Undissolved Ilmenite Mud from TiO2 Production—Waste or a Valuable Addition to Portland Cement Composites?
Abstract
1. Introduction
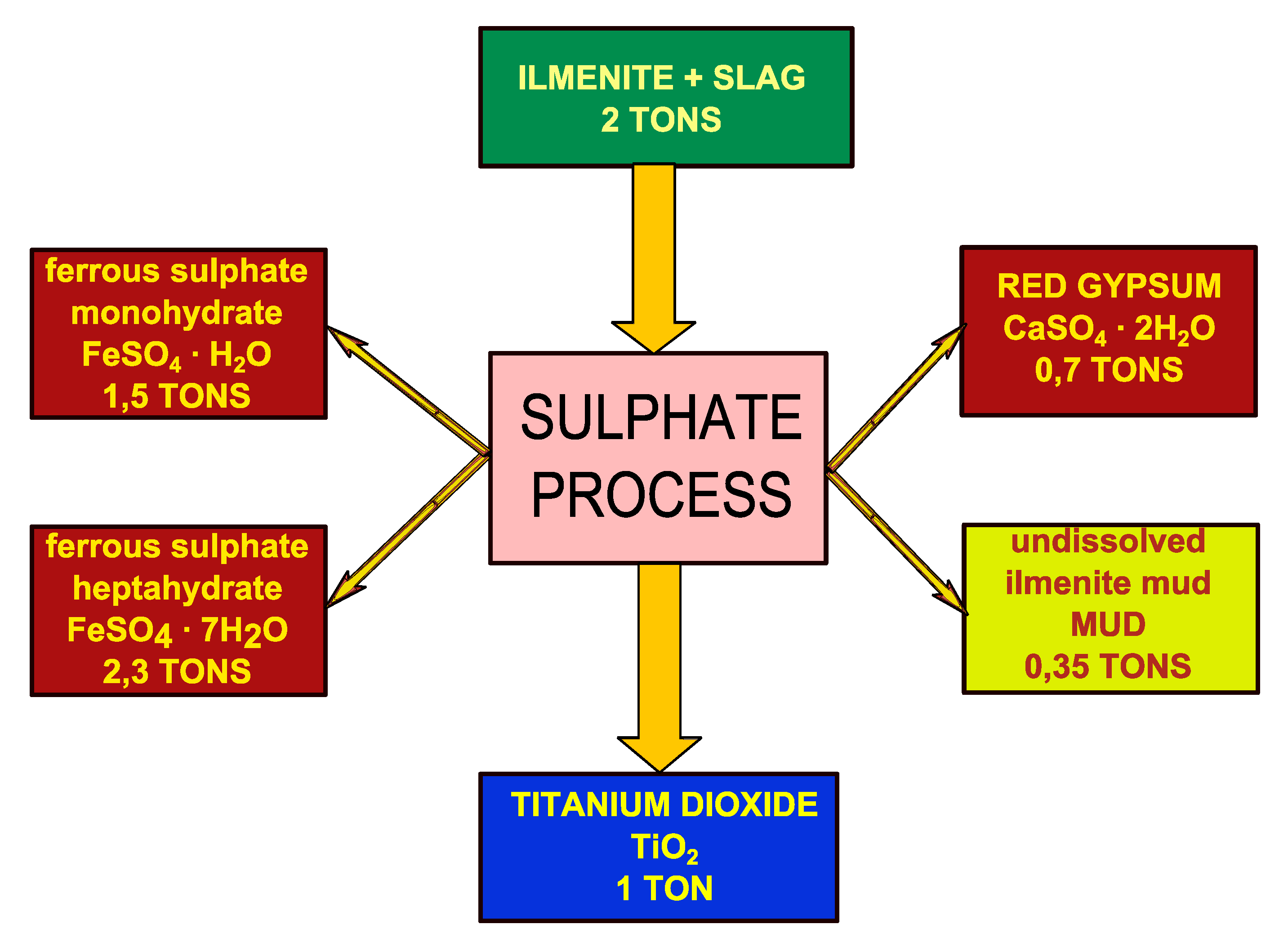
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sulphuric Acid Equivalent, Acidity Number and Sulphate Content
2.2.2. Major and Trace Constituents
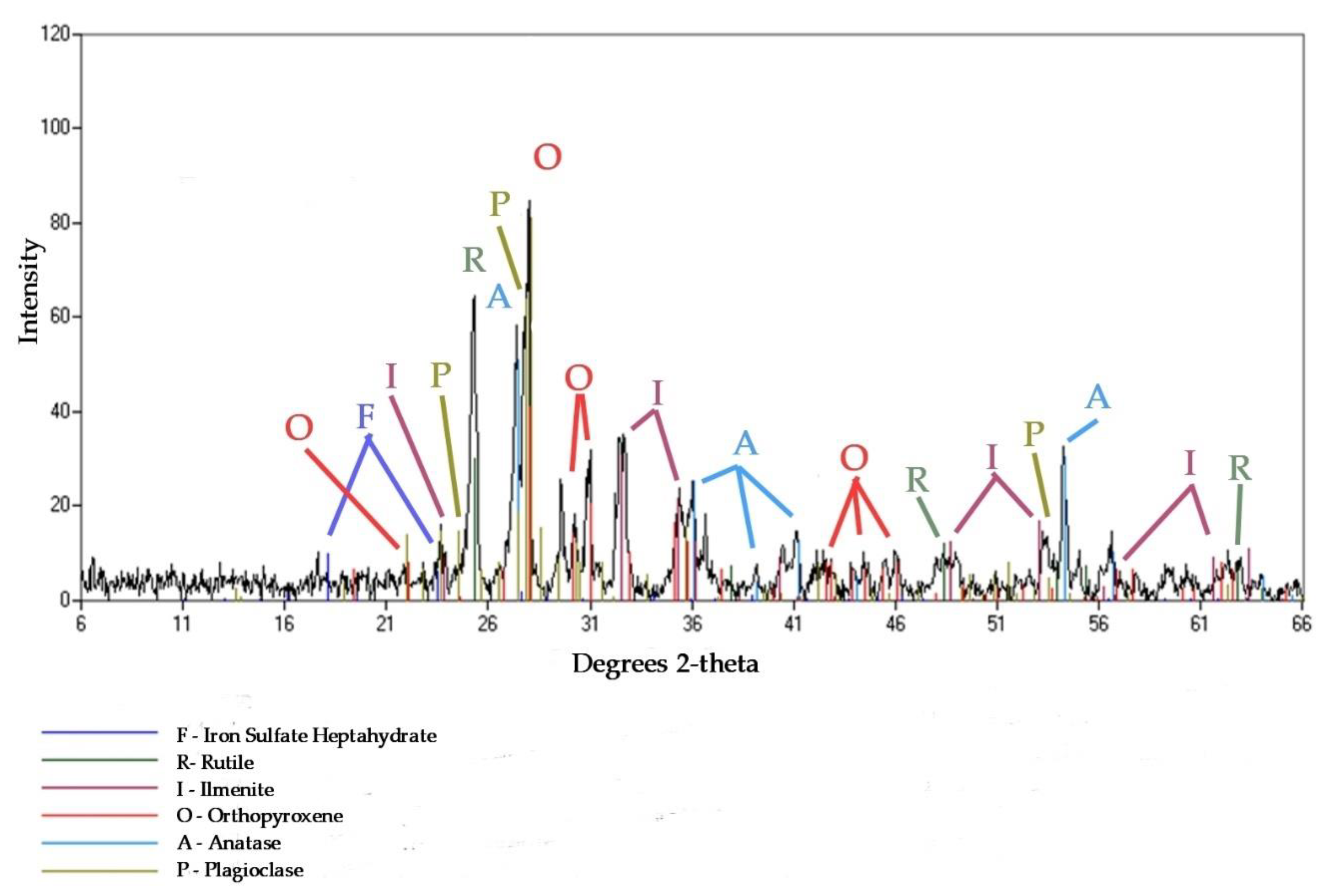
2.2.3. XRD
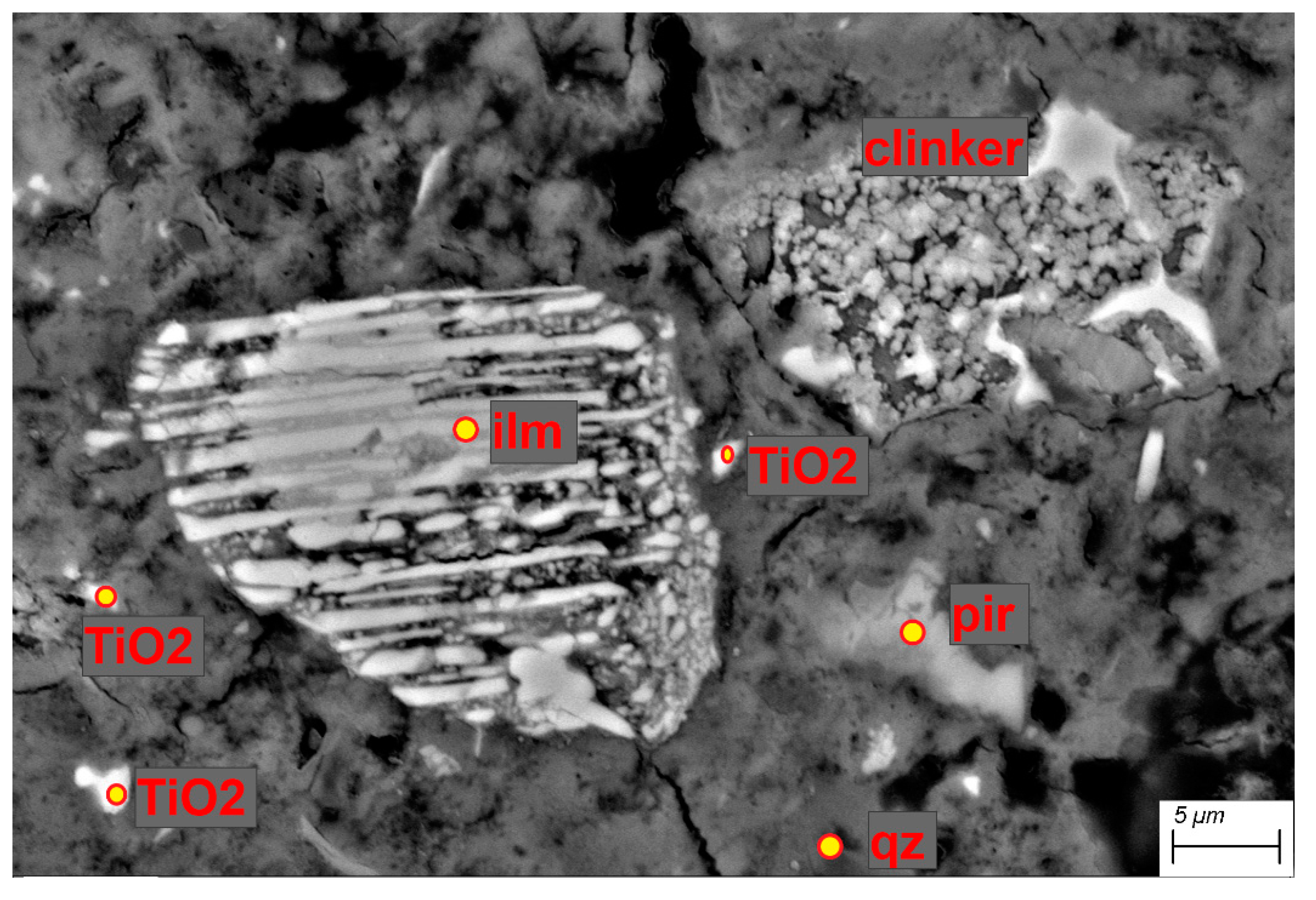
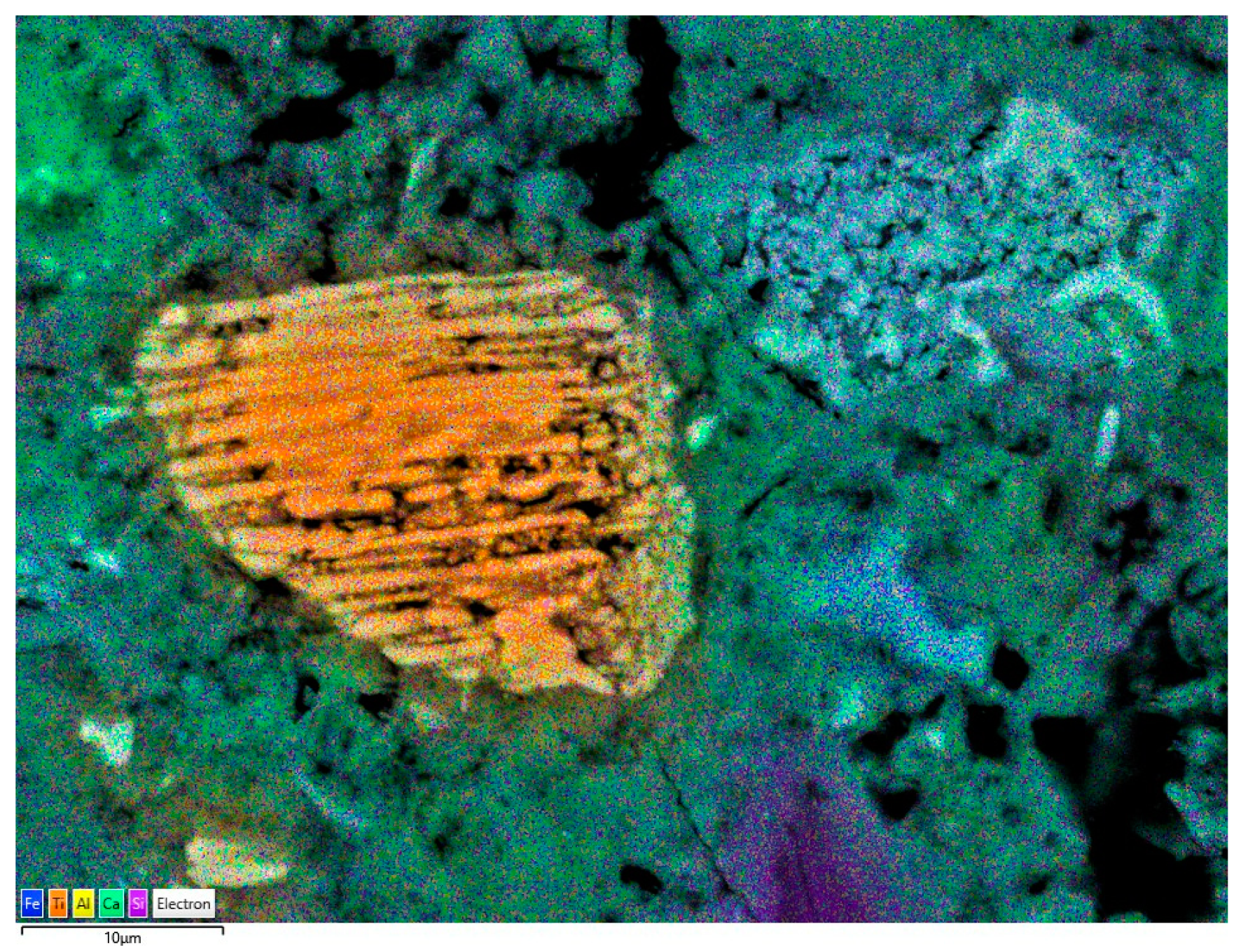
2.2.4. Scanning Electron Microscopy (SEM) with X-ray Microanalysis
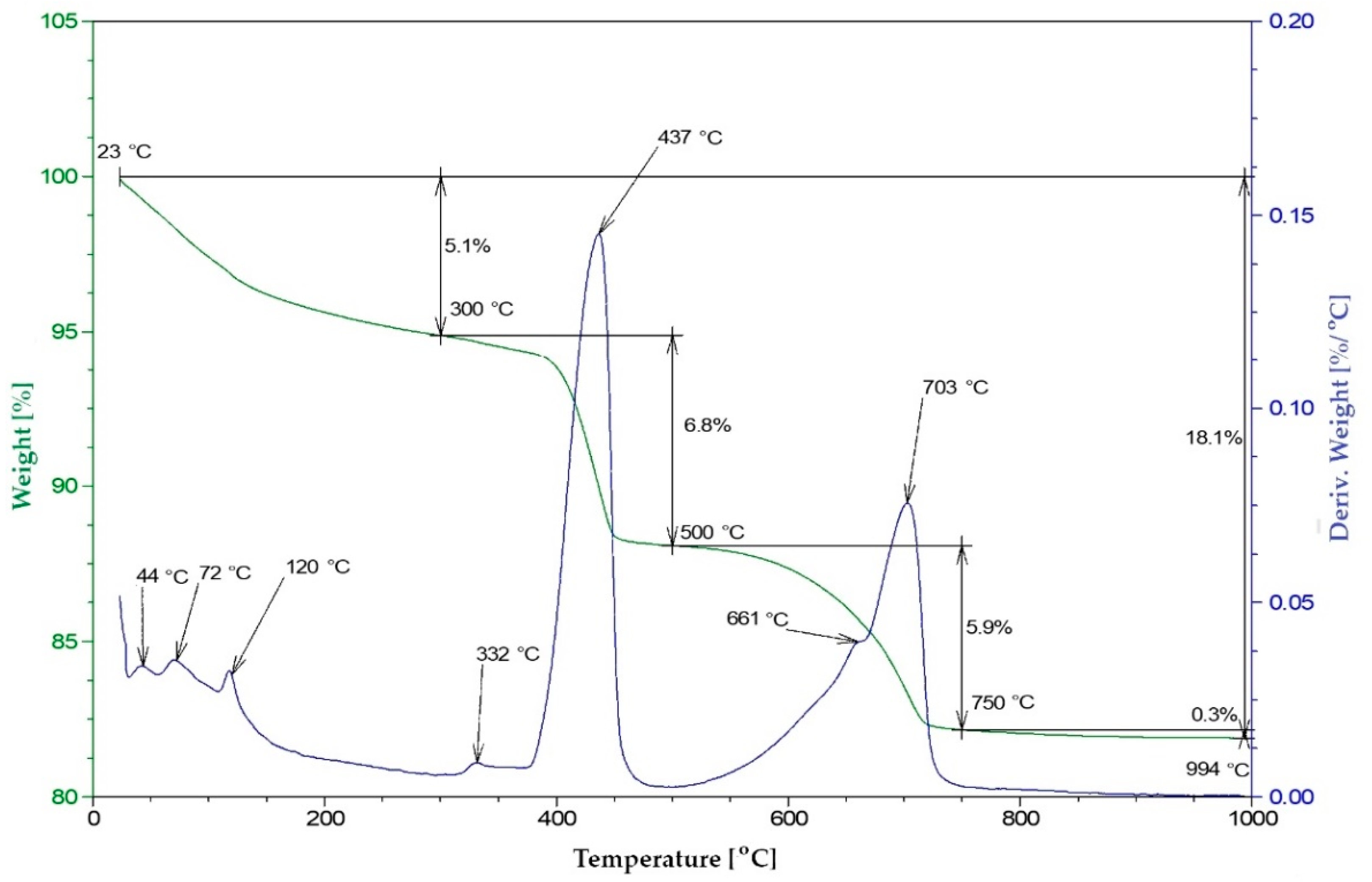
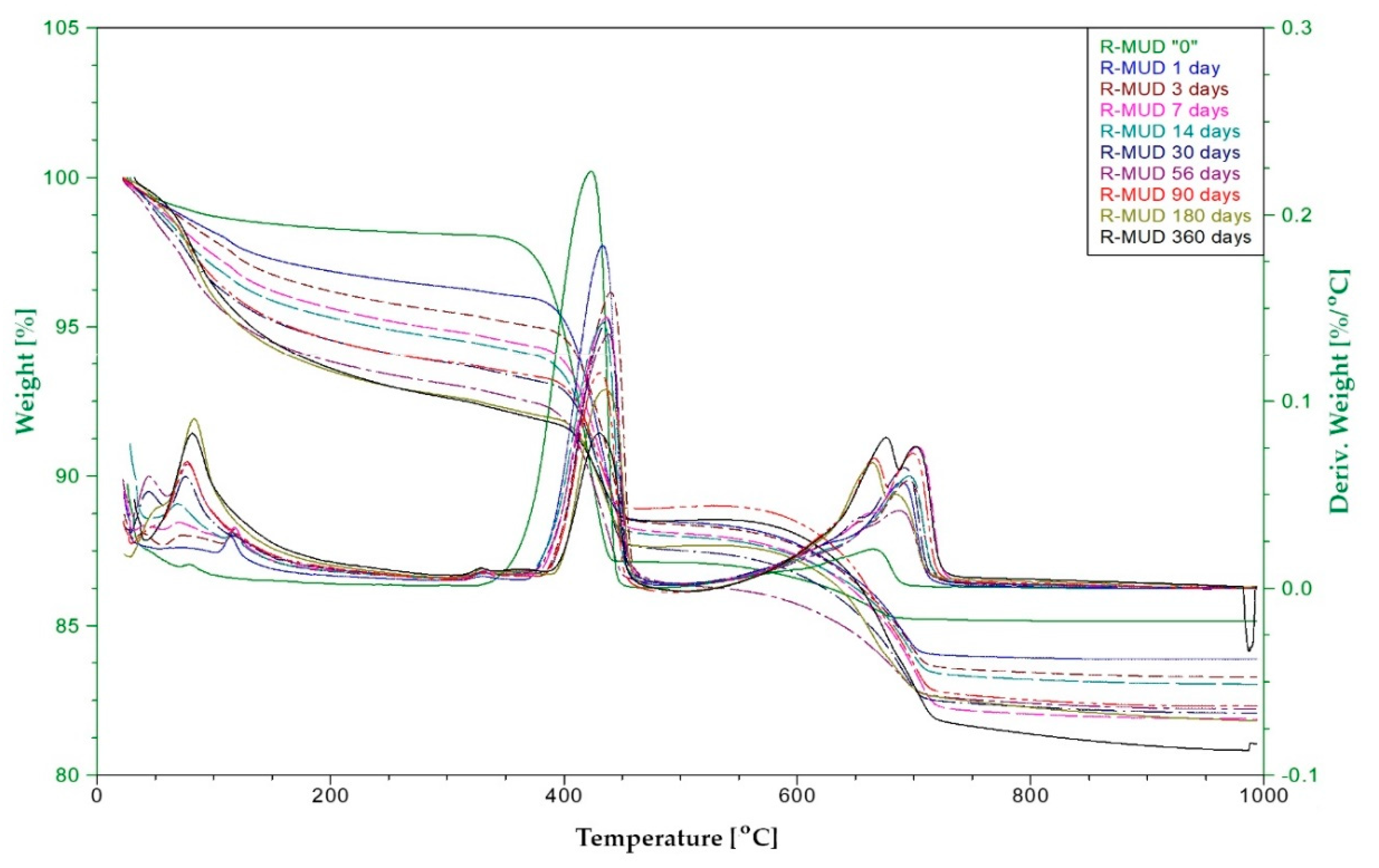
2.2.5. Thermal Analysis—Pozzolanic Activity
2.2.6. Reactive Silicone Dioxide
2.2.7. Calorimetric Tests
- 8 g samples (S) of cement and appropriate mixtures of cement and pozzolana were placed in special calorimetric vessels;
- 4 g of water (W) were placed in syringes;
- after thermostatting was finished, the measurement was started and water was introduced through injection needles into the measurement chambers.
3. Results and Discussion
3.1. Acid and Sulphate Content
3.2. Major and Trace Elements (XRF and TMA)
3.3. Mineral Composition
3.4. Scanning Electron Microscopy (SEM)
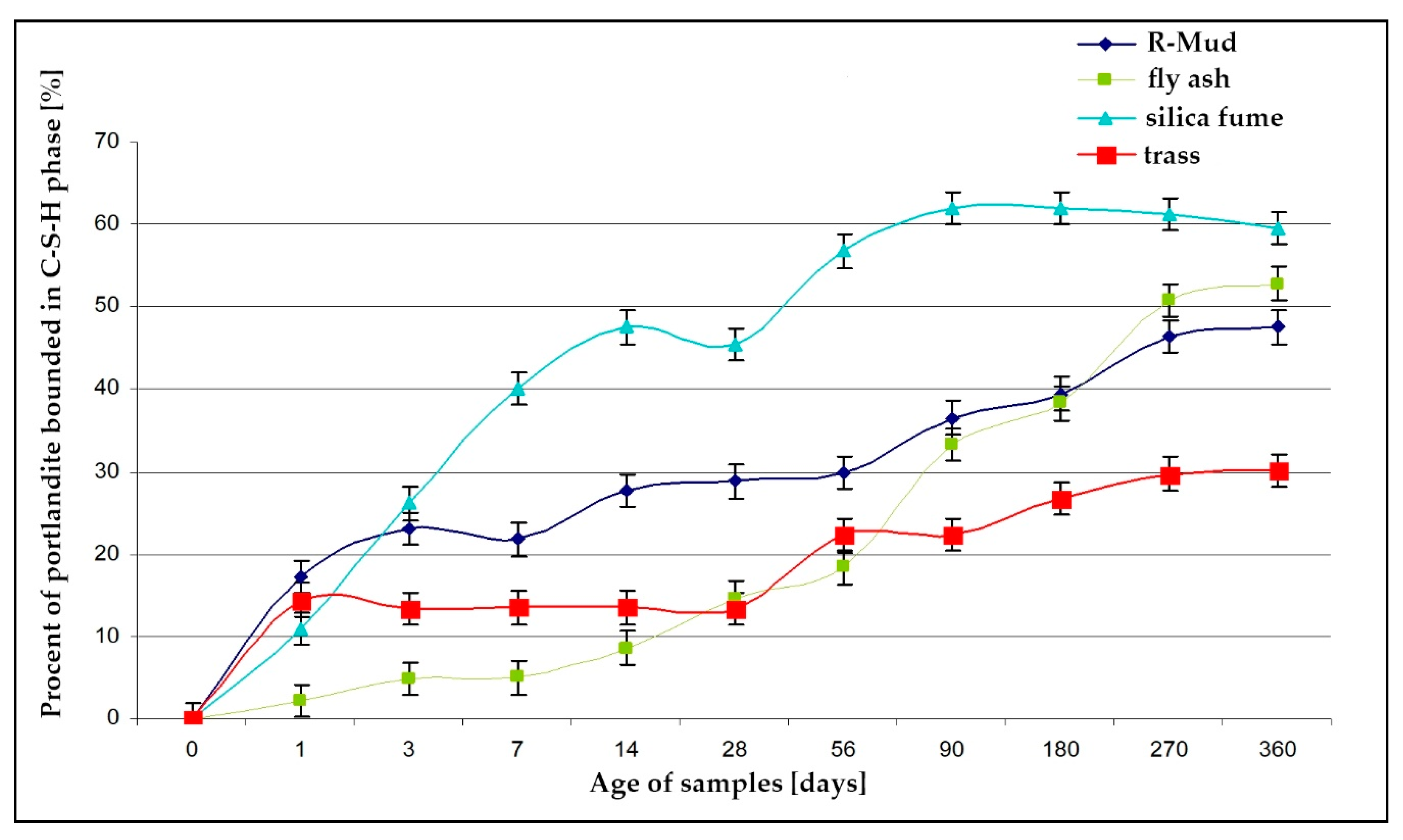
3.5. Pozzolanic Activity (DTA)
3.6. Reactive Silicon Dioxide
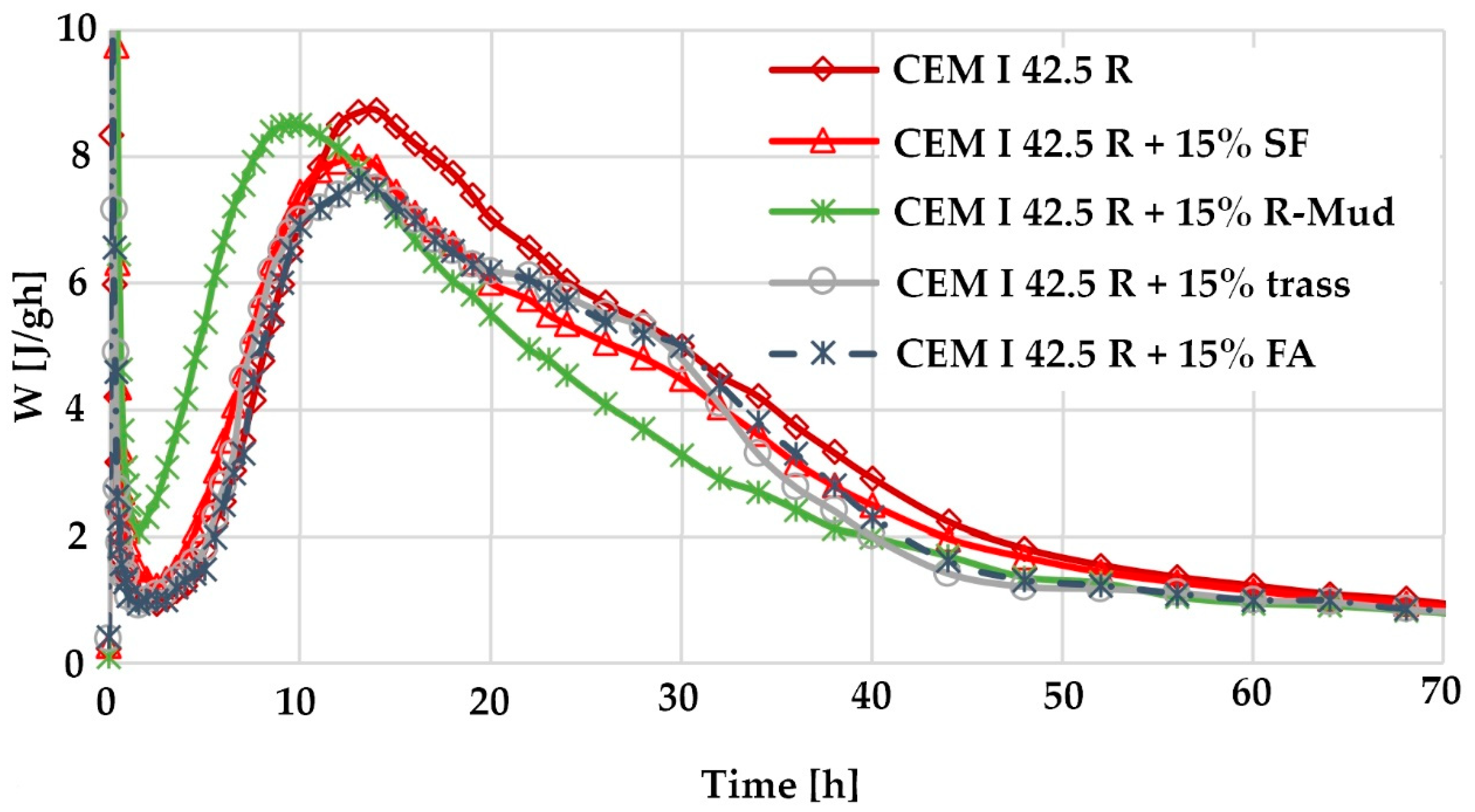
3.7. Calorimetric Test Results
4. Conclusions
- ▪
- the correction of the sulphuric acid content and the washing out of some of the soluble salts allows to obtain a new quality of waste—R-MUD, which is less hazardous than MUD and allows to consider other possibilities of application. Moreover, the resulting filtrate is a valuable raw material for further production of TiO2;
- ▪
- it is justified to expect that minimising the sulphuric acid content in R-MUD to a level below 1% will not threaten the durability of cement composites [4];
- ▪
- ▪
- R-MUD has a relatively low content of silicon dioxide (about 35%) and reactive silicon dioxide (about 10%) with surprisingly high pozzolanic activity. This allows for the effective use of this material as a reactive admixture to the concrete substitute part of the cement;
- ▪
- R-MUD most likely gains in reactivity as a result of aggressive chemical, thermal and mechanical treatment and may contain nano-silica and nano-TiO2 [20]. The latter conclusion, however, needs to be confirmed by further studies;
- ▪
- the results of calorimetric and thermogravimetric tests confirm the high activity of R-MUD and the ability to form the C-S-H phase responsible for the microstructure of cement grouts.
Author Contributions
Funding
Conflicts of Interest
References
- Titanium Dioxide (TiO2) Market 2019 Global Industry Analysis By Key Players, Share, Revenue, Trends, Organizations Size, Growth, Opportunities, and Regional Forecast to 2025. Available online: https://www.grandviewresearch.com/industry-analysis/titanium-dioxide-industry (accessed on 23 June 2020).
- Contreras, M.; Martín, M.I.; Gázquez, M.J.; Romero, M.; Bolívar, J.P. Valorisation of ilmenite mud waste in the manufacture of commercial ceramic. Constr. Build. Mater. 2014, 72, 31–40. [Google Scholar] [CrossRef]
- Contreras, M.; Teixeira, S.R.; Lucas, M.C.; Lima, L.C.; Cardoso, D.S.; Da Silva, G.A.; Gregório, G.C.; De Souza, A.E.; Dos Santos, A. Recycling of construction and demolition waste for producing new construction material (Brazil case-study). Constr. Build. Mater. 2016, 123, 594–600. [Google Scholar] [CrossRef]
- Chyliński, F.; Kuczyński, K.; Łukowski, P. Application of ilmenite mud waste as an addition to concrete. Materials 2020, 13, 866. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Mantero, J.; Bolívar, J.P.; García-Tenorio, R.; Vaca, F.; Lozano, R.L. Physico-chemical and radioactive characterization of TiO2 undissolved mud for its valorization. J. Hazard. Mater. 2011, 191, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Bobrowicz, J.; Chyliński, F. The influence of ilmenite mud waste on the hydration process of Portland cement. J. Therm. Anal. Calorim. 2016, 126, 493–498. [Google Scholar] [CrossRef]
- Contreras, M.; Martín, M.I.; Gázquez, M.J.; Romero, M.; Bolívar, J.P. Manufacture of ceramic bodies by using a mud waste from the TiO2 pigment industry. Key Eng. Mater. 2016, 663, 75–85. [Google Scholar] [CrossRef]
- Llanes, M.C.; González, M.J.G.; Moreno, S.M.P.; Raya, J.P.B. Recovery of ilmenite mud as an additive in commercial Portland cements. Environ. Sci. Pollut. Res. 2018, 25, 24695–24703. [Google Scholar] [CrossRef] [PubMed]
- Harasymiuk, J.; Rudziński, A. Old dumped fly ash as a sand replacement in cement composites. Buildings 2020, 10, 67. [Google Scholar] [CrossRef]
- Fan, C.C.; Huang, R.; Hwang, H.; Chao, S.J. The effects of different fine recycled concrete aggregates on the properties of Mortar. Materials 2015, 8, 2658–2672. [Google Scholar] [CrossRef]
- Chung, S.Y.; Elrahman, M.A.; Sikora, P.; Rucinska, T.; Horszczaruk, E.; Stephan, D. Evaluation of the effects of crushed and expanded waste glass aggregates on the material properties of lightweight concrete using image-based approaches. Materials 2017, 10, 1354. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O. Durability and compressive strength of high cement replacement ratio self-consolidating concrete. Buildings 2018, 8, 153. [Google Scholar] [CrossRef]
- Konkol, J. Fracture Toughness and fracture surface morphology of concretes modified with selected additives of pozzolanic properties. Buildings 2019, 9, 174. [Google Scholar] [CrossRef]
- Moghaddam, F.; Sirivivatnanon, V.; Vessalas, K. The effect of fly ash fineness on heat of hydration, microstructure, flow and compressive strength of blended cement pastes. Case Stud. Constr. Mater. 2019, 10, e00218. [Google Scholar] [CrossRef]
- Karthiyaini, S. Physicochemical propertiesof alkali activated fly ash based geopolymer concrete: A review. Int. J. Earth Sci. Eng. 2016, 9, 2419–2426. [Google Scholar]
- Czarnecki, L.; Kapron, M. Sustainable construction as a research area. Int. J. Soc. Mater. Eng. Resour. 2010, 7, 99–106. [Google Scholar] [CrossRef]
- Marthong, C.; Agrawal, T.P. Effect of fly ash additive on concrete properties. Int. J. Eng. Res. Appl. 2012, 2, 1986–1991. [Google Scholar]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Michalik, A.; Babińska, J.; Chyliński, F.; Piekarczuk, A. Ammonia in fly ashes from flue gas denitrification process and its impact on the properties of cement composites. Buildings 2019, 9, 225. [Google Scholar] [CrossRef]
- Sikora, P.; Augustyniak, A.; Cendrowski, K.; Horszczaruk, E.; Rucinska, T.; Nawrotek, P.; Mijowska, E. Characterization of mechanical and bactericidal properties of cement mortars containingwaste glass aggregate and nanomaterials. Materials 2016, 9, 701. [Google Scholar] [CrossRef]
- EN 206+A1:2016-12. Concrete-Specification, Performance, Production and Conformity; European Committee for Standardization: Brussels, Belgium, 2016. [Google Scholar]
- Horoszczaruk, E. Role of nanosilica in the formation of the properties of cement composites, state of the art. Cem. Wapno Bet. 2018, 6, 487–495. [Google Scholar]
- D’Alessandro, A.; Materazzi, A.L.; Ubertini, F. Nanotechnology in Cement-Based Construction, 1st ed.; Jenny, S., Ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Czarnecki, L.; Schorn, H. Nanomonitoring of polymer cement concrete microstructure. Restor. Build. Monum. 2014, 13, 141–152. [Google Scholar] [CrossRef]
- Kurdowski, W. Cement and Concrete Chemistry; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Bautista-Gutierrez, K.P.; Herrera-May, A.L.; Santamaría-López, J.M.; Honorato-Moreno, A.; Zamora-Castro, S.A. Recent progress in nanomaterials for modern concrete infrastructure: Advantages and challenges. Materials 2019, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Liang, Y.; Cheng, H.; Guo, S.; Jiao, C. Preparation and photocatalytic properties of a bagasse. Materials 2020, 13, 1645. [Google Scholar] [CrossRef] [PubMed]
- Ferone, C.; Colangelo, F.; Messina, F.; Santoro, L.; Cioffi, R. Recycling of pre-washed municipal solid waste incinerator fly ash in the manufacturing of low temperature setting geopolymer materials. Materials 2013, 6, 3420–3437. [Google Scholar] [CrossRef]
- Wahlström, M.; Laine-Ylijoki, J.; Wik, O.; Oberender, A.; Hjelmar, O. Hazardous Waste Classification; Nordic Council of Ministers: Copenhagen, Denmark, 2016. [Google Scholar]
- Tavakoli, D.; Hashempour, M.; Heidari, A. Use of waste materials in concrete: A review. Pertanika J. Sci. Technol. 2018, 26, 499–522. [Google Scholar]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Comparison of test methods to assess pozzolanic activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Kramar, S.; Ducman, V. Evaluation of ash pozzolanic activity by means of the strength activity index test, frattini test and DTA/TG analysis. Teh. Vjesn. 2018, 25, 1746–1752. [Google Scholar]
- EN 450-1:2012. Fly Ash for Concrete-Part. 1: Definition, Specifications and Conformity Criteria; European Committee for Standardization: Brussels, Belgium, 2012. [Google Scholar]
- EN 13263-1+A1:2009. Silica Fume for Concrete-Part. 1: Definitions, Requirements and Conformity Criteria; European Committee for Standardization: Brussels, Belgium, 2009. [Google Scholar]
- Klepka, M.; Lawniczak-Jablonska, K.; Jablonski, M.; Wolska, A.; Minikayev, R.; Paszkowicz, W.; Przepiera, A.; Spolnik, Z.; Van Grieken, R. Combined XRD, EPMA and X-ray absorption study of mineral ilmenite used in pigments production. J. Alloys Compd. 2005, 401, 281–288. [Google Scholar] [CrossRef]
- Diot, H.; Bolle, O.; Lambert, J.-M.; Launeau, P.; Duchesne, J.-C. The tellnes ilmenite deposit (Rogaland, South Norway): Magnetic and petrofabric evidence for emplacement of a Ti-enriched noritic crystal mush in a fracture zone. J. Struct. Geol. 2003, 25, 481–501. [Google Scholar] [CrossRef]
- Sunde, M. Organic Binder as a Substitute for Bentonite in Ilmenite Pelletization. Master’s Thesis, Institutt for Materialteknologi, Trondheim, Norway, 2012. [Google Scholar]
- Samal, S.; Mohapatra, B.K.; Mukherjee, P.S.; Chatterjee, S.K. Integrated XRD, EPMA and XRF study of ilmenite and titania slag used in pigment production. J. Alloys Compd. 2009, 474, 484–489. [Google Scholar] [CrossRef]
- Bobrowicz, J.; Chyliński, F. Comparison of pozzolanic activity of ilmenite MUD waste to other pozzolans used as an additive for concrete production. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Chyliński, F.; Kuczyński, K. Ilmenite mud waste as an additive for frost resistance in sustainable concrete. Materials 2020, 13, 2904. [Google Scholar] [CrossRef] [PubMed]
- EN 196-2:2013-11. Method of Testing Cement-Part. 2: Chemical Analysis of Cement; European Committee for Standardization: Brussels, Belgium, 2013. [Google Scholar]
- Roszczynialski, W.; Stępień, W.; Tkaczewska, P.; Roszczynialski, E. The reliability of pozzolanic activity assessment methods in testing different fly ashes. Cem. Wapno Bet. 2014, 5, 323–333. [Google Scholar]
- Mertens, G.; Snellings, R.; van Balen, K.; Bicer-Simsir, B.; Verlooy, P.; Elsen, J. Pozzolanic reactions of common natural zeolites with lime and parameters affecting their reactivity. Cem. Concr. Res. 2009, 39, 233–240. [Google Scholar] [CrossRef]
- Broekmans, M.A.T.M.; Pöllmann, H. Applied Mineralogy of Cement & Concrete; Mineralogical Society of America: Chantilly, VA, USA, 2018. [Google Scholar]
- Witakowski, P.; Czamarska, D.; Bobrowicz, J. Skoputeryzowany układ do pomiarów kalorymetrycznych Część I Aparatura. C Ement. Waspno. Gips. 1991, 7, 182–186. [Google Scholar]
- Nocuń-Wczelik, W.; Małolepszy, J. Application of calorimetry in studies of the immobilization of heavy metals in cementitious materials. Thermochim. Acta 1995, 269–270, 613–619. [Google Scholar] [CrossRef]
- Giergiczny, Z.; Król, A. Immobilization of heavy metals (Pb, Cu, Cr, Zn, Cd, Mn) in the mineral additions containing concrete composites. J. Hazard. Mater. 2008, 160, 247–255. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef]
- Habib, M.A.; Bahadur, N.M.; Mahmood, A.J.; Islam, M.A. Immobilization of heavy metals in cementitious matrices. J. Saudi Chem. Soc. 2012, 16, 263–269. [Google Scholar] [CrossRef]
- Chyliński, F.; Łukowski, P. Management of hazardous waste from the production of titanium dioxide as a substitute for part of cement in cement composites. Mater. Bud. 2016, 530, 18–20. [Google Scholar]
- Ramachandran, V.S.; Paroli, R.M.; Beaudoin, J.J.; Delgado, A.H. The Handbook of Thermal Analysis of Construction Materials; William Andrew: Norwich, UK, 2009. [Google Scholar]
- Han, F.; Zhang, Z.; Liu, J.; Yan, P. Effect of water-to-binder ratio on the hydration kinetics of composite binder containing slag or fly ash. J. Therm. Anal. Calorim. 2017, 128, 855–865. [Google Scholar] [CrossRef]
- Alahrache, S.; Winnefeld, F.; Champenois, J.B.; Hesselbarth, F.; Lothenbach, B. Chemical activation of hybrid binders based on siliceous fly ash and Portland cement. Cem. Concr. Compos. 2016, 66, 10–23. [Google Scholar] [CrossRef]
- Ercikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H.; Alp, Ì. Effect of natural pozzolans as mineral admixture on the performance of cemented-paste backfill of sulphide-rich tailings. Waste Manag. Res. 2010, 28, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Ramakokovhu, M.M.; Mojisola, T.; Mbaya, R.K.K.; Olubambi, P.A. An electrochemical study and thermodynamic quantification of pre-oxidized ilmenite metastable phases. In Proceedings of the 11th International Heavy Minerals Conference, Western Cape, South Africa, 5–6 August 2019. [Google Scholar]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Li, M. Influence of Nano-SiO2 on the consistency, setting time, early-age strength, and shrinkage of composite cement pastes. Adv. Mater. Sci. Eng. 2016, 2016, 1–8. [Google Scholar]
- Indukuri, C.S.R.; Nerella, R.; Madduru, S.R.C. Effect of graphene oxide on microstructure and strengthened properties of fly ash and silica fume based cement composites. Constr. Build. Mater. 2019, 229, 116863. [Google Scholar] [CrossRef]
- Rossen, J.E.; Lothenbach, B.; Scrivener, K.L. Composition of C-S-H in pastes with increasing levels of silica fume addition. Cem. Concr. Res. 2015, 75, 14–22. [Google Scholar] [CrossRef]


| Elements | CEM I 42.5R | R-MUD | Silica Fume | Trass | Fly Ash |
|---|---|---|---|---|---|
| SiO2 | 20.60 | 35.07 | 91.40 | 77.05 | 51.51 |
| CaO | 64.41 | 3.09 | 0.09 | 0.30 | 3.82 |
| TiO2 | – | 33.05 | <0.01 | 0.09 | 1.09 |
| Al2O3 | 4.13 | 5.53 | 0.15 | 12.35 | 25.71 |
| Fe2O3 | 3.38 | 9.65 | 0.65 | 0.07 | 8.51 |
| MgO | 0.89 | 7.26 | 1.51 | 0.06 | 2.53 |
| Na2O | 0.24 | 1.10 | 1.02 | 3.25 | 1.37 |
| K2O | 0.56 | 0.26 | 1.07 | 4.85 | 2.73 |
| MnO | – | 0.53 | 0.06 | 0.01 | 0.10 |
| P2O5 | – | 0.01 | 0.13 | 0.02 | 0.31 |
| (SO3) | 2.97 | 0.05 | 0.01 | <0.01 | 0.48 |
| (Cl) | 0.070 | 0.017 | 0.115 | 0.011 | 0.015 |
| Specific surface area 1 [cm2/g] | 4060 | 8390 | 23,160 | 6030 | 4020 |
| Element | SiO2 | TiO2 | Fe2O3 | MgO | Al2O3 | CaO | Na2O | MnO | K2O | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| MUD | 23.56 | 27.20 | 16.65 | 5.39 | 4.08 | 2.49 | 0.82 | 0.33 | 0.13 | 0.01 |
| R-MUD | 35.07 | 33.05 | 9.65 | 7.26 | 5.53 | 3.09 | 1.10 | 0.53 | 0.26 | 0.01 |
| Element | As | Br | Ce | Co | Cr | Cu | Ga | Hf | La | Mo | Nb | Ni | Pb |
| MUD | 18 | 4 | 21 | 77 | 253 | 313 | 11 | 19 | 29 | 3 | 57 | 194 | <3 |
| R-MUD | 22 | 5 | 38 | 79 | 294 | 201 | 7 | 24 | 38 | 4 | 137 | 151 | <3 |
| Element | Rb | Sr | Bi | Th | U | V | Y | Zn | Zr | Cd | Sn | Hg | |
| MUD | 3 | 155 | 4 | 6 | 7 | 558 | 11 | 519 | 1065 | 10 | 10 | 0.014 | |
| R-MUD | 11 | 220 | 3 | 28 | 5 | 795 | 19 | 504 | 1296 | 11 | <2 | 0.025 |
| Time [h] | Total Heat of Hydration [J/g] | ||||
|---|---|---|---|---|---|
| CEM I 42.5R | CEM I 42.5R + 15% Trass | CEM I 42.5 R + 15% Silica Fume | CEM I 42.5R + 15% Fly Ash | CEM I 42.5R + 15% R-MUD | |
| 0.5 | 6.6 | 1.4 | 7.0 | 3.9 | 15.5 |
| 1 | 7.8 | 1.7 | 8.4 | 5.0 | 17.4 |
| 2 | 9.2 | 3.1 | 9.9 | 6.1 | 19.6 |
| 4 | 13.0 | 5.5 | 12.7 | 8.3 | 25.9 |
| 6 | 20.1 | 8.9 | 17.7 | 11.8 | 36.9 |
| 12 | 62.6 | 43.5 | 55.8 | 43.8 | 85.5 |
| 18 | 110.0 | 88.8 | 100.1 | 89.8 | 127.9 |
| 24 | 147.2 | 125.7 | 135.6 | 126.2 | 159.5 |
| 36 | 196.2 | 178.3 | 188.2 | 179.4 | 199.8 |
| 48 | 221.8 | 203.1 | 215.5 | 204.0 | 221.4 |
| 60 | 237.1 | 218.2 | 231.9 | 218.9 | 235.0 |
| 72 | 248.6 | 228.9 | 243.7 | 229.5 | 245.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chyliński, F.; Bobrowicz, J.; Łukowski, P. Undissolved Ilmenite Mud from TiO2 Production—Waste or a Valuable Addition to Portland Cement Composites? Materials 2020, 13, 3555. https://doi.org/10.3390/ma13163555
Chyliński F, Bobrowicz J, Łukowski P. Undissolved Ilmenite Mud from TiO2 Production—Waste or a Valuable Addition to Portland Cement Composites? Materials. 2020; 13(16):3555. https://doi.org/10.3390/ma13163555
Chicago/Turabian StyleChyliński, Filip, Jan Bobrowicz, and Paweł Łukowski. 2020. "Undissolved Ilmenite Mud from TiO2 Production—Waste or a Valuable Addition to Portland Cement Composites?" Materials 13, no. 16: 3555. https://doi.org/10.3390/ma13163555
APA StyleChyliński, F., Bobrowicz, J., & Łukowski, P. (2020). Undissolved Ilmenite Mud from TiO2 Production—Waste or a Valuable Addition to Portland Cement Composites? Materials, 13(16), 3555. https://doi.org/10.3390/ma13163555






