Leachable Poly(Trimethylene Carbonate)/CaCO3 Composites for Additive Manufacturing of Microporous Vascular Structures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Functionalization of Three-Armed PTMC
2.3. Preparation and Characterization of PTMC-MA/CaCO3 Films
2.4. Leaching of CaCO3 Particles and Characterization of Microporous PTMC Films
2.5. Water Flux
2.6. Mechanical Properties
2.7. Additive Manufacturing of Microvascular Structures Using PTMC/CaCO3 Resin
2.8. Scanning Electron Microscopy (SEM) and Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Characterization of PTMC-MA, CaCO3 Particles and PTMC-MA/CaCO3 Composite Films
3.2. Characterization of Leached PTMC-MA/CaCO3 Composite Films
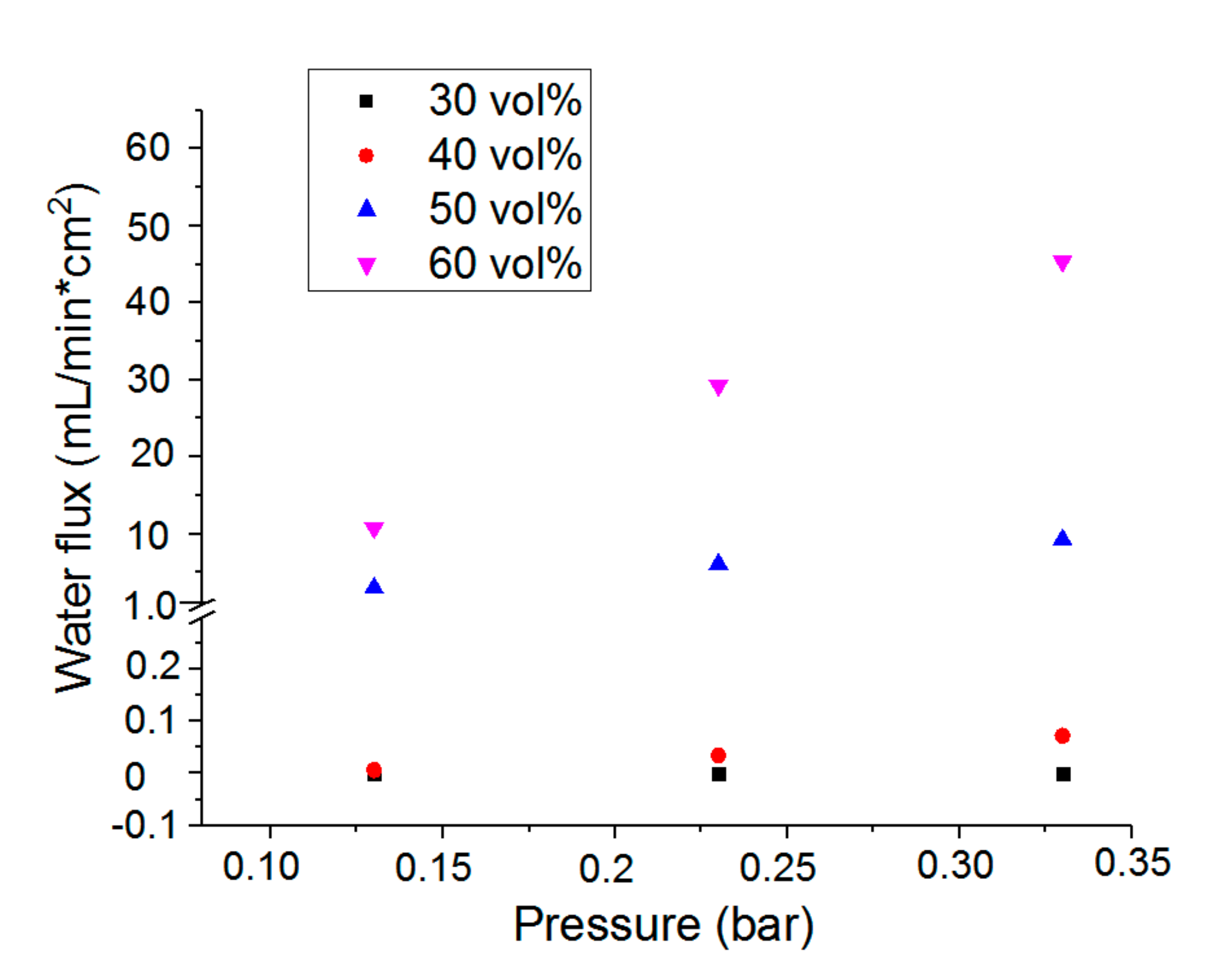
3.3. Water Permeability of the Microporous PTMC Films
3.4. Mechanical Properties of the Microporous PTMC Films
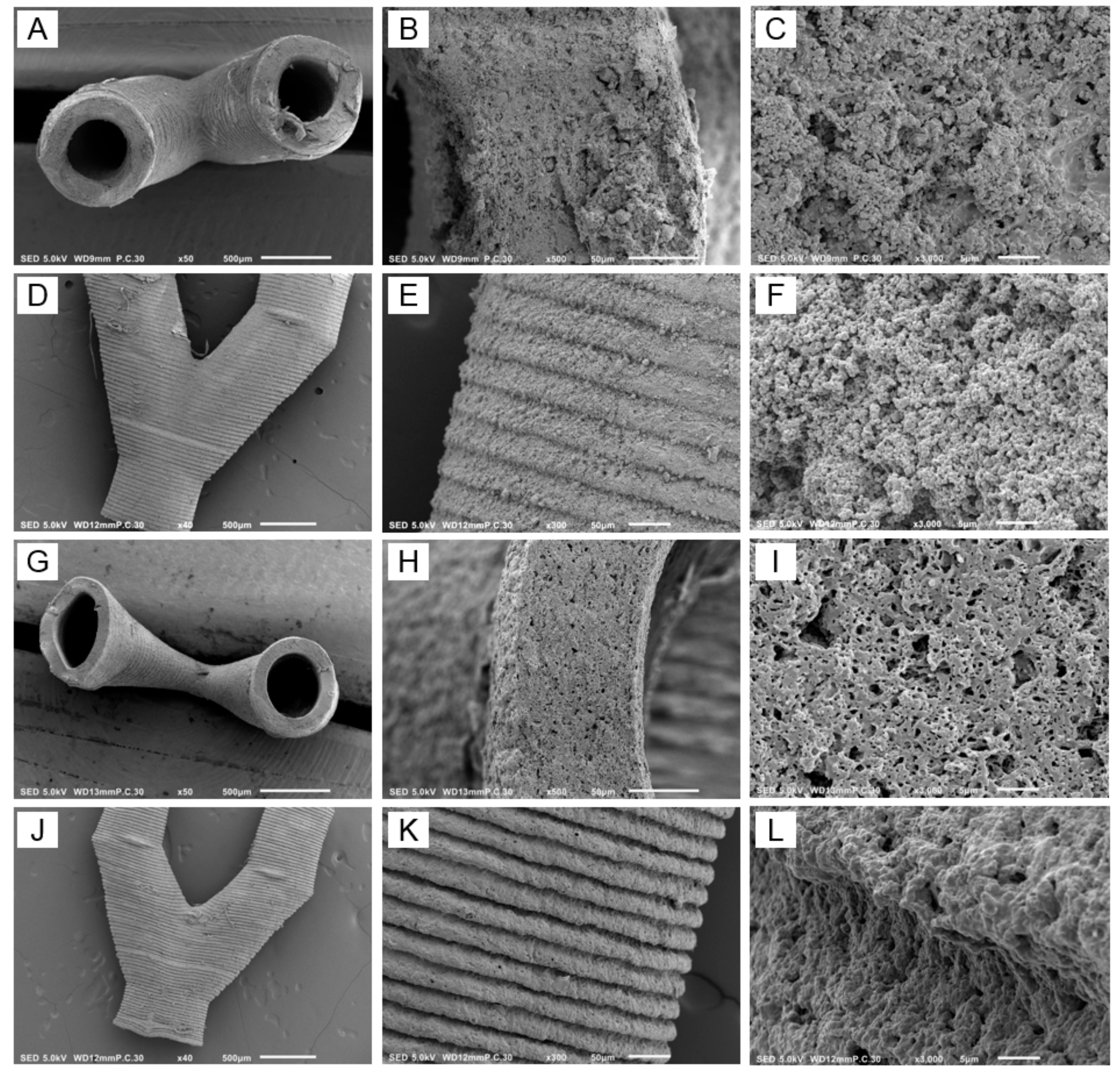
3.5. Structure Design, Resin Formulation and SLA
3.6. Characterization of the Branched Vascular PTMC Structures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bae, H.; Puranik, A.S.; Gauvin, R.; Edalat, F.; Carrillo-Conde, B.; Peppas, N.A.; Khademhosseini, A. Building Vascular Networks. Sci. Transl. Med. 2012, 4, 160ps23. [Google Scholar] [CrossRef] [PubMed]
- Tocchio, A.; Tamplenizza, M.; Martello, F.; Gerges, I.; Rossi, E.; Argentiere, S.; Rodighiero, S.; Zhao, W.; Milani, P.; Lenardi, C. Versatile fabrication of vascularizable scaffolds for large tissue engineering in bioreactor. Biomaterials 2015, 45, 124–131. [Google Scholar] [CrossRef] [PubMed]
- West, J.L.; Moon, J.J. Vascularization of Engineered Tissues: Approaches to Promote Angio-genesis in Biomaterials. Curr. Top. Med. Chem. 2008, 8, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.F.; Toh, J.K.C.; Du, C.; Narayanan, K.; Lu, H.F.; Lim, T.C.; Wan, A.C.A.; Ying, J.Y. Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres. Nat. Commun. 2013, 4, 2353. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013, 13, 1489. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K. Tissue engineering for clinical applications. Biotechnol. J. 2010, 5, 1309–1323. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2010, 6, 13–22. [Google Scholar]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Jain, R.K.; Au, P.; Tam, J.; Duda, D.G.; Fukumura, D. Engineering vascularized tissue. Nat. Biotechnol. 2005, 23, 821–823. [Google Scholar] [CrossRef]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Landau, S.; Guo, S.; Levenberg, S. Localization of Engineered Vasculature within 3D Tissue Constructs. Front. Bioeng. Biotechnol. 2018, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Vyas, C.; Pereira, R.; Huang, B.; Liu, F.; Wang, W.; Bartolo, P. Engineering the vasculature with additive manufacturing. Curr. Opin. Biomed. Eng. 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Sarker, M.D.; Naghieh, S.; Sharma, N.K.; Chen, X. 3D biofabrication of vascular networks for tissue regeneration: A report on recent advances. J. Pharm. Anal. 2018, 8, 277–296. [Google Scholar] [CrossRef]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef]
- Pimentel, R.; Ko, S.K.; Caviglia, C.; Wolff, A.; Emnéus, J.; Keller, S.S.; Dufva, M. Three-dimensional fabrication of thick and densely populated soft constructs with complex and actively perfused channel network. Acta Biomater. 2018, 65, 174–184. [Google Scholar] [CrossRef]
- Lei, D.; Yang, Y.; Liu, Z.; Yang, B.; Gong, W.; Chen, S.; Wang, S.; Sun, L.; Song, B.; Xuan, H.; et al. 3D printing of biomimetic vasculature for tissue regeneration. Mater. Horizons 2019, 6, 1197–1206. [Google Scholar] [CrossRef]
- Stampfl, J.; Baudis, S.; Heller, C.; Liska, R.; Neumeister, A.; Kling, R.; Ostendorf, A.; Spitzbart, M. Photopolymerization with tunable mechanical properties processed by laser-based high-resolution stereolithography. J. Micromech. Microeng. 2008, 18, 125014. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold design for tissue engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Zondervan, G.J.; Hoppen, H.J.; Pennings, A.J.; Fritschy, W.; Wolters, G.; van Schilfgaard, R. Design of a polyurethane membrane for the encapsulation of islets of Langerhans. Biomaterials 1992, 13, 136–144. [Google Scholar] [CrossRef]
- Guillaume, O.; Geven, M.A.; Grijpma, D.W.; Tang, T.T.; Qin, L.; Lai, Y.X.; Yuan, H.; Richards, R.G.; Eglin, D. Poly(trimethylene carbonate) and nano-hydroxyapatite porous scaffolds manufactured by stereolithography. Polym. Adv. Technol. 2017, 28, 1219–1225. [Google Scholar] [CrossRef]
- Pateman, C.J.; Harding, A.J.; Glen, A.; Taylor, C.S.; Christmas, C.R.; Robinson, P.P.; Rimmer, S.; Boissonade, F.M.; Claeyssens, F.C.; Haycock, J.W. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials 2015, 49, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Kokkari, A.; Närhi, T.; Seppälä, J.V. Porous 3D modeled scaffolds of bioactive glass and photocrosslinkable poly(ε-caprolactone) by stereolithography. Compos. Sci. Technol. 2013, 74, 99–106. [Google Scholar] [CrossRef]
- Song, Y.; Kamphuis, M.M.; Zhang, Z.; Sterk, L.T.; Vermes, I.; Poot, A.A.; Feijen, J.; Grijpma, D.W. Flexible and elastic porous poly(trimethylene carbonate) structures for use in vascular tissue engineering. Acta Biomater. 2010, 6, 1269–1277. [Google Scholar] [CrossRef]
- Guo, Z.; Grijpma, D.W.; Poot, A.A. Preparation and characterization of flexible and elastic porous tubular PTMC scaffolds for vascular tissue engineering. Polym. Adv. Technol. 2016, 28, 1239–1244. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, S.; Vancso, G.J.; Grijpma, D.W.; Feijen, J. Enzymatic Surface Erosion of Poly(trimethylene carbonate) Films Studied by Atomic Force Microscopy. Biomacromolecules 2005, 6, 3404–3409. [Google Scholar] [CrossRef]
- Rongen, J.; Van Bochove, B.; Hannink, G.; Grijpma, D.W.; Buma, P. Degradation behavior of, and tissue response to photo-crosslinked poly(trimethylene carbonate) networks. J. Biomed. Mater. Res. Part A 2016, 104, 2823–2832. [Google Scholar] [CrossRef]
- Van Bochove, B.; Grijpma, D.W. Photo-crosslinked synthetic biodegradable polymer networks for biomedical applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 77–106. [Google Scholar] [CrossRef]
- Schüller-Ravoo, S.; Feijen, J.; Grijpma, D.W. Flexible, elastic and tear-resistant networks prepared by photo-crosslinking poly(trimethylene carbonate) macromers. Acta Biomater. 2012, 8, 3576–3585. [Google Scholar] [CrossRef]
- Schüller-Ravoo, S.; Zant, E.; Feijen, J.; Grijpma, D.W. Preparation of a Designed Poly(trimethylene carbonate) Microvascular Network by Stereolithography. Adv. Health Mater. 2014, 3, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Pennella, P.; Cerino, G.; Massai, D.; Gallo, D.; Falvo D’Urso Labate, G.; Schiavi, A.; Deriu, M.A.; Audenino, A.; Morbiducci, U. A survey of methods for the evaluation of tissue engineering scaffold permeability. Ann. Biomed. Eng. 2013, 41, 2027–2041. [Google Scholar] [CrossRef] [PubMed]
- Chooi, K.Y.; Comerford, A.; Sherwin, S.J.; Weinberg, P.D. Intimal and medial contributions to the hydraulic reisistance of the arterial wall at different pressures: A combined computational and experimental study. J. R. Soc. Interface 2016, 13, 20160234. [Google Scholar] [CrossRef] [PubMed]
- Van Bochove, B.; Hannink, G.; Grijpma, D.W.; Buma, P. Preparation of Designed Poly(trimethylene carbonate) Meniscus Implants by Stereolithography: Challenges in Stereolithography. Macromol. Biosci. 2016, 16, 1853–1863. [Google Scholar] [CrossRef]
- Blanquer, S.; Gebraad, A.; Miettinen, S.; Poot, A.A.; Grijpma, D.W.; Haimi, S.P. Differentiation of adipose stem cells seeded towards annulus fibrosus cells on a designed poly(trimethylene carbonate) scaffold prepared by stereolithography. J. Tissue Eng. Regen. Med. 2016, 11, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Blanquer, S.B.G.; Haimi, S.P.; Poot, A.A.; Grijpma, D.W. Effect of Pore Characteristics on Mechanical Properties and Annulus Fibrosus Cell Seeding and Proliferation in Designed PTMC Tissue Engineering Scaffolds. Macromol. Symp. 2013, 334, 75–81. [Google Scholar] [CrossRef]
- Bat, E.; Plantinga, J.A.; Harmsen, M.C.; van Luyn, M.J.A.; Zhang, Z.; Grijpma, D.W.; Feijen, J. Trimethylene carbonate and ε-caprolactone based (co)polymer networks: Mechanical properties and enzymatic degradation. Biomacromolecules 2008, 9, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Bat, E.; Plantinga, J.A.; Harmsen, M.C.; van Luyn, M.J.A.; Feijen, J.; Grijpma, D.W. In Vivo behavior of trimethylene carbonate and ε-caprolactone based (co)polymer networks: Degradation and tissue response. J. Biomed. Mater. Res. Part A 2010, 95A, 940–949. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, T.; Zhang, L.; Gong, W.; Sun, W. Biomimetic design and fabrication of scaffolds integrating oriented micro-pores with branched channel networks for myocardial tissue engineering. Biofabrication 2019, 11, 035004. [Google Scholar] [CrossRef]
- Mu, X.; Bertron, T.; Dunn, C.; Qiao, H.; Wu, J.; Zhao, Z.; Saldana, C.; Qi, H.J. Porous polymeric materials by 3D printing of photocurable resin. Mater. Horizons 2017, 4, 442–449. [Google Scholar] [CrossRef]
- Huber, B.; Engelhardt, S.; Meyer, W.; Kruger, H.; Wenz, A.; Schönhaar, V.; Tovar, G.E.M.; Kluger, P.J.; Borchers, K. Blood-Vessel Mimicking Structures by Stereolithographic Fabrication of Small Porous Tubes Using Cytocompatible Polyacrylate Elastomers, Biofunctionalization and Endothelialization. J. Funct. Biomater. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed]
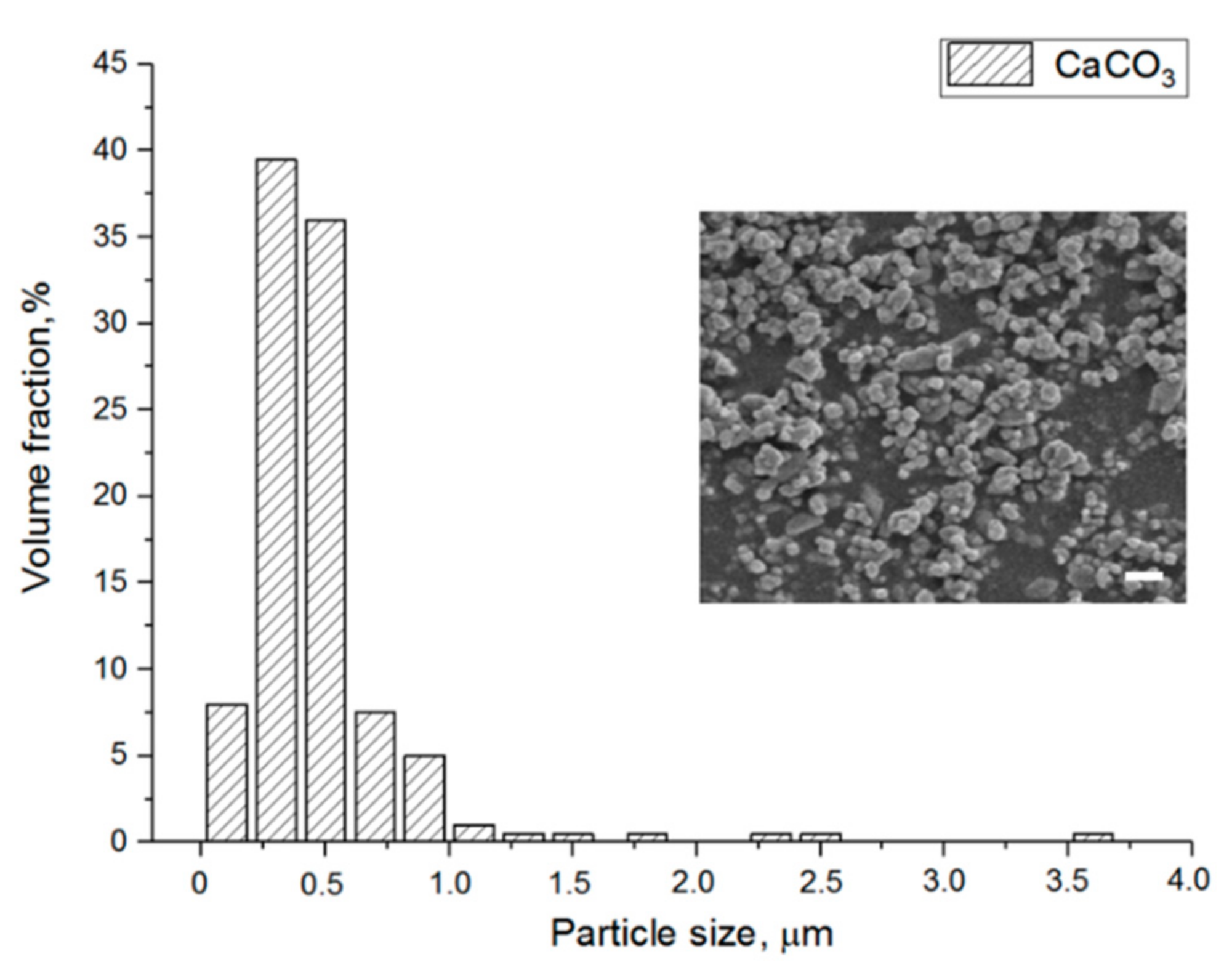
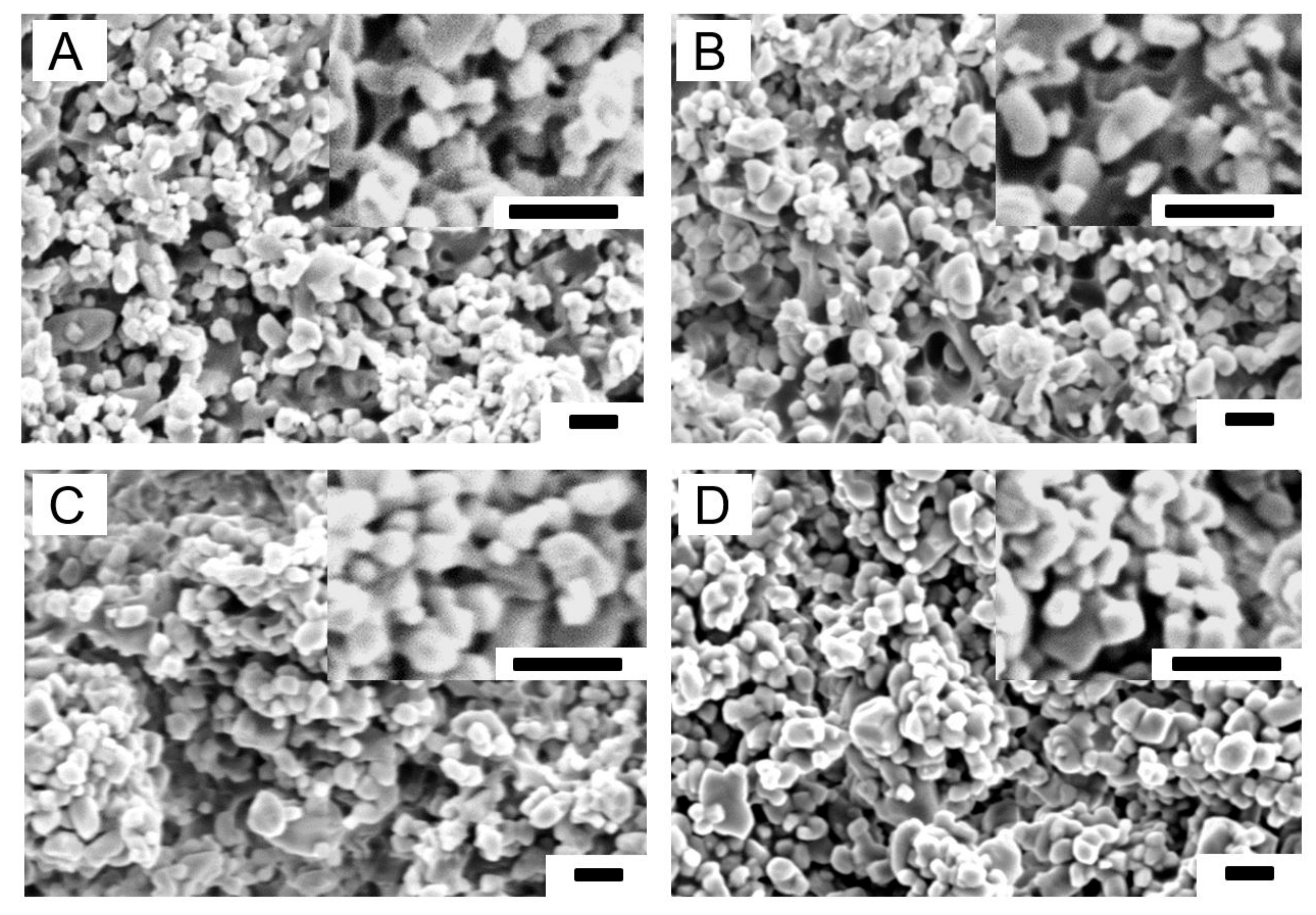
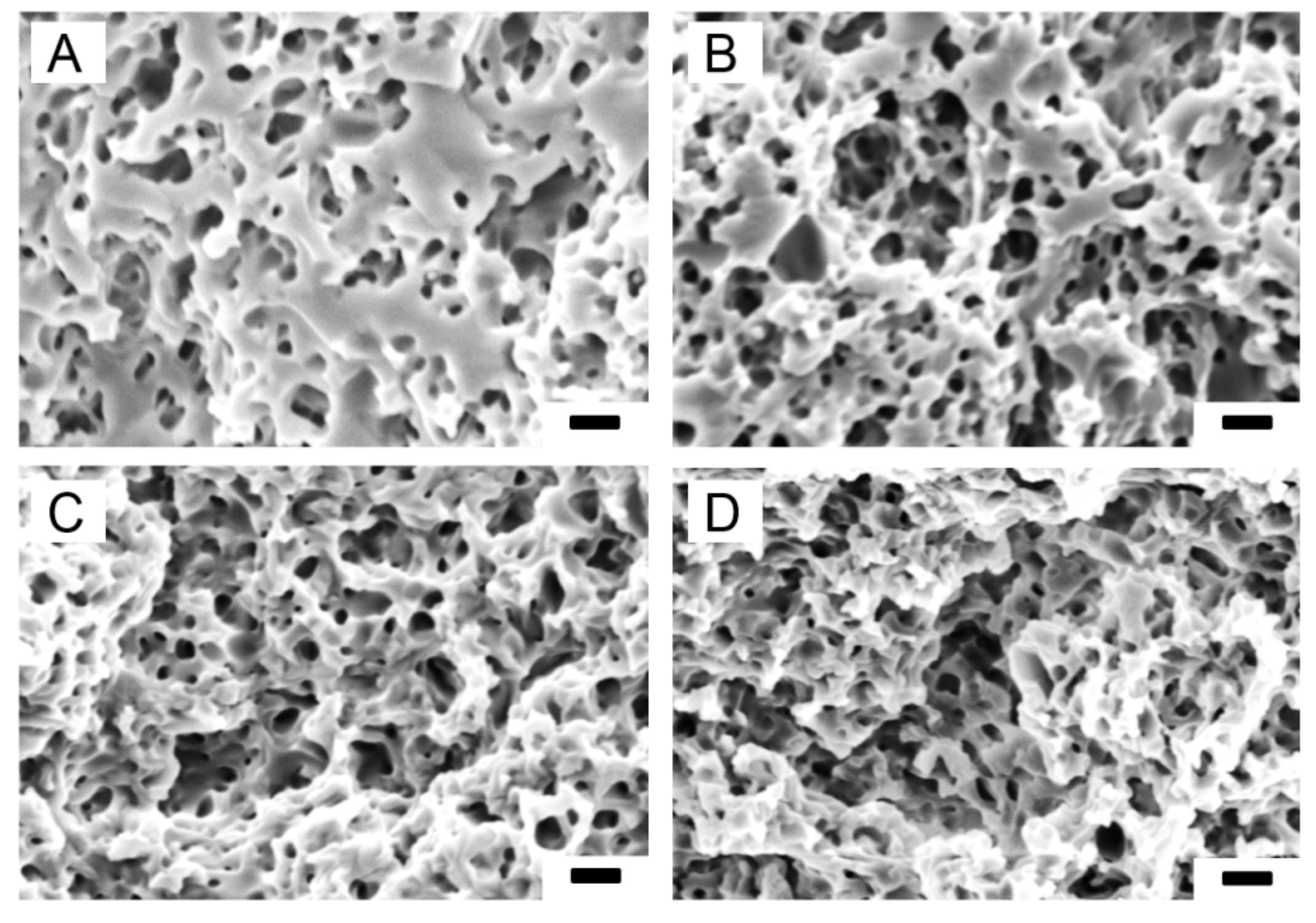
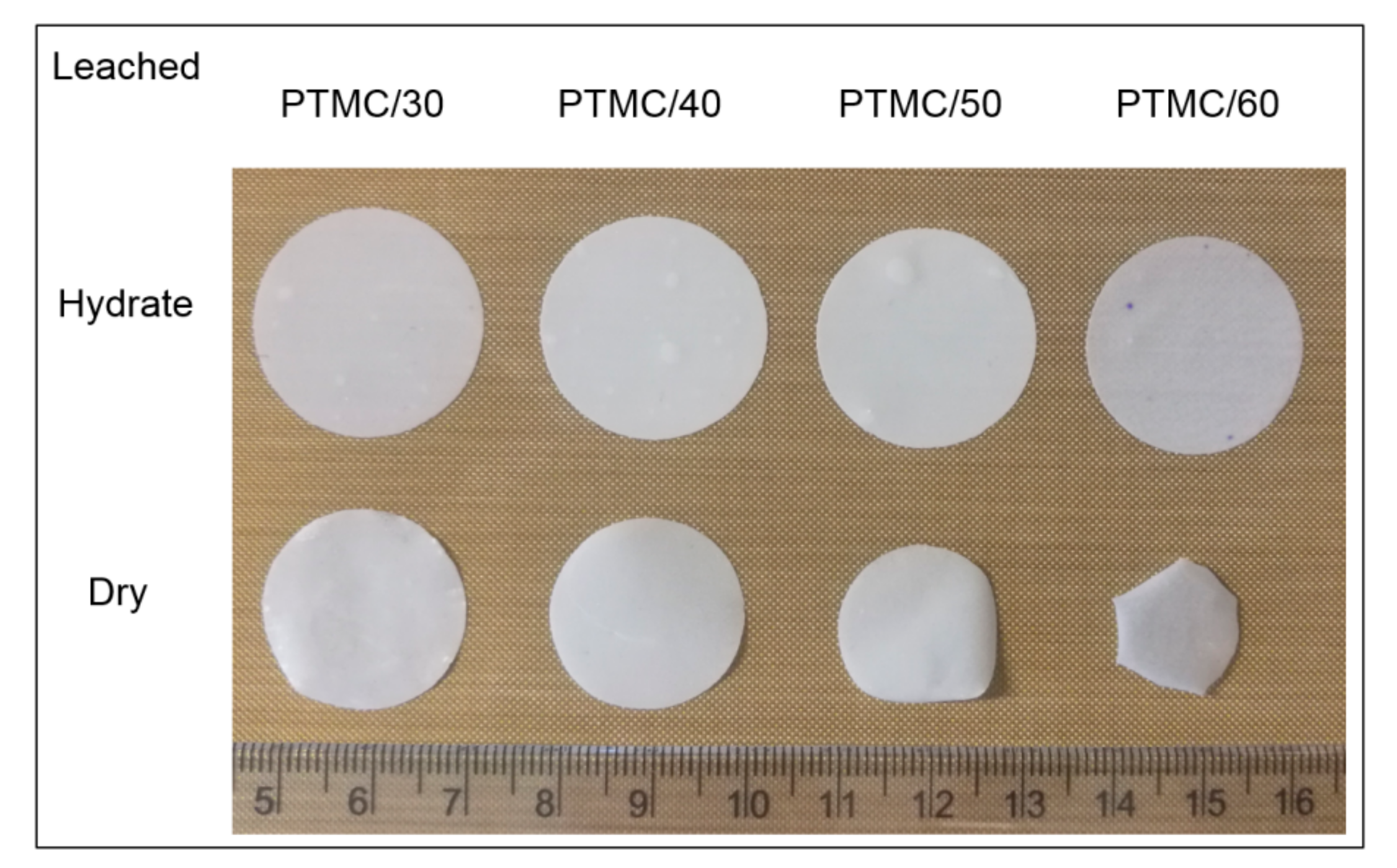

| Component | Weight (g) | Content (%) |
|---|---|---|
| PTMC-MA | 46.5 | 22.1 |
| CaCO3 | 78.7 | 37.4 |
| Propylene carbonate | 85.0 | 40.5 |
| TPO-L | 2.3 | 5 * |
| Orasol Orange G | 0.07 | 0.15 * |
| Sample Code | CaCO3 Loading, vol % | Gel Content, % |
|---|---|---|
| PTMC | 0 | 98.1 ± 0.3 |
| PTMC/30 | 30 | 97.2 ± 1.1 |
| PTMC/40 | 40 | 97.4 ± 0.7 |
| PTMC/50 | 50 | 96.6 ± 0.6 |
| PTMC/60 | 60 | 94.9 ± 2.1 |
| Sample Code | Porosity, % Hydrated | Porosity, % Dry | Pore Size, μm |
|---|---|---|---|
| PTMC/30 | 33.2 ± 1.9 | 31.4 ± 2.2 | 0.49 ± 0.27 |
| PTMC/40 | 43.1 ± 2.4 | 41.9 ± 1.4 | 0.50 ± 0.21 |
| PTMC/50 | 57.3 ± 3.7 | 52.1 ± 4.3 | 0.45 ± 0.36 |
| PTMC/60 | 71.7 ± 5.1 | 51.3 ± 3.9 | 0.46 ± 0.29 |
| Hydrated | Dry | |||||
|---|---|---|---|---|---|---|
| Sample Code | Emod, MPa | Fmax, MPa | Elongation at Break, % | Emod, MPa | Fmax, MPa | Elongation at Break, % |
| PTMC | 8.16 ± 0.43 | 4.81 ± 0.89 | 67.1 ± 10.9 | 9.07 ± 0.34 | 6.57 ± 0.14 | 83.6 ± 4.2 |
| PTMC/30 | 3.52 ± 0.16 | 3.28 ± 0.72 | 89.6 ± 12.5 | 7.76 ± 0.15 | 5.76 ± 1.21 | 121.7 ± 12.1 |
| PTMC/40 | 2.72 ± 0.14 | 2.79 ± 0.52 | 109.4 ± 11.7 | 6.95 ± 0.17 | 4.58 ± 1.79 | 131.5 ± 14.2 |
| PTMC/50 | 1.13 ± 0.05 | 1.48 ± 0.21 | 103.5 ± 8.1 | 5.82 ± 0.18 | 2.70 ± 0.54 | 143.3 ± 24.1 |
| PTMC/60 | 0.30 ± 0.06 | 0.54 ± 0.04 | 104.1 ± 10.2 | 6.13 ± 0.16 | 2.16 ± 0.11 | 86.8 ± 19.9 |
| Non-Leached | Leached, Hydrated | Leached, Dry | |
|---|---|---|---|
| Inner diameter, μm | 480.2 ± 10.9 | 482 ± 10.2 | 416.3 ± 7.5 |
| Wall thickness, μm | 162.5 ± 4.3 | 146.0 ± 6.1 | 90.1 ± 3.8 |
| Pore size, μm | - | - | 0.40 ± 0.27 |
| CaCO3 content, vol % | 50.2 ± 2.9 | - | - |
| Porosity, % | - | 59 ± 3 | 35 ± 4 |
| Water flux at 0.16 bar, mL/min·cm2 | - | 0.09 ± 0.02 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Grijpma, D.; Poot, A. Leachable Poly(Trimethylene Carbonate)/CaCO3 Composites for Additive Manufacturing of Microporous Vascular Structures. Materials 2020, 13, 3435. https://doi.org/10.3390/ma13153435
Guo Z, Grijpma D, Poot A. Leachable Poly(Trimethylene Carbonate)/CaCO3 Composites for Additive Manufacturing of Microporous Vascular Structures. Materials. 2020; 13(15):3435. https://doi.org/10.3390/ma13153435
Chicago/Turabian StyleGuo, Zhengchao, Dirk Grijpma, and André Poot. 2020. "Leachable Poly(Trimethylene Carbonate)/CaCO3 Composites for Additive Manufacturing of Microporous Vascular Structures" Materials 13, no. 15: 3435. https://doi.org/10.3390/ma13153435
APA StyleGuo, Z., Grijpma, D., & Poot, A. (2020). Leachable Poly(Trimethylene Carbonate)/CaCO3 Composites for Additive Manufacturing of Microporous Vascular Structures. Materials, 13(15), 3435. https://doi.org/10.3390/ma13153435




