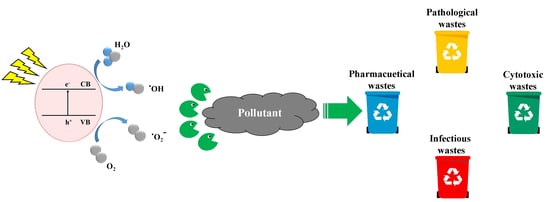
Biomedical Waste Management by Using Nanophotocatalysts: The Need for New Options
Abstract
1. Introduction
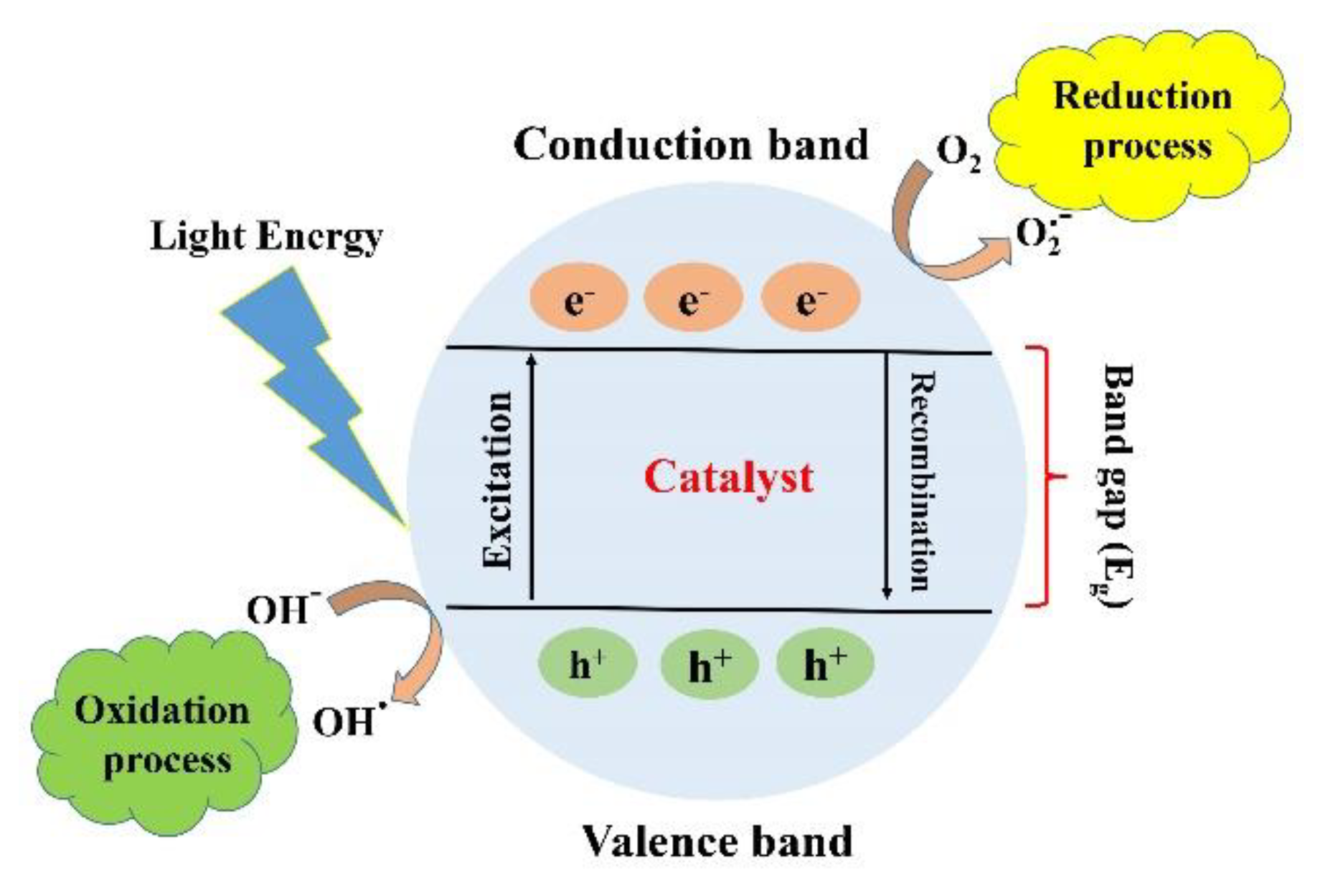
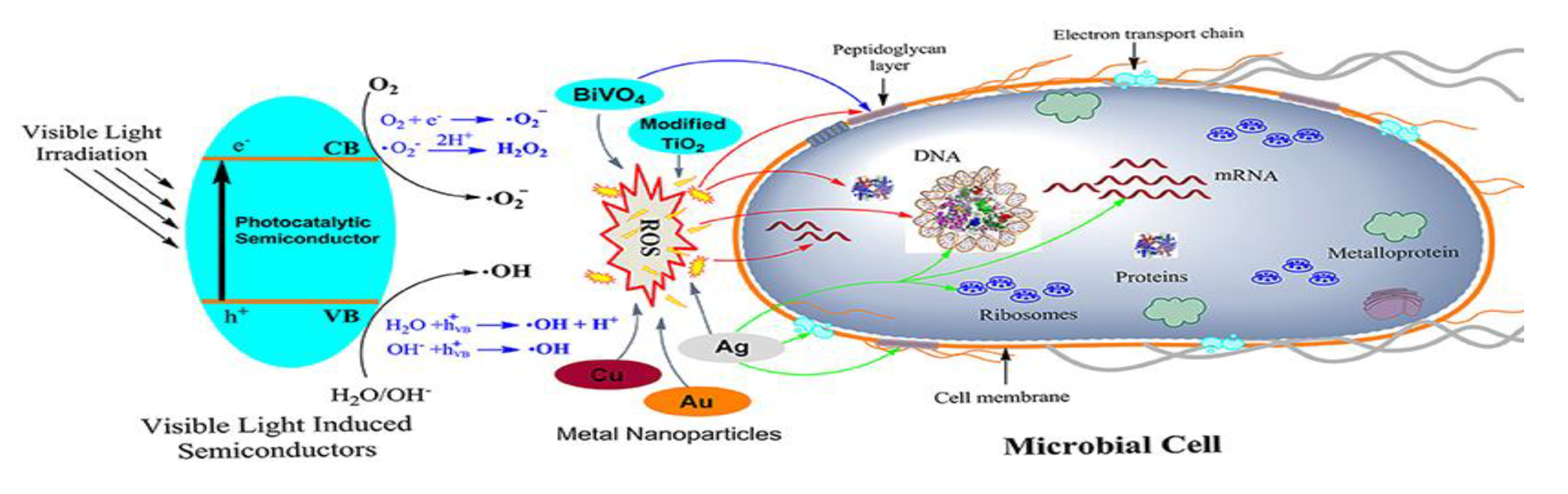
2. Fundamentals and Mechanism of Photocatalytic Reactions
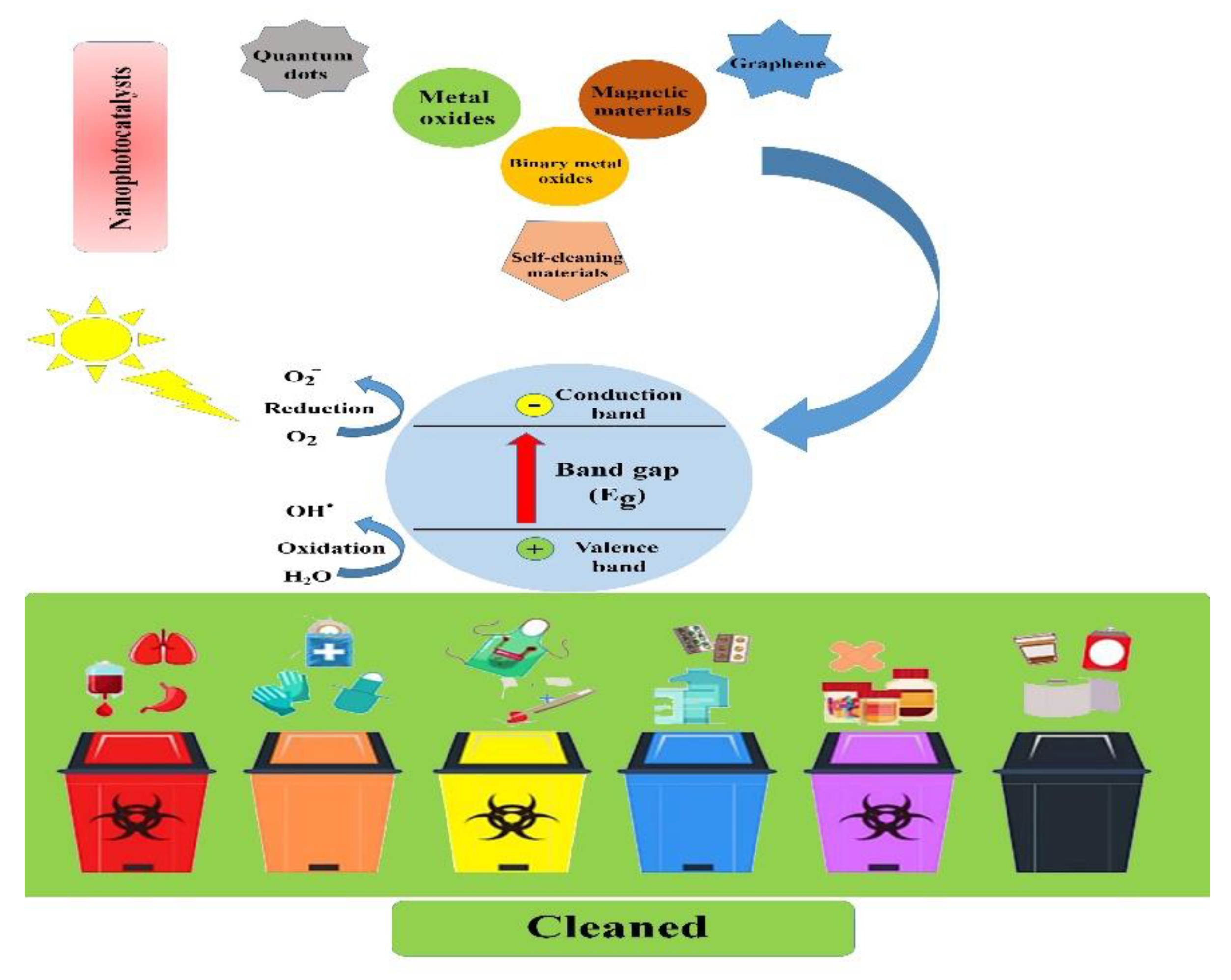
3. Types and Characteristics of Nanophotocatalysts
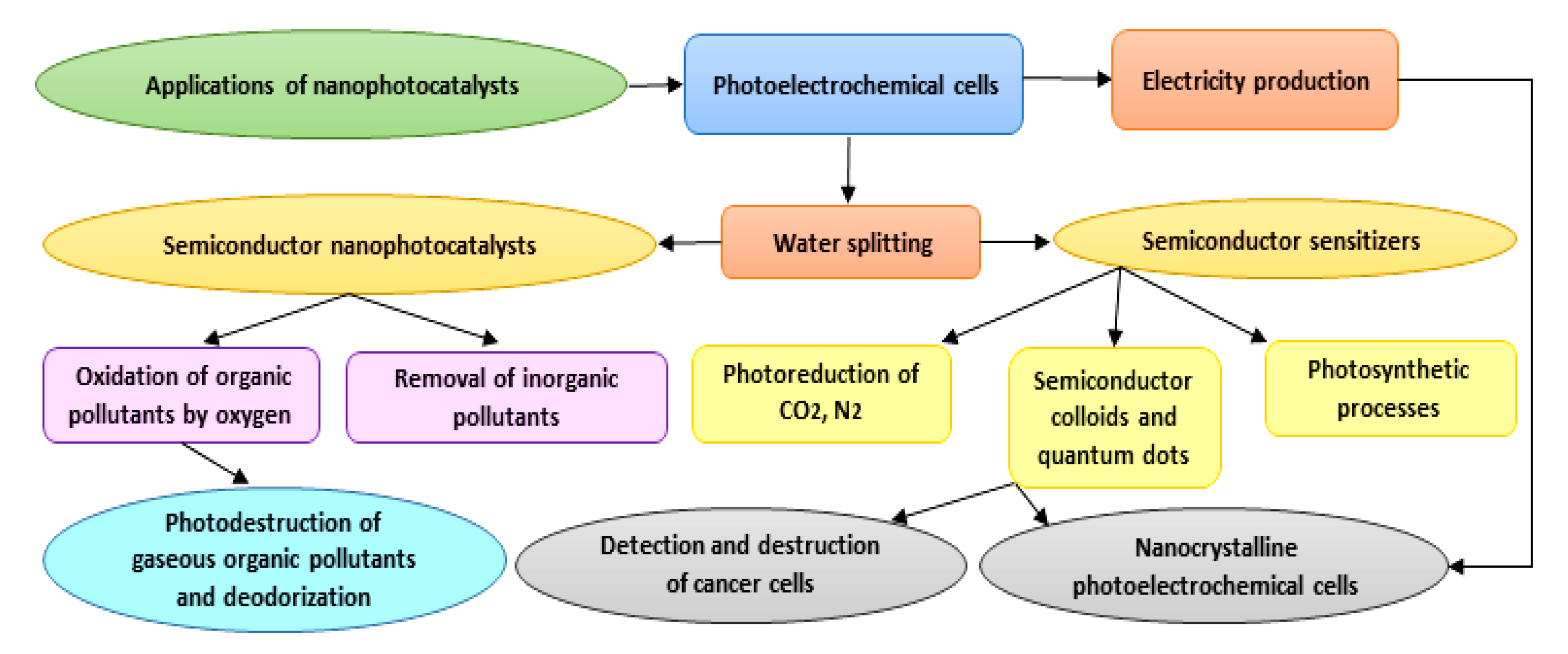
4. Biomedical Applications of Nanophotocatalysts
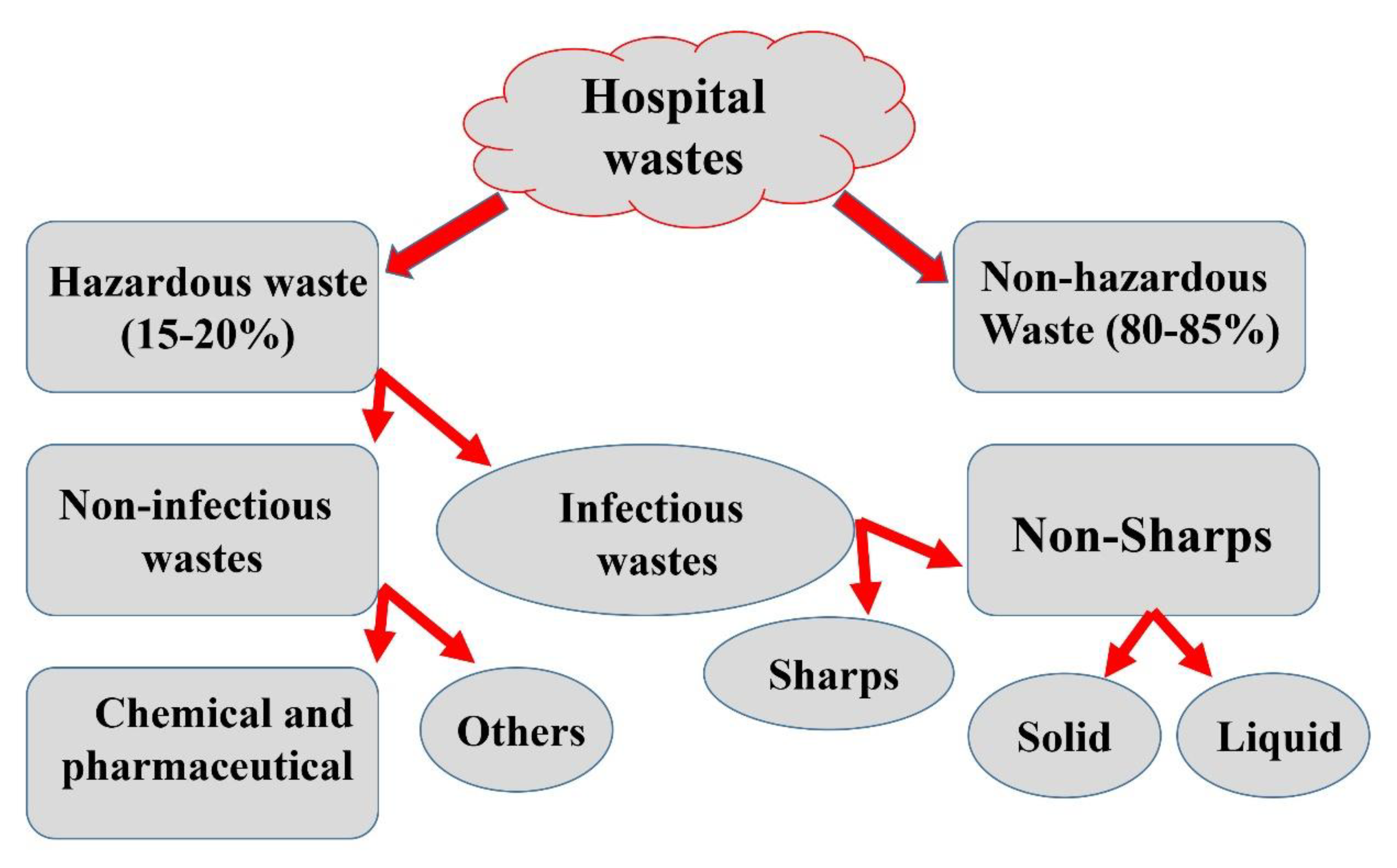
4.1. Laboratory and Hospital
4.1.1. Basics and Fundamentals
4.1.2. Photocatalytic Point of View
4.2. Biological and Medical
4.2.1. Categories and Conventional Methods
4.2.2. Photocatalytic Strategies
Metal Oxides
- Titanium Dioxide (TiO2)
- 2.
- Zinc Oxide (ZnO)
- 3.
- Iron Oxide (Fe2O3)
- 4.
- Gadolinium Oxide (Gd2O3)
- 5.
- Antimony Oxide (Sb2O4)
Binary Metal Oxides
Metal Sulfides
Magnetic Nanophotocatalysts
Graphene
Quantum Dots
Smart Materials (Self-Cleaning)
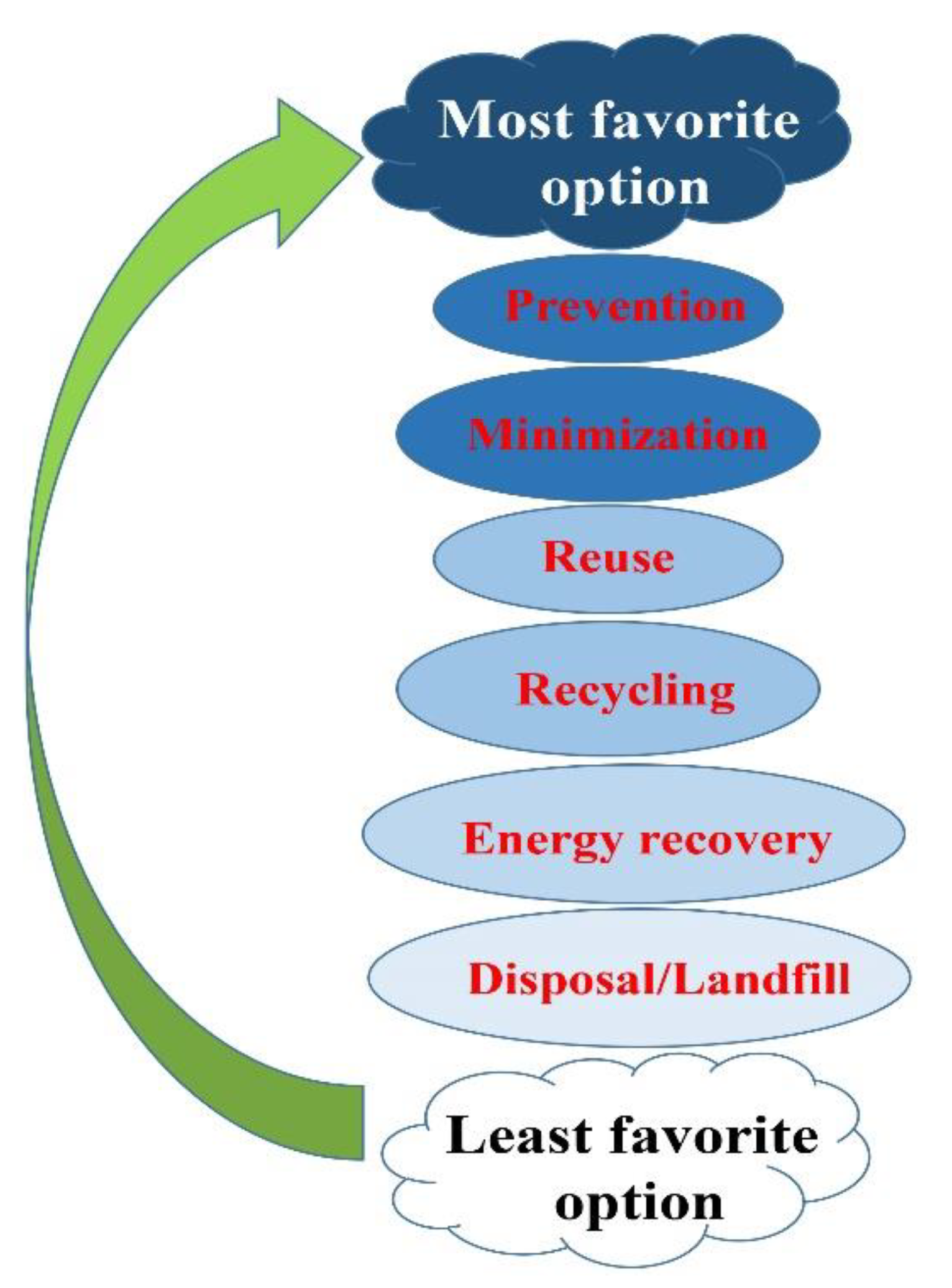
5. Future Prospects, and Concluding Remarks
6. Importance of Waste Management as a Crucial Public Service during the COVID-19 Outbreak
Author Contributions
Funding
Conflicts of Interest
References
- Babatunde, B.; Vincent-Akpu, I.; Woke, G.; Atarhinyo, E.; Aharanwa, U.; Green, A.; Isaac-Joe, O. Comparative analysis of municipal solid waste (MSW) composition in three local government areas in Rivers State, Nigeria. Afr. J. Environ. Sci. Technol. 2013, 7, 874–881. [Google Scholar]
- Pasupathi, P.; Sindhu, S.; Ponnusha, B.S. Biomedical waste management for health care industry: A review. Int. J. Biol. Med. Res. 2011, 2, 472–486. [Google Scholar]
- Ugwuishiwu, B.; Owoh, I.; Udom, I. Solar Energy Application in Waste Treatment A Review. Niger. J. Technol. 2016, 35, 432–440. [Google Scholar] [CrossRef]
- Ryu, C. Potential of municipal solid waste for renewable energy production and reduction of greenhouse gas emissions in South Korea. J. Air Waste Manag. Assoc. 2010, 60, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Chen, W.; Xuan, D.; Cheng, H.; Poon, C.S.; Tsang, D.C.W. Immobilization of hazardous municipal solid waste incineration fly ash by novel alternative binders derived from cementitious waste. J. Hazard. Mater. 2020, 393, 122386. [Google Scholar] [CrossRef] [PubMed]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; World Bank: Washington, DC, USA, 2012; Volume 15. [Google Scholar]
- Fraiese, A.; Naddeo, V.; Uyguner-Demirel, C.; Prado, M.; Cesaro, A.; Zarra, T.; Liu, H.; Belgiorno, V.; Ballesteros, F., Jr. Removal of Emerging Contaminants in Wastewater by Sonolysis, Photocatalysis and Ozonation. Glob. NEST J. 2019, 21, 98–105. [Google Scholar]
- Jin, S.-E.; Jin, J.E.; Hwang, W.; Hong, S.W. Photocatalytic antibacterial application of zinc oxide nanoparticles and self-assembled networks under dual UV irradiation for enhanced disinfection. Int. J. Nanomed. 2019, 14, 1737. [Google Scholar] [CrossRef]
- Gauvin, F.; Caprai, V.; Yu, Q.; Brouwers, H. Effect of the morphology and pore structure of porous building materials on photocatalytic oxidation of air pollutants. Appl. Catal. B Environ. 2018, 227, 123–131. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, L.; Wang, W.; Shao, D. Internal Electric Field Assisted Photocatalytic Generation of Hydrogen Peroxide over BiOCl with HCOOH. ACS Sustain. Chem. Eng. 2018, 6, 8704–8710. [Google Scholar] [CrossRef]
- Jens, H. Industrial Catalysis: A Practical Approach; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Tahir, M.B.; Kiran, H.; Iqbal, T. The detoxification of heavy metals from aqueous environment using nano-photocatalysis approach: A review. Environ. Sci. Pollut. Res. 2019, 26, 10515–10528. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Feng, C. Application of photocatalytic technology in environmental safety. Procedia Eng. 2012, 45, 993–997. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.; Gohar, N. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Keshavarz, M.; Wales, D.J.; Seichepine, F.; Abdelaziz, M.E.M.K.; Kassanos, P.; Li, Q.; Temelkuran, B.; Shen, H.; Yang, G.-Z. Induced neural stem cell differentiation on a drawn fiber scaffold—Toward peripheral nerve regeneration. Biomed. Mater. 2020, 15, 055011. [Google Scholar] [CrossRef]
- Jiang, C.; Jia, J.; Zhai, S. Mechanistic understanding of toxicity from nanocatalysts. Int. J. Mol. Sci. 2014, 15, 13967–13992. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwar, K.; Osugi, M.; Chanmanee, W.; Chenthamarakshan, C.; Zanoni, M.V.B.; Kajitvichyanukul, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 171–192. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Biaduń, E.; Nowak, N.; Kowalska, J.; Miecznikowski, K.; Krasnodębska-Ostręga, B. Organic matter decomposition before arsenic speciation analysis of water sample—“Soft decomposition” using nano-photocatalysts. Chemosphere 2018, 207, 481–488. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal Oxides as Photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Lee, K.M.; Hamid, S.B.A. Simple Response Surface Methodology: Investigation on Advance Photocatalytic Oxidation of 4-Chlorophenoxyacetic Acid Using UV-Active ZnO Photocatalyst. Materials 2015, 8, 339–354. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Soni, M.; Raizada, P.; Hosseini-Bandegharaei, A.; Saini, A.K.; Singh, P. Fabrication of fluorine doped graphene and SmVO4 based dispersed and adsorptive photocatalyst for abatement of phenolic compounds from water and bacterial disinfection. J. Clean. Prod. 2018, 203, 386–399. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Soni, M.; Raizada, P.; Lim, J.H.; Jeong, D.Y.; Dewedi, R.P.; Saini, A.K.; Singh, P. Islanding of EuVO4 on high-dispersed fluorine doped few layered graphene sheets for efficient photocatalytic mineralization of phenolic compounds and bacterial disinfection. J. Taiwan Inst. Chem. Eng. 2018, 93, 528–542. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Sudhaik, A.; Soni, M.; Raizada, P.; Saini, A.K.; Singh, P. GdVO4 modified fluorine doped graphene nanosheets as dispersed photocatalyst for mitigation of phenolic compounds in aqueous environment and bacterial disinfection. Sep. Purif. Technol. 2019, 210, 804–816. [Google Scholar] [CrossRef]
- Singh, P.; Shandilya, P.; Raizada, P.; Sudhaik, A.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A. Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab. J. Chem. 2020, 13, 3498–3520. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Singh, P.; Saini, R.V.; Saini, A.K.; Hosseini-Bandegharaei, A. Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J. 2020, 391, 123496. [Google Scholar] [CrossRef]
- Saravanan, R.; Gracia, F.; Stephen, A. Basic Principles, Mechanism, and Challenges of Photocatalysis. In Nanocomposites for Visible Light-Induced Photocatalysis; Khan, M.M., Pradhan, D., Sohn, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–40. [Google Scholar]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Label-Free SERS Quantum Semiconductor Probe for Molecular-Level and in Vitro Cellular Detection: A Noble-Metal-Free Methodology. ACS Appl. Mater. Interfaces 2018, 10, 34886–34904. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Multiplex Photoluminescent Silicon Nanoprobe for Diagnostic Bioimaging and Intracellular Analysis. Adv. Sci. 2018, 5, 1700548. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A. Photocatalytic degradation of organic pollutants in water. In Organic Pollutants-Monitoring, Risk and Treatment; IntechOpen: Rijeka, Coratia, 2013; Volume 8, pp. 196–197. [Google Scholar]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Jeong, D.Y.; Lim, J.H.; Singh, P. Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 2018, 67, 28–51. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Singh, P. Magnetically recoverable graphitic carbon nitride and NiFe2O4 based magnetic photocatalyst for degradation of oxytetracycline antibiotic in simulated wastewater under solar light. J. Environ. Chem. Eng. 2018, 6, 3874–3883. [Google Scholar] [CrossRef]
- Hasija, V.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P. Carbon quantum dots supported AgI/ZnO/phosphorus doped graphitic carbon nitride as Z-scheme photocatalyst for efficient photodegradation of 2,4-dinitrophenol. J. Environ. Chem. Eng. 2019, 7, 103272. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.; Brown, R.; Hashib, M. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Radhika, N.; Selvin, R.; Kakkar, R.; Umar, A. Recent advances in nano-photocatalysts for organic synthesis. Arab. J. Chem. 2019, 12, 4550–4578. [Google Scholar] [CrossRef]
- Poole, C.P., Jr.; Owens, F.J. Introduction to Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kumar, P.; Singh, P.K.; Bhattacharya, B. Study of nano-CdS prepared in methanolic solution and polymer electrolyte matrix. Ionics 2011, 17, 721–725. [Google Scholar] [CrossRef]
- Townsend, T.K.; Browning, N.D.; Osterloh, F.E. Nanoscale strontium titanate photocatalysts for overall water splitting. ACS Nano 2012, 6, 7420–7426. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Cell Selective Apoptosis Induced by Polymorphic Alteration of Self-Assembled Silica Nanowebs. ACS Appl. Mater. Interfaces 2017, 9, 6292–6305. [Google Scholar] [CrossRef]
- Kaur, P.; Bansal, P.; Sud, D. Heterostructured nanophotocatalysts for degradation of organophosphate pesticides from aqueous streams. J. Korean Chem. Soc. 2013, 57, 382–388. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Castillo-Ledezma, J.; Salas, J.S.; López-Malo, A.; Bandala, E. Effect of pH, solar irradiation, and semiconductor concentration on the photocatalytic disinfection of Escherichia coli in water using nitrogen-doped TiO2. Eur. Food Res. Technol. 2011, 233, 825. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Lou, L.; Yang, S.; Chen, Y. UV or visible light induced photodegradation of AO7 on TiO2 particles: The influence of inorganic anions. J. Photochem. Photobiol. A Chem. 2004, 165, 201–207. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, P.R.; Sharma, G.; Naushad, M.; Rana, A.; Mola, G.T.; Stadler, F.J. Carbon nitride, metal nitrides, phosphides, chalcogenides, perovskites and carbides nanophotocatalysts for environmental applications. Environ. Chem. Lett. 2019, 17, 655–682. [Google Scholar] [CrossRef]
- Gamage, J.; Zhang, Z. Applications of photocatalytic disinfection. Int. J. Photoenergy 2010, 2010, 764870. [Google Scholar] [CrossRef]
- Ramos-Delgado, N.A.; Gracia-Pinilla, M.Á.; Mangalaraja, R.V.; O’Shea, K.; Dionysiou, D.D. Industrial synthesis and characterization of nanophotocatalysts materials: Titania. Nanotechnol. Rev. 2016, 5, 467–479. [Google Scholar]
- Radha, K.; Kalaivani, K.; Lavanya, R. A case study of biomedical waste management in hospitals. Glob. J. Health Sci. 2009, 1, 82–88. [Google Scholar]
- Runcie, H. Sort your waste! An audit on the use of clinical waste bins and its implications. Futur. Heal. J. 2018, 5, 203. [Google Scholar] [CrossRef]
- Rudraswamy, S. Hospital Waste Management Training among the Staff of Dental Teaching Hospitals in Bangalore City: Hospital Waste Management; Anchor Academic Publishing (aap_verlag): Hamburg, Germany, 2014. [Google Scholar]
- Kühn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W.; Sonntag, H.-G.; Erdinger, L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef]
- Evans, P.; English, T.; Hammond, D.; Pemble, M.; Sheel, D. The role of SiO2 barrier layers in determining the structure and photocatalytic activity of TiO2 films deposited on stainless steel. Appl. Catal. A Gen. 2007, 321, 140–146. [Google Scholar] [CrossRef]
- Regmi, C.; Joshi, B.; Ray, S.K.; Gyawali, G.; Pandey, R.P. Understanding mechanism of photocatalytic microbial decontamination of environmental wastewater. Front. Chem. 2018, 6, 33. [Google Scholar] [CrossRef]
- Caballero, L.; Whitehead, K.; Allen, N.; Verran, J. Inactivation of Escherichia coli on immobilized TiO2 using fluorescent light. J. Photochem. Photobiol. A Chem. 2009, 202, 92–98. [Google Scholar] [CrossRef]
- Evans, P.; Sheel, D. Photoactive and antibacterial TiO2 thin films on stainless steel. Surf. Coat. Technol. 2007, 201, 9319–9324. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.; Lin, J.; Yip, H.; Wong, P.K. Photocatalytic activity, antibacterial effect, and photoinduced hydrophilicity of TiO2 films coated on a stainless steel substrate. Environ. Sci. Technol. 2003, 37, 2296–2301. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Bactericidal activity of copper-deposited TiO2 thin film under weak UV light illumination. Environ. Sci. Technol. 2003, 37, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.-S.; Chu, W.-C.; Sun, D.-S.; Huang, H.-S.; Chen, J.-H.; Tsai, P.-J.; Lin, N.-T.; Yu, M.-S.; Hsu, S.-F.; Wang, S.-L. Visible-light-induced bactericidal activity of a nitrogen-doped titanium photocatalyst against human pathogens. Appl. Environ. Microbiol. 2006, 72, 6111–6116. [Google Scholar] [CrossRef] [PubMed]
- Rengifo-Herrera, J.; Mielczarski, E.; Mielczarski, J.; Castillo, N.; Kiwi, J.; Pulgarin, C. Escherichia coli inactivation by N, S co-doped commercial TiO2 powders under UV and visible light. Appl. Catal. B Environ. 2008, 84, 448–456. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.; Pierzchała, K.; Sienkiewicz, A.; Forro, L.; Kiwi, J.; Pulgarin, C. Abatement of organics and Escherichia coli by N, S co-doped TiO2 under UV and visible light. Implications of the formation of singlet oxygen (1O2) under visible light. Appl. Catal. B Environ. 2009, 88, 398–406. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.; Kiwi, J.; Pulgarin, C. N, S co-doped and N-doped Degussa P-25 powders with visible light response prepared by mechanical mixing of thiourea and urea. Reactivity towards E. coli inactivation and phenol oxidation. J. Photochem. Photobiol. A Chem. 2009, 205, 109–115. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chan, R.C.; Wong, P. Disinfection of Legionella pneumophila by photocatalytic oxidation. Water Res. 2007, 41, 842–852. [Google Scholar] [CrossRef]
- Lee, C.; Choi, H.; Lee, C.; Kim, H. Photocatalytic properties of nano-structured TiO2 plasma sprayed coating. Surf. Coat. Technol. 2003, 173, 192–200. [Google Scholar] [CrossRef]
- Kern, P.; Schwaller, P.; Michler, J. Electrolytic deposition of titania films as interference coatings on biomedical implants: Microstructure, chemistry and nano-mechanical properties. Thin Solid Films 2006, 494, 279–286. [Google Scholar] [CrossRef]
- Lee, S.-H.; Pumprueg, S.; Moudgil, B.; Sigmund, W. Inactivation of bacterial endospores by photocatalytic nanocomposites. Colloids Surf. B Biointerfaces 2005, 40, 93–98. [Google Scholar] [CrossRef]
- Pathakoti, K.; Morrow, S.; Han, C.; Pelaez, M.; He, X.; Dionysiou, D.D.; Hwang, H.-M. Photoinactivation of Escherichia coli by sulfur-doped and nitrogen–fluorine-codoped TiO2 nanoparticles under solar simulated light and visible light irradiation. Environ. Sci. Technol. 2013, 47, 9988–9996. [Google Scholar] [CrossRef] [PubMed]
- Szczawiński, J.; Tomaszewski, H.; Jackowska-Tracz, A.; Szczawińska, M. Effect of UV radiation on survival of Salmonella Enteritidis on the surface of ceramic tiles coated with TiO2. Bull Vet. Inst. Pulawy. 2010, 54, 479–483. [Google Scholar]
- Keshavarz, M.; Kassanos, P.; Tan, B.; Venkatakrishnan, K. Metal-oxide surface-enhanced Raman biosensor template towards point-of-care EGFR detection and cancer diagnostics. Nanoscale Horiz. 2020, 5, 294–307. [Google Scholar] [CrossRef]
- Renigo-Herrera, J.; Sienkiewicz, A.; Forro, L.; Kiwi, J.; Moser, J.; Pulgarin, C. New evidence for the nature of the N, S, co-doped TiO2 sited under visible light leading to E. coli inactivation. Catalyst characterization. J. Phys. Chem. 2010, 114, 2717–2723. [Google Scholar]
- Walther, B.A.; Ewald, P.W. Pathogen survival in the external environment and the evolution of virulence. Biol. Rev. 2004, 79, 849–869. [Google Scholar] [CrossRef]
- Chen, K.-T.; Chen, P.-Y.; Tang, R.-B.; Huang, Y.-F.; Lee, P.-I.; Yang, J.-Y.; Chen, H.-Y.; Bresee, J.; Hummelman, E.; Glass, R. Sentinel hospital surveillance for rotavirus diarrhea in Taiwan, 2001–2003. J. Infect. Dis. 2005, 192, S44–S48. [Google Scholar] [CrossRef]
- Alkhuraiji, T.S. Advanced oxidation process based on water radiolysis to degrade and mineralize diclofenac in aqueous solutions. Sci. Total Environ. 2019, 688, 708–717. [Google Scholar] [CrossRef]
- Chianese, S.; Iovino, P.; Leone, V.; Musmarra, D.; Prisciandaro, M. Photodegradation of Diclofenac Sodium Salt in Water Solution: Effect of HA, NO3− and TiO2 on Photolysis Performance. Water Air Soil Pollut. 2017, 228, 270. [Google Scholar] [CrossRef]
- Son, H.S.; Ko, G.; Zoh, K.D. Kinetics and mechanism of photolysis and TiO2 photocatalysis of triclosan. J. Hazard. Mater. 2009, 166, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Chartier, Y. Safe Management of Wastes from Health-Care Activities; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Harhay, M.O.; Halpern, S.D.; Harhay, J.S.; Olliaro, P.L. Health care waste management: A neglected and growing public health problem worldwide. Trop. Med. Int. Health 2009, 14, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Capoor, M.R.; Bhowmik, K.T. Current perspectives on biomedical waste management: Rules, conventions and treatment technologies. Indian J. Med Microbiol. 2017, 35, 157. [Google Scholar]
- Widmer, A.F.; Frei, R. Decontamination, disinfection, and sterilization. In Manual of Clinical Microbiology, 10th ed.; American Society of Microbiology: Washington, DC, USA, 2011; pp. 143–173. [Google Scholar]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y. Key factors influencing the environmental performance of pyrolysis, gasification and incineration Waste-to-Energy technologies. Energy Convers. Manag. 2019, 196, 497–512. [Google Scholar] [CrossRef]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: Butterworth-Heinemann, UK, 2010. [Google Scholar]
- Nema, S.; Ganeshprasad, K. Plasma pyrolysis of medical waste. Curr. Sci. 2002, 271–278. [Google Scholar]
- Rubin, E.; Burhan, Y. Noncombustion technologies for remediation of persistent organic pollutants in stockpiles and soil. Remediat. J. 2006, 16, 23–42. [Google Scholar] [CrossRef]
- Shiraishi, K.; Koseki, H.; Tsurumoto, T.; Baba, K.; Naito, M.; Nakayama, K.; Shindo, H. Antibacterial metal implant with a TiO2-conferred photocatalytic bactericidal effect against Staphylococcus aureus. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Films 2009, 41, 17–22. [Google Scholar]
- Bogdan, J.; Jackowska-Tracz, A.; Zarzyńska, J.; Pławińska-Czarnak, J. Chances and limitations of nanosized titanium dioxide practical application in view of its physicochemical properties. Nanoscale Res. Lett. 2015, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Sichel, C.; Tello, J.; De Cara, M.; Fernández-Ibáñez, P. Effect of UV solar intensity and dose on the photocatalytic disinfection of bacteria and fungi. Catal. Today 2007, 129, 152–160. [Google Scholar] [CrossRef]
- Gupta, K.; Singh, R.; Pandey, A.; Pandey, A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus. P. aeruginosa and E. coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Szczawiński, J.; Tomaszewski, H.; Jackowska-Tracz, A.; Szczawińska, M. Survival of Staphylococcus aureus exposed to UV radiation on the surface of ceramic tiles coated with TiO2. Pol. J. Vet. Sci. 2011, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, H.; Jach, K. Influence of deposition conditions of titania thin films by magnetron sputtering on catalytic, hydrophilic and bactericidal properties of the layers. Mater. Ceram. Ceram. Mater. 2012, 64, 11–21. [Google Scholar]
- Mohamed, R.; McKinney, D.; Sigmund, W. Enhanced nanocatalysts. Mater. Sci. Eng. R Rep. 2012, 73, 1–13. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Flores, P.; Hellín, P.; Vela, N.; Navarro, G.; García-García, J.; Navarro, S. Implementation of a new modular facility to detoxify agro-wastewater polluted with neonicotinoid insecticides in farms by solar photocatalysis. Energy 2019, 175, 722–729. [Google Scholar] [CrossRef]
- Ikram, S. Role of Nanomaterials and their Applications as Photo-catalyst and Senors: A Review. Nano Res. 2016, 2, 10. [Google Scholar]
- Batchu, S.R.; Panditi, V.R.; O’Shea, K.E.; Gardinali, P.R. Photodegradation of antibiotics under simulated solar radiation: Implications for their environmental fate. Sci. Total Environ. 2014, 470, 299–310. [Google Scholar] [CrossRef]
- Soares, P.A.; Silva, T.F.; Manenti, D.R.; Souza, S.M.; Boaventura, R.A.; Vilar, V.J. Insights into real cotton-textile dyeing wastewater treatment using solar advanced oxidation processes. Environ. Sci. Pollut. Res. 2014, 21, 932–945. [Google Scholar] [CrossRef]
- Mueses, M.A.; Machuca-Martinez, F.; Puma, G.L. Effective quantum yield and reaction rate model for evaluation of photocatalytic degradation of water contaminants in heterogeneous pilot-scale solar photoreactors. Chem. Eng. J. 2013, 215, 937–947. [Google Scholar] [CrossRef]
- Sharma, V.K.; Triantis, T.M.; Antoniou, M.G.; He, X.; Pelaez, M.; Han, C.; Song, W.; O’Shea, K.E.; Armah, A.; Kaloudis, T. Destruction of microcystins by conventional and advanced oxidation processes: A review. Sep. Purif. Technol. 2012, 91, 3–17. [Google Scholar] [CrossRef]
- Velegraki, T.; Hapeshi, E.; Fatta-Kassinos, D.; Poulios, I. Solar-induced heterogeneous photocatalytic degradation of methyl-paraben. Appl. Catal. B Environ. 2015, 178, 2–11. [Google Scholar] [CrossRef]
- Gimeno, O.; Rivas, F.J.; Beltrán, F.J.; Carbajo, M. Photocatalytic ozonation of winery wastewaters. J. Agric. Food Chem. 2007, 55, 9944–9950. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Tanaka, M.; Shinohara, S.; Gotoh, M.; Karube, I. Development of a self-sterilizing lancet coated with a titanium dioxide photocatalytic nano-layer for self-monitoring of blood glucose. Biosens. Bioelectron. 2007, 22, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Yao, Y.; Ohko, Y.; Tanaka, K.; Ishido, T.; Fujishima, A.; Kubota, Y. Self-sterilizing catheters with titanium dioxide photocatalyst thin films for clean intermittent catheterization: Basis and study of clinical use. Int. J. Urol. 2007, 14, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Marchi, J.; Amorim, E.M.; Lazar, D.R.; Ussui, V.; Bressiani, A.H.A.; Cesar, P.F. Physico-chemical characterization of zirconia–titania composites coated with an apatite layer for dental implants. Dent. Mater. 2013, 29, 954–962. [Google Scholar] [CrossRef]
- Sawase, T.; Jimbo, R.; Wennerberg, A.; Suketa, N.; Tanaka, Y.; Atsuta, M. A novel characteristic of porous titanium oxide implants. Clin. Oral Implant. Res. 2007, 18, 680–685. [Google Scholar] [CrossRef]
- Singer, J.; Merz, A.; Frommelt, L.; Fink, B. High rate of infection control with one-stage revision of septic knee prostheses excluding MRSA and MRSE. Clin. Orthop. Relat. Res® 2012, 470, 1461–1471. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, S.C.; Lee, Y.-C.; Son, B.; Park, S.Y.; Lee, J.W.; Oh, Y.-K.; Kim, Y.; Choi, S.; Lee, Y.-S. Innovative three-dimensional (3D) eco-TiO2 photocatalysts for practical environmental and bio-medical applications. Sci. Rep. 2014, 4, 6740. [Google Scholar] [CrossRef]
- Xie, J.; Li, Y.; Zhao, W.; Bian, L.; Wei, Y. Simple fabrication and photocatalytic activity of ZnO particles with different morphologies. Powder Technol. 2011, 207, 140–144. [Google Scholar] [CrossRef]
- Bolink, H.J.; Coronado, E.; Orozco, J.; Sessolo, M. Efficient polymer light-emitting diode using air-stable metal oxides as electrodes. Adv. Mater. 2009, 21, 79–82. [Google Scholar] [CrossRef]
- Emeline, A.; Kataeva, G.; Panasuk, A.; Ryabchuk, V.; Sheremetyeva, N.; Serpone, N. Effect of surface photoreactions on the photocoloration of a wide band gap metal oxide: Probing whether surface reactions are photocatalytic. J. Phys. Chem. B 2005, 109, 5175–5185. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17. [Google Scholar] [CrossRef]
- Chu, D.; Masuda, Y.; Ohji, T.; Kato, K. Formation and photocatalytic application of ZnO nanotubes using aqueous solution. Langmuir 2009, 26, 2811–2815. [Google Scholar] [CrossRef]
- Lupan, O.; Pauporté, T.; Le Bahers, T.; Viana, B.; Ciofini, I. Wavelength-emission tuning of ZnO nanowire-based light-emitting diodes by Cu doping: Experimental and computational insights. Adv. Funct. Mater. 2011, 21, 3564–3572. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, X.; Bando, Y.; Gautam, U.K.; Zhai, T.; Fang, X.; Liu, B.; Golberg, D. Template Deformation-Tailored ZnO Nanorod/Nanowire Arrays: Full Growth Control and Optimization of Field-Emission. Adv. Funct. Mater. 2009, 19, 3165–3172. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Rout, C.S.; Krishna, S.H.; Vivekchand, S.; Govindaraj, A.; Rao, C. Hydrogen and ethanol sensors based on ZnO nanorods, nanowires and nanotubes. Chem. Phys. Lett. 2006, 418, 586–590. [Google Scholar] [CrossRef]
- Shen, G.; Bando, Y.; Liu, B.; Golberg, D.; Lee, C.J. Characterization and Field-Emission Properties of Vertically Aligned ZnO Nanonails and Nanopencils Fabricated by a Modified Thermal-Evaporation Process. Adv. Funct. Mater. 2006, 16, 410–416. [Google Scholar] [CrossRef]
- Gautam, U.K.; Panchakarla, L.; Dierre, B.; Fang, X.; Bando, Y.; Sekiguchi, T.; Govindaraj, A.; Golberg, D.; Rao, C. Solvothermal Synthesis, Cathodoluminescence, and Field-Emission Properties of Pure and N-Doped ZnO Nanobullets. Adv. Funct. Mater. 2009, 19, 131–140. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Zeng, B.-Q.; Yang, J.; Poudel, B.; Huang, J.; Naughton, M.J.; Ren, Z. Aligned ultralong ZnO nanobelts and their enhanced field emission. Adv. Mater. 2006, 18, 3275–3278. [Google Scholar] [CrossRef]
- Wei, A.; Sun, X.W.; Xu, C.; Dong, Z.; Yu, M.; Huang, W. Stable field emission from hydrothermally grown ZnO nanotubes. Appl. Phys. Lett. 2006, 88, 213102. [Google Scholar] [CrossRef]
- Wang, N.; Cao, X.; Guo, L. Facile one-pot solution phase synthesis of SnO2 nanotubes. J. Phys. Chem. C 2008, 112, 12616–12622. [Google Scholar] [CrossRef]
- Tseng, Y.K.; Huang, C.J.; Cheng, H.M.; Lin, I.N.; Liu, K.S.; Chen, I.C. Characterization and field-emission properties of needle-like zinc oxide nanowires grown vertically on conductive zinc oxide films. Adv. Funct. Mater. 2003, 13, 811–814. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Sun, X.; Feng, S.; Xu, J.; Zhao, Q.; Xiang, B.; Wang, R.; Yu, D. Efficient field emission from ZnO nanoneedle arrays. Appl. Phys. Lett. 2003, 83, 144–146. [Google Scholar] [CrossRef]
- Xu, C.; Sun, X. Field emission from zinc oxide nanopins. Appl. Phys. Lett. 2003, 83, 3806–3808. [Google Scholar] [CrossRef]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Asiri, A.M.; Abdullah, M. Synthesis, characterizations, photocatalytic and sensing studies of ZnO nanocapsules. Appl. Surf. Sci. 2011, 258, 672–677. [Google Scholar] [CrossRef]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Abdullah, M. Fabrication of ZnO nanoparticles based sensitive methanol sensor and efficient photocatalyst. Appl. Surf. Sci. 2012, 258, 7515–7522. [Google Scholar] [CrossRef]
- Kaur, J.; Bansal, S.; Singhal, S. Photocatalytic degradation of methyl orange using ZnO nanopowders synthesized via thermal decomposition of oxalate precursor method. Phys. B Condens. Matter 2013, 416, 33–38. [Google Scholar] [CrossRef]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Asiri, A.M.; Abdullah, M. Smart chemical sensor and active photo-catalyst for environmental pollutants. Chem. Eng. J. 2011, 173, 178–184. [Google Scholar] [CrossRef]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Akhtar, K.; Abdullah, M. Role of ZnO-CeO2 nanostructures as a photo-catalyst and chemi-sensor. J. Mater. Sci. Technol. 2011, 27, 594–600. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Z.; Li, Z.; Zhou, Q.; Zhu, Y. Enhanced photocatalytic activity of porous α-Fe2O3 films prepared by rapid thermal oxidation. J. Phys. D Appl. Phys. 2008, 41, 202002. [Google Scholar] [CrossRef]
- Barroso, M.; Cowan, A.J.; Pendlebury, S.R.; Grätzel, M.; Klug, D.R.; Durrant, J.R. The role of cobalt phosphate in enhancing the photocatalytic activity of α-Fe2O3 toward water oxidation. J. Am. Chem. Soc. 2011, 133, 14868–14871. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-W.; Zhu, Y.-J. Hierarchically nanostructured α-Fe2O3 hollow spheres: Preparation, growth mechanism, photocatalytic property, and application in water treatment. J. Phys. Chem. C 2008, 112, 6253–6257. [Google Scholar] [CrossRef]
- Li, L.; Chu, Y.; Liu, Y.; Dong, L. Template-free synthesis and photocatalytic properties of novel Fe2O3 hollow spheres. J. Phys. Chem. C 2007, 111, 2123–2127. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Amaresh, S.; Lee, S.N.; Aravindan, V.; Lee, Y.S. Fluorine-Doped Fe2O3 as High Energy Density Electroactive Material for Hybrid Supercapacitor Applications. Chem. Asian J. 2014, 9, 852–857. [Google Scholar] [CrossRef]
- Iturrondobeitia, A.; Goñi, A.; Orue, I.; Gil de Muro, I.; Lezama, L.; Doeff, M.; Rojo, T. Effect of Carbon coating on the physicochemical and electrochemical properties of Fe2O3 nanoparticles for anode application in high performance lithium ion batteries. Inorg. Chem. 2015, 54, 5239–5248. [Google Scholar] [CrossRef]
- Gu, S.; Lou, Z.; Li, L.; Chen, Z.; Ma, X.; Shen, G. Fabrication of flexible reduced graphene oxide/Fe2O3 hollow nanospheres based on-chip micro-supercapacitors for integrated photodetecting applications. Nano Res. 2016, 9, 424–434. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, N.; Hu, J.; Li, S.; Qin, G. Photocatalytic degradation properties of α-Fe2O3 nanoparticles for dibutyl phthalate in aqueous solution system. Royal Soc. Open Sci. 2018, 5, 172196. [Google Scholar] [CrossRef]
- Karunakaran, C.; Senthilvelan, S. Fe2O3-photocatalysis with sunlight and UV light: Oxidation of aniline. Electrochem. Commun. 2006, 8, 95–101. [Google Scholar] [CrossRef]
- Aroutiounian, V.M.; Arakelyan, V.M.; Shahnazaryan, G.E.; Aleksanyan, M.S.; Hernadi, K.; Nemeth, Z.; Berki, P.; Papa, Z.; Toth, Z.; Forro, L. The ethanol sensors made from α-Fe2O3 decorated with multiwall carbon nanotubes. Adv. Nano Res. 2015, 3, 1–11. [Google Scholar] [CrossRef][Green Version]
- Zhang, A.; Zhang, J. Visible-light activities of Gd2O3/BiVO4 composite photocatalysts. J. Mater. Sci. 2010, 45, 4040–4045. [Google Scholar] [CrossRef]
- Abdullah, M.; Rahman, M.M.; Faisal, M.; Khan, S.B.; Singh, P.; Rub, M.A.; Azum, N.; Khan, A.; Khan, A.A.P.; Hasmuddin, M. Fabrication of ethanol chemical sensors based on as-prepared Gd2O3 nanorods by facile hydrothermal routes. J. Colloid Sci. Biotechnol. 2013, 2, 322–327. [Google Scholar] [CrossRef]
- Abdullah, M.; Rahman, M.M.; Bouzid, H.; Faisal, M.; Khan, S.B.; Al-Sayari, S.; Ismail, A.A. Sensitive and fast response ethanol chemical sensor based on as-grown Gd2O3 nanostructures. J. Rare Earths 2015, 33, 214–220. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, L.; Yao, C.; Zhang, F.; Zhang, J.; Li, C. Synthesis and characterization of Gd2O3 hollow microspheres using a template-directed method. Materials 2016, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Barrera, A.; Tzompantzi, F.; Campa-Molina, J.; Casillas, J.; Pérez-Hernández, R.; Ulloa-Godinez, S.; Velasquez, C.; Arenas-Alatorre, J. Photocatalytic activity of Ag/Al2O3–Gd2O3 photocatalysts prepared by the sol–gel method in the degradation of 4-chlorophenol. RSC Adv. 2018, 8, 3108–3119. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Kumar, P.S.; Karuthapandian, S.; Muthuraj, V.; Prithivikumaran, N. Design of Gd2O3 nanorods: A challenging photocatalyst for the degradation of neurotoxicity chloramphenicol drug. J. Mater. Sci. Mater. Electron. 2019, 30, 3744–3752. [Google Scholar] [CrossRef]
- Jamal, A.; Raahman, M.M.; Khan, S.B.; Abdullah, M.M.; Faisaal, M.; Asiri, A.M.; Aslam, A.; Khan, P.; Akhtar, K. Simple growth and characterization of α-Sb2O4: Evaluation of their photo-catalytic and chemical sensing applications. J. Chem. Soc. Pak. 2013, 35, 570. [Google Scholar]
- Agarwal, H.; Sharma, D.; Sindhu, S.K.; Tyagi, S.; Ikram, S. Removal of mercury from wastewater use of green adsorbents—A review. Electron. J. Environ. Agric. Food Chem. 2010, 9, 1155–1558. [Google Scholar]
- Tyagi, S.; Agarwal, H.; Ikram, S.; Gupta, M.K.; Singh, S. Uranyl Selective Polymeric Membrane Sensor Based on P-Tert-Butyl-Biscalix [4] Arene. Anal. Bioanal. Electrochem. 2011, 3, 350–364. [Google Scholar]
- Tyagi, S.; Agarwal, H.; Ikram, S.; Gupta, M.K.; Singh, S. A Polymeric Membrane Electrode Based on p-tert-butyl-thiacalix [4] arene Derivative for Thorium Determination. Anal. Bioanal. Electrochem 2011, 3, 436–449. [Google Scholar]
- Konstantinou, I.K.; Sakkas, V.A.; Albanis, T.A. Photocatalytic degradation of propachlor in aqueous TiO2 suspensions. Determination of the reaction pathway and identification of intermediate products by various analytical methods. Water Res. 2002, 36, 2733–2742. [Google Scholar] [CrossRef]
- Sreethawong, T.; Ngamsinlapasathian, S.; Suzuki, Y.; Yoshikawa, S. Nanocrystalline mesoporous Ta2O5-based photocatalysts prepared by surfactant-assisted templating sol-gel process for photocatalytic H2 evolution. J. Mol. Catal. A Chem. 2005, 235, 1–11. [Google Scholar] [CrossRef]
- Jing, D.; Guo, L. Hydrogen production over Fe-doped tantalum oxide from an aqueous methanol solution under the light irradiation. J. Phys. Chem. Solids 2007, 68, 2363–2369. [Google Scholar] [CrossRef]
- Arora, A.K.; Jaswal, V.S.; Singh, K.; Singh, R. Applications of Metal/Mixed Metal Oxides as Photocatalyst: (A Review). Orient. J. Chem. 2016, 32, 2035. [Google Scholar] [CrossRef]
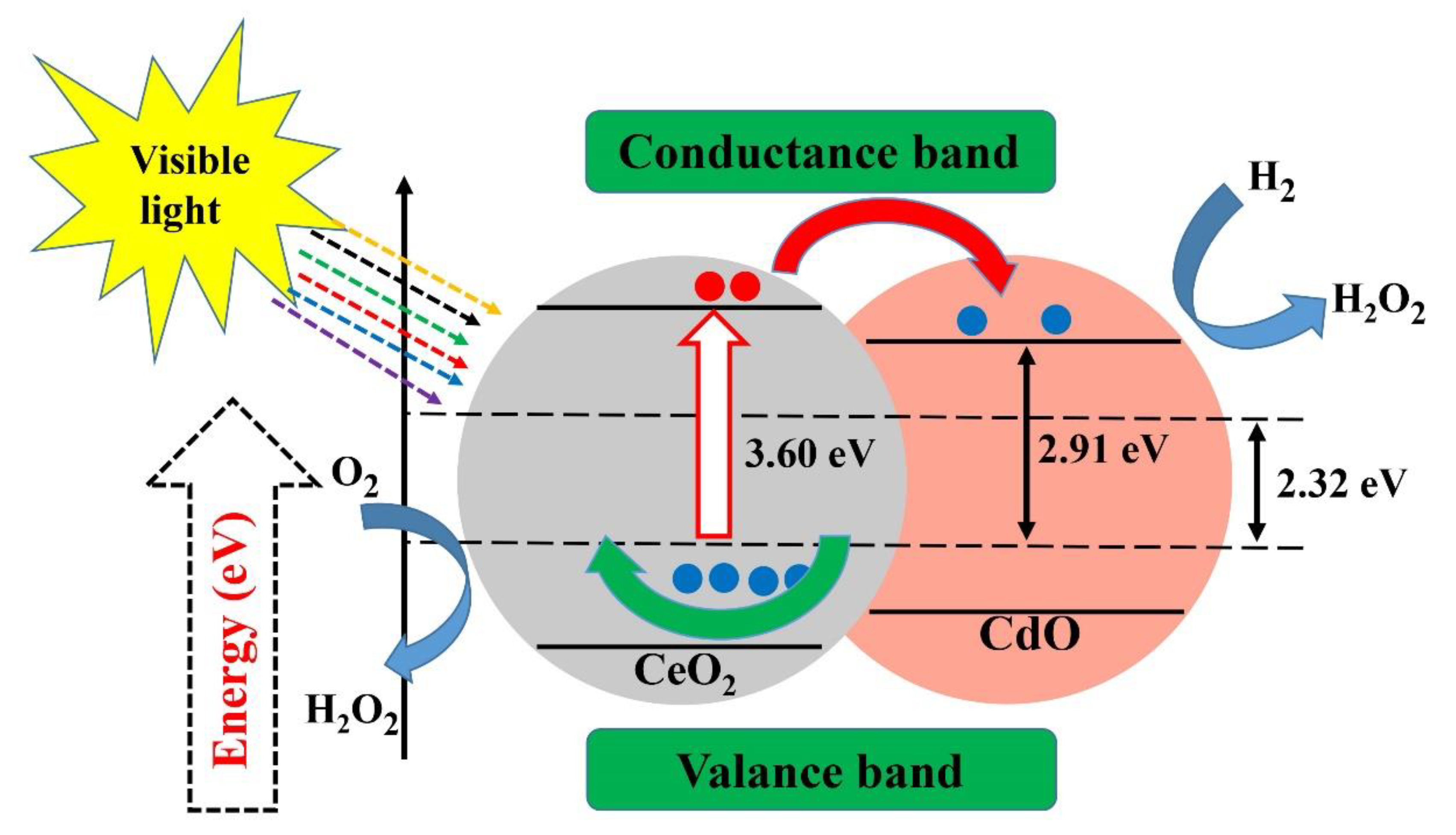
- Magdalane, C.M.; Kaviyarasu, K.; Vijaya, J.J.; Siddhardha, B.; Jeyaraj, B. Photocatalytic activity of binary metal oxide nanocomposites of CeO2/CdO nanospheres: Investigation of optical and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2016, 163, 77–86. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R.; Campelo, J.M.; Colmenares, F.; Karpiński, Z.; Romero, A.A. Nanostructured photocatalysts and their applications in the photocatalytic transformation of lignocellulosic biomass: An overview. Materials 2009, 2, 2228–2258. [Google Scholar] [CrossRef]
- Jang, J.S.; Joshi, U.A.; Lee, J.S. Solvothermal synthesis of CdS nanowires for photocatalytic hydrogen and electricity production. J. Phys. Chem. C 2007, 111, 13280–13287. [Google Scholar] [CrossRef]
- Janet, C.; Viswanath, R. Large scale synthesis of CdS nanorods and its utilization in photo-catalytic H2 production. Nanotechnology 2006, 17, 5271. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanath, R. Photocatalytic generation of hydrogen over mesoporous CdS nanoparticle: Effect of particle size, noble metal and support. Catal. Today 2007, 129, 421–427. [Google Scholar] [CrossRef]
- Bao, N.; Shen, L.; Takata, T.; Domen, K. Self-templated synthesis of nanoporous CdS nanostructures for highly efficient photocatalytic hydrogen production under visible light. Chem. Mater. 2007, 20, 110–117. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.L.; Clark, J.H. Tuneable Mesoporous Materials from α-d-Polysaccharides. ChemSusChem Chem. Sustain. Energy Mater. 2008, 1, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, I.; Kato, H.; Kobayashi, H.; Kudo, A. Photocatalytic H2 evolution reaction from aqueous solutions over band structure-controlled (AgIn)xZn2(1-x)S2 solid solution photocatalysts with visible-light response and their surface nanostructures. J. Am. Chem. Soc. 2004, 126, 13406–13413. [Google Scholar] [CrossRef] [PubMed]
- Linley, S.; Leshuk, T.; Gu, F.X. Magnetically separable water treatment technologies and their role in future advanced water treatment: A patent review. CLEAN Soil Air Water 2013, 41, 1152–1156. [Google Scholar] [CrossRef]
- Yao, Y.R.; Huang, W.Z.; Zhou, H.; Zheng, Y.F.; Song, X.C. Self-assembly of dandelion-like Fe3O4@ C@ BiOCl magnetic nanocomposites with excellent solar-driven photocatalytic properties. J. Nanoparticle Res. 2014, 16, 2451. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Zhao, Q.; Wang, L. A general, one-step and template-free synthesis of sphere-like zinc ferrite nanostructures with enhanced photocatalytic activity for dye degradation. J. Colloid Interface Sci. 2011, 358, 102–108. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Gong, X.; Yan, F.; Zhang, Z. Sonochemical synthesis and characterization of magnetic separable Fe3O4–TiO2 nanocomposites and their catalytic properties. Int. J. Smart Nano Mater. 2010, 1, 278–287. [Google Scholar] [CrossRef]
- Fisli, A.; Saridewi, R.; Dewi, S.H.; Gunlazuardi, J. Preparation and Characterization of Fe3O4/TiO2 Composites by Heteroagglomeration. In Proceedings of the 2012 International Conference on Advanced Materials Engineering and Technology, ICAMET 2012, Penang, Malaysia, 28–30 November 2012; Advanced Materials Research. Trans Tech Publ.: Penang, Malaysia, 2013; pp. 131–137. [Google Scholar]
- Jing, J.; Li, J.; Feng, J.; Li, W.; William, W.Y. Photodegradation of quinoline in water over magnetically separable Fe3O4/TiO2 composite photocatalysts. Chem. Eng. J. 2013, 219, 355–360. [Google Scholar] [CrossRef]
- Rana, S.; Srivastava, R.; Sorensson, M.; Misra, R. Synthesis and characterization of nanoparticles with magnetic core and photocatalytic shell: Anatase TiO2–NiFe2O4 system. Mater. Sci. Eng. B 2005, 119, 144–151. [Google Scholar] [CrossRef]
- Fu, W.; Yang, H.; Li, M.; Li, M.; Yang, N.; Zou, G. Anatase TiO2 nanolayer coating on cobalt ferrite nanoparticles for magnetic photocatalyst. Mater. Lett. 2005, 59, 3530–3534. [Google Scholar] [CrossRef]
- Cheng, P.; Li, W.; Zhou, T.; Jin, Y.; Gu, M. Physical and photocatalytic properties of zinc ferrite doped titania under visible light irradiation. J. Photochem. Photobiol. A Chem. 2004, 168, 97–101. [Google Scholar] [CrossRef]
- Fu, W.; Yang, H.; Li, M.; Chang, L.; Yu, Q.; Xu, J.; Zou, G. Preparation and photocatalytic characteristics of core-shell structure TiO2/BaFe12O19 nanoparticles. Mater. Lett. 2006, 60, 2723–2727. [Google Scholar] [CrossRef]
- Fu, W.; Yang, H.; Chang, L.; Li, M.; Zou, G. Anatase TiO2 nanolayer coating on strontium ferrite nanoparticles for magnetic photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2006, 289, 47–52. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2009, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qin, J.; Jiang, Y.; Chen, K.; Yan, X.; Zhang, D.; Li, R.; Tang, H. Fabrication of P25/Ag3PO4/graphene oxide heterostructures for enhanced solar photocatalytic degradation of organic pollutants and bacteria. Appl. Catal. B Environ. 2015, 166, 231–240. [Google Scholar] [CrossRef]
- Zhou, K.; Zhu, Y.; Yang, X.; Jiang, X.; Li, C. Preparation of graphene—TiO2 composites with enhanced photocatalytic activity. New J. Chem. 2011, 35, 353–359. [Google Scholar] [CrossRef]
- Du, J.; Lai, X.; Yang, N.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Mesoporous TiO2 graphene composite films: Improved mass transfer, reduced charge recombination, and their enhanced photocatalytic activities. ACS Nano 2011, 5, 590–596. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, P.; Du, G.; Chen, Z.; Oh, K.; Pan, D.; Jiao, Z. A facile one-step synthesis of TiO2/graphene composites for photodegradation of methyl orange. Nano Res. 2011, 4, 274–283. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Casalongue, H.S.; Chen, Z.; Dai, H. TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res. 2010, 3, 701–705. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Zhao, X. Graphene-metal-oxide composites for the degradation of dyes under visible light irradiation. J. Mater. Chem. 2011, 21, 3634–3640. [Google Scholar] [CrossRef]
- Gao, E.; Wang, W.; Shang, M.; Xu, J. Synthesis and enhanced photocatalytic performance of graphene-Bi2WO6 composite. Phys. Chem. Chem. Phys. 2011, 13, 2887–2893. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B Environ. 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, X. Magnetically separable ZnFe2O4—Graphene catalyst and its high photocatalytic performance under visible light irradiation. Ind. Eng. Chem. Res. 2011, 50, 7210–7218. [Google Scholar] [CrossRef]
- Ng, Y.H.; Iwase, A.; Kudo, A.; Amal, R. Reducing graphene oxide on a visible-light BiVO4 photocatalyst for an enhanced photoelectrochemical water splitting. J. Phys. Chem. Lett. 2010, 1, 2607–2612. [Google Scholar] [CrossRef]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Rajabi, H.R. Study of photocatalytic activity of ZnS quantum dots as efficient nanoparticles for removal of methyl violet: Effect of ferric ion doping. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 260–267. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Farsi, M. Effect of transition metal ion doping on the photocatalytic activity of ZnS quantum dots: Synthesis, characterization, and application for dye decolorization. J. Mol. Catal. A Chem. 2015, 399, 53–61. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rajabi, H.R.; Khani, O. Pure and Fe3+-doped ZnS quantum dots as novel and efficient nanophotocatalysts: Synthesis, characterization and use for decolorization of Victoria blue R. Mater. Sci. Semicond. Process. 2013, 16, 1154–1161. [Google Scholar] [CrossRef]
- Pouretedal, H.; Keshavarz, M. Study of Congo red photodegradation kinetic catalyzed by Zn1-XCuXS and Zn1-XNiXS nanoparticles. Int. J. Phys. Sci. 2011, 6, 6268–6279. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Norozi, A.; Keshavarz, M.H.; Semnani, A. Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes. J. Hazard. Mater. 2009, 162, 674–681. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Keshavarz, M.H.; Yosefi, M.H.; Shokrollahi, A.; Zali, A. Photodegradation of HMX and RDX in the presence of nanocatalyst of zinc sulfide doped with copper. Iran. J. Chem. Chem. Eng. IJCCE 2009, 28, 13–19. [Google Scholar]
- Roushani, M.; Mavaei, M.; Rajabi, H.R. Graphene quantum dots as novel and green nano-materials for the visible-light-driven photocatalytic degradation of cationic dye. J. Mol. Catal. A Chem. 2015, 409, 102–109. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, R.; Liu, Y.; Ding, H.; Zhang, Y.-L. Graphene quantum dots prepared from chemical exfoliation of multiwall carbon nanotubes: An efficient photocatalyst promoter. Catal. Commun. 2016, 74, 104–109. [Google Scholar] [CrossRef]
- Fan, T.; Li, Y.; Shen, J.; Ye, M. Novel GQD-PVP-CdS composite with enhanced visible-light-driven photocatalytic properties. Appl. Surf. Sci. 2016, 367, 518–527. [Google Scholar] [CrossRef]
- Xia, J.; Di, J.; Li, H.; Xu, H.; Li, H.; Guo, S. Ionic liquid-induced strategy for carbon quantum dots/BiOX (X = Br, Cl) hybrid nanosheets with superior visible light-driven photocatalysis. Appl. Catal. B Environ. 2016, 181, 260–269. [Google Scholar] [CrossRef]
- Muthulingam, S.; Lee, I.-H.; Uthirakumar, P. Highly efficient degradation of dyes by carbon quantum dots/N-doped zinc oxide (CQD/N-ZnO) photocatalyst and its compatibility on three different commercial dyes under daylight. J. Colloid Interface Sci. 2015, 455, 101–109. [Google Scholar] [CrossRef]
- Su, J.; Zhu, L.; Chen, G. Ultrasmall graphitic carbon nitride quantum dots decorated self-organized TiO2 nanotube arrays with highly efficient photoelectrochemical activity. Appl. Catal. B Environ. 2016, 186, 127–135. [Google Scholar] [CrossRef]
- Samadi-Maybodi, A.; Sadeghi-Maleki, M.-R. In-situ synthesis of high stable CdS quantum dots and their application for photocatalytic degradation of dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 156–164. [Google Scholar] [CrossRef]
- Yue-Sheng, L.; Zhi-Yong, S.; Jiang-Tao, Q.; HUANG, H.-T.; Yan, H. Study on Photocatalytic Degradation of Core/Shell CdSe/ZnS Quantum Dots with Nano-TiO2 by Fluorescent Spectrometric Methods. Chin. J. Anal. Chem. 2016, 44, 61–67. [Google Scholar]
- Sood, S.; Kumar, S.; Umar, A.; Kaur, A.; Mehta, S.K.; Kansal, S.K. TiO2 quantum dots for the photocatalytic degradation of indigo carmine dye. J. Alloy Compd. 2015, 650, 193–198. [Google Scholar] [CrossRef]
- Kaur, A.; Umar, A.; Kansal, S.K. Sunlight-driven photocatalytic degradation of non-steroidal anti-inflammatory drug based on TiO2 quantum dots. J. Colloid Interface Sci. 2015, 459, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Lan, W.; Yang, Z.; Zhao, X.; Chen, Y.; Wang, J.; Zhang, X.; Zhang, Y.; Su, Q.; Xie, E. A simple method for preparing ZnO foam/carbon quantum dots nanocomposite and their photocatalytic applications. Mater. Sci. Semicond. Process. 2016, 47, 25–31. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, S.; Liu, L.; Hu, J.; Cui, W. Oil-in-water self-assembled Ag@ AgCl QDs sensitized Bi2WO6: Enhanced photocatalytic degradation under visible light irradiation. Appl. Catal. B Environ. 2015, 164, 192–203. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Farsi, M. Quantum dot based photocatalytic decolorization as an efficient and green strategy for the removal of anionic dye. Mater. Sci. Semicond. Process. 2015, 31, 478–486. [Google Scholar] [CrossRef]
- Carneiro, J.; Teixeira, V.; Portinha, A.; Magalhaes, A.; Coutinho, P.; Tavares, C.; Newton, R. Iron-doped photocatalytic TiO2 sputtered coatings on plastics for self-cleaning applications. Mater. Sci. Eng. B 2007, 138, 144–150. [Google Scholar] [CrossRef]
- Galkina, O.; Vinogradov, V.; Vinogradov, A.; Agafonov, A. Development of the low-temperature sol-gel synthesis of TiO2 to provide self-cleaning effect on the textile materials. Nano Technol. Russ. 2012, 7, 604–614. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.-J.; Chou, H.-H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Reduction of graphene oxide with substituted borohydrides. J. Mater. Chem. A 2013, 1, 1892–1898. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kim, J.-H. Green chemistry approach for the synthesis of biocompatible graphene. Int. J. Nanomed. 2013, 8, 2719. [Google Scholar] [CrossRef]
- Sher Shah, M.S.A.; Park, A.R.; Zhang, K.; Park, J.H.; Yoo, P.J. Green synthesis of biphasic TiO2—Reduced graphene oxide nanocomposites with highly enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2012, 4, 3893–3901. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhong, M.; Yang, Y.; Wen, Y.; Wang, M. A green and ultrafast approach to the synthesis of scalable graphene nanosheets with Zn powder for electrochemical energy storage. J. Mater. Chem. 2011, 21, 15449–15455. [Google Scholar] [CrossRef]
- Gupta, S.S.; Sreeprasad, T.S.; Maliyekkal, S.M.; Das, S.K.; Pradeep, T. Graphene from sugar and its application in water purification. ACS Appl. Mater. Interfaces 2012, 4, 4156–4163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Yin, J. Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. ACS Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, J.; Zeng, J.; Ou, J.; Li, Z.; Liu, X.; Yang, S. “Green” electrochemical synthesis of Pt/graphene sheet nanocomposite film and its electrocatalytic property. J. Power Sources 2010, 195, 4628–4633. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Green reduction of graphene oxide by aqueous phytoextracts. Carbon 2012, 50, 5331–5339. [Google Scholar] [CrossRef]
- Singh, V.V.; Gupta, G.; Batra, A.; Nigam, A.K.; Boopathi, M.; Gutch, P.K.; Tripathi, B.K.; Srivastava, A.; Samuel, M.; Agarwal, G.S. Greener electrochemical synthesis of high quality graphene nanosheets directly from pencil and its SPR sensing application. Adv. Funct. Mater. 2012, 22, 2352–2362. [Google Scholar] [CrossRef]
- Liu, J.; Fu, S.; Yuan, B.; Li, Y.; Deng, Z. Toward a universal “adhesive nanosheet” for the assembly of multiple nanoparticles based on a protein-induced reduction/decoration of graphene oxide. J. Am. Chem. Soc. 2010, 132, 7279–7281. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zeng, L.; Song, S.; Liu, D. Green and controlled synthesis of Cu2O—Graphene hierarchical nanohybrids as high-performance anode materials for lithium-ion batteries via an ultrasound assisted approach. Dalton Trans. 2012, 41, 4316–4319. [Google Scholar] [CrossRef]
- Chen, X.; Su, B.; Wu, G.; Yang, C.J.; Zhuang, Z.; Wang, X.; Chen, X. Platinum nanoflowers supported on graphene oxide nanosheets: Their green synthesis, growth mechanism, and advanced electrocatalytic properties for methanol oxidation. J. Mater. Chem. 2012, 22, 11284–11289. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Shamsipur, M.; Samari, F.; Rajabi, H.R. Study of the interaction between human serum albumin and Mn-doped ZnS quantum dots. J. Iran. Chem. Soc. 2015, 12, 1729–1738. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rajabi, H.R. Pure zinc sulfide quantum dot as highly selective luminescent probe for determination of hazardous cyanide ion. Mater. Sci. Eng. C 2014, 36, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.S.; Yeong Wu, T.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Schmidt, M.; Amrhein, K.; Braun, T.; Glotzbach, C.; Kamaruddin, S.; Tänzer, R. Nanotechnological improvement of structural materials–impact on material performance and structural design. Cem. Concr. Compos. 2013, 36, 3–7. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2014, 71, 193–203. [Google Scholar]
- Coronado, J.M.; Fresno, F.; Hernández-Alonso, M.D.; Portela, R. Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Springer: Cham, Switzerland, 2013; ISBN 978-1-4471-5061-9. [Google Scholar]
- Kitano, M.; Matsuoka, M.; Ueshima, M.; Anpo, M. Recent developments in titanium oxide-based photocatalysts. Appl. Catal. A Gen. 2007, 325, 1–14. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
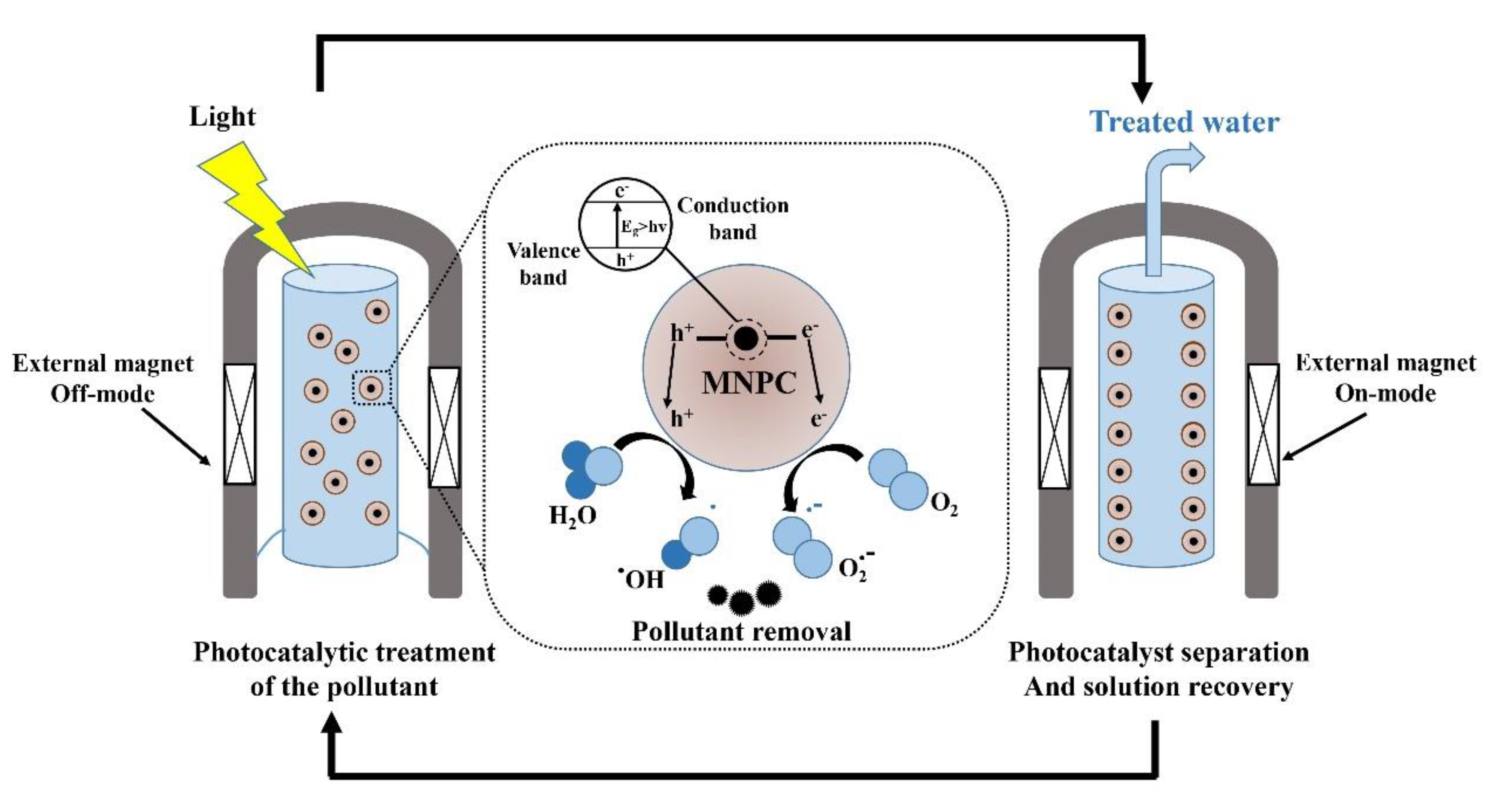
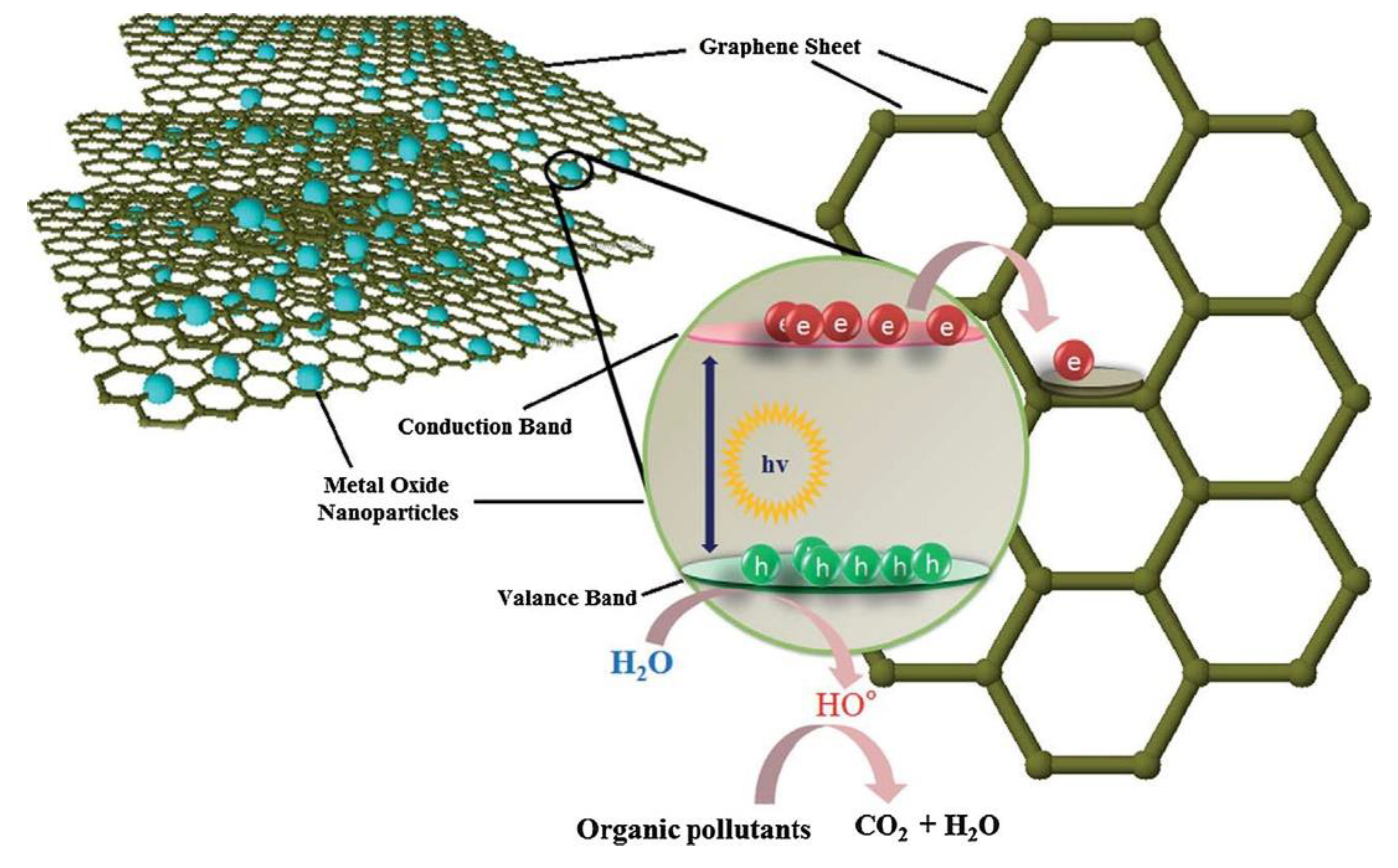
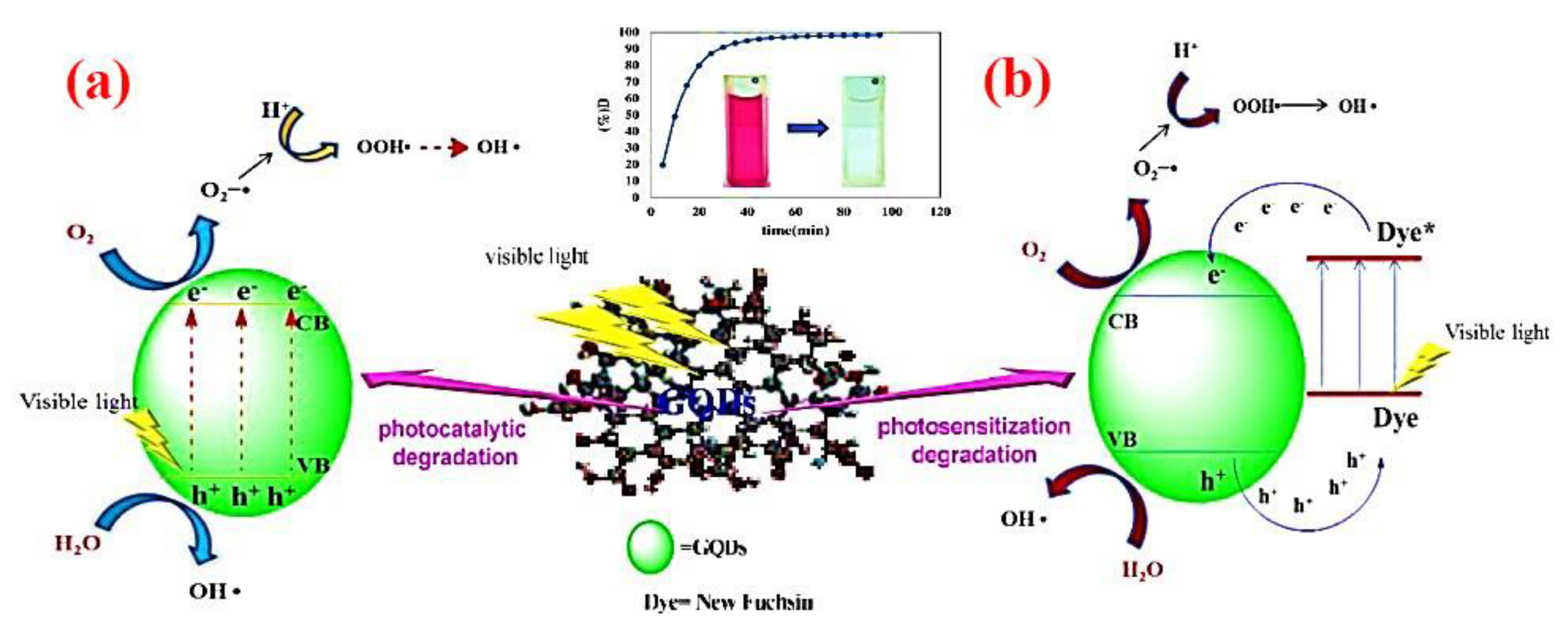
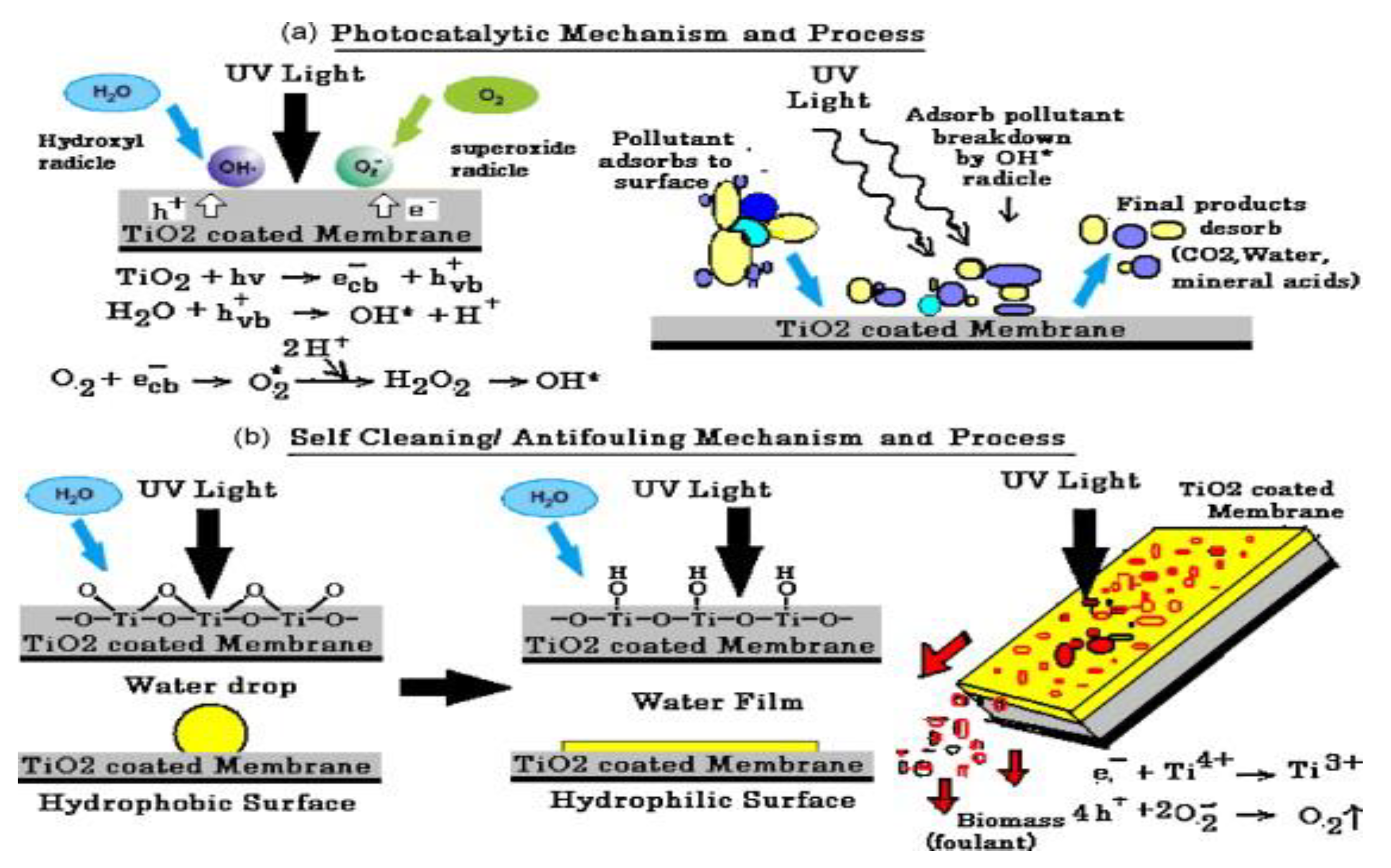
| Nanophotocatalyst | Contaminant Conversion | Operative Conditions | Duration/Performance of Nanophotocatalyst | By-Products | Ref. |
|---|---|---|---|---|---|
| TiO2@Plexiglas | E. coli, P. aeruginosa, S. aureus and E. faecium | Photocatalysis on TiO2-coated Plexiglas with indirect UVA light irradiation (60 min) with and without time resolution | Reduction efficiency more than 6 log10 steps in 60 min in disinfecting the surfaces | Oxygen radicals and hydrogen peroxide | [53] |
| TTIP and TiCl4/TiO2 films@stainless steel and @SiO2 layers | Destruction of stearic acid layers | Photocatalytic activities of TTIP and TiCl4 TiO2 films (156 nm) deposited onto stainless steel, silica (30 nm) and (120 nm) under 365 nm UV irradiation | Photocatalytic activity of 7.6 × 10−4 and 9.0 × 10−5 cm−1 min−1 for the TTIP and TiCl4-grown films, respectively | Iron and chromium | [54] |
| TiO2 PC105 (15–25 nm with a nanoanatase phase of 100%) | Inactivation of E. coli | Irradiation with fluorescent light produced by eight 8 W lamps with visible light and UV (290–400 nm) at 0.05–0.12 Wm−2 intensity | With loadings ranging from 520 to 15,590 mgm−2, E. coli inactivation as a function of time was monitored for up to 120 min | Hydroxyl and hydroperoxyl radicals | [56] |
| TiO2 thin films on stainless steel | Destruction of stearic acid layers, monitored using FT-IR spectroscopy | Pre-Activation of the sample using UVA (365 nm) radiation (2.24 mW cm−2, 24 h), exposure to the same UVA radiation for timed intervals | Biocidally effective against E. coli with a 100% kill (6 log reduction) in less than 3 h | HCl | [57] |
| Transparent TiO2 films on stainless steel | Oxidation of acetone in air at ambient temperature using a 7000 mL reactor | UV illumination by a 15 W 365 nm UV lamp with a UV intensity of 540 (10 íW/cm2 in an ambient condition (22 °C, RH 80%, in air) | Excellent photoinduced hydrophilicity and antibacterial effect for Bacillus pumilus on the TiO2 films with 3 h calcination under UV illumination decreases to 50% within 2 h | Fe3+ ion, carbon dioxide | [58] |
| Copper-Deposited TiO2 Thin Film | Two types of E. coli cells (IFO 3301 strain and 53TNE007 strain) | Fluorescence intensities at 460 nm under various conditions were compared to that observed from the TiO2 film after UV light illumination with intensity of 1 mW/cm2 for 20 min. | Efficient bactericidal function for various bacteria compared to the conventional copper system in an ordinary living space with very weak UV intensity | Cu+, hydroxyl radicals, 7-hydroxycoumarin | [59] |
| Nitrogen-Doped TiO2 substrates | Pathogens, including Shigella flexneri, Listeria monocytogenes, Vibrio parahaemolyticus, S. aureus, Streptococcus pyogenes, and Acinetobacter baumannii | Visible light illumination under the incandescent lamp for 5 min at 4 °C at a distance of 5 cm, corresponding to an illumination density of 3 × 104 lux. | Superior visible-light-induced bactericidal activity against E. coli compared to pure TiO2 and carbon-doped TiO2 substrates | ROS, exotoxin | [60] |
| N, S co-doped commercial TiO2 powders (Tayca TKP101, TKP102) | E. coli | Illumination during 2 h and samples (1.0 mL) were taken at different time intervals. | Suitable photocatalytic activity under UV illumination towards E. coli inactivation and also under visible light irradiation (400–500 nm) | ROS, inactivate bacterial cells, pyrosulfate, SO42− and SO2 | [61] |
| N, S co-doped TiO2 (Tayca) | Phenol, Dichloroacetate, E. coli | Inactivation were achieved under UV illumination (pH 6.0, UV intensity 30 Wm−2 and concentration of N, S TiO2 of 1 g L−1) and visible light irradiation (400–500 nm) for 16 min | Strong photocatalytic activity towards the photo-degradation of phenol and dichloroacetate, and inactivation of E. coli under exposure to UV light | Hydroxyl and O2− radicals, 1O2, p-benzoquinone | [62] |
| N, S co-doped and N-doped Degussa P-25 powders | E. coli inactivation and phenol oxidation | Photocatalytic activities of the powders were tested using phenol and E. coli cells under UV intensity of 38 W m−2 (320–380 nm) and visible light (400–500 nm) | The highest photocatalytic E. coli inactivation under visible light with Degussa P-25 | P-benzoquinone, Hydroxyl and O2− radicals, 1O2 | [63] |
| TiO2 (P25 formulation; Degussa) | Inactivation of Legionella pneumophila | Inactivation of the viability of selected L. pneumophila strains and controls (with initial cell concentration of 107 cfu/mL) at different time intervals of PCO using 1000 mg/L of TiO2 and 108 µW/cm2 of UV365 nm | Total mineralization of bacterial cell with prolonged photocatalytic oxidation treatment with the highest inactivation efficiency (IE, log-reduction) after 90 min | OH radicals | [64] |
| TiO2 plasma sprayed coating on stainless steel 304 | Methylene blue aqueous solution decomposition | Irradiation using ultraviolet rays (390 nm) lamp which excites electrons and forms holes in TiO2 coatings | A lower heat input resulted in a higher anatase phase fraction and smaller anatase grain size and the best photodecomposition efficiency | Superoxide ions and hydroxyl radicals | [65] |
| TiO2 films electrolytically deposited on AISI 316L stainless steel and Ti6Al4V substrates | Vanadium, aluminum, sulfur and phosphorus | Scratch tests on electrolytic TiO2 deposited −75 mA cm−2/8 C on AISI 316 L after annealing | Excellent adhesion and very ductile behavior were found from nanoindentation and scratch tests | Fe (3–4 at. %) and Cr (1 at. %), peroxo-complex | [66] |
| Needle-Like shaped uniform anatase TiO2 coatings on MWNTs | Bacterial endospores (Bacillus cereus) | UV lamps were stabilized for 30 min to obtain constant intensity (92 W/m2) before each test | 90% inactivation of spores (LD90) and also in terms of time required to achieve a 1.0 log10 reduction of spores in the tail region of the inactivation curve | Hydroxyl radicals | [67] |
| Sulfur-Doped and Nitrogen-Fluorine-codoped TiO2 | Photoinactivation of E. coli | Under solar simulated light (UVA 3 mW/cm2; 162,370 lx) and visible light (162,370 lx) irradiation for 30 min | S-TiO2 photocatalysts did not show any enhancement in photocatalytic activity toward E. coli inactivation under visible light irradiation | ROS, OH and O2− radicals | [68] |
| Ceramic tiles coated with TiO2 | Salmonella Enteritidis | Radiation of UV-C of 253.7 nm wavelength for 0, 60, 90, and 120 s. | Bactericidal action of UV radiation is much stronger on the surfaces of TiO2-coated tiles than on the uncovered tiles | pyrimidine dimers | [69] |
| Type | Pollutant | Catalyst | |
|---|---|---|---|
| Organic pollutants | Dye wastewater | Methyl orange | Y-TiO2-HPW |
| Alkaline red dye | TiO2-Fenton | ||
| Rhodamine 6G | TiO2 | ||
| Anthraquinone dye | N-TiO2 | ||
| Pharmaceutical wastewater | Amoxicillin, Penbritin | TiO2 | |
| Cloxacillin, Oxolinic acid | TiO2 | ||
| Pesticide wastewater | Kappa furan pesticides | TiO2 | |
| Armour mix phosphorus | TiO2 | ||
| Alon | TiO2-SBA | ||
| Organophosphorus pesticide | TiO2 | ||
| Explosives wastewater | TNT, RDX, HMX | TiO2 | |
| Chlorine hydroxybenzene wastewater | Chlorinated phenol | TiO2 | |
| Nitrobenzene wastewater | Nitrobenzene | H3PW12O40/TiO2 | |
| Inorganic pollutants | Heavy metal pollutants | Hg (II) | TiO2 |
| Cr (VI) | ZrO2 | ||
| Mn (II), Ti (I) | TiO2 | ||
| Cyanide-containing waste | CN− | TiO2 | |
| NO−2 containing waste | NO−2 | Fe3+/TiO2/SiO2 | |
| Type of Nanophotocatalyst | Photocatalytic Activity | Ref (s) | |
|---|---|---|---|
| Metal oxides | TiO2 | Degradation of expired drugs and pharmaceutical compounds, dyes in textile industries, pesticides, cyanobacterial toxin microcystin-LR, parabens. Photocatalytic films covering scalpels, surgical masks, and catheters | [96,102] |
| ZnO | Photocatalytic degradation of acridine orange, methyl orange (MO), methylene blue (MB) | [126,127] | |
| Fe2O3 | Photodegradation of dibutyl phthalate in wastewater, Photocatalytic oxidation of aniline to azobenzene | [138,140] | |
| Gd2O3 | Photodegradation of MO, 4-chlorophenol, neurotoxicity chloramphenicol drug | [144] | |
| Sb2O4 | Photodegradation of acridine orange, Removal of heavy metals (e.g., mercury) from wastewater | [147] | |
| Binary metal oxides | ZnO-CeO2 | Photodegradation for MB and acridine orange | [130] |
| CuxS-TiO2 | Photodegradation of dyes | [154] | |
| CeO2-CdO | Antimicrobial activity of bacteria and fungi | [155] | |
| Metal sulfides | ZnS, CdS | Visible light assisted water splitting | [156] |
| Magnetic nanophotocatalysts | Fe3O4@TiO2 | Degradation of rhodamine B (RhB), MB, Quinoline | [166,167,168] |
| NiFe2O4@TiO2 | Degradation of MO | [169] | |
| CoFe2O4@TiO2 | Degradation of procion red MX-5B (PR) | [170] | |
| ZnFe2O4@TiO2 | Degradation of MO | [171] | |
| BaFe12O19@TiO2 | Degradation of PR | [172] | |
| SrFe12O19@TiO2 | Degradation of PR | [173] | |
| Graphene | P25–G | Decomposing MB under UV and visible light, Decomposing benzene (gas phase) under UV light | [174,175] |
| TiO2–G | Decomposing MB under sunlight light, Decomposing MB under UV light, Decomposing MO under UV light, Decomposing rhodamine B (RhB) under UV light, Decomposing RhB under visible light | [176,177,178,179] | |
| SnO2–G | Decomposing RhB under visible light | [180] | |
| Bi2WO6–G | Decomposing RhB under visible light | [181] | |
| ZnO–G | Decomposing MB under UV light | [182] | |
| ZnFe2O4–G | Decomposing MB under visible light | [183] | |
| BiVO4–G | Photoelectrochemical water splitting | [184] | |
| CdS–G | Photocatalytic H2 evolution under visible light | [185] | |
| Quantum dots | ZnS QDs | Degradation of Methyl violet, Victoria blue, Malachite green, Thymol blue, Congo red, Safranin, MB, HMX (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine), RDX (hexahydro-1,3,5- trinitro-1,3,5-triazine) | [186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204] |
| Graphene QDs | Degradation of New fuchsin, RhB, MO | [192,193,194] | |
| Carbon QDs/BiOX (X = Br, Cl) | Degradation of Phenol RhB, Ciprofloxacin, Bisphenol A (BPA) | [195] | |
| Carbon QD/NZnO | Degradation of Malachite green, MB, Fluorescein | [196] | |
| Graphitic carbon nitride QDs | Degradation of RhB | [197] | |
| CdS QDs | Degradation of Alizarin, Acid violet, Mordant red, Thymol blue | [198] | |
| CdSe/ZnS QDs | Degradation of Methyl green | [199] | |
| TiO2 QDs | Degradation of Indigo carmine, Ketorolac tromethamine | [200,201] | |
| ZnO foam/carbon QDs | Degradation of RhB, MO, MB | [202] | |
| Ag@AgCl QDs Sensitized Bi2WO6 | Degradation of RhB | [203] | |
| Smart materials (self-cleaning) | ZnO | Decomposition of organic contaminants | [205] |
| TiO2 | Self-decontamination textiles, the antibacterial activity of UV shielding | [206] | |
| PVDF/TiO2 | Antifouling/self-cleaning, photoactive, and bactericidal | [207] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooshmand, S.; Kargozar, S.; Ghorbani, A.; Darroudi, M.; Keshavarz, M.; Baino, F.; Kim, H.-W. Biomedical Waste Management by Using Nanophotocatalysts: The Need for New Options. Materials 2020, 13, 3511. https://doi.org/10.3390/ma13163511
Hooshmand S, Kargozar S, Ghorbani A, Darroudi M, Keshavarz M, Baino F, Kim H-W. Biomedical Waste Management by Using Nanophotocatalysts: The Need for New Options. Materials. 2020; 13(16):3511. https://doi.org/10.3390/ma13163511
Chicago/Turabian StyleHooshmand, Sara, Saeid Kargozar, Ahmad Ghorbani, Majid Darroudi, Meysam Keshavarz, Francesco Baino, and Hae-Won Kim. 2020. "Biomedical Waste Management by Using Nanophotocatalysts: The Need for New Options" Materials 13, no. 16: 3511. https://doi.org/10.3390/ma13163511
APA StyleHooshmand, S., Kargozar, S., Ghorbani, A., Darroudi, M., Keshavarz, M., Baino, F., & Kim, H.-W. (2020). Biomedical Waste Management by Using Nanophotocatalysts: The Need for New Options. Materials, 13(16), 3511. https://doi.org/10.3390/ma13163511