CrossRef]
- Mao, W.; Jia, Z.; Bai, Y.; Qin, Y.; Wang, B.; Han, S.; Zhang, W.; Kou, L.; Lu, J.; Kemnitz, E. Fe/Hollow nano-MgF2: A green and highly-efficient alternative to classical Cr-based catalysts for the gas-phase fluorination reaction. Catal. Sci. Technol. 2019, 9, 3015–3019. [Google Scholar] [CrossRef]
- Jia, Z.; Mao, W.; Bai, Y.; Wang, B.; Ma, H.; Li, C.; Lu, J. Hollownano-MgF2 supported catalysts: Highly active and stable in gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane. Appl. Catal. B Environ. 2018, 238, 599–608. [Google Scholar] [CrossRef]
- Kemnitz, E.; Wuttke, S.; Coman, S.M. Tailor-made MgF2-based catalysts by Sol-Gel synthesis. Eur. J. Inorg. Chem. 2011, 2011, 4773–4794. [Google Scholar] [CrossRef]
- Dreger, M.; Scholz, G.; Kemnitz, E. An easy access to nanocrystalline alkaline earth metal fluorides-just by shaking. Solid State Sci. 2012, 14, 528–534. [Google Scholar] [CrossRef]
- Arkhipenko, S.Y.; Fedorova, A.A.; Morozov, I.V.; Shaporev, A.S. Preparation of calcium and magnesium fluorides with extended surface areas using β-cyclodextrin as a structure-forming agent. Mendeleev Commun. 2012, 22, 25–26. [Google Scholar] [CrossRef]
- Astruc, A.; Cochon, C.; Dessources, S.; Célérier, S.; Brunet, S. High specific surface area metal fluorides as catalysts for the fluorination of 2-chloropyridine by HF. Appl. Catal. A Gen. 2013, 453, 20–27. [Google Scholar] [CrossRef]
- Rywak, A.A.; Burlitch, J.M. Sol−Gel synthesis of nanocrystalline magnesium fluoride: Its use in the preparation of MgF2 films and MgF2−SiO2 composites. Chem. Mater. 1996, 8, 60–67. [Google Scholar] [CrossRef]
- Murata, T.; Ishizawa, H.; Motoyama, I.; Tanaka, A. Investigations of MgF2 optical thin films prepared from autoclaved sol. J. Sol Gel Sci. Technol. 2004, 32, 161–165. [Google Scholar] [CrossRef]
- Krishna Murthy, J.; Groß, U.; Rüdiger, S.; Kemnitz, E.; Winfield, J.M. Sol-Gel-Fluorination synthesis of amorphous magnesium fluoride. J. Solid State Chem. 2006, 179, 739–746. [Google Scholar] [CrossRef]
- Wuttke, S.; Coman, S.M.; Scholz, G.; Kirmse, H.; Vimont, A.; Daturi, M.; Schroeder, S.L.M.; Kemnitz, E. Novel Sol-Gel synthesis of acidic MgF2-x(OH)x materials. Chem. Eur. J. 2008, 14, 11488–11499. [Google Scholar] [CrossRef]
- Teinz, K.; Wuttke, S.; Börno, F.; Eicher, J.; Kemnitz, E. Highly selective metal fluoride catalysts for the dehydrohalogenation of 3-chloro-1,1,1,3-tetrafluorobutane. J. Catal. 2011, 282, 175–182. [Google Scholar] [CrossRef]
- Krüger, H.; Kemnitz, E.; Hertwig, A.; Beck, U. Transparent MgF2-films by Sol-Gel coating: Synthesis and optical properties. Thin Solid Films 2008, 516, 4175–4177. [Google Scholar] [CrossRef]
- Sevonkaev, I.; Matijević, E. Formation of magnesium fluoride particles of different morphologies. Langmuir 2009, 25, 10534–10539. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Czajka, B.; Pietrowski, M.; Zieliński, M. MgF2 as a non-conventional catalytic support. Surface and structure characterization. Catal. Lett. 2000, 66, 147–153. [Google Scholar] [CrossRef]
- Lellouche, J.; Kahana, E.; Elias, S.; Gedanken, A.; Banin, E. Antibiofilm activity of nanosized magnesium fluoride. Biomaterials 2009, 30, 5969–5978. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Iskandar, F.; Ogi, T.; Okuyama, K. Nanometer to submicrometer magnesium fluoride particles with controllable morphology. Langmuir 2010, 26, 12260–12266. [Google Scholar] [CrossRef] [PubMed]
- Lellouche, J.; Friedman, A.; Lellouche, J.P.; Gedanken, A.; Banin, E. Improved antibacterial and antibiofilm activity of magnesium fluoride nanoparticles obtained by water-based ultrasound chemistry. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, Y.; Qi, Y.; Guo, C.; Hu, C. Synthesis and characterization of MgF2 and KMgF3 nanorods. J. Solid State Chem. 2004, 177, 2205–2209. [Google Scholar] [CrossRef]
- Bas, S.; Chatterjee, U.; Soucek, M.D. Synthesis of amphiphilic triblock copolymers for the formation of magnesium fluoride (MgF2) nanoparticles serkan. J. Appl. Polym. Sci. 2012, 116, 998–1007. [Google Scholar] [CrossRef]
- Clarenc, R. Synthèse et Caractérisation de Composés Fluorés pour le Piégeage de Fluorures Gazeux. Thèse de Doctorat, Université de Bordeaux I, Bordeaux, France, 2020. [Google Scholar]
- Demourgues, A.; Penin, N.; Dambournet, D.; Clarenc, R.; Tressaud, A.; Durand, E. About MX3 and MX2 (Mn+ = Mg2+, Al3+, Ti4+, Fe3+; Xp− = F−, O2−, OH−) nanofluorides. J. Fluor. Chem. 2012, 134, 35–43. [Google Scholar] [CrossRef]
- Murata, T.; Hieda, J.; Saito, N.; Takai, O. Wettability characterization of transparent MgF2 nanoparticle coatings with SiO2 binder covered with fluoroalkylsilane self-assembled monolayers. J. Sol-Gel Sci. Technol. 2011, 60, 125–130. [Google Scholar] [CrossRef]
- Ji, Z.; Hao, L.; Wang, H.; Chen, R. Analysis and research on the formative factors and properties of nano-MgF2 crystals with different morphologies. Polyhedron 2019, 157, 136–145. [Google Scholar] [CrossRef]
- Pietrowski, M.; Wojciechowska, M. Microwave-assisted synthesis of spherical monodispersed magnesium fluoride. J. Fluor. Chem. 2007, 128, 219–223. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; Del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-Initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of debye–scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Langford, J.I. A rapid method for analysing the breadths of diffraction and spectral lines using the Voigt function. J. Appl. Cryst. 1978, 11, 10–14. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 12 August 2020).
- Astruc, A.; Célérier, S.; Pavon, E.; Mamede, A.-S.; Delevoye, L.; Brunet, S. Mixed Ba1−xLaxF2+x fluoride materials as catalyst for the gas phase fluorination of 2-chloropyridine by HF. Appl. Catal. B Environ. 2017, 204, 107–118. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Wuttke, S.; Scholz, G.; Rüdiger, S.; Kemnitz, E. Variation of Sol–Gel synthesis parameters and their consequence for the surface area and structure of magnesium fluoride. J. Mater. Chem. 2007, 17, 4980. [Google Scholar] [CrossRef]
- De La Hoz, A. Microwave Heating as a tool for dustainable chemistry. ChemSusChem 2011, 4, 666. [Google Scholar] [CrossRef]
- Krishnan, K.; Krishnan, R.S. Raman and infrared spectra of ethylene glycol. Proc. Indian Acad. Sci. Sect. A 1966, 64, 111–121. [Google Scholar] [CrossRef]
- Lhoste, J.; Jouanneaux, A.; Fayon, F.; Body, M.; Kodjikian, S.; Leblanc, M.; Wirth, E.; Bobet, J.-L.; Legein, C.; Maisonneuve, V. Microwave-assisted synthesis, characterization, and hydrogen adsorption of nanostructured β-AlF3-x(OH)X. Chem. Mater. (under review).
- Rouquerol, J.; Llewellyn, P.; Denoyel, R. Texture des matériaux divisés—Taille de pores des matériaux nanoporeux par adsorption d’azote. Tech. l’Ingénieur 2017, 1–15. [Google Scholar]
- Rouquerol, F.; Rouquerol, J.; Llewellyn, P.; Denoyel, R. Texture des matériaux divisés-aire spécifique des matériaux pulvérulents ou nanoporeux. Tech. l’Ingénieur 2017, 50–54. [Google Scholar]
- Baur, W.H. Rutile-type compounds. V. refinement of MnO2 and MgF2. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1976, 32, 2200–2204. [Google Scholar] [CrossRef]
- Sadoc, A.; Body, M.; Legein, C.; Biswal, M.; Fayon, F.; Rocquefelte, X.; Boucher, F. NMR Parameters in alkali, alkaline earth and rare earth fluorides from first principle calculations. Phys. Chem. Chem. Phys. 2011, 13, 18539–18550. [Google Scholar] [CrossRef]
- Clavier, B. Synthèse, Caractérisations et Évaluation de l’Activité Bactéricide de Composés Inorganiques à Base de Cuivre. Ph.D. Thesis, Le Mans Université, Mans, France, 2019. [Google Scholar]
- Prescott, H.A.; Li, Z.-J.; Kemnitz, E.; Deutsch, J.; Lieske, H. New magnesium oxide fluorides with hydroxy groups as catalysts for michael additions. J. Mater. Chem. 2005, 15, 4616–4628. [Google Scholar] [CrossRef]
- Sideris, P.J.; Nielsen, U.G.; Gan, Z.; Grey, C.P. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science 2008, 321, 113–117. [Google Scholar] [CrossRef]
- Wuttke, S.; Vimont, A.; Lavalley, J.-C.; Daturi, M.; Kemnitz, E. Infrared investigation of the acid and basic properties of a Sol-Gel prepared MgF2. J. Phys. Chem. C 2010, 114, 5113–5120. [Google Scholar] [CrossRef]
| Solvent | ε′ | ε″ | tan δ |
|---|---|---|---|
| MeOH | 34.0 | 21.5 | 0.632 |
| H2O | 80.4 | 9.9 | 0.123 |
| iPrOH | 18.3 | 14.6 | 0.798 |
| EtOH | 24.3 | 22.9 | 0.942 |
| EG | 37.0 | 49.9 | 1.349 |
| DMSO | 45.0 | 37.1 | 0.824 |
| Solvent | SBET (m2·g−1) | DXRD (nm) | Yield (%) |
|---|---|---|---|
| MeOH | 345 | 4 | ~100 |
| H2O | 237 | 11 | ~100 |
| iPrOH | 325 | 7 | ~95 |
| EtOH | 334 | 6 | ~45 |
| EG | 267 | 6 | ~30 |
| DMSO | 340 | 5 | ~20 |
| Sample | Acetate | Carbonate | Chloride | Nitrate | |
|---|---|---|---|---|---|
| As-synthesized | Before HF | 372 | 327 | 237 | 240 |
| After HF | 33 | 49 | 47 | 59 | |
| Air | Before HF | 62 | 71 | 47 | 63 |
| After HF | 28 | 49 | 35 | 32 | |
| O2 | Before HF | 37 | 82 | 40 | 61 |
| After HF | 33 | 48 | 35 | 49 | |
| Sample | Acetate | Carbonate | Chloride | Nitrate | |||||
|---|---|---|---|---|---|---|---|---|---|
| DXRD | DTEM | DXRD | DTEM | DXRD | DTEM | DXRD | DTEM | ||
| As-synthesized | Before HF | 4 | 5 | 4 | 5 | 8 | 5 | 7 | 5 |
| After HF | 35 | 36 | 19 | 21 | 19 | 2 | 27 | 28 | |
| Air | Before HF | 14 | - | 9 | - | 15 | - | 14 | - |
| After HF | 33 | - | 15 | - | 28 | - | 27 | - | |
| O2 | Before HF | 20 | 21 | 9 | - | 16 | - | 14 | 16 |
| After HF | 27 | 28 | 16 | - | 25 | - | 24 | 27 | |
| Sample | Acetate | Carbonate | Chloride | Nitrate | ||||
|---|---|---|---|---|---|---|---|---|
| Qsites | Csites | Qsites | Csites | Qsites | Csites | Qsites | Csites | |
| As-synthesized | 64 | 1.9 | 87 | 1.8 | 177 | 2.4 | 106 | 1.8 |
| Air | 87 | 3.1 | 156 | 3.2 | 79 | 2.3 | 90 | 2.8 |
| O2 | 68 | 2.1 | 151 | 1.8 | 78 | 1.7 | 96 | 2.0 |
| Sample | Acetate | Carbonate | Chloride | Nitrate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | TOF | A | TOF | A | TOF | A | TOF | |||||
| (a) | (b) | - | (a) | (b) | - | (a) | (b) | - | (a) | (b) | - | |
| As-synthesized | 26 | 0.75 | 406 | 32 | 0.66 | 370 | 30 | 0.64 | 170 | 29 | 0.49 | 270 |
| Air | 29 | 1.02 | 330 | 32 | 0.67 | 210 | 30 | 0.87 | 380 | 31 | 0.63 | 340 |
| O2 | 25 | 0.77 | 370 | 32 | 0.83 | 210 | 31 | 0.89 | 400 | 31 | 0.63 | 320 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gohari Bajestani, Z.; Lhoste, J.; Auguste, S.; Hémon-Ribaud, A.; Body, M.; Legein, C.; Maisonneuve, V.; Guiet, A.; Brunet, S. The Effects of Various Parameters of the Microwave-Assisted Solvothermal Synthesis on the Specific Surface Area and Catalytic Performance of MgF2 Nanoparticles. Materials 2020, 13, 3566. https://doi.org/10.3390/ma13163566
Wang Y, Gohari Bajestani Z, Lhoste J, Auguste S, Hémon-Ribaud A, Body M, Legein C, Maisonneuve V, Guiet A, Brunet S. The Effects of Various Parameters of the Microwave-Assisted Solvothermal Synthesis on the Specific Surface Area and Catalytic Performance of MgF2 Nanoparticles. Materials. 2020; 13(16):3566. https://doi.org/10.3390/ma13163566
Chicago/Turabian StyleWang, Yawen, Zahra Gohari Bajestani, Jérôme Lhoste, Sandy Auguste, Annie Hémon-Ribaud, Monique Body, Christophe Legein, Vincent Maisonneuve, Amandine Guiet, and Sylvette Brunet. 2020. "The Effects of Various Parameters of the Microwave-Assisted Solvothermal Synthesis on the Specific Surface Area and Catalytic Performance of MgF2 Nanoparticles" Materials 13, no. 16: 3566. https://doi.org/10.3390/ma13163566
APA StyleWang, Y., Gohari Bajestani, Z., Lhoste, J., Auguste, S., Hémon-Ribaud, A., Body, M., Legein, C., Maisonneuve, V., Guiet, A., & Brunet, S. (2020). The Effects of Various Parameters of the Microwave-Assisted Solvothermal Synthesis on the Specific Surface Area and Catalytic Performance of MgF2 Nanoparticles. Materials, 13(16), 3566. https://doi.org/10.3390/ma13163566








