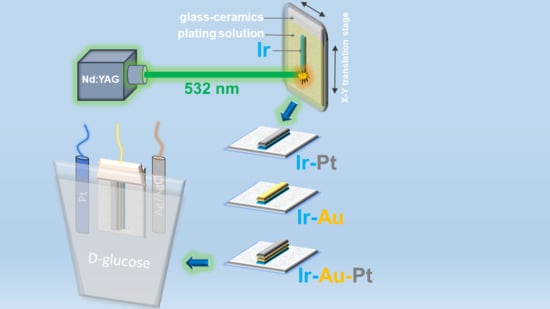
Laser-Induced Synthesis of Composite Materials Based on Iridium, Gold and Platinum for Non-Enzymatic Glucose Sensing
Abstract
1. Introduction
2. Materials and Methods
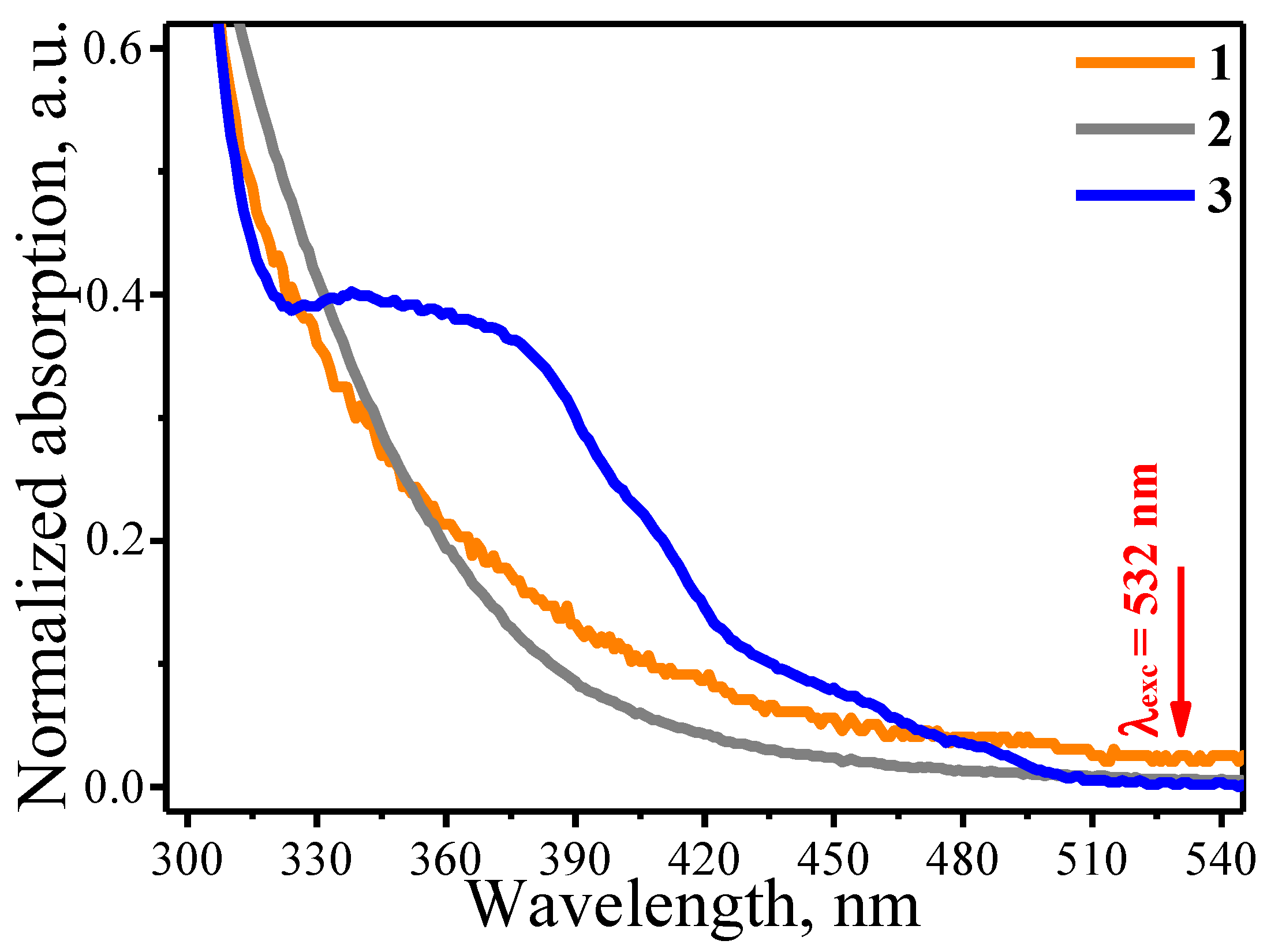
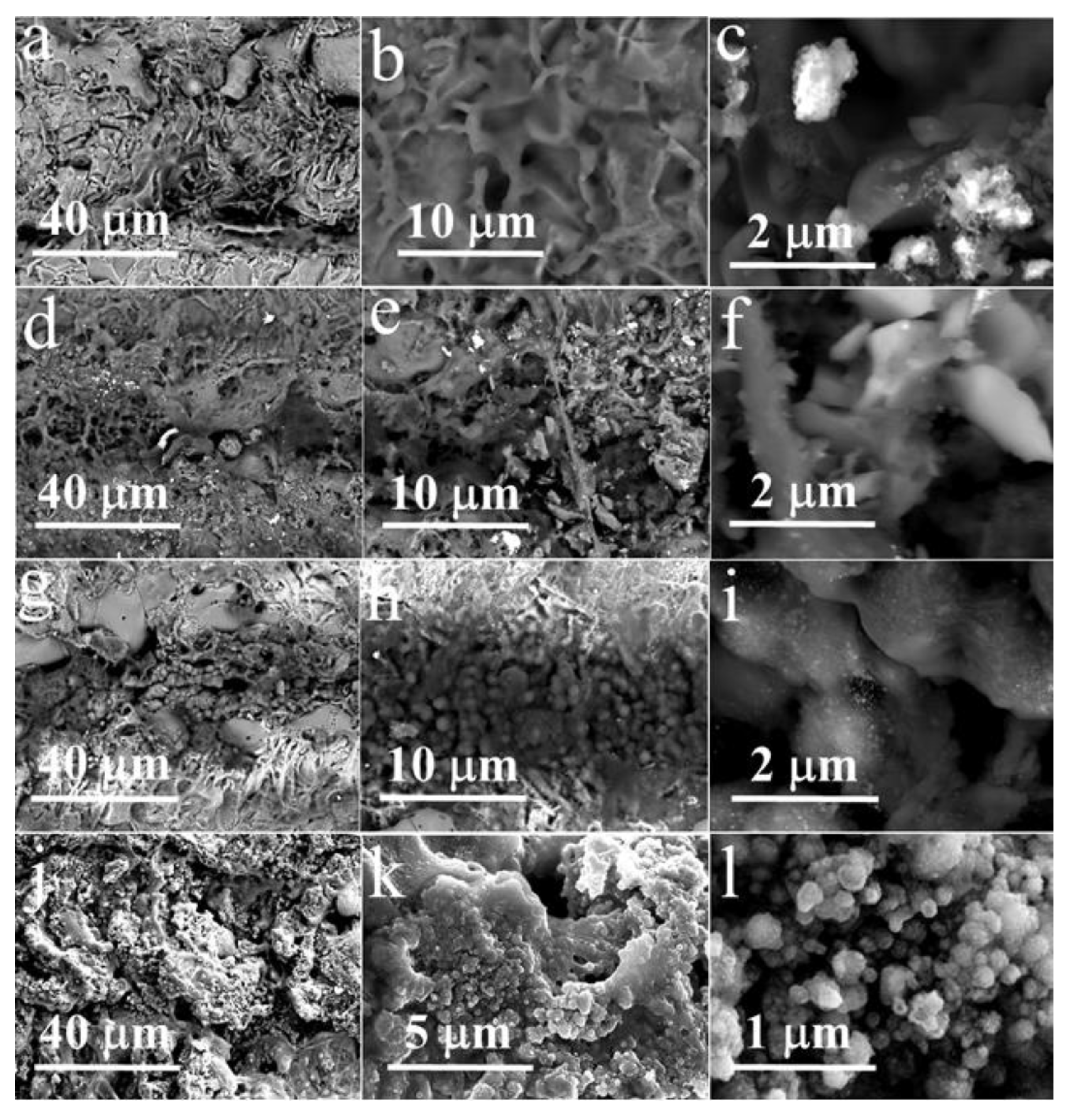
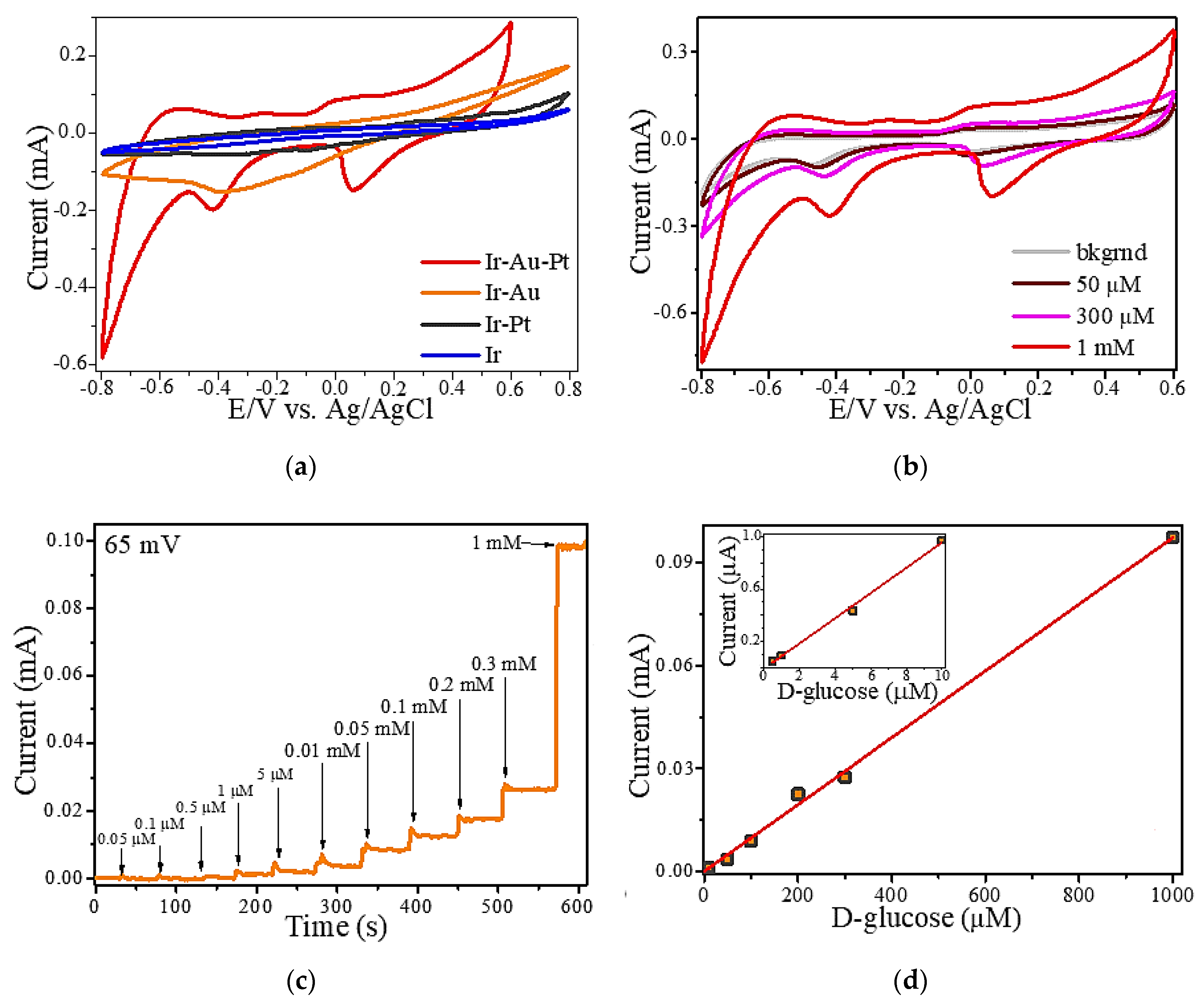
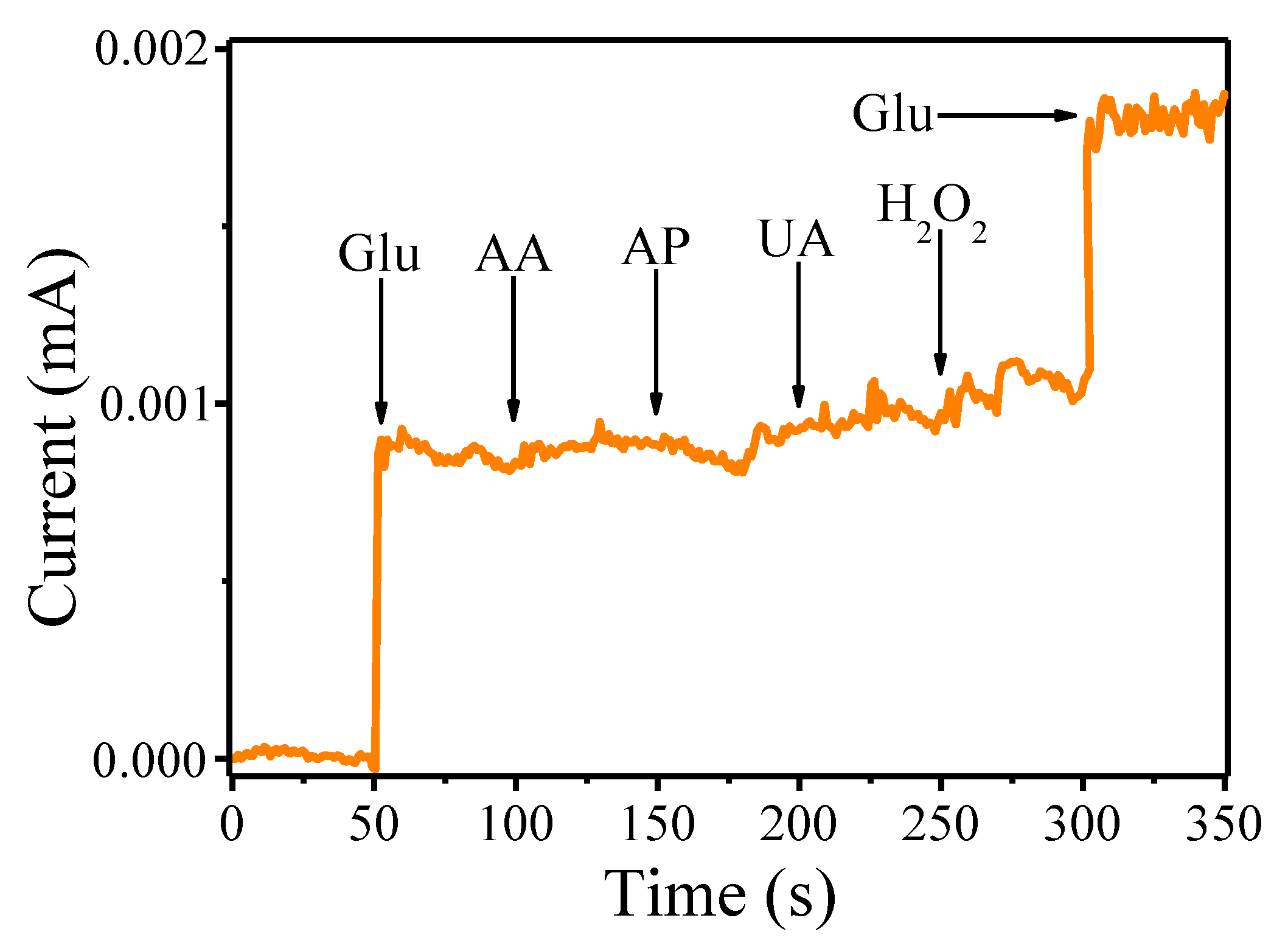
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hahn, Y.B.; Ahmad, R.; Tripathy, N. Chemical and biological sensors based on metal oxide nanostructures. Chem. Commun. 2012, 48, 10369–10385. [Google Scholar] [CrossRef]
- Trial, C. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995, 47, 1703–1720. [Google Scholar] [CrossRef]
- Bakker, E. Electrochemical sensors. Anal. Chem. 2004, 76, 3285–3298. [Google Scholar] [CrossRef]
- Saei, A.A.; Dolatabadi, J.E.N.; Najafi-Marandi, P.; Abhari, A.; de la Guardia, M. Electrochemical biosensors for glucose based on metal nanoparticles. TrAC Trends Anal. Chem. 2013, 42, 216–227. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab. Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Ferri, S.; Kojima, K.; Sode, K. Review of glucose oxidases and glucose dehydrogenases: A bird’s eye view of glucose sensing enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef]
- Cheng, T.M.; Huang, T.K.; Lin, H.K.; Tung, S.P.; Chen, Y.L.; Lee, C.Y.; Chiu, H.T. (110)-Exposed gold nanocoral electrode as low onset potential selective glucose sensor. ACS Appl. Mater. Interfaces 2010, 2, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Lay, B.; Coyle, V.E.; Kandjani, A.E.; Amin, M.H.; Sabri, Y.M.; Bhargava, S.K. Nickel-gold bimetallic monolayer colloidal crystals fabricated: Via galvanic replacement as a highly sensitive electrochemical sensor. J. Mater. Chem. B 2017, 5, 5441–5449. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Yamauchi, Y. Electrochemical deposition of mesoporous Pt-Au alloy films in aqueous surfactant solutions: Towards a highly sensitive amperometric glucose sensor. Chem.-A Eur. J. 2013, 19, 2242–2246. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Su, F.; Liu, A.; Qiu, H. Nanoporous PtAg and PtCu alloys with hollow ligaments for enhanced electrocatalysis and glucose biosensing. Biosens. Bioelectron. 2011, 27, 160–166. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Kondo, Y. Direct writing of two- and three-dimensional Cu-based microstructures by femtosecond laser reductive sintering of the Cu2O nanospheres. Opt. Mater. Express 2019, 9, 2828–2837. [Google Scholar] [CrossRef]
- Kwon, J.; Cho, H.; Suh, Y.D.; Lee, J.; Lee, H.; Jung, J.; Kim, D.; Lee, D.; Hong, S.; Ko, S.H. Flexible and Transparent Cu Electronics by Low-Temperature Acid-Assisted Laser Processing of Cu Nanoparticles. Adv. Mater. Technol. 2017, 2, 1600222. [Google Scholar] [CrossRef]
- Kochemirovsky, V.A.; Skripkin, M.Y.; Tveryanovich, Y.S.; Mereshchenko, A.S.; Gorbunov, A.O.; Panov, M.S.; Tumkin, I.I.; Safonov, S.V. Laser-induced copper deposition from aqueous and aqueous–organic solutions: State of the art and prospects of research. Russ. Chem. Rev. 2015, 84, 1059–1075. [Google Scholar] [CrossRef]
- Panov, M.S.; Tumkin, I.I.; Mironov, V.S.; Khairullina, E.M.; Smikhovskaia, A.V.; Ermakov, S.S.; Kochemirovsky, V.A. Sensory properties of copper microstructures deposited from water-based solution upon laser irradiation at 532 nm. Opt. Quantum Electron. 2016, 48, 490. [Google Scholar] [CrossRef]
- Baranauskaite, V.E.; Novomlinskii, M.O.; Tumkin, I.I.; Khairullina, E.M.; Mereshchenko, A.S.; Balova, I.A.; Panov, M.S.; Kochemirovsky, V.A. In situ laser-induced synthesis of gas sensing microcomposites based on molybdenum and its oxides. Compos. Part B Eng. 2019, 157, 322–330. [Google Scholar] [CrossRef]
- Smikhovskaia, A.V.; Panov, M.S.; Tumkin, I.I.; Khairullina, E.M.; Ermakov, S.S.; Balova, I.A.; Ryazantsev, M.N.; Kochemirovsky, V.A. In situ laser-induced codeposition of copper and different metals for fabrication of microcomposite sensor-active materials. Anal. Chim. Acta 2018, 1044, 138–146. [Google Scholar] [CrossRef]
- Smikhovskaia, A.V.; Andrianov, V.S.; Khairullina, E.M.; Lebedev, D.V.; Ryazantsev, M.N.; Panov, M.S.; Tumkin, I.I. In situ laser-induced synthesis of copper-silver microcomposite for enzyme-free d-glucose and l-alanine sensing. Appl. Surf. Sci. 2019, 488, 531–536. [Google Scholar] [CrossRef]
- Panov, M.; Aliabev, I.; Khairullina, E.; Mironov, V.; Tumkin, I. Fabrication of nickel-gold microsensor using in situ laser-induced metal deposition technique. J. Laser Micro Nanoeng. 2019, 14, 266–269. [Google Scholar] [CrossRef]
- Panov, M.S.; Vereshchagina, O.A.; Ermakov, S.S.; Tumkin, I.I.; Khairullina, E.M.; Skripkin, M.Y.; Mereshchenko, A.S.; Ryazantsev, M.N.; Kochemirovsky, V.A. Non-enzymatic sensors based on in situ laser-induced synthesis of copper-gold and gold nano-sized microstructures. Talanta 2017, 167, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Andriianov, V.S.; Mironov, V.S.; Smikhovskaia, A.V.; Khairullina, E.M.; Tumkin, I.I. Laser-induced synthesis of carbon-based electrode materials for non-enzymatic glucose detection. Opt. Quantum Electron. 2020, 52, 45. [Google Scholar] [CrossRef]
- Shishov, A.; Gordeychuk, D.; Logunov, L.; Tumkin, I. High rate laser deposition of conductive copper microstructures from deep eutectic solvents. Chem. Commun. 2019, 55, 9626–9628. [Google Scholar] [CrossRef] [PubMed]
- Gorski, W.; Kennedy, R.T. Electrocatalyst for non-enzymatic oxidation of glucose in neutral saline solutions. J. Electroanal. Chem. 1997, 424, 43–48. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Miao, X.; Yang, C.; Leung, C.H.; Ma, D.L. Application of iridium(III) complex in label-free and non-enzymatic electrochemical detection of hydrogen peroxide based on a novel “on-off-on” switch platform. Sci. Rep. 2016, 6, 25774. [Google Scholar] [CrossRef]
- Holt-Hindle, P.; Nigro, S.; Asmussen, M.; Chen, A. Amperometric glucose sensor based on platinum-iridium nanomaterials. Electrochem. Commun. 2008, 10, 1438–1441. [Google Scholar] [CrossRef]
- Dong, Q.; Song, D.; Huang, Y.; Xu, Z.; Chapman, J.H.; Willis, W.S.; Li, B.; Lei, Y. High-temperature annealing enabled iridium oxide nanofibers for both non-enzymatic glucose and solid-state pH sensing. Electrochim. Acta 2018, 281, 117–126. [Google Scholar] [CrossRef]
- Glebov, E.M.; Plyusnin, V.F.; Grivin, V.P.; Krupoder, S.A.; Liskovskaya, T.I.; Danilovich, V.S. Photochemistry of copper(II) polyfluorocarboxylates and copper(II) acetate as their hydrocarbon analogues. J. Photochem. Photobiol. A Chem. 2000, 133, 177–183. [Google Scholar] [CrossRef]
- Khan, M.; Bouet, G.; Vierling, F.; Meullemeestre, J.; Schwing, M.J. Formation of cobalt(II), nickel(II) and copper(II) chloro complexes in alcohols and the Irving-Williams order of stabilities. Transit. Met. Chem. 1996, 21, 231–234. [Google Scholar] [CrossRef]
- El-Khoury, P.Z.; Tarnovsky, A.N.; Schapiro, I.; Ryazantsev, M.N.; Olivucci, M. Structure of the photochemical reaction path populated via promotion of CF2I2 into its first excited state. J. Phys. Chem. A 2009, 113, 10767–10771. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumoto, A. Topics in Current Chemistry Editorial Board; Springer: Cham, Switzerland, 2005; ISBN 9783540733461. [Google Scholar]
- Xu, Y.; Zhang, B. Recent advances in porous Pt-based nanostructures: Synthesis and electrochemical applications. Chem. Soc. Rev. 2014, 43, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Dolinska, J.; Kannan, P.; Sobczak, J.W.; Opallo, M. Glucose Electrooxidation in Bimetallic Suspensions of Nanoparticles in Alkaline Media. ChemElectroChem 2015, 2, 1199–1205. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, Y.; Kwon, Y.; Han, S.W. Noble-Metal Nanocrystals with Controlled Facets for Electrocatalysis. Chem.-Asian J. 2016, 11, 2224–2239. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Q.; Yuan, M.; Guo, X.; Zheng, S.; Pang, H. Synthesis of Co0.5Mn0.1Ni0.4C2O4·n H2O Micropolyhedrons: Multimetal Synergy for High-Performance Glucose Oxidation Catalysis. Chem.—Asian J. 2019, 14, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Pasta, M.; La Mantia, F.; Cui, Y. Mechanism of glucose electrochemical oxidation on gold surface. Electrochim. Acta 2010, 55, 5561–5568. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, J.B.; Nguyen, Q.N.; Lee, J.M.; Park, S.; Chung, T.D.; Byun, J.Y. Nanoporous platinum thin films synthesized by electrochemical dealloying for nonenzymatic glucose detection. Phys. Chem. Chem. Phys. 2013, 15, 5782–5787. [Google Scholar] [CrossRef]
- Shen, J.; Dudik, L.; Liu, C.C. An iridium nanoparticles dispersed carbon based thick film electrochemical biosensor and its application for a single use, disposable glucose biosensor. Sens. Actuators B Chem. 2007, 125, 106–113. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, C.; Song, J.; Engelhard, M.; Xia, H.; Du, D.; Lin, Y. PdCuPt Nanocrystals with Multibranches for Enzyme-Free Glucose Detection. ACS Appl. Mater. Interfaces 2016, 8, 22196–22200. [Google Scholar] [CrossRef]
- Hsu, C.W.; Su, F.C.; Peng, P.Y.; Young, H.T.; Liao, S.; Wang, G.J. Highly sensitive non-enzymatic electrochemical glucose biosensor using a photolithography fabricated micro/nano hybrid structured electrode. Sens. Actuators B Chem. 2016, 230, 559–565. [Google Scholar] [CrossRef]
- Qiu, H.; Huang, X. Effects of Pt decoration on the electrocatalytic activity of nanoporous gold electrode toward glucose and its potential application for constructing a nonenzymatic glucose sensor. J. Electroanal. Chem. 2010, 643, 39–45. [Google Scholar] [CrossRef]
- Singh, B.; Dempsey, E.; Dickinson, C.; Laffir, F. Inside/outside Pt nanoparticles decoration of functionalised carbon nanofibers (Pt19.2/f-CNF80.8) for sensitive non-enzymatic electrochemical glucose detection. Analyst 2012, 137, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, D.; Xu, C. Nonenzymatic electrochemical sensor for glucose based on nanoporous platinum-gold alloy. J. Nanosci. Nanotechnol. 2016, 16, 7145–7150. [Google Scholar] [CrossRef]


| Component | Concentration (mM) | Solvent |
|---|---|---|
| tris(2-phenylpyridine)iridium(III) | 3 | DMF |
| chloro(triphenylphosphine)gold(I) | 3 | DMF |
| dichloro(dicyclopentadienyl)platinum(II) | 3 | DMF |
| Electrode Material | Sensitivity (μA/mM × cm2) | Linear Range (mM) | Limit of Detection (μM) | Ref. |
|---|---|---|---|---|
| This work (Ir-Au-Pt) | 9960 | 0.0005–1 | 0.12 | - |
| IrO2 NFs Nafion/GCE | 22.22 | 0–16 | 2.93 | [28] |
| Ir-carbon | 48.83 | 0–50 | 28,000 | [39] |
| Pt-Ir | 93.7 | 0–10 | N/A | [27] |
| Nanoporous Pt | 10.0 | 1–10 | 50 | [40] |
| Pt NP | 22.7 | 0–10 | 0.42 | [44] |
| Pd-Cu-Pt | 553 | 1–10 | 1.29 | [41] |
| Pt-Au nanoporous film | 12.85 | 0.2–4.8 | 1.3 | [45] |
| Pt-nanoporous Au | 145.7 | 0.5–10 | 0.6 | [43] |
| Au nanocorals | 22.6 | 0.0005–0.3 | 10 | [7] |
| Au NP film | 749.2 | 0.0556–13.89 | 9 | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
S. Panov, M.; M. Khairullina, E.; S. Vshivtcev, F.; N. Ryazantsev, M.; I. Tumkin, I. Laser-Induced Synthesis of Composite Materials Based on Iridium, Gold and Platinum for Non-Enzymatic Glucose Sensing. Materials 2020, 13, 3359. https://doi.org/10.3390/ma13153359
S. Panov M, M. Khairullina E, S. Vshivtcev F, N. Ryazantsev M, I. Tumkin I. Laser-Induced Synthesis of Composite Materials Based on Iridium, Gold and Platinum for Non-Enzymatic Glucose Sensing. Materials. 2020; 13(15):3359. https://doi.org/10.3390/ma13153359
Chicago/Turabian StyleS. Panov, Maxim, Evgeniia M. Khairullina, Filipp S. Vshivtcev, Mikhail N. Ryazantsev, and Ilya I. Tumkin. 2020. "Laser-Induced Synthesis of Composite Materials Based on Iridium, Gold and Platinum for Non-Enzymatic Glucose Sensing" Materials 13, no. 15: 3359. https://doi.org/10.3390/ma13153359
APA StyleS. Panov, M., M. Khairullina, E., S. Vshivtcev, F., N. Ryazantsev, M., & I. Tumkin, I. (2020). Laser-Induced Synthesis of Composite Materials Based on Iridium, Gold and Platinum for Non-Enzymatic Glucose Sensing. Materials, 13(15), 3359. https://doi.org/10.3390/ma13153359