Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors
Abstract
:1. Introduction
2. Chemiresistive Gas Sensors
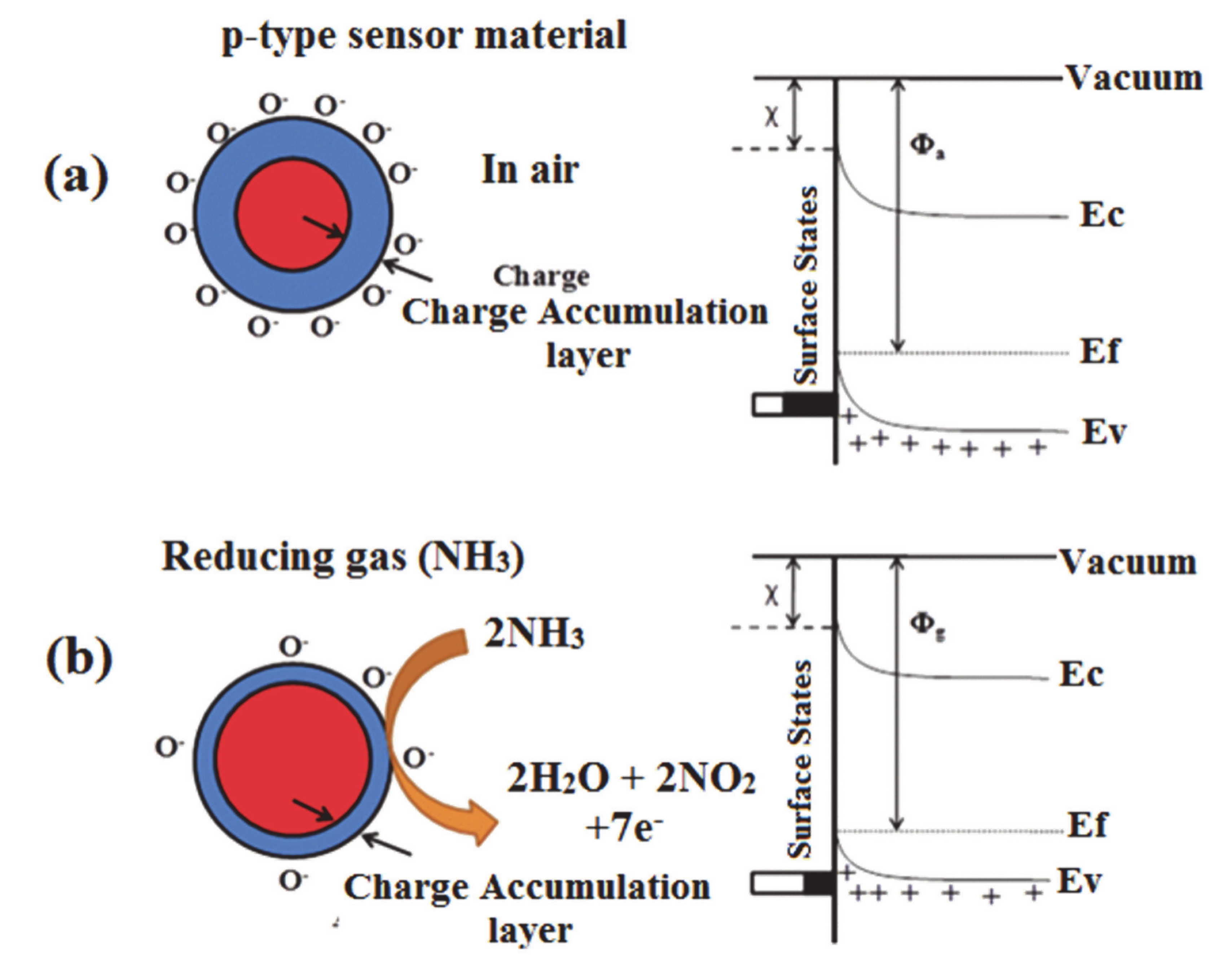
2.1. The Detection Mechanism of Chemiresistive Sensors
2.2. Gas-Sensing Performance Parameters
2.3. Sensing Material
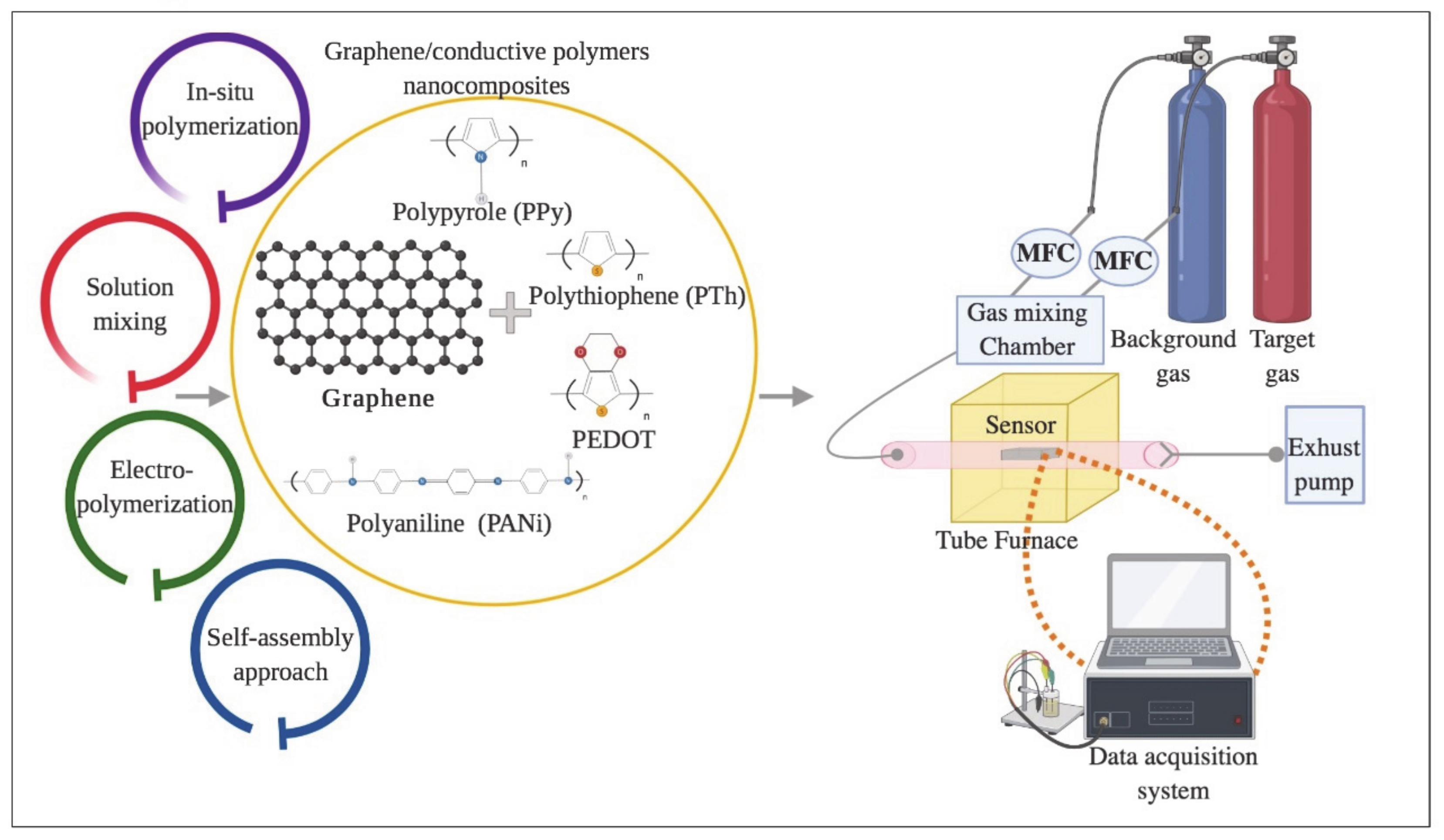
3. Graphene/Conducting Polymer Nanocomposites for Chemiresistive Gas Sensor Application
3.1. Graphene/Polyaniline (PANi) Nanocomposites
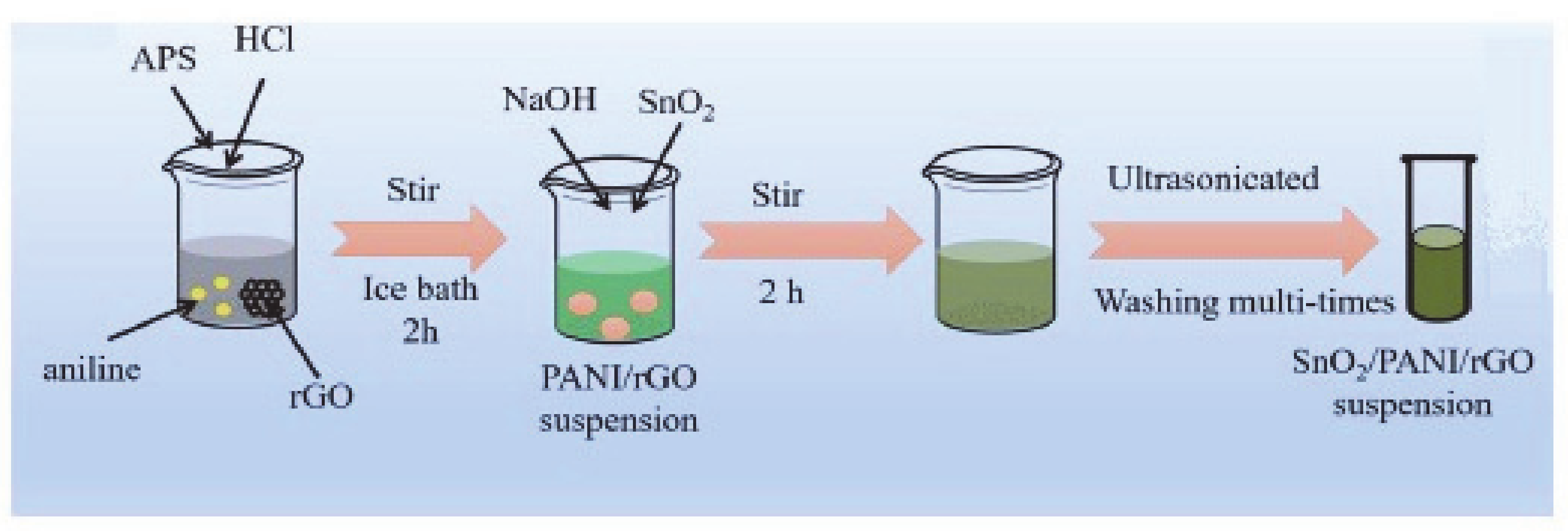
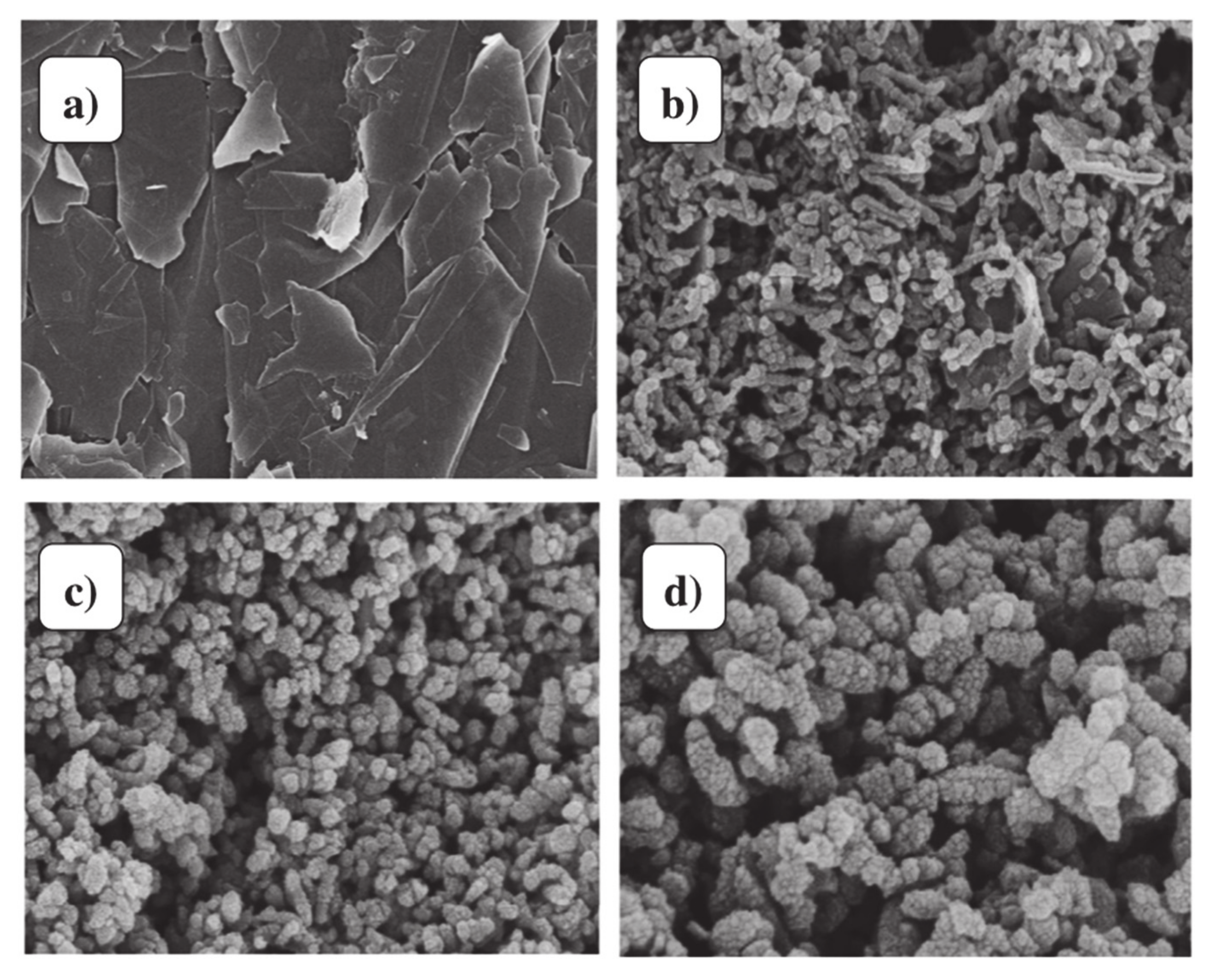
3.1.1. Preparation of Graphene/Polyaniline Nanocomposites
3.1.2. The Sensing Performance of Gas Sensors Based on Graphene/PANi Nanocomposites
3.2. Graphene/Poly (3,4 Ethyldioxythiophene) (PEDOT) Nanocomposite
3.2.1. Preparation of Graphene/PEDOT Nanocomposite
3.2.2. The Sensing Performance of Gas Sensors Based on Graphene/PEDOT Nanocomposites
3.3. Graphene/Polypyrrole (PPy) Nanocomposites
3.3.1. Preparation of Graphene/PPy Nanocomposites
3.3.2. The Sensing Performance of Gas Sensors Based on Graphene/PPy Nanocomposites
3.4. Preparation and Sensing Performance of Gas Sensors Based on Other Graphene-Based Polymer Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. TrAC Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Arsat, R.; Breedon, M.; Shafiei, M.; Spizziri, P.; Gilje, S.; Kaner, R.; Kalantar-Zadeh, K.; Wlodarski, W. Graphene-like nano-sheets for surface acoustic wave gas sensor applications. Chem. Phys. Lett. 2009, 467, 344–347. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078. [Google Scholar] [CrossRef]
- Olsen, T.; Yan, J.; Mortensen, J.J.; Thygesen, K.S. Dispersive and Covalent Interactions between Graphene and Metal Surfaces from the Random Phase Approximation. Phys. Rev. Lett. 2011, 107, 156401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abergel, D.; Apalkov, V.; Berashevich, J.; Ziegler, K.; Chakraborty, T. Properties of graphene: a theoretical perspective. Adv. Phys. 2010, 59, 261–482. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Li, Z.; Liu, Z.; Zhou, G.; Hao, S.; Wu, J.; Gu, B.-L.; Duan, W. Adsorption of Gas Molecules on Graphene Nanoribbons and Its Implication for Nanoscale Molecule Sensor. J. Phys. Chem. C 2008, 112, 13442–13446. [Google Scholar] [CrossRef] [Green Version]
- Ko, G.; Kim, H.-Y.; Ahn, J.; Park, Y.-M.; Lee, K.-Y.; Kim, J. Graphene-based nitrogen dioxide gas sensors. Curr. Appl. Phys. 2010, 10, 1002–1004. [Google Scholar] [CrossRef]
- Hill, E.; Vijayaraghavan, A.; Novoselov, K.S. Graphene Sensors. IEEE Sensors J. 2011, 11, 3161–3170. [Google Scholar] [CrossRef]
- Wehling, T.O.; Novoselov, K.S.; Morozov, S.V.; Vdovin, E.E.; Katsnelson, M.I.; Geim, A.K.; Lichtenstein, A.I. Molecular Doping of Graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Wang, Y.; Chai, J.; Gao, R.; Yang, Z.; Kong, E.S.-W.; Zhang, Y. Gas sensor based on p-phenylenediamine reduced graphene oxide. Sensors Actuators B: Chem. 2012, 163, 107–114. [Google Scholar] [CrossRef]
- Meng, F.; Guo, Z.; Huang, X.-J. Graphene-based hybrids for chemiresistive gas sensors. TrAC Trends Anal. Chem. 2015, 68, 37–47. [Google Scholar] [CrossRef]
- Dong, L.; Chen, Z.; Zhao, X.; Ma, J.; Lin, S.; Li, M.; Bao, Y.; Chu, L.; Leng, K.; Lu, H.; et al. A non-dispersion strategy for large-scale production of ultra-high concentration graphene slurries in water. Nat. Commun. 2018, 9, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef]
- Vaia, R.A.; Wagner, H.D. Framework for nanocomposites. Mater. Today 2004, 7, 32–37. [Google Scholar] [CrossRef]
- Verdejo, R.; Bernal, M.M.; Romasanta, J.L.; Lopez-Manchado, M.A. Graphene filled polymer nanocomposites. J. Mater. Chem. 2011, 21, 3301–3310. [Google Scholar] [CrossRef] [Green Version]
- Galpaya, D.; Wang, M.; Liu, M.; Motta, N.; Waclawik, E.; Yan, C. Recent Advances in Fabrication and Characterization of Graphene-Polymer Nanocomposites. Graphene 2012, 1, 30–49. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.; Si, W.; Xu, Y.; Gu, Z.; Hao, Q. Conducting polymer composites with graphene for use in chemical sensors and biosensors. Microchim. Acta 2014, 181, 707–722. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.S. Conducting Polymers Directly Coated on Reduced Graphene Oxide Sheets as High-Performance Supercapacitor Electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Y.; Su, Z.; Wei, G. Recent advances in the synthesis and applications of graphene–polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A Review on Graphene-Based Gas/Vapor Sensors with Unique Properties and Potential Applications. Nano-Micro Lett. 2015, 8, 95–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Mitra, A.; Mukhopadhyay, S. Graphene and its sensor-based applications: A review. Sensors Actuators A: Phys. 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sensors Actuators B: Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.; Baharuddin, A.A.; Wong, Y.H. Review—Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. J. Electrochem. Soc. 2019, 167, 037503. [Google Scholar] [CrossRef]
- Hur, J.; Park, S.; Bae, J. Elaborate Chemical Sensors Based on Graphene/Conducting Polymer Hybrids. Curr. Org. Chem. 2015, 19, 1117–1133. [Google Scholar] [CrossRef]
- Arafat, M.M.; Haseeb, A.; Akbar, S.; Quadir, Z. In-situ fabricated gas sensors based on one dimensional core-shell TiO2-Al2O3 nanostructures. Sensors Actuators B: Chem. 2017, 238, 972–984. [Google Scholar] [CrossRef]
- Basu, S.; Bhattacharyya, P. Recent developments on graphene and graphene oxide based solid state gas sensors. Sensors Actuators B: Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Wang, J.; Adelodun, A.A.; Oh, J.M.; Jo, Y.-M. TEPA impregnation of electrospun carbon nanofibers for enhanced low-level CO2 adsorption. Nano Converg. 2020, 7, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Tripathi, S.N.; Choudhary, V.; Gupta, B.D. SPR based fibre optic ammonia gas sensor utilizing nanocomposite film of PMMA/reduced graphene oxide prepared by in situ polymerization. Sensors Actuators B: Chem. 2014, 199, 190–200. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Chen, F.; Sun, X.; Wan, P. Hierarchical Graphene–Polyaniline Nanocomposite Films for High-Performance Flexible Electronic Gas Sensors. Nanoscale 2016, 8, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Dua, V.; Surwade, S.P.; Ammu, S.; Agnihotra, S.R.; Jain, S.; Roberts, K.E.; Park, S.; Ruoff, R.S.; Manohar, S.K. All-Organic Vapor Sensor Using Inkjet-Printed Reduced Graphene Oxide. Angew. Chem. Int. Ed. 2010, 49, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [Green Version]
- Eisele, I.; Doll, T.; Burgmair, M. Low power gas detection with FET sensors. Sensors Actuators B: Chem. 2001, 78, 19–25. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, J.; Im, J.P.; Lim, S.Y.; Kwon, J.Y.; Moon, S.E. MEMS-based NO2 gas sensor using ZnO nano-rods for low-power IoT application. J. Korean Phys. Soc. 2017, 70, 924–928. [Google Scholar] [CrossRef]
- Qazi, M.; Vogt, T.; Koley, G. Trace gas detection using nanostructured graphite layers. Appl. Phys. Lett. 2007, 91, 233101. [Google Scholar] [CrossRef]
- Bănică, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Aswal, D.K.; Gupta, S.K. Science and Technology of Chemiresistor Gas Sensors; Nova Publishers: Hauppauge, NY, USA, 2007. [Google Scholar]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sensors Actuators B: Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Miasik, J.J.; Hooper, A.; Tofield, B.C. Conducting polymer gas sensors. J. Chem. Soc. Faraday Trans. 1: Phys. Chem. Condens. Phases 1986, 82, 1117. [Google Scholar] [CrossRef]
- Hangarter, C.; Chartuprayoon, N.; Hernández, S.C.; Choa, Y.-H.; Myung, N.V. Hybridized conducting polymer chemiresistive nano-sensors. Nano Today 2013, 8, 39–55. [Google Scholar] [CrossRef]
- Miller, D.; Akbar, S.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sensors Actuators B: Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Tanguy, N.R.; Wiltshire, B.; Arjmand, M.; Zarifi, M.H.; Yan, N. Highly Sensitive and Contactless Ammonia Detection Based on Nanocomposites of Phosphate-Functionalized Reduced Graphene Oxide/Polyaniline Immobilized on Microstrip Resonators. ACS Appl. Mater. Interfaces 2020, 12, 9746–9754. [Google Scholar] [CrossRef]
- Jin, L.; Chen, W.; Zhang, Y. Application of Graphene Hybrid Materials in Fault Characteristic Gas Detection of Oil-Immersed Equipment. Front. Chem. 2018, 6, 399. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor Gas Sensors: Dry Synthesis and Application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef]
- Pandey, S.; Nanda, K. One-dimensional nanostructure based chemiresistor sensor. Nanotechnology 2013, 10, 1–17. [Google Scholar]
- Balamurugan, C.; Song, S.-J.; Kim, H.-S. Enhancing Gas Response Characteristics of Mixed Metal Oxide Gas Sensors. J. Korean Ceram. Soc. 2018, 55, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, H.; Chen, J.; Jing, Q.; Zhou, Y.; Wen, X.; Wang, Z.L. Single-Electrode-Based Sliding Triboelectric Nanogenerator for Self-Powered Displacement Vector Sensor System. ACS Nano 2013, 7, 7342–7351. [Google Scholar] [CrossRef]
- Khani, H.; Rofouei, M.K.; Arab, P.; Gupta, V.K.; Vafaei, Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion(II). J. Hazard. Mater. 2010, 183, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Tomchenko, A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting metal oxide sensor array for the selective detection of combustion gases. Sensors Actuators B: Chem. 2003, 93, 126–134. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Linb, Y. A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta 2010, 81, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline Nanofiber Gas Sensors: Examination of Response Mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon Nanotube Sensors for Gas and Organic Vapor Detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Kanan, S.M.; El-Kadri, O.M.; Abu-Yousef, I.A.; Kanan, M.C. Semiconducting Metal Oxide Based Sensors for Selective Gas Pollutant Detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef] [Green Version]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [Green Version]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide Materials for Development of Integrated Gas Sensors—A Comprehensive Review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Govardhan, K.; Grace, A.N. Metal/Metal Oxide Doped Semiconductor Based Metal Oxide Gas Sensors—A Review. Sens. Lett. 2016, 14, 741–750. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D. Development of nanocrystalline TiO2–Er2O3 and TiO2–Ta2O5 thin film gas sensors: Controlling the physical and sensing properties. Sensors Actuators B: Chem. 2009, 141, 76–84. [Google Scholar] [CrossRef]
- Yavari, F.; Chen, Z.; Thomas, A.V.; Ren, W.; Cheng, H.-M.; Koratkar, N. High Sensitivity Gas Detection Using a Macroscopic Three-Dimensional Graphene Foam Network. Sci. Rep. 2011, 1, 166. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Nylander, C.; Armgarth, M.; Lundström, I. An ammonia detector based on a conducting polymer. In Proceedings of the International Meeting on Chemical Sensors, Fukuoka, Japan, 19–22 September 1983; pp. 203–207. [Google Scholar]
- Zakrzewska, K. Mixed oxides as gas sensors. Thin Solid Films 2001, 391, 229–238. [Google Scholar] [CrossRef]
- Dubbe, A. Fundamentals of solid state ionic micro gas sensors. Sensors Actuators B: Chem. 2003, 88, 138–148. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Berg, A.V.D. Ammonia sensors and their applications—a review. Sensors Actuators B: Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Lee, Y.R.; Raghu, A.V.; Jeong, H.M.; Kim, B.K. Properties of Waterborne Polyurethane/Functionalized Graphene Sheet Nanocomposites Prepared by an in situ Method. Macromol. Chem. Phys. 2009, 210, 1247–1254. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Liang, J.; Huang, Y.; Ma, Y.; Wan, X.; Chen, Y. A hybrid material of graphene and poly (3,4-ethyldioxythiophene) with high conductivity, flexibility, and transparency. Nano Res. 2009, 2, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Quan, H.; Zhang, B.; Zhao, Q.; Yuen, K.K.R.; Li, R.K. Facile preparation and thermal degradation studies of graphite nanoplatelets (GNPs) filled thermoplastic polyurethane (TPU) nanocomposites. Compos. Part A: Appl. Sci. Manuf. 2009, 40, 1506–1513. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Shin, K.; Kalantar-Zadeh, K.; Plessis, J.D.; Han, S.H.; Kojima, R.W.; Kaner, R.B.; Li, D.; Gou, X.; Ippolito, S.J.; et al. Graphene/Polyaniline Nanocomposite for Hydrogen Sensing. J. Phys. Chem. C 2010, 114, 16168–16173. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, D.; Koo, H.Y.; Maeng, S. Chemically modified graphene/PEDOT:PSS nanocomposite films for hydrogen gas sensing. Carbon 2015, 81, 54–62. [Google Scholar] [CrossRef]
- Xiang, C.; Jiang, D.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Sun, L.; Zheng, L. Ammonia sensor based on polypyrrole–graphene nanocomposite decorated with titania nanoparticles. Ceram. Int. 2015, 41, 6432–6438. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sensors Actuators B: Chem. 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Dehsari, H.S.; Hasani, A.; Mahyari, M.; Shalamzari, E.K.; Salehi, A.; Afshar-Taromi, F.; Gavgani, J.N. Copper( ii ) phthalocyanine supported on a three-dimensional nitrogen-doped graphene/PEDOT-PSS nanocomposite as a highly selective and sensitive sensor for ammonia detection at room temperature. RSC Adv. 2015, 5, 79729–79737. [Google Scholar] [CrossRef]
- Yong, Y.-C.; Dong, X.-C.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and Monolithic Anode Based on Polyaniline Hybridized Three-Dimensional Graphene for High-Performance Microbial Fuel Cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Shao, B.; Fan, Z.; Qian, W.; Zhang, M.; Wei, F. Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance. Carbon 2010, 48, 487–493. [Google Scholar] [CrossRef]
- Radhapyari, K.; Kotoky, P.; Das, M.R.; Khan, R. Graphene–polyaniline nanocomposite based biosensor for detection of antimalarial drug artesunate in pharmaceutical formulation and biological fluids. Talanta 2013, 111, 47–53. [Google Scholar] [CrossRef]
- Tabr, F.A.; Salehiravesh, F.; Adelnia, H.; Gavgani, J.N.; Mahyari, M.; Tabar, F.A. High sensitivity ammonia detection using metal nanoparticles decorated on graphene macroporous frameworks/polyaniline hybrid. Talanta 2019, 197, 457–464. [Google Scholar] [CrossRef]
- Ma, B.; Zhou, X.; Bao, H.; Li, X.; Wang, G. Hierarchical composites of sulfonated graphene-supported vertically aligned polyaniline nanorods for high-performance supercapacitors. J. Power Sources 2012, 215, 36–42. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors Based on Flexible Graphene/Polyaniline Nanofiber Composite Films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-F.; Zhang, H.; Liu, Q.; Sun, L.; Stanciu, L.; Xie, J. Fabrication of High-Surface-Area Graphene/Polyaniline Nanocomposites and Their Application in Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, C.; Tjiu, W.W.; Pramoda, K.P.; He, C.; Liu, T. Graphene-Wrapped Polyaniline Hollow Spheres As Novel Hybrid Electrode Materials for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2013, 5, 3382–3391. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Huang, K.-C.; Chung, P.-H.; Wang, C.-C.; Chen, C.-Y.; Vittal, R.; Wu, C.-G.; Chiu, W.-Y.; Ho, K.-C. Graphene-modified polyaniline as the catalyst material for the counter electrode of a dye-sensitized solar cell. J. Power Sources 2012, 217, 152–157. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Lei, S.; Song, Y. Graphene-based polyaniline nanocomposites: preparation, properties and applications. J. Mater. Chem. A 2014, 2, 4491–4509. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, W.; Yuan, W.; Xu, J.; Jiang, Y. In Situ Polymerization Deposition of Porous Conducting Polymer on Reduced Graphene Oxide for Gas Sensor. ACS Appl. Mater. Interfaces 2014, 6, 13807–13814. [Google Scholar] [CrossRef]
- Verma, D.; Goh, K.L. Functionalized Graphene-Based Nanocomposites for Energy Applications; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 219–243. [Google Scholar]
- Verdejo, R.; Tapiador, F.J.; Helfen, L.; Bernal, M.M.; Bitinis, N.; Lopez-Manchado, M.A. Fluid dynamics of evolving foams. Phys. Chem. Chem. Phys. 2009, 11, 10860. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Lam, K.-H.; Fan, J.T.; Chan, L.H. Improved dielectric properties for chemically functionalized exfoliated graphite nanoplates/syndiotactic polystyrene composites prepared by a solution-blending method. Carbon 2014, 80, 496–503. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Ramedani, A.; Abrishamkar, A.; Milan, P.B.; Moghadan, Z.S.; Chauhan, N.P.S.; Sefat, F.; Mozafari, M. Synthetic route of PANI (V): Electrochemical polymerization; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 105–119. [Google Scholar]
- Gao, C.; Li, W.; Morimoto, H.; Nagaoka, Y.; Maekawa, T. Magnetic Carbon Nanotubes: Synthesis by Electrostatic Self-Assembly Approach and Application in Biomanipulations. J. Phys. Chem. B 2006, 110, 7213–7220. [Google Scholar] [CrossRef]
- Smith, K.H.; Tejeda-Montes, E.; Poch, M.; Mata, A. Integrating top-down and self-assembly in the fabrication of peptide and protein-based biomedical materials. Chem. Soc. Rev. 2011, 40, 4563–4577. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Q.; Huang, X.; Bai, H.; Wang, J.-J. A self-assembly route to porous polyaniline/reduced graphene oxide composite materials with molecular-level uniformity for high-performance supercapacitors. Energy Environ. Sci. 2018, 11, 1280–1286. [Google Scholar] [CrossRef]
- Tjong, S.C. Polymer composites with graphene nanofillers: electrical properties and applications. J. Nanosci. Nanotechnol. 2014, 14, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hu, N.; Zhang, L.; Wei, L.; Wei, H.; Zhang, Y. The NH3 sensing properties of gas sensors based on aniline reduced graphene oxide. Synth. Met. 2013, 185, 25–30. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Room Temperature Methane Sensor Based on Graphene Nanosheets/Polyaniline Nanocomposite Thin Film. IEEE Sensors J. 2012, 13, 777–782. [Google Scholar] [CrossRef]
- Bai, S.; Zhao, Y.; Sun, J.; Tian, Y.; Luo, R.; Li, D.; Chen, A. Ultrasensitive room temperature NH 3 sensor based on a graphene–polyaniline hybrid loaded on PET thin film. Chem. Commun. 2015, 51, 7524–7527. [Google Scholar] [CrossRef]
- Huang, X.L.; Hu, N.T.; Wang, Y.Y.; Zhang, Y.F. Ammonia Gas Sensor Based on Aniline Reduced Graphene Oxide. Adv. Mater. Res. 2013, 669, 79–84. [Google Scholar] [CrossRef]
- Konwer, S.; Guha, A.K.; Dolui, S.K. Graphene oxide-filled conducting polyaniline composites as methanol-sensing materials. J. Mater. Sci. 2012, 48, 1729–1739. [Google Scholar] [CrossRef]
- Al-Hartomy, O.A.; Khasim, S.; Roy, A.; Pasha, A. Highly conductive polyaniline/graphene nano-platelet composite sensor towards detection of toluene and benzene gases. Appl. Phys. A 2018, 125, 12. [Google Scholar] [CrossRef]
- Lee, C.-T.; Wang, Y.-S. High-performance room temperature NH3 gas sensors based on polyaniline-reduced graphene oxide nanocomposite sensitive membrane. J. Alloy. Compd. 2019, 789, 693–696. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Hasani, A.; Nouri, M.; Mahyari, M.; Salehi, A. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sensors Actuators B: Chem. 2016, 229, 239–248. [Google Scholar] [CrossRef]
- Hakimi, M.; Salehi, A.; Boroumand, F.; Mosleh, N. Fabrication of a Room Temperature Ammonia Gas Sensor Based on Polyaniline With N-Doped Graphene Quantum Dots. IEEE Sensors J. 2018, 18, 2245–2252. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Tai, H.; Guo, N.; Xie, G.; Yuan, Z. The investigation of reduced graphene oxide@ SnO2–polyaniline composite thin films for ammonia detection at room temperature. J. Mater. Sci. Mater. Electron. 2014, 26, 833–841. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Navazani, S.; Shokuhfar, A.; Hassanisadi, M.; Di Carlo, A.; Shahcheraghi, N. Fabrication and characterization of a sensitive, room temperature methane sensor based on SnO2@reduced graphene oxide-polyaniline ternary nanohybrid. Mater. Sci. Semicond. Process. 2018, 88, 139–147. [Google Scholar] [CrossRef]
- Tian, J.; Su, F.; Zhang, Z.; Yang, G.; Jiang, D. A hybrid material consisting of bulk-reduced TiO2, graphene oxide and polyaniline for resistance based sensing of gaseous ammonia at room temperature. Microchim. Acta 2016, 183, 2871–2878. [Google Scholar] [CrossRef]
- An, J.; Liu, J.; Zhou, Y.; Zhao, H.; Ma, Y.; Li, M.; Yu, M.; Li, S. Polyaniline-Grafted Graphene Hybrid with Amide Groups and Its Use in Supercapacitors. J. Phys. Chem. C 2012, 116, 19699–19708. [Google Scholar] [CrossRef]
- Ansari, M.O.; Yadav, S.K.; Cho, J.W.; Mohammad, F. Thermal stability in terms of DC electrical conductivity retention and the efficacy of mixing technique in the preparation of nanocomposites of graphene/polyaniline over the carbon nanotubes/polyaniline. Compos. Part B: Eng. 2013, 47, 155–161. [Google Scholar] [CrossRef]
- Parmar, M.; Balamurugan, C.; Lee, D.-W. PANI and Graphene/PANI Nanocomposite Films — Comparative Toluene Gas Sensing Behavior. Sensors 2013, 13, 16611–16624. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Yang, Z.; He, J.; Wang, J.; Zhao, L.; Lu, H.; Tian, T.; Liu, F.; Sun, P.; et al. Room temperature high performance NH3 sensor based on GO-rambutan-like polyaniline hollow nanosphere hybrid assembled to flexible PET substrate. Sensors Actuators B: Chem. 2018, 273, 726–734. [Google Scholar] [CrossRef]
- Huang, X.; Hu, N.; Gao, R.; Yu, Y.; Wang, Y.; Yang, Z.; Kong, E.S.-W.; Wei, H.; Zhang, Y. Reduced graphene oxide–polyaniline hybrid: Preparation, characterization and its applications for ammonia gas sensing. J. Mater. Chem. 2012, 22, 22488–22495. [Google Scholar] [CrossRef]
- Ly, T.N.; Park, S. Highly sensitive ammonia sensor for diagnostic purpose using reduced graphene oxide and conductive polymer. Sci. Rep. 2018, 8, 18030. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Yang, W.; Li, S.; Xu, J.; Jiang, Y. Porous conducting polymer and reduced graphene oxide nanocomposites for room temperature gas detection. RSC Adv. 2014, 4, 42546–42553. [Google Scholar] [CrossRef]
- Han, T.H.; Huang, Y.-K.; Tan, A.T.; Dravid, V.P.; Huang, J. Steam Etched Porous Graphene Oxide Network for Chemical Sensing. J. Am. Chem. Soc. 2011, 133, 15264–15267. [Google Scholar] [CrossRef] [PubMed]
- Dunst, K.; Jurkow, D.; Jasiński, P. Laser patterned platform with PEDOT–graphene composite film for NO 2 sensing. Sensors Actuators B: Chem. 2016, 229, 155–165. [Google Scholar] [CrossRef]
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-cost and flexible printed graphene–PEDOT:PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Timpanaro, S.; Kemerink, M.; Touwslager, F.; De Kok, M.; Schrader, S. Morphology and conductivity of PEDOT/PSS films studied by scanning–tunneling microscopy. Chem. Phys. Lett. 2004, 394, 339–343. [Google Scholar] [CrossRef]
- Nardes, A.; Kemerink, M.; De Kok, M.; Vinken, E.; Maturova, K.; Janssen, R. Conductivity, work function, and environmental stability of PEDOT:PSS thin films treated with sorbitol. Org. Electron. 2008, 9, 727–734. [Google Scholar] [CrossRef]
- Hakimi, M.; Salehi, A.; Boroumand, F. Fabrication and Characterization of an Ammonia Gas Sensor Based on PEDOT-PSS With N-Doped Graphene Quantum Dots Dopant. IEEE Sensors J. 2016, 16, 6149–6154. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, S.; Wan, J.; Tang, H.; Chang, L.; He, L.; Zhao, H.; Gao, Y.; Tang, Z. Three-Dimensional Graphene/Metal Oxide Nanoparticle Hybrids for High-Performance Capacitive Deionization of Saline Water. Adv. Mater. 2013, 25, 6270–6276. [Google Scholar] [CrossRef]
- Dunst, K.; Trzciński, K.; Scheibe, B.; Sawczak, M.; Jasiński, P. Study of the NO2 sensing mechanism of PEDOT-RGO film using in situ Raman Spectroscopy. Sensors Actuators B: Chem. 2018, 260, 1025–1033. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Ratinac, K.; Yang, W.; Gooding, J.J.; Thordarson, P.; Braet, F. Graphene and Related Materials in Electrochemical Sensing. Electroanalysis 2011, 23, 803–826. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Chen, Y.; Yu, P.; Wang, C.; Ma, Y.-W. Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors. J. Power Sources 2011, 196, 5990–5996. [Google Scholar] [CrossRef]
- Lin, W.-D.; Chang, H.-M.; Wu, R.-J. Applied novel sensing material graphene/polypyrrole for humidity sensor. Sensors Actuators B: Chem. 2013, 181, 326–331. [Google Scholar] [CrossRef]
- Ruangchuay, L.; Sirivat, A.; Schwank, J. Polypyrrole/poly(methylmethacrylate) blend as selective sensor for acetone in lacquer. Talanta 2003, 60, 25–30. [Google Scholar] [CrossRef]
- Sahoo, S.; Karthikeyan, G.; Nayak, G.C.; Das, C.K. Electrochemical characterization of in situ polypyrrole coated graphene nanocomposites. Synth. Met. 2011, 161, 1713–1719. [Google Scholar] [CrossRef]
- Naikoo, R.A.; Tomar, R. Fabrication of a novel Zeolite-X/Reduced graphene oxide/Polypyrrole nanocomposite and its role in sensitive detection of CO. Mater. Chem. Phys. 2018, 211, 225–233. [Google Scholar] [CrossRef]
- Tien, H.N.; Hur, S.H. One-step synthesis of a highly conductive graphene-polypyrrole nanofiber composite using a redox reaction and its use in gas sensors. Phys. Status solidi (RRL) - Rapid Res. Lett. 2012, 6, 379–381. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, S.; Fu, J. Examining the use of TiO2 to enhance the NH3 sensitivity of polypyrrole films. J. Appl. Polym. Sci. 2010, 118, 3351–3356. [Google Scholar] [CrossRef]
- Liu, N.; Luo, F.; Wu, H.; Liu, Y.; Zhang, C.; Chen, J. One-Step Ionic-Liquid-Assisted Electrochemical Synthesis of Ionic-Liquid-Functionalized Graphene Sheets Directly from Graphite. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Graphene-based Composite Thin Films for Electronics. Nano Lett. 2009, 9, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, X.; Wang, J.; Wan, L.; Liu, F.; Zheng, H.; Chen, R.; Xu, C. Preparation and properties of graphene nanosheets–polystyrene nanocomposites via in situ emulsion polymerization. Chem. Phys. Lett. 2010, 484, 247–253. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Hu, P.A.; Zhang, J.; Wang, L.; Feng, W.; Lei, S.; Yang, B.; Cao, W. Colorimetric Sensor Based on Self-Assembled Polydiacetylene/Graphene-Stacked Composite Film for Vapor-Phase Volatile Organic Compounds. Adv. Funct. Mater. 2013, 23, 6044–6050. [Google Scholar] [CrossRef]
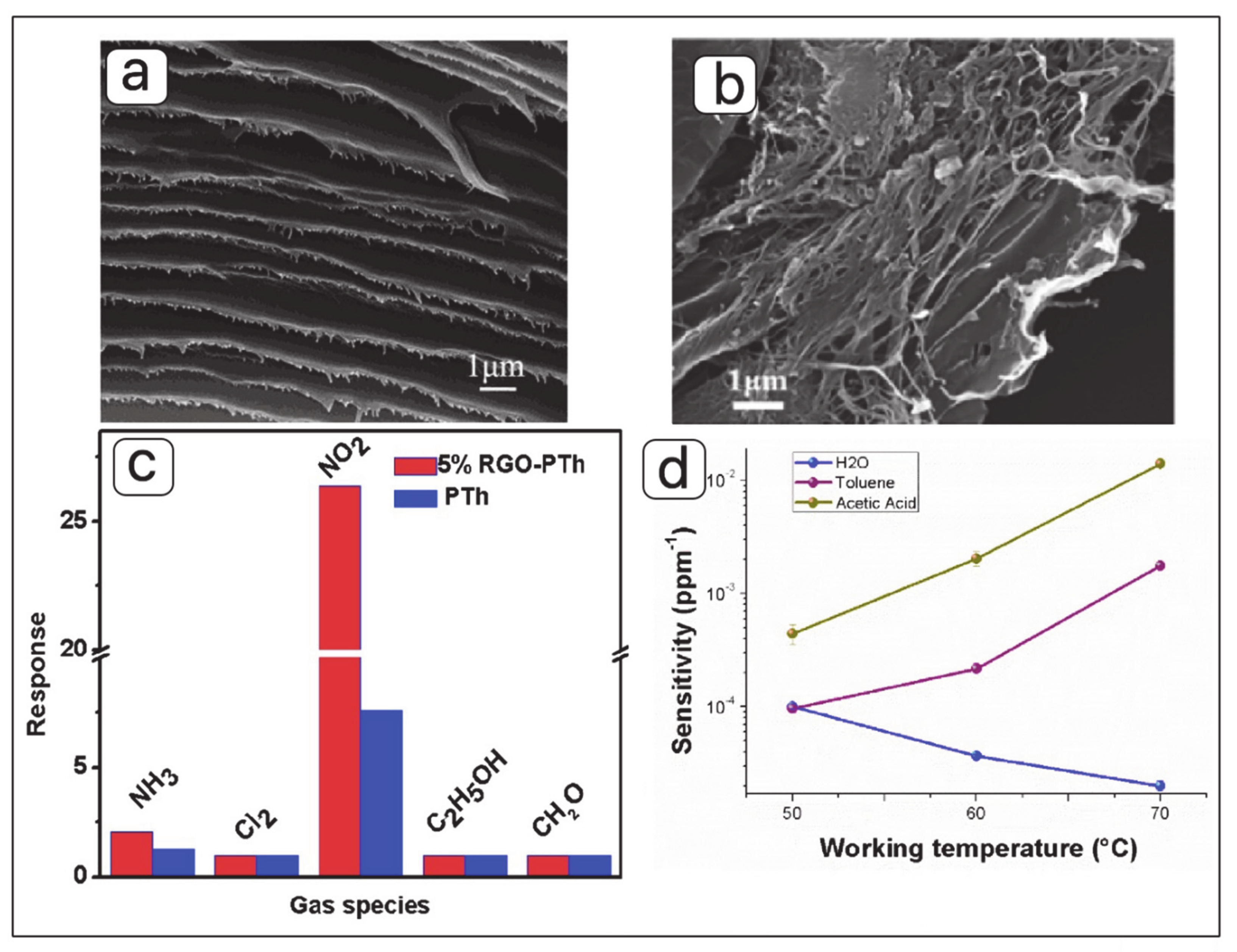
- Bai, S.; Guo, J.; Sun, J.; Tang, P.; Chen, A.; Luo, R.; Li, D. Enhancement of NO2-Sensing Performance at Room Temperature by Graphene-Modified Polythiophene. Ind. Eng. Chem. Res. 2016, 55, 5788–5794. [Google Scholar] [CrossRef]
- Avossa, J.; Paolesse, R.; Di Natale, C.; Zampetti, E.; Bertoni, G.; De Cesare, F.; Scarascia-Mugnozza, G.; Macagnano, A. Electrospinning of Polystyrene/Polyhydroxybutyrate Nanofibers Doped with Porphyrin and Graphene for Chemiresistor Gas Sensors. Nanomaterials 2019, 9, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dua, V.; Surwade, S.P.; Ammu, S.; Zhang, X.; Jain, S.; Manohar, S.K. Chemical Vapor Detection Using Parent Polythiophene Nanofibers. Macromolecules 2009, 42, 5414–5415. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Wang, S.; Guo, X.; Xia, H.; Wang, Y.; Zhang, S.; Huang, W.; Wu, S. Gas sensing properties of SnO2 hollow spheres/polythiophene inorganic–organic hybrids. Sensors Actuators B: Chem. 2010, 146, 8–13. [Google Scholar] [CrossRef]
- Navale, S.; Mane, A.; Khuspe, G.; Chougule, M.; Patil, V.B. Room temperature NO2 sensing properties of polythiophene films. Synth. Met. 2014, 195, 228–233. [Google Scholar] [CrossRef]
- Guo, X.-Z.; Kang, Y.; Yang, T.-L.; Wang, S.-R. Low-temperature NO2 sensors based on polythiophene/WO3 organic-inorganic hybrids. Trans. Nonferrous Met. Soc. China 2012, 22, 380–385. [Google Scholar] [CrossRef]
- Che, J.; Shen, L.; Xiao, Y. A new approach to fabricate graphene nanosheets in organic medium: combination of reduction and dispersion. J. Mater. Chem. 2010, 20, 1722. [Google Scholar] [CrossRef]
- Uyar, T.; Besenbacher, F. Electrospinning of uniform polystyrene fibers: The effect of solvent conductivity. Polymers 2008, 49, 5336–5343. [Google Scholar] [CrossRef]
- Bychuk, M.A.; Kil’Deeva, N.R.; Kurinova, M.A.; Bogdanov, N.V.; Kalinin, M.V.; Novikov, A.V.; Vikhoreva, G.A. Electrospinning of Biodegradable Polymer Scaffolds. Fibre Chem. 2015, 46, 345–348. [Google Scholar] [CrossRef]
- Ranola, R.A.; Ferroni, M.; Sangaletti, L.; Comini, E.; Concina, I.; Sevilla, F.B.; Sberveglieri, G. Room temperature trimethylamine gas sensor based on aqueous dispersed graphene. In Proceedings of the 2015 XVIII AISEM Annual Conference, Trento, Italy, 3–5 February 2015; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2015; pp. 1–4. [Google Scholar]
- Popov, A.; Brasiunas, B.; Mikoliunaite, L.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. Comparative study of polyaniline (PANI), poly(3,4-ethylenedioxythiophene) (PEDOT) and PANI-PEDOT films electrochemically deposited on transparent indium thin oxide based electrodes. Polymers 2019, 172, 133–141. [Google Scholar] [CrossRef]
- Yoon, H. Current Trends in Sensors Based on Conducting Polymer Nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [Green Version]
- Rajesh; Ahuja, T.; Kumar, D. Recent progress in the development of nano-structured conducting polymers/nanocomposites for sensor applications. Sensors Actuators B: Chem. 2009, 136, 275–286. [Google Scholar] [CrossRef]


| Year of Publication | Title of Paper | Main Emphasis | References |
|---|---|---|---|
| 2019 | Review—Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review | Conducting polymers | [28] |
| 2018 | A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. | 2D transition metal dichalcogenides, metal oxide nanomaterials, and graphene | [24] |
| 2018 | Research progress of gas sensor based on graphene and its derivatives: a review. | Graphene and its derivatives | [25] |
| 2018 | Graphene and its sensor-based applications: A review. | Graphene and its application in 3 different sensor applications such as electrochemical, strain and electrical sensors | [26] |
| 2017 | Chemo-electrical gas sensors based on conducting polymer hybrids. | Conducting polymers and conducting polymer hybrids for chemo-electrical gas sensors | [21] |
| 2016 | A review on graphene-based gas/vapor sensors with unique properties and potential applications. | Graphene | [23] |
| 2015 | Graphene–metal oxide nanohybrids for toxic gas sensor: A review. | Graphene and metal oxide hybrids | [27] |
| 2015 | Graphene-based hybrids for chemiresistive gas sensors | Graphene and graphene-based hybrids | [11] |
| 2015 | Elaborate chemical sensors based on graphene/conducting polymer hybrids. | Graphene and conducting polymer hybrids for chemical sensors | [29] |
| 2014 | Conducting polymer composites with graphene for use in chemical sensors and biosensors. | Chemical sensors and biosensors | [19] |
| Fabrication Methods | Advantages | Disadvantages |
|---|---|---|
| In situ polymerization | Highly effective and a high level dispersion | Needs highly cost reactants and the processing steps are complicated |
| Solution mixing | Versatile and a good dispersion | Solvent removal is a critical issue |
| Electro-polymerization | Short process, easier to control, and eco-friendly | Available monomers are less |
| Self-assembly approach | Efficient way to precisely control the composition of a composite | High costs, purification limitations, and difficult to achieve a high quantity of materials |
| Target Gas | Sensing Material | Response | LOD | Response Time | Recovery Time | Nanocomposite Preparation Method | Ref |
|---|---|---|---|---|---|---|---|
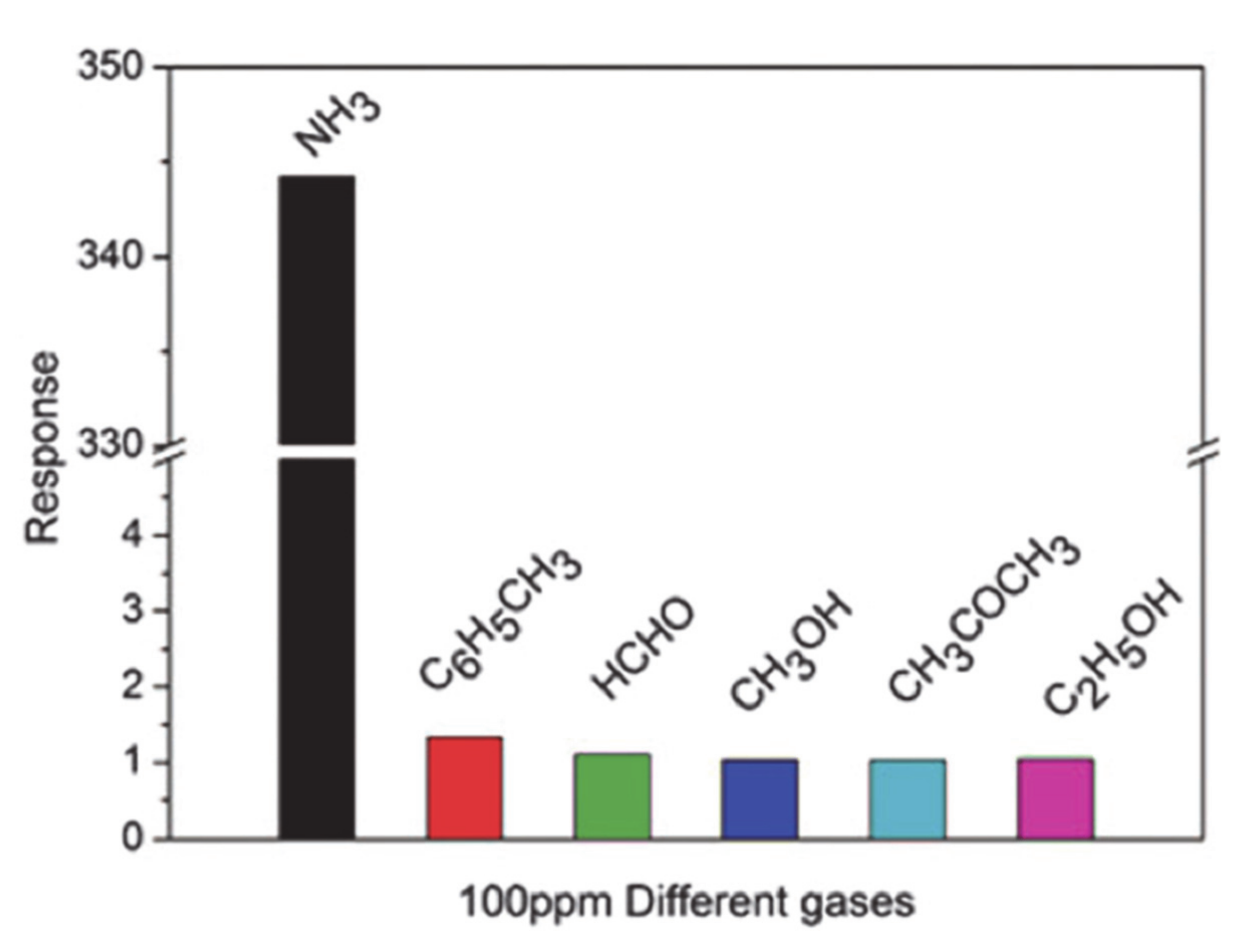
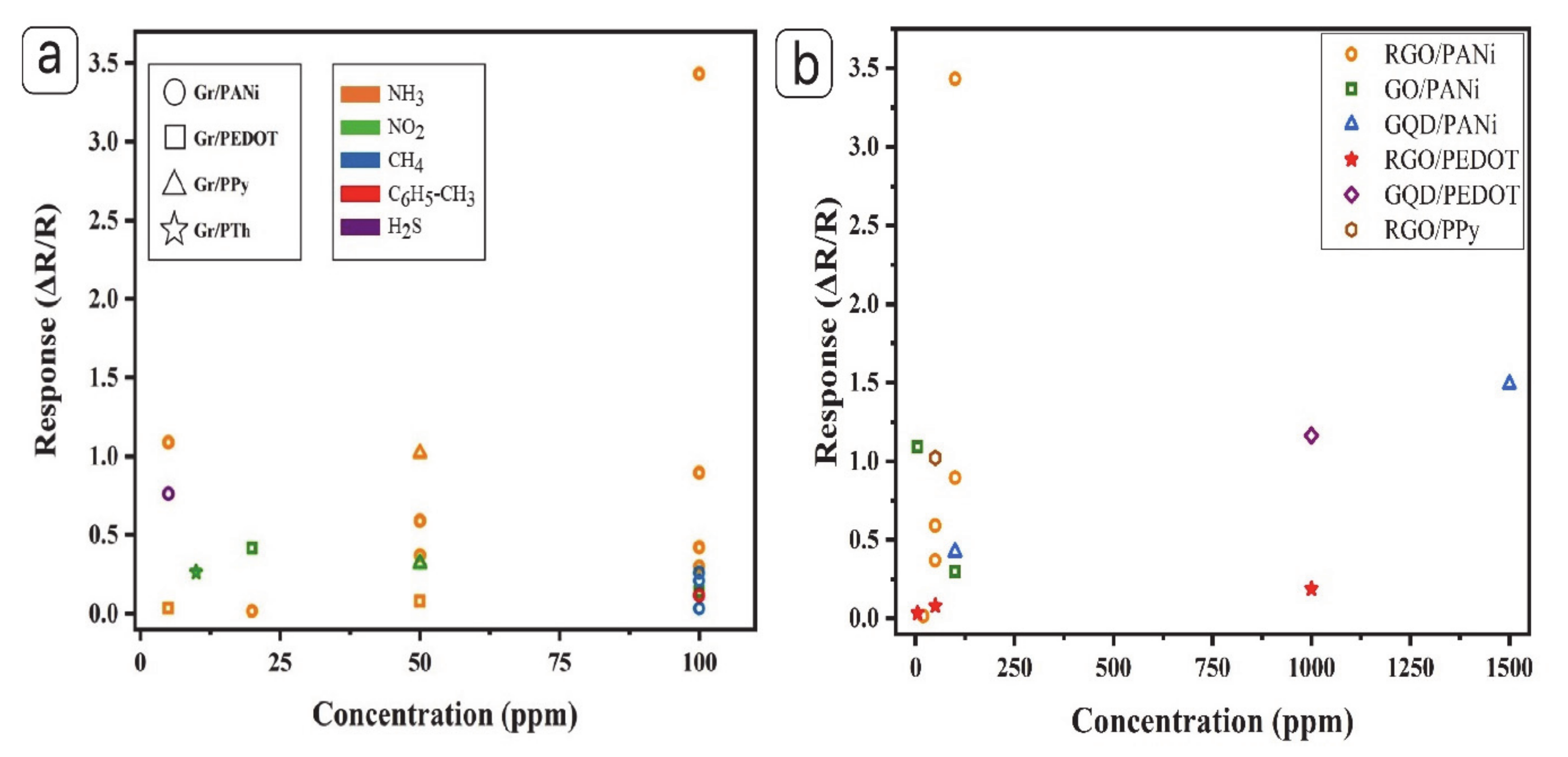
| NH3 | CRG/PANi | 37.1% (50 ppm) | - | 18 min | - | In-situ polymerization | [99] |
| RGO–PANi | 59.2% (50 ppm) | - | 18 min | 4 min | [116] | ||
| (S, N: GQDs)/PANi | 42.3% (100 ppm) | 1–1000 (ppm) | 115 s | 44 s | [106] | ||
| GS–PANi | 1.6 (20 ppm) | 71 ppb | 11 min | - | [108] | ||
| GO-PANi | Rg/Ra = 30.8 (100 ppm) | 50 ppb | 102 s | 186 s | [115] | ||
| TiO2/GO/PANi | Rg/Ra = 110 (100 ppm) | 5 ppm | 32 s | 17 s | [111] | ||
| N-GQDs/PANi | Rg/Ra = 150.09 (1500 ppm) | - | 366 s | 4.98 s | [107] | ||
| Py-RGO/PANi | 59.1% (50 ppm) | 0.2 ppm | - | - | [117] | ||
| rGO–PANi | Rg/Ra = 344.2 (100 ppm) | - | 20 s | 27 s | [101] | ||
| NiNPs@3D-(N)GFs | 750.2% (1000 ppm) | 45 ppb | 95 s | 32 s | [83] | ||
| CH4 | PANi/GO | 20.9 (100 ppm) | - | 3–120 s | 3–120 s | [103] | |
| SnO2@rGO/PANi | 26.1% (100 ppm) | - | - | - | [110] | ||
| G/PANi-C15 | 3.25% (100 ppm) | 10–1600 (ppm) | 85 s | 45 s | [100] | ||
| C6H5–CH3 | C-PANi | 11.6% (100 ppm) | - | 8 min | 22 min | Solution mixing | [114] |
| PANi–G | 90% (5000 ppm) | - | 8.6 s | 16 s | In-situ polymerization | [104] | |
| Benzene | PANi–G | 80% (5000 ppm) | - | 16.25 s | 18.5 s | ||
| H2S | SnO2/rGO/PANi | 76.25% (5 ppm) | 50 ppb | 80 s | 88 s | [109] |
| Target Gas | Sensing Material | Response | LOD | Response Time | Recovery Time | Nanocomposite Preparation Method | Ref |
|---|---|---|---|---|---|---|---|
| NH3 | PEDOT/RGO | 3.43% (5 ppm) | 200 ppb | 90–100 s | 180 s | In situ polymerization | [118] |
| G/PEDOT:PSS | 18.9% (1000 ppm) | 10 ppm | 3 min | 5 min | Solution mixing | [121] | |
| GQDs/PEDOT-PSS | 116.38% (1000 ppm) | - | 7.7 min | 10 min | [125] | ||
| CuTSPc@3D-(N)GF/PEDOT-PSS | 8% (50 ppm) | 1–1000 ppm | 138 s | 63 s | [122] | ||
| NO2 | PEDOT/RGO | 41.7% (20 ppm) | - | 170–180 s | 70 s | In situ polymerization | [90] |
| 14% (100 ppm) | - | 8.3 min | 16.3 min | Electro-polymerization | [120] |
| Target Gas | Sensing Material | Response | LOD | Response Time | Recovery Time | Nanocomposite Preparation Method | Ref. |
|---|---|---|---|---|---|---|---|
| NO2 | RGO/Polypyrrole (PPy) | 32% (50 ppm) | - | - | - | In situ polymerization | [135] |
| Humidity | RGO/PPy | 138% | - | 15 s | 20 s | [131] | |
| NH3 | TiO2@PPy–GN | 102.2% (50 ppm) | 1 ppm | 36 s | 16 s | [77] | |
| CO | Na-X/rGO/PPy | 14.9% (5 ppm) | - | 600 s | 358 s | [134] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamiri, G.; Haseeb, A.S.M.A. Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors. Materials 2020, 13, 3311. https://doi.org/10.3390/ma13153311
Zamiri G, Haseeb ASMA. Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors. Materials. 2020; 13(15):3311. https://doi.org/10.3390/ma13153311
Chicago/Turabian StyleZamiri, Golnoush, and A. S. M. A. Haseeb. 2020. "Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors" Materials 13, no. 15: 3311. https://doi.org/10.3390/ma13153311
APA StyleZamiri, G., & Haseeb, A. S. M. A. (2020). Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors. Materials, 13(15), 3311. https://doi.org/10.3390/ma13153311






