Experimental Study on the Evaluation of Physical Performance and Durability of Cement Mortar Mixed with Water Repellent Impregnated Natural Zeolite
Abstract
1. Introduction
2. Materials and Specimens
2.1. Materials
2.2. Mortar Specimens Mix Proportion
2.3. Specimens
2.3.1. Specimen for Compressive Strength, measurement of Contact angle and Mercury Intrusion Porosimetry (MIP)
2.3.2. Specimen for Chloride Ion Migration Coefficient and Evaluation
2.3.3. Accelerated Carbonation Test
2.3.4. Curing Method
3. Experimental Method
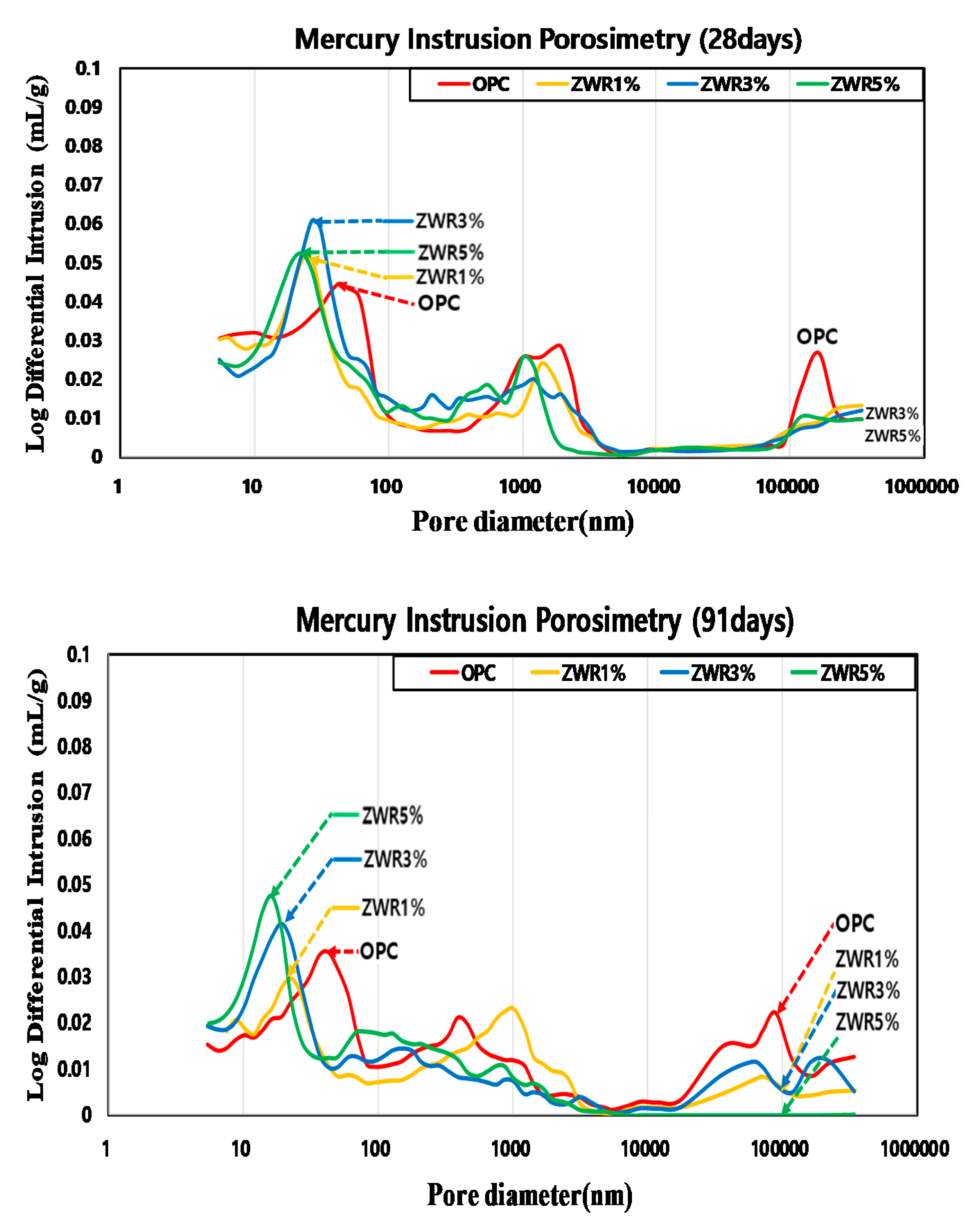
3.1. Mercury Intrusion Porosimetry (MIP)
3.2. Evaluation of Specimen Compressive Strength
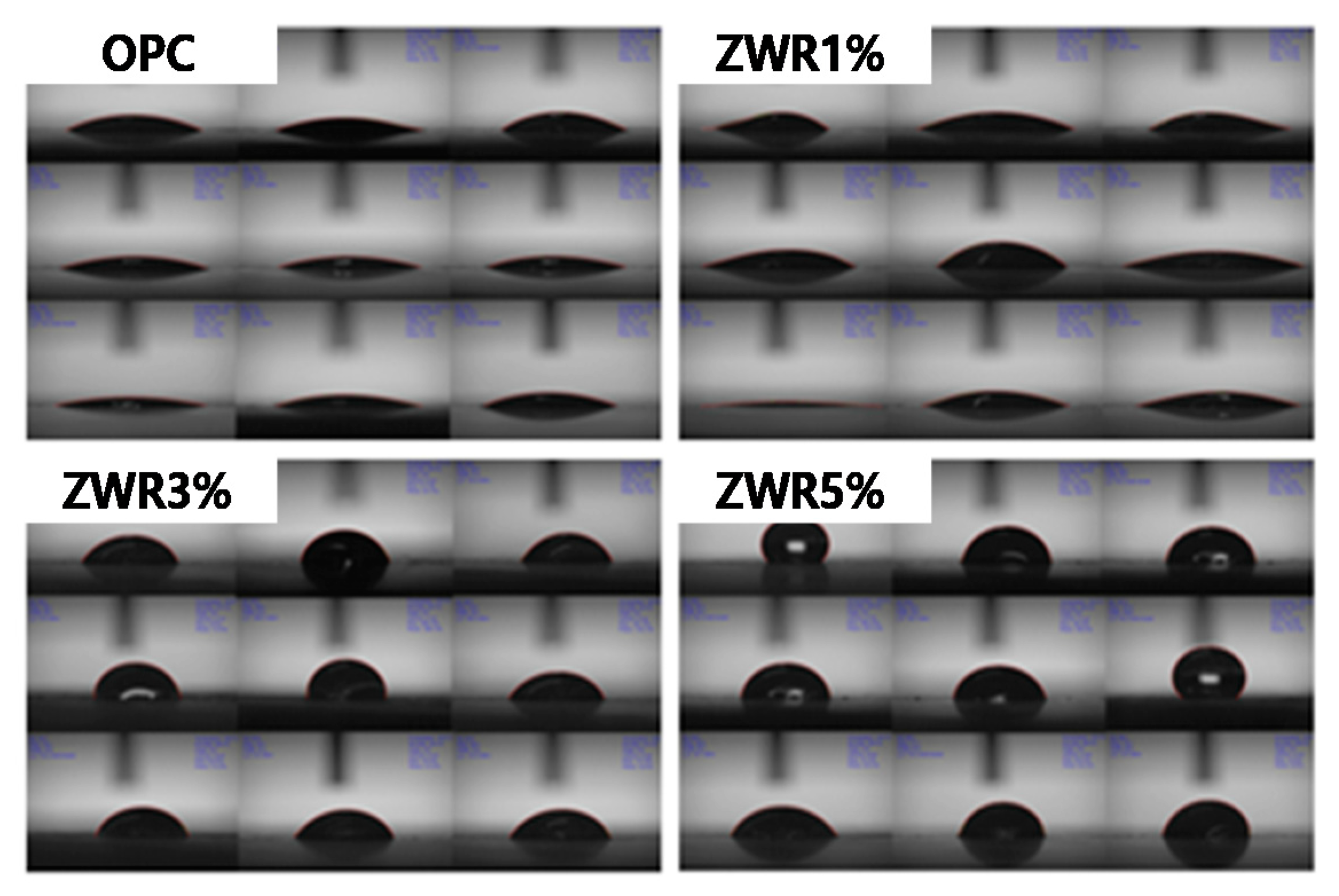
3.3. Evaluation of Contact Angle
3.4. Evaluation of Chloride Ion Migration Coefficient
- Dnssm—is a non-steady-state migration coefficient (m2/s)
- R—gas constant, R = 8.314 J(K·mol)
- F—faraday constant = 9.648 × 104 J(V·mol)
- U—absolute value of the applied voltage (V)
- T—average of the initial and final temperatures in the anolyte solution (°C)
- L—thickness of the specimen (mm)
- —average value of the penetration depths (mm)
- t—test duration (hour)
- Cd—chloride concentration at which the color changes
3.5. Accelerated Carbonation Test
3.6. Water Penetration Test
4. Results and Discussion
4.1. Mercury Intrusion Porosimetry (MIP)
4.2. Result of Concrete Compressive Strength
4.3. Result of Evaluation of Contact Angle
4.4. Evaluation of Chloride Ion Migration Coefficient
4.5. Accelerated Carbonation Test
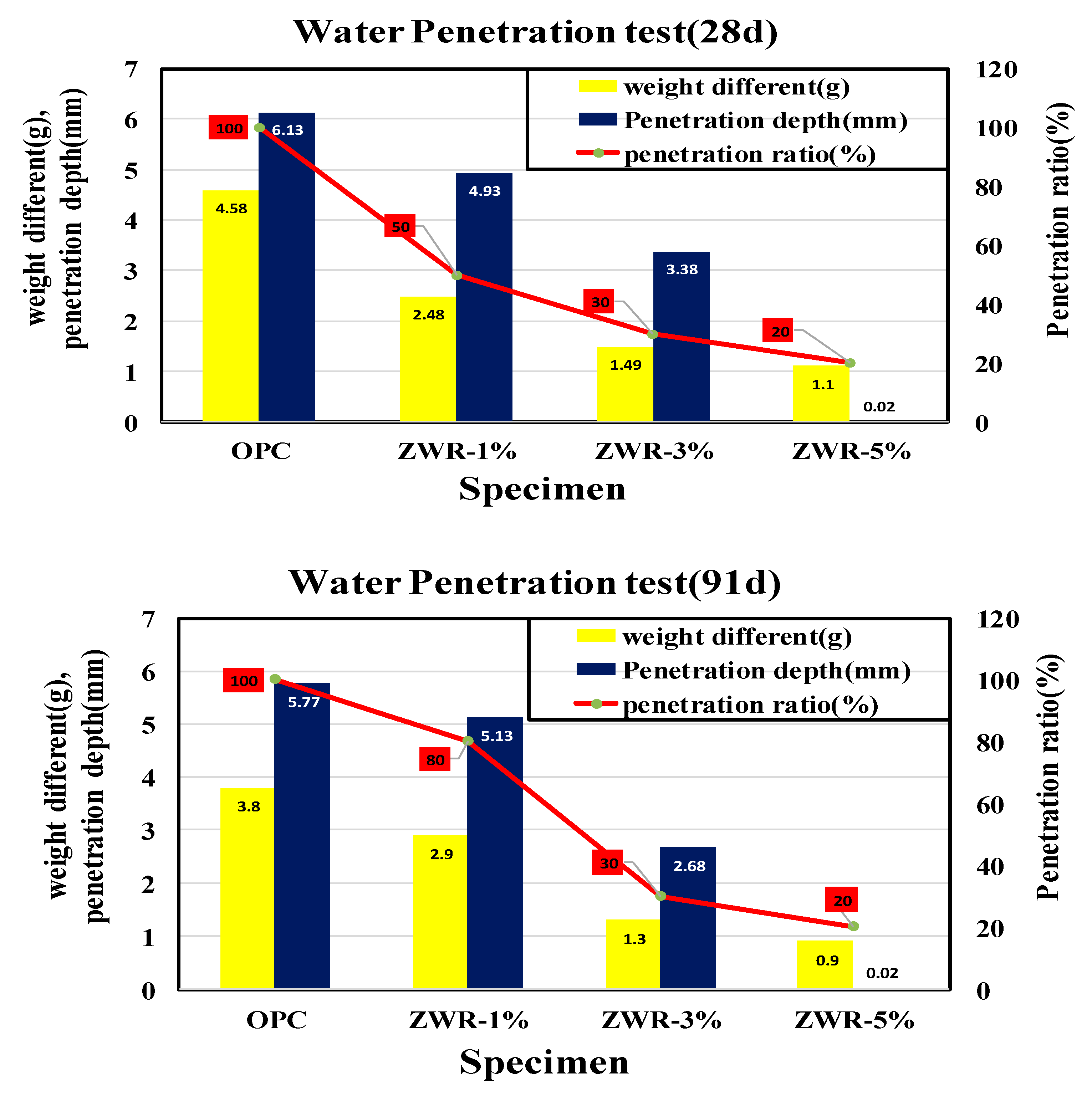
4.6. Water Penetration Test
5. Conclusions
- The evaluation results of contact angle and compressive strength for the ZWR 5% specimen showed a compressive strength of 82% of a standard mortar and a contact angle more than 5 times. The ZWR-incorporating specimens appeared to have increased air content due to the effect of some silane-siloxane particles not bound to the cement particles. It is considered that the cement and mortar sand and paste were delayed in bonding, the adhesive strength was reduced, and the compressive strength was also reduced. In addition, it is considered that the ZWR-filled sample has excellent moisture permeation resistance, but its compressive strength decreases due to the cushioning effect.
- It was confirmed that the penetration resistance performance of water for the ZWR 5% test specimen was the best among the test specimens. As the curing days of the material passed, the hydration product and ZWR were filled into the pores to increase the water tightness, and the greater the amount of ZWR mixed in, the more the resistance to water absorption increased.
- In the chloride penetration resistance test and chloride diffusion coefficient tests, it was observed that the penetration and migration of chloride-containing water was higher in the test specimen containing ZWR than in OPC. In comparison to OPC, it was found that generally, the amount of charge and the diffusion coefficient due to the penetration of water from the specimen mixed with ZWR were inversely proportional.
- The MIP test results showed that the pore size and cumulative pore tended to decrease with curing days. In the case of the test specimen containing ZWR, it is considered that the hydration product of cement has the same effect as that of filling the pores in the interior and reducing the pores size and the pore ratio. In addition, compared with OPC, the size of the pores and the cumulative pores of the specimen with ZWR 5% were about 64%. Based on the test results, the increase of contact angle could be evaluated due to the decrease of chloride diffusion coefficient [39], the decrease of CO2 penetration depth in the carbonation accelerated test, and the decrease of water absorption.
- It was confirmed that the greater the amount of ZWR mixed, the more the compression strength tended to decrease, but the durability performance improved. It can be judged from this experiment that the optimum ZWR mixing ratio for improving the durability of concrete due to the penetration of water and imparting water repellency to the inside of concrete is 5%.
- It is considered that additional physical and durability experiments are needed to evaluate the resistance to moisture penetration inside the concrete.
Author Contributions
Funding
Conflicts of Interest
References
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I. The development of compressive strength of ground granulated blast furnace slag-palm oil fuel ash-fly ash based geopolymer mortar. Mater. Des. 2014, 56, 833–841. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. Strength and Permeation Properties of Slag Blended Fly Ash Based Geopolymer Concrete. Adv. Mat. Res. 2013, 651, 168–173. [Google Scholar]
- Biondini, F.; Frangopol, D.M. Life-Cycle Design, Assessment, and Maintenance of Structures and Infrastructure Systems; American Society of Civil Engineers: Reston, VA, USA, 2019. [Google Scholar]
- Shim, H.B.; Lee, M.S. An Experimental Study on Water Resistance of Penetrating Water Repellency of Emulsified Silicon Type Exposed to The Outdoor Environment. J. Korea Concr. Inst. 2004, 16, 477–484. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P. Hydraulic Lime Mortars with Siloxane for Waterproofing Historic Masonry. Cem. Concr. Res. 2007, 37, 283–290. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Sudbrink, B.; TylerLey, M. Determining the Effective Service Life of Silane Treatments in Concrete Bridge Decks. Constr. Build. Mater. 2016, 116, 121–127. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Vogel, M.; Muller, H.S.; Zhao, T. Influence of Freeze-thaw Cycles on Capillary Absorption and Chloride Penetration into Concrete. Cem. Concr. Res. 2017, 100, 60–67. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Kou, S.C.; Poon, C.S.; Dai, J.G.; Li, Q.Y. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Hasan, M.S.; Nosonovsky, M. Lotus Effect and Friction: Does Nonsticky Mean Slippery? Biomimetics 2020, 5, 28. [Google Scholar] [CrossRef]
- Lee, J.S. Comparative study on repellent ability of silane repellent according to type and treatment method. Master’s Thesis, University of Ulsan, Ulsan, Korea, 2004. [Google Scholar]
- De Vries, J.; Polder, R.B. Hydrophobic treatment of concrete. Constr. Build. Mater. 1997, 11, 259–265. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. Chloride Diffusion in Concrete Protected with a Silane-Based Corrosion Inhibitor. Materials 2020, 13, 2001. [Google Scholar] [CrossRef]
- Dai, J.G.; Akira, Y.; Wittmann, F.H.; Yokota, H.; Zhang, P. Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks. Cem. Concr. Compos. 2010, 32, 101–109. [Google Scholar] [CrossRef]
- Baerlocher, C.; Mccusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Naiqian, F.; Changchen, M.; Xihuang, J. Natural zeolite for preventing expansion due to alkali-aggregate reaction. Cem. Concr. Aggreg. 1992, 14. [Google Scholar]
- Agrawal, U.S.; Wanjari, S.P.; Naresh, D.N. Impact of replacement of natural river sand with geopolymer fly ash sand on hardened properties of concrete. Constr. Build. Mater. 2019, 209, 499–507. [Google Scholar] [CrossRef]
- Park, J.H.; Joh, S.H.; Lee, H.S. Effect of Curing Condition on Resistance to Chloride Ingress in Concrete Using Ground Granulated Blast Furnace Slag. Materials 2019, 12, 3233. [Google Scholar] [CrossRef] [PubMed]
- Korean Industrial Standards. KS E. 3076, Methods for X-ray Fluorescence Spectrometric Analysis of Silica Stone and Silica Sand; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2017.
- Massazza, F. Pozzolana and pozzolanic cements. In Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier: Oxford, UK, 1998. [Google Scholar]
- Shi, H.S.; Xu, B.W.; Zhou, X.C. Influence of mineral admixtures on compressive strength, gas permeability and carbonation of high performance concrete. Constr. Build. Mater. 2009, 23, 1980–1985. [Google Scholar] [CrossRef]
- Vejmelková, E.; Koňáková, D.; Kulovaná, T.; Keppert, M.; Žumár, J.; Rovnaníková, P.; Černý, R. Engineering properties of concrete containing natural zeolite as supplementary cementitious material: Strength, toughness, durability, and hygrothermal performance. Cem. Concr. Compos. 2015, 55, 259–267. [Google Scholar] [CrossRef]
- Canpolat, F.; Yılmaz, K.; Köse, M.M.; Sümer, M.; Yurdusev, M.A. Use of zeolite, coal bottom ash and fly ash as replacement materials in cement production. Cem. Concr. Res. 2004, 34, 731–735. [Google Scholar] [CrossRef]
- Valipour, M.; Pargar, F.; Shekarchi, M.; Khani, S. Comparing a natural pozzolan, zeolite, to metakaolin and silica fume in terms of their effect on the durability characteristics of concrete: A laboratory study. Constr. Build. Mater. 2013, 41, 879–888. [Google Scholar] [CrossRef]
- ASTM International. ASTM A C39 Standard Test Method for Compressive Strength of Concrete; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Korean Industrial Standards. KS F. 2584, Standard Test Method for Accelerated Carbonation of Concrete; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2015.
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley Interscience: New York, NY, USA, 1967. [Google Scholar]
- Korean Industrial Standards. KS L. 5105, Testing Method for Compressive Strength of Hydraulic Cement Mortar; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2017.
- Uelzen, T.; Muller, J. Wettability enhancement by rough surfaces generated by thin film technology. Thin Solid Films 2003, 434, 311–315. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, K.I.; Ahn, S.H. Transactions: Feasibility Study of Laser Contact Angle Measurement for Nano-fiber Characterization. J. Korean Soc. Cloth. Text. 2003, 27, 554–559. [Google Scholar]
- ASTM International. ASTM C 1202 Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration; Annual Book of American Society for Testing Materials Standards, Vol. C04.02; ASTM International: West Conshohocken, PA, USA, 1993. [Google Scholar]
- Nordtest. Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-steady-State Migration Experiments; N.T. Build 492; Nordtest: Taastrup, Denmark, 1999. [Google Scholar]
- Tang, L. Electrically accelerated methods for determining chloride diffusivity in concrete—current development. Mag. Concr. Res. 1996, 48, 173–179. [Google Scholar] [CrossRef]
- Lee, B.; Kim, G.; Nam, J.; Cho, B.; Hama, Y.; Kim, R. Compressive strength, resistance to chloride-ion penetration and freezing/thawing of slag-replaced concrete and cementless slag concrete containing desulfurization slag activator. Constr. Build. Mater. 2016, 128, 341–348. [Google Scholar] [CrossRef]
- Korean Standards Association. KS F. 4919, Cement-Polymer Modified Waterproof Coatings; Korean Standards Association: Seoul, Korea, 2008.
- Jain, J.A.; Neithalath, N. Chloride transport in fly ash and glass powder modified concretes–influence of test methods on microstructure. Cem. Concr. Compos. 2010, 32, 148–156. [Google Scholar] [CrossRef]
- Zhao, Z.; Qu, X.; Li, J. Application of polymer modified cementitious coatings (PCCs) for impermeability enhancement of concrete. Constr. Build. Mater. 2020, 249, 118769. [Google Scholar] [CrossRef]
- Yoo, S.W.; Bang, K.S.; Jung, S.H.; Chang, S.P. Study on the Carbonation Properties of Fly Ash Concrete by the New Rapid Carbonation Experimental System. J. Korean Soc. Civ. Eng. 2007, 27, 601–607. [Google Scholar]
- Ghrici, M.; Kenai, S.; Meziane, E. Mechanical and durability properties of cement mortar with Algerian natural pozzolana. J. Mater. Sci. 2006, 41, 6965–6972. [Google Scholar] [CrossRef]
- Youm, K.S.; Moon, J.; Cho, J.Y.; Kim, J.J. Experimental study on strength and durability of lightweight aggregate concrete containing silica fume. Constr. Build. Mater. 2016, 114, 517–527. [Google Scholar] [CrossRef]

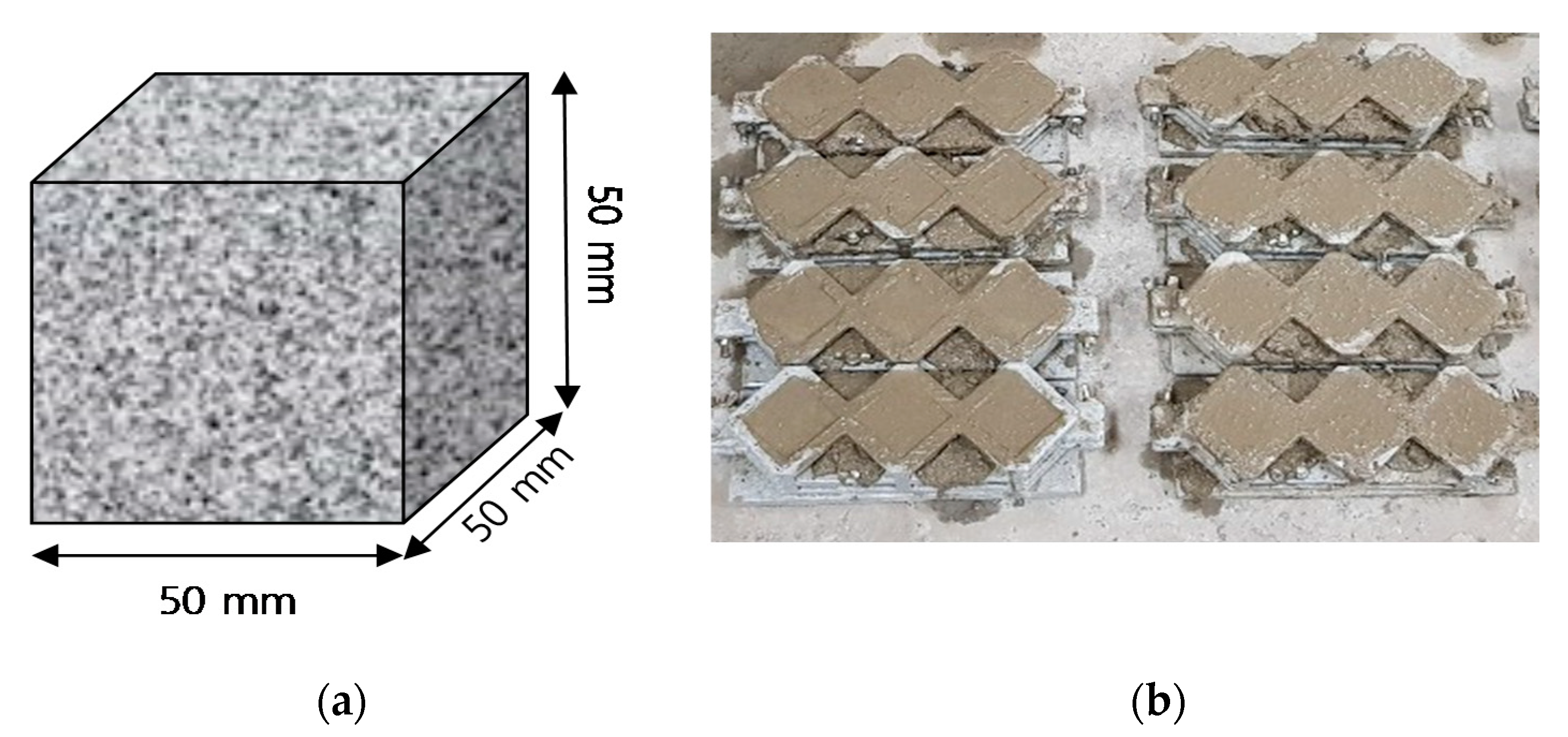



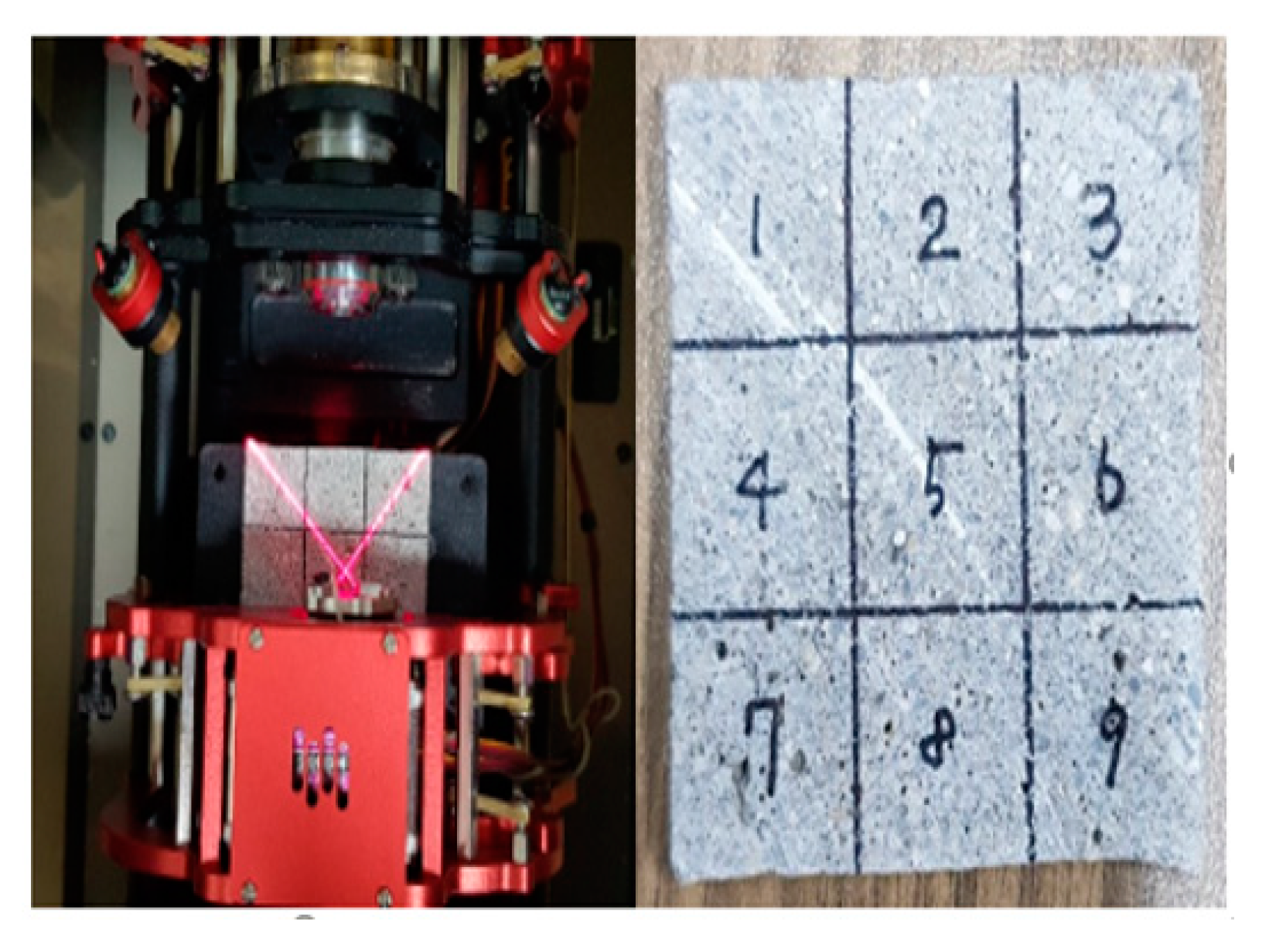
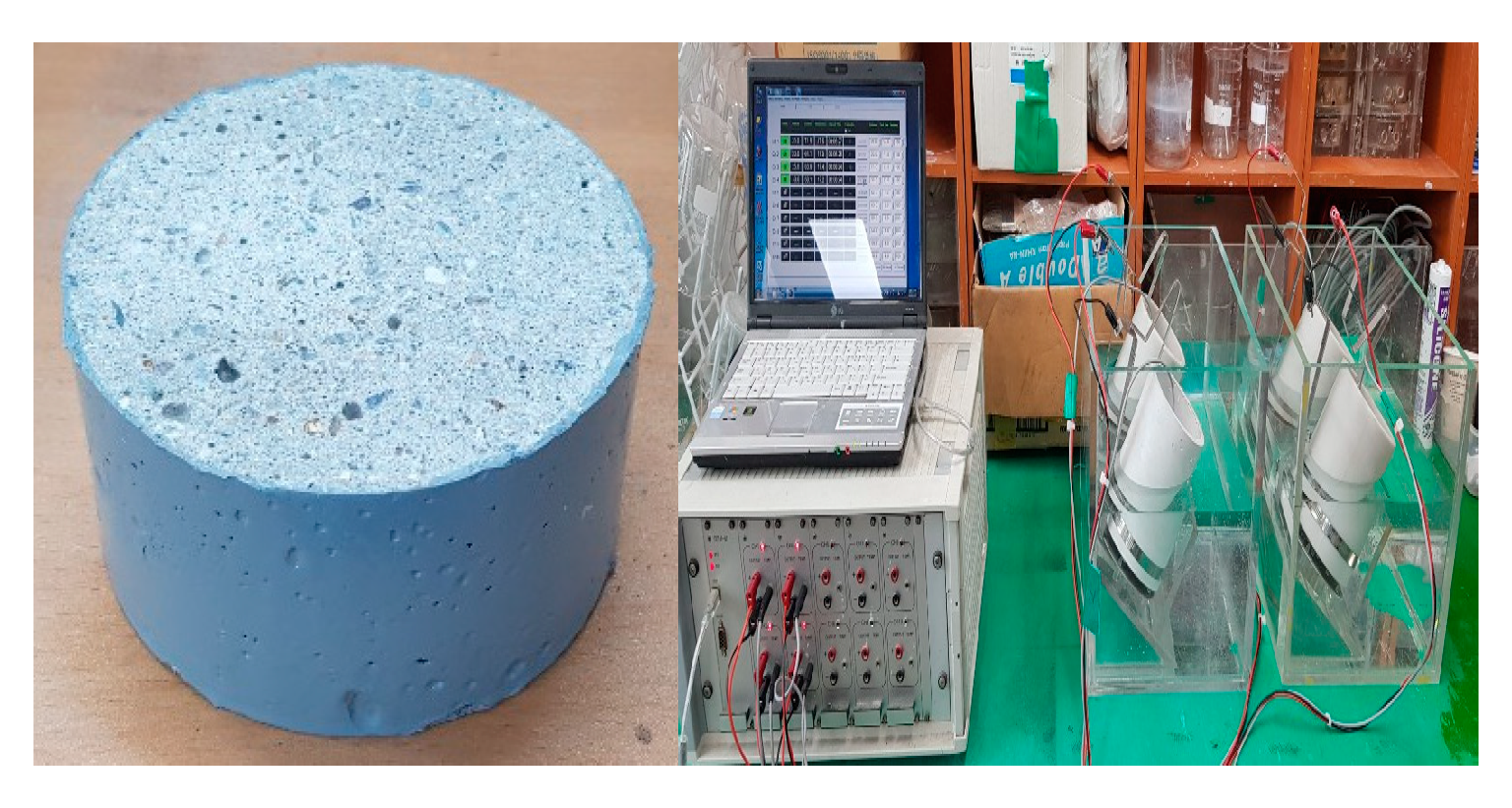


| Name | Chemical Compositions (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | TiO2 | Fe2O3 | CaO | MgO | SO3 | K2O | Etc. /Lg. Loss | L.O.I | |
| OPC | 19.74 | 5.33 | 0.30 | 2.93 | 61.74 | 3.78 | 2.47 | 0.89 | 2.82 | 2.3 |
| Name | Chemical Compositions (%) | ||||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | K2O | Na2O | CEC | Etc./Lg. Loss | |
| Zeolite | 66.8 | 13.2 | 1.68 | 3.02 | 1.16 | 106 | 14.14 |
| Name | Chemical Compositions (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| As | Cd | Hg | Pb | Cr | Cu | Ni | Zn | |
| Limit | 20 | 2 | 1 | 50 | 90 | 120 | 20 | 400 |
| Result | 0.64 | 7.87 | 4.37 | 3.75 | 23.81 | |||
| Name | Chemical Compositions (%) | |||||
|---|---|---|---|---|---|---|
| Color | Kind | Effective Ratio (%) | Diluent | Freeze Stability | pH | |
| Water Repellent | White | Silane - Siloxane | 50 | Water | 12 | |
| Name | W/B (%) | Unit Weight (kg/m3) | |||||
|---|---|---|---|---|---|---|---|
| C | W | Sand | Impregnation Admixture = ZWR | Addition Ratio | |||
| Zeolite | WR1 | ||||||
| OPC | 40% | 510 | 204 | 1530 | |||
| ZWR1% | 40% | 510 | 208 | 1530 | 5.1 | 5.1 | 1% |
| ZWR3% | 40% | 510 | 216.2 | 1530 | 15.3 | 15.3 | 3% |
| ZWR5% | 40% | 510 | 224.4 | 1530 | 25.5 | 25.5 | 5% |
| Surface Contact Angle (θ) | Permeability |
|---|---|
| >130° | Very good repellency |
| 110–130° | Good repellency |
| 90–110° | Slight wetting |
| 30–90° | Pronounced wetting |
| <30° | Surface completely wet |
| Initial Current I30V (with 30V) (mA) | Applied Voltage U (after Adjustment) (V) | Possible New Initial Current I0 (mA) | Test Duration (h) |
|---|---|---|---|
| I0 < 5 | 60 | I0 < 10 | 96 |
| 5 ≤ I0 < 10 | 60 | 10 ≤ I0 < 20 | 48 |
| 10 ≤ I0 < 15 | 60 | 20 ≤ I0 < 30 | 24 |
| 15 ≤ I0 < 20 | 50 | 25 ≤ I0 < 35 | 24 |
| 20 ≤ I0 < 30 | 40 | 25 ≤ I0 < 40 | 24 |
| 30 ≤ I0 < 40 | 35 | 35 ≤ I0 < 50 | 24 |
| 40 ≤ I0 < 60 | 30 | 40 ≤ I0 < 60 | 24 |
| 60 ≤ I0 < 90 | 25 | 50 ≤ I0 < 75 | 24 |
| 90 ≤ I0 < 120 | 20 | 60 ≤ I0 < 80 | 24 |
| 120 ≤ I0 < 180 | 15 | 60 ≤ I0 < 90 | 24 |
| 180 ≤ I0 < 360 | 10 | 60 ≤ I0 < 120 | 24 |
| I0 ≥ 360 | 10 | I0 ≥ 120 | 6 |
| Name | Porosity (%) | OPC 91D Porosity Ratio (%) | |
|---|---|---|---|
| 28 d | 91 d | ||
| OPC | 16.2 | 13.5 | 100 |
| ZWR1% | 15.8 | 12.0 | 88.8 |
| ZWR3% | 12.8 | 10.9 | 80.7 |
| ZWR5% | 11.4 | 8.7 | 64.4 |
| Compressive Strength (MPa) | |||||
|---|---|---|---|---|---|
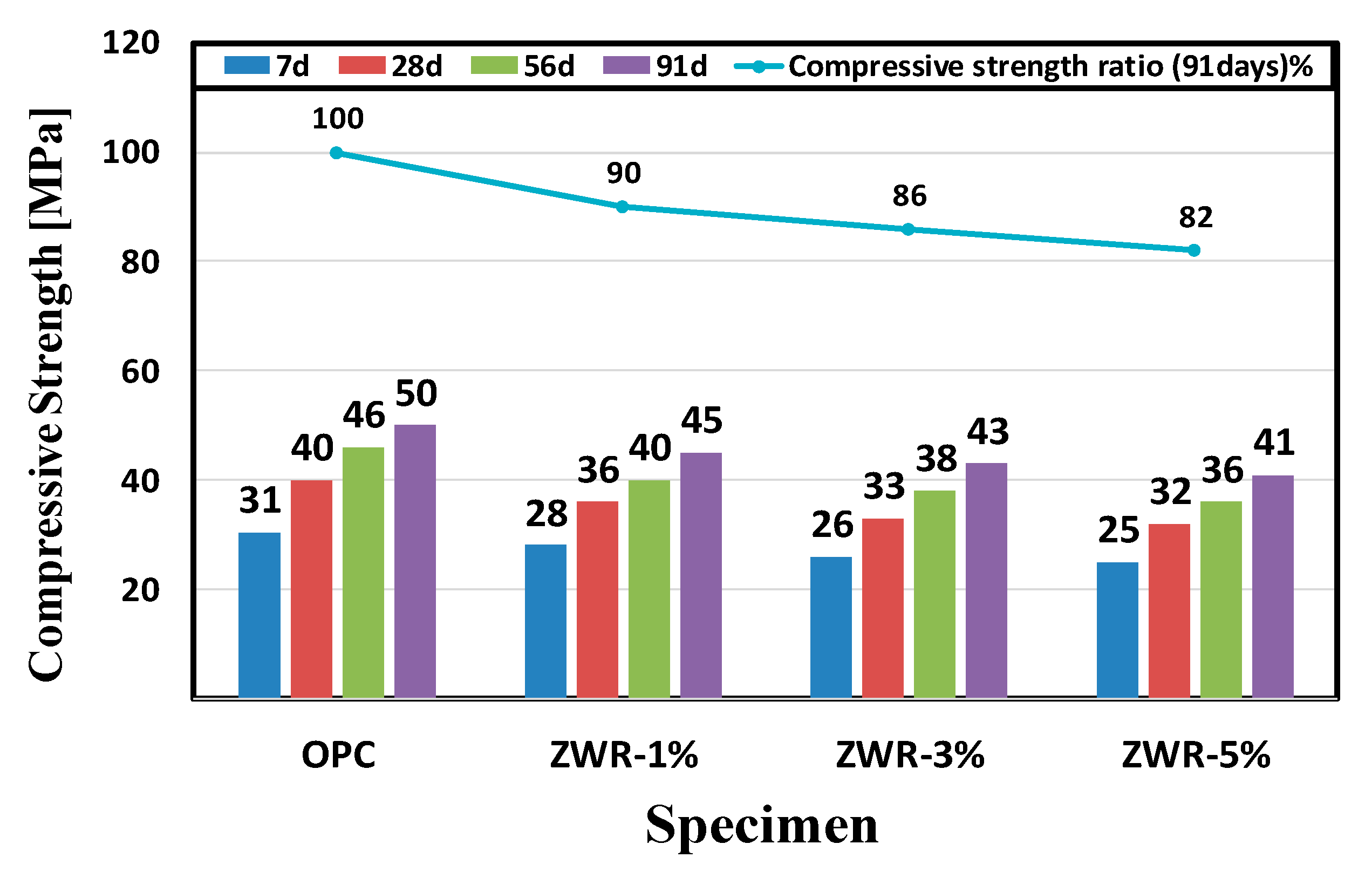
| Specimen | 7 Days | 28 Days | 56 Days | 91 Days | 91 Days Ratio (%) |
| OPC | 31 | 40 | 46 | 50 | 100 |
| ZWR1% | 28 | 36 | 40 | 45 | 90 |
| ZWR3% | 26 | 33 | 38 | 43 | 86 |
| ZWR5% | 25 | 32 | 36 | 41 | 82 |
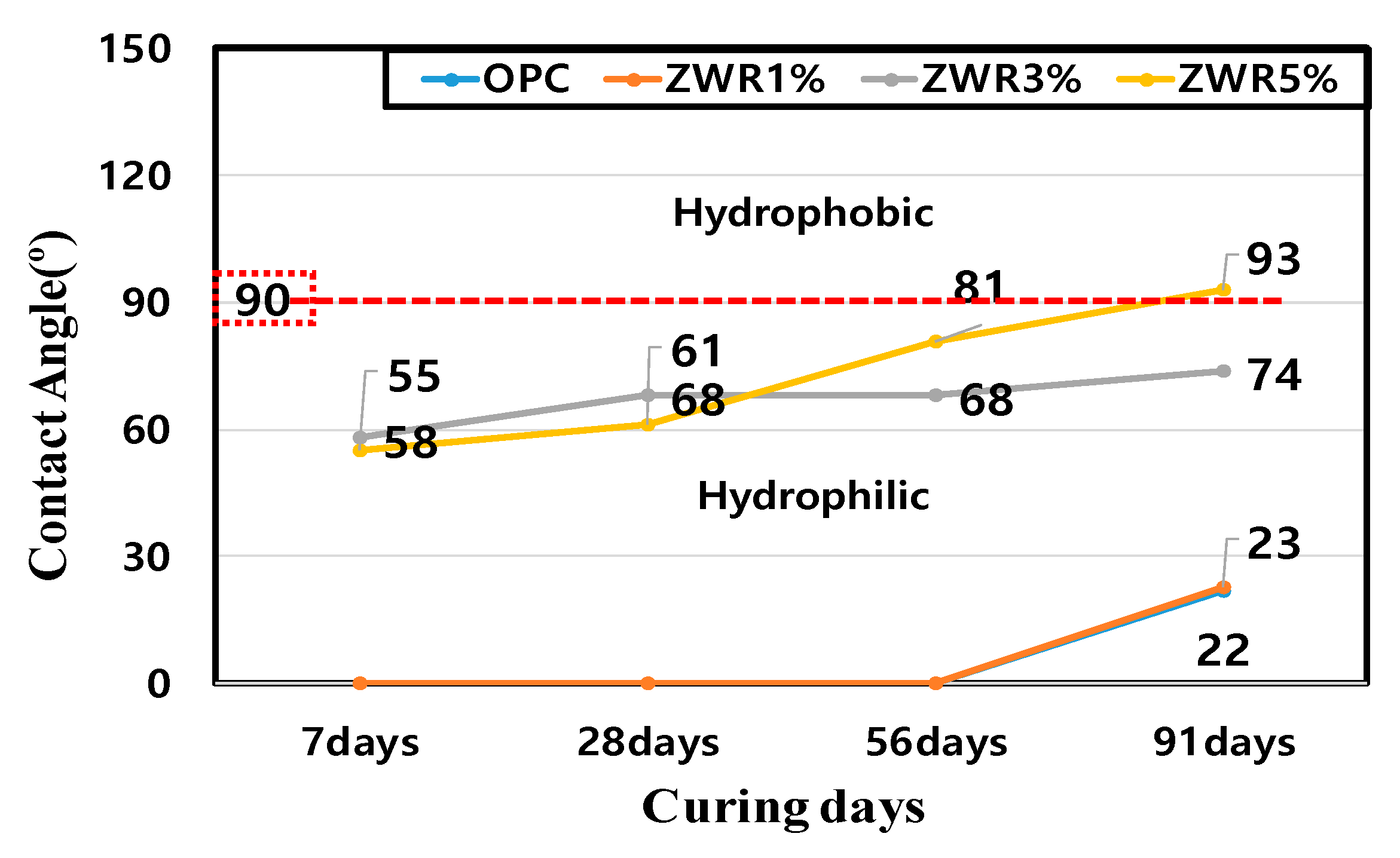
| Name | Contact Angle (°) | |||
|---|---|---|---|---|
| 7d | 28d | 56d | 91d | |
| OPC | 22 | |||
| ZWR1% | 23 | |||
| ZWR3% | 58 | 68 | 68 | 74 |
| ZWR5% | 55 | 61 | 81 | 93 |
| Name | Chloride Penetration Average Depth (mm) | Chloride Ion Diffusion Coefficient (× 10−12m2/s) | ||
|---|---|---|---|---|
| 28 d | 91 d | 28 d | 91 d | |
| OPC | 20.46 | 10.17 | 13.3 | 13.1 |
| ZWR1% | 19.46 | 10.91 | 13.14 | 9.51 |
| ZWR3% | 16.50 | 9.82 | 8.8 | 6.36 |
| ZWR5% | 16.78 | 9.15 | 7.39 | 4.77 |
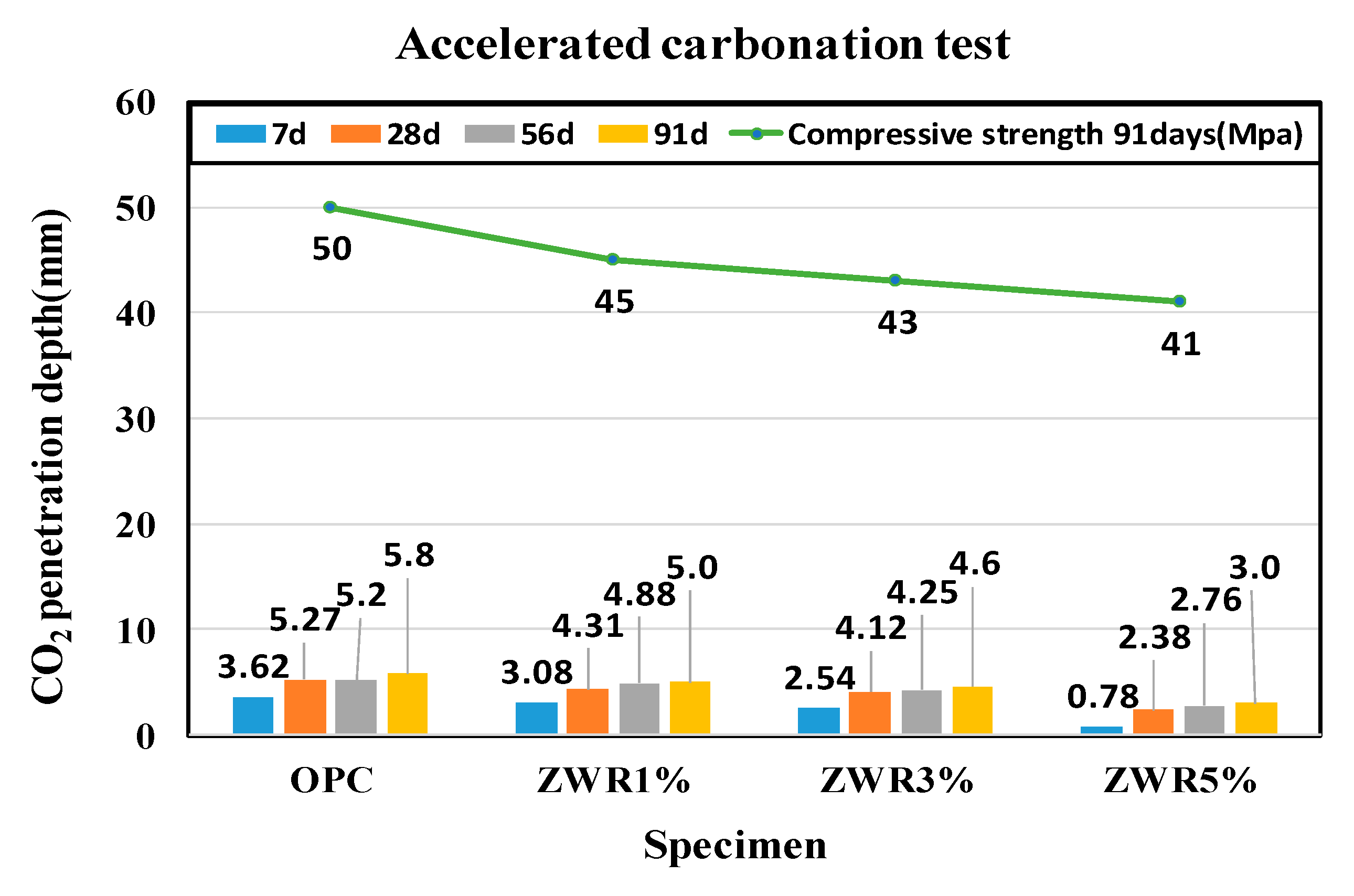
| Name | CO2 Penetration Average Depth (mm) | OPC 91D Penetration Ratio (%) | |||
|---|---|---|---|---|---|
| 7d | 28d | 56d | 91d | ||
| OPC | 3.62 | 5.27 | 5.2 | 5.8 | 100 |
| ZWR1% | 3.08 | 4.31 | 4.88 | 5.0 | 86 |
| ZWR3% | 2.54 | 4.12 | 4.25 | 4.6 | 79 |
| ZWR5% | 0.78 | 2.38 | 2.76 | 3.0 | 51 |
| Name | Water Penetration Test (28 d) | ||
|---|---|---|---|
| Weight Difference (g) | Penetration Depth (mm) | Penetration Ratio (%) | |
| OPC | 4.58 | 6.13 | 100 |
| ZWR1% | 2.48 | 4.93 | 50 |
| ZWR3% | 1.49 | 3.38 | 30 |
| ZWR5% | 1.1 | 0.02 | 20 |
| Name | Water Penetration Test (91 d) | ||
|---|---|---|---|
| Weight Difference (g) | Penetration Depth (mm) | Penetration Ratio (%) | |
| OPC | 3.8 | 5.77 | 100 |
| ZWR1% | 2.9 | 5.13 | 80 |
| ZWR3% | 1.3 | 2.68 | 30 |
| ZWR5% | 0.9 | 0.02 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, C.B.; Lee, H.S. Experimental Study on the Evaluation of Physical Performance and Durability of Cement Mortar Mixed with Water Repellent Impregnated Natural Zeolite. Materials 2020, 13, 3288. https://doi.org/10.3390/ma13153288
Yoon CB, Lee HS. Experimental Study on the Evaluation of Physical Performance and Durability of Cement Mortar Mixed with Water Repellent Impregnated Natural Zeolite. Materials. 2020; 13(15):3288. https://doi.org/10.3390/ma13153288
Chicago/Turabian StyleYoon, Chang Bok, and Han Seung Lee. 2020. "Experimental Study on the Evaluation of Physical Performance and Durability of Cement Mortar Mixed with Water Repellent Impregnated Natural Zeolite" Materials 13, no. 15: 3288. https://doi.org/10.3390/ma13153288
APA StyleYoon, C. B., & Lee, H. S. (2020). Experimental Study on the Evaluation of Physical Performance and Durability of Cement Mortar Mixed with Water Repellent Impregnated Natural Zeolite. Materials, 13(15), 3288. https://doi.org/10.3390/ma13153288





