Mechanochemical Synthesis of BaTiO3 Powders and Evaluation of Their Acrylic Dispersions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
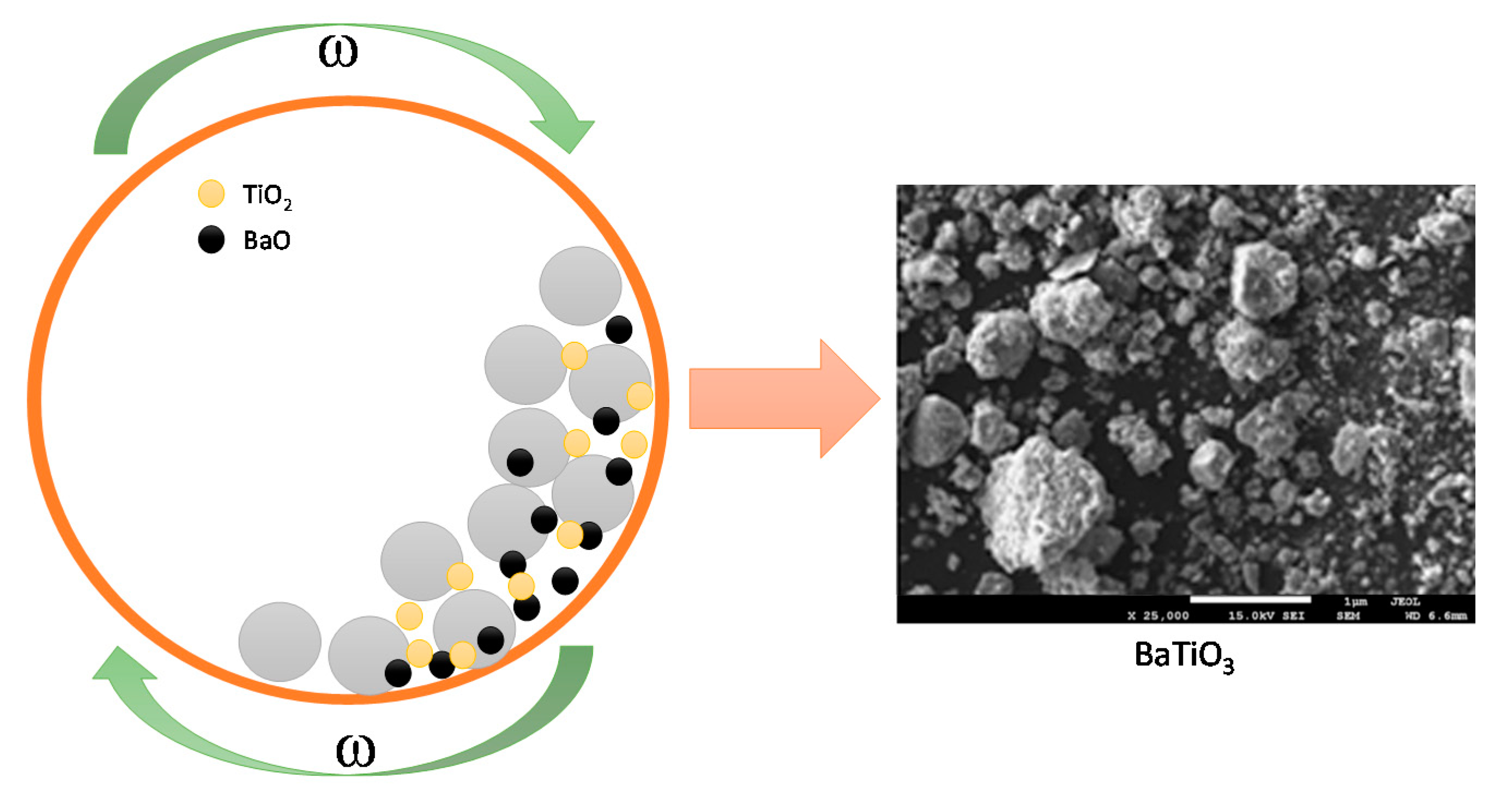
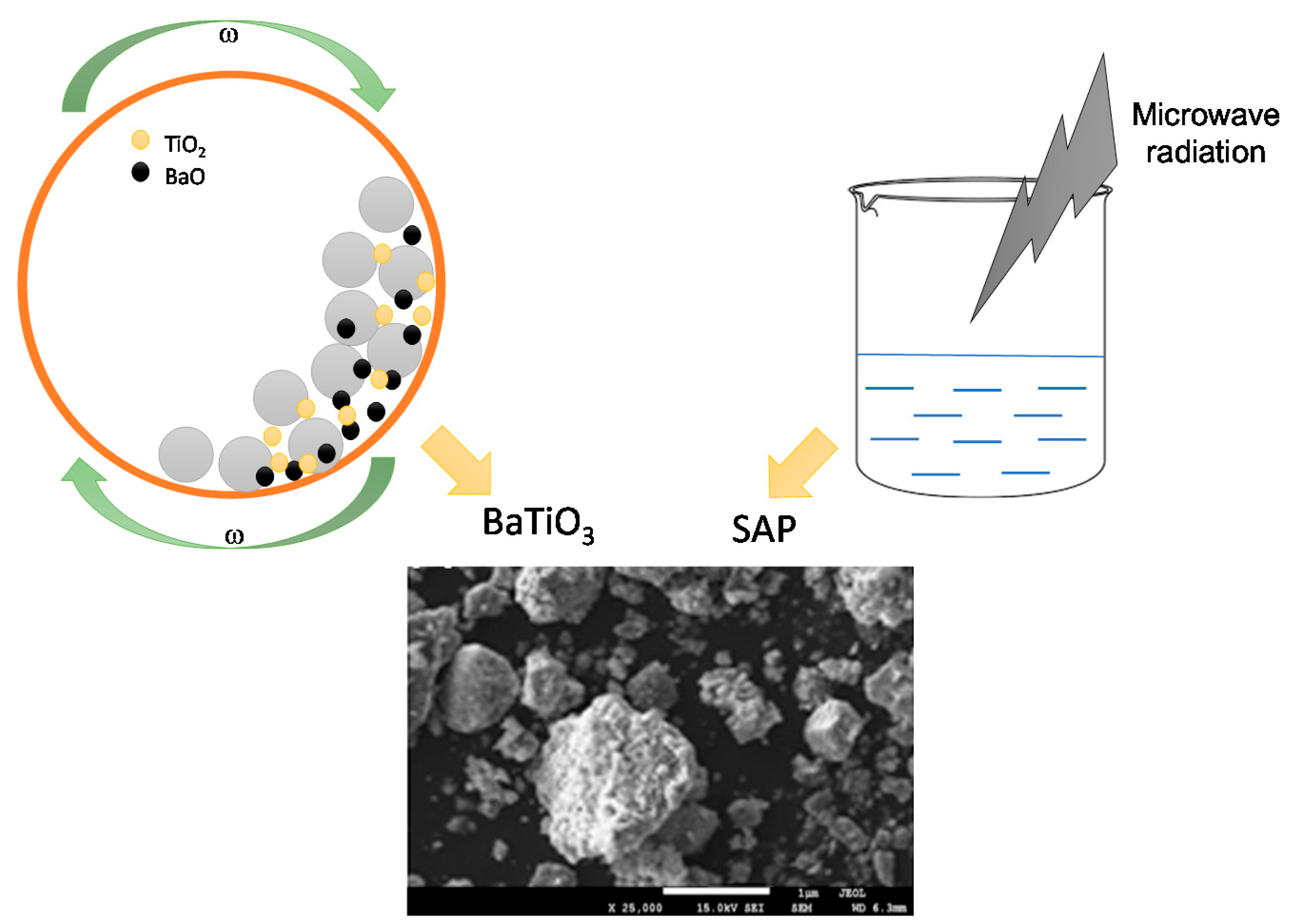
2.2. Applied Methodology
3. Results and Discussion
3.1. Analysis of Barium Titanate Powder Using X-ray Diffraction (XRD) Technique
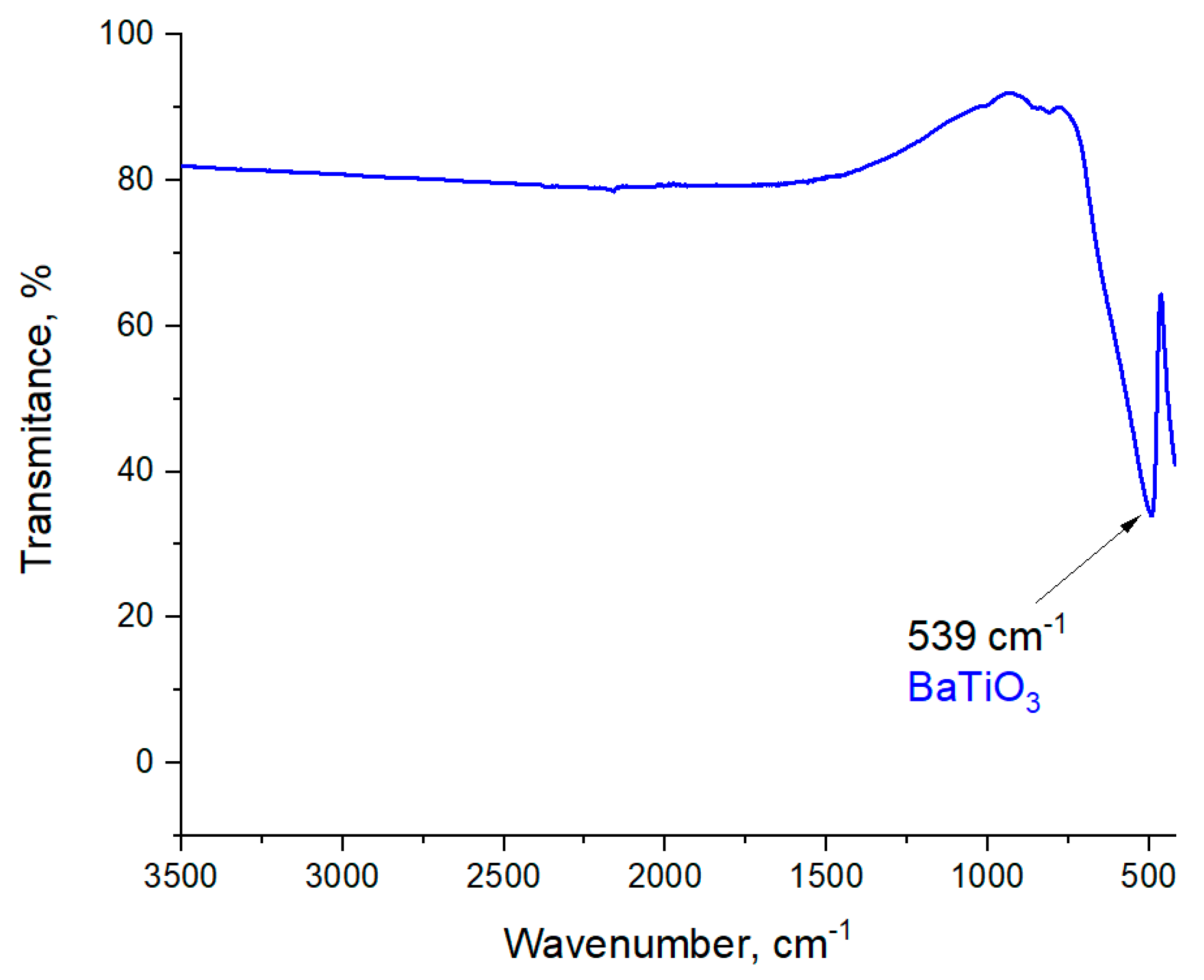
3.2. Analysis of the Structure of Obtained Powder by Fourier Transform Infrared (FT-IR) Spectroscopy
3.3. Determination of the Specific Surface Area of the BaTiO3 Powders
- S—the external surface area of the solid substance.
- m—the mass of the substance.
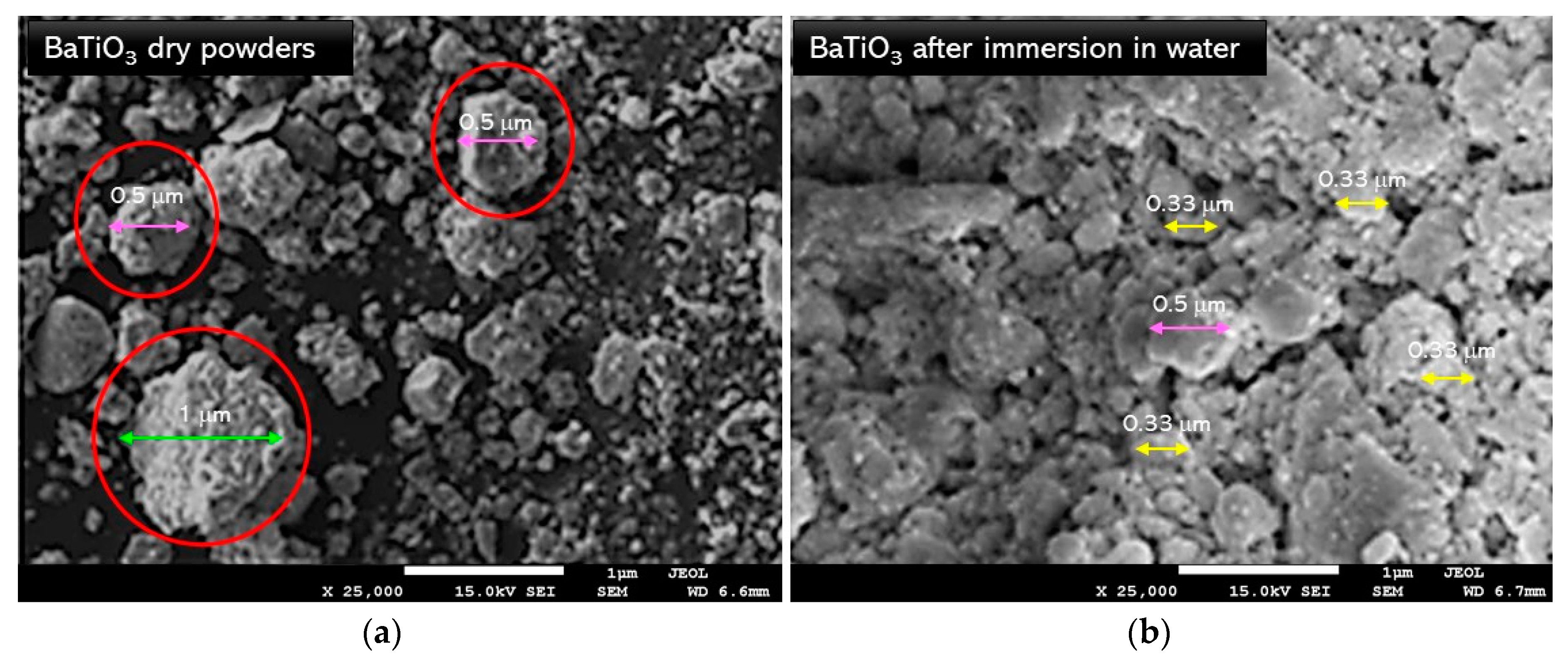
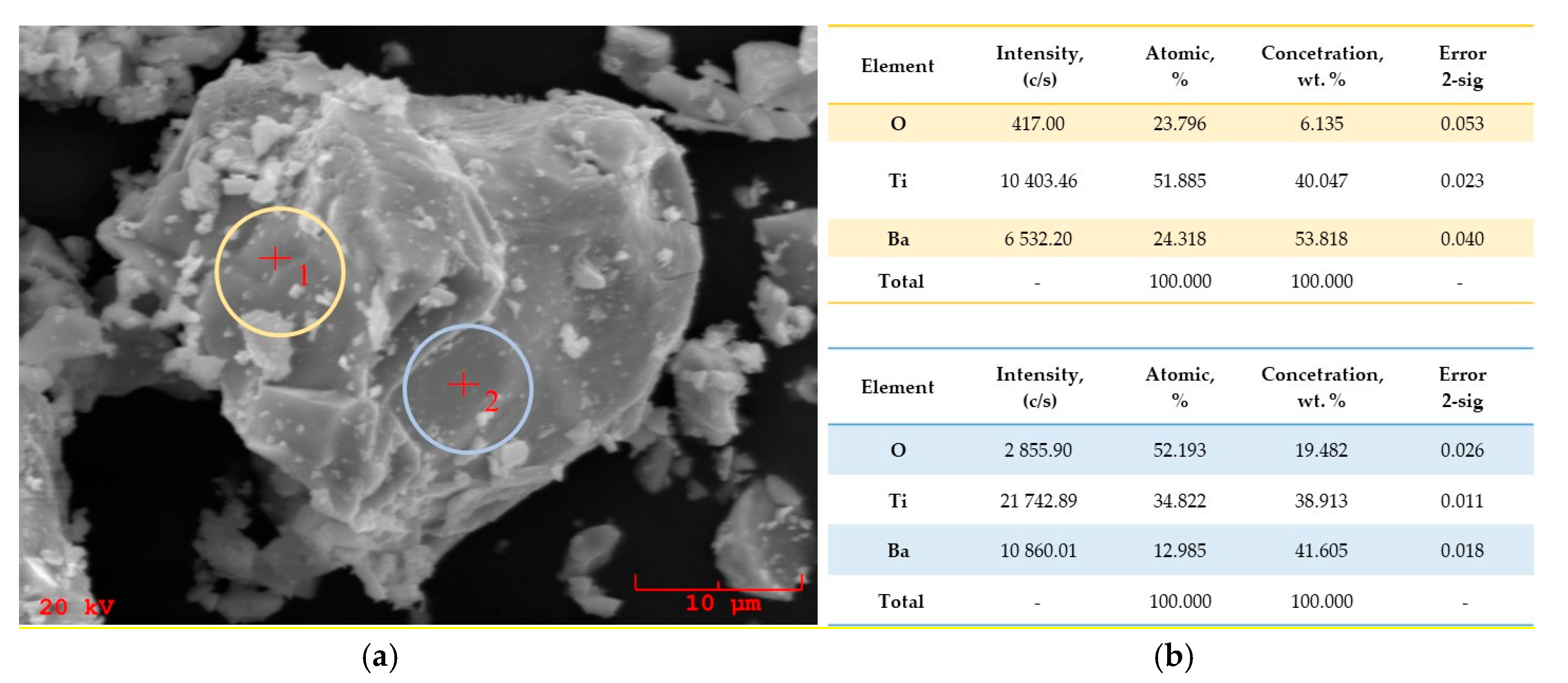
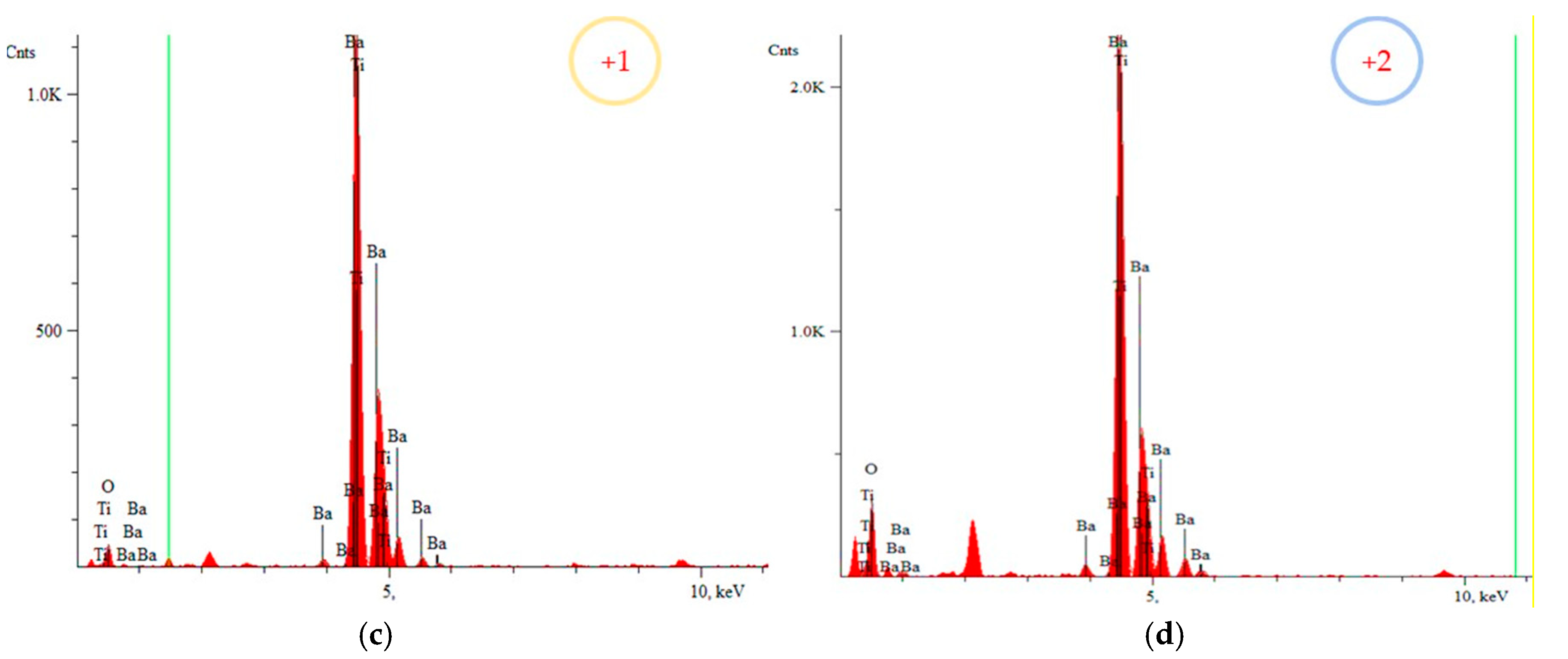
3.4. Scanning Electron Microscope with Energy Dispersive X-ray (SEM-EDS) Spectroscopy
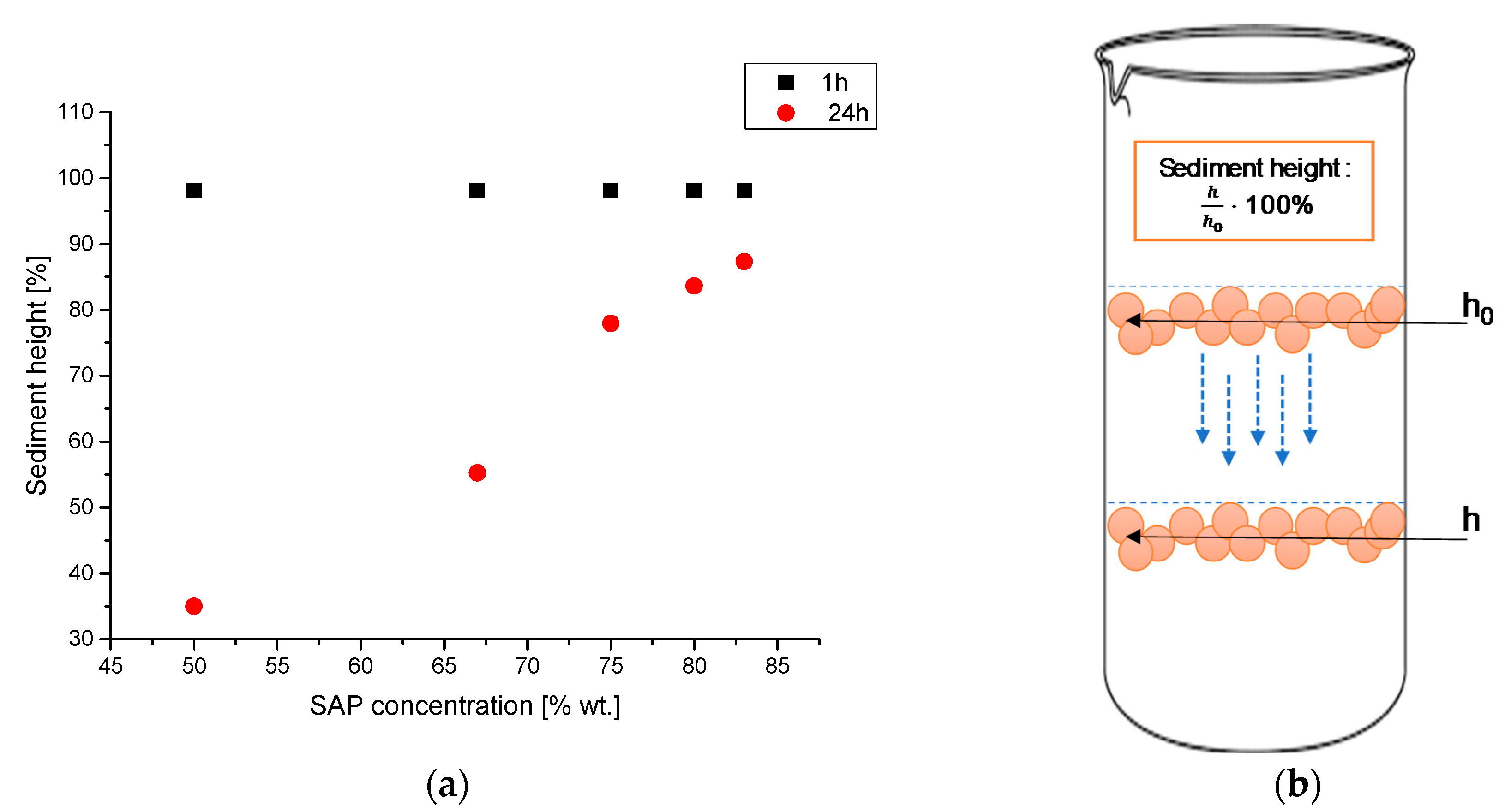
3.5. Investigations on the Sedimentation Process of Barium Titanate Particles in Obtained Suspenions
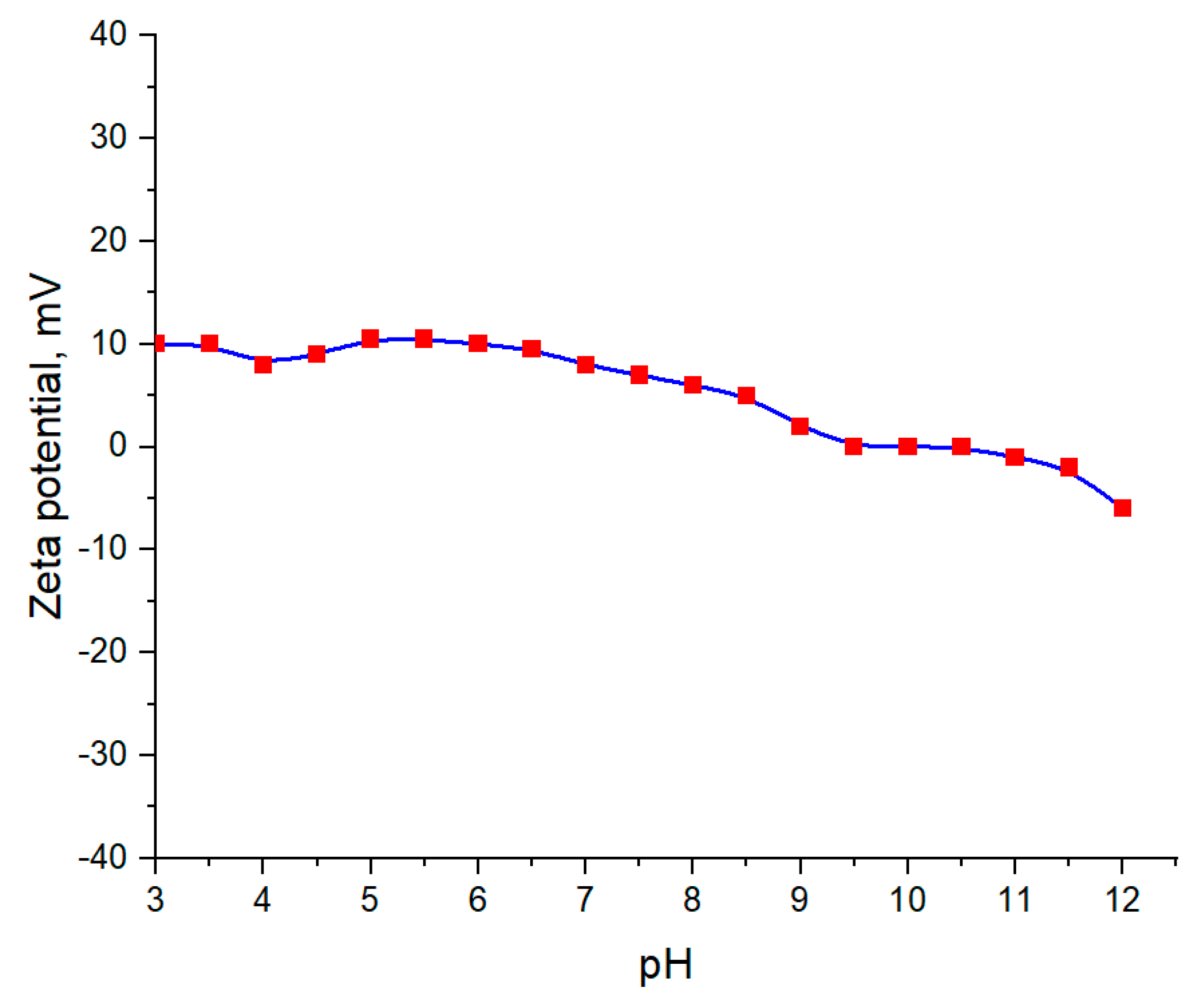
3.6. pH Investigations
Measurement of pH and Zeta Potential of Aqueous Suspension of Barium Titanate
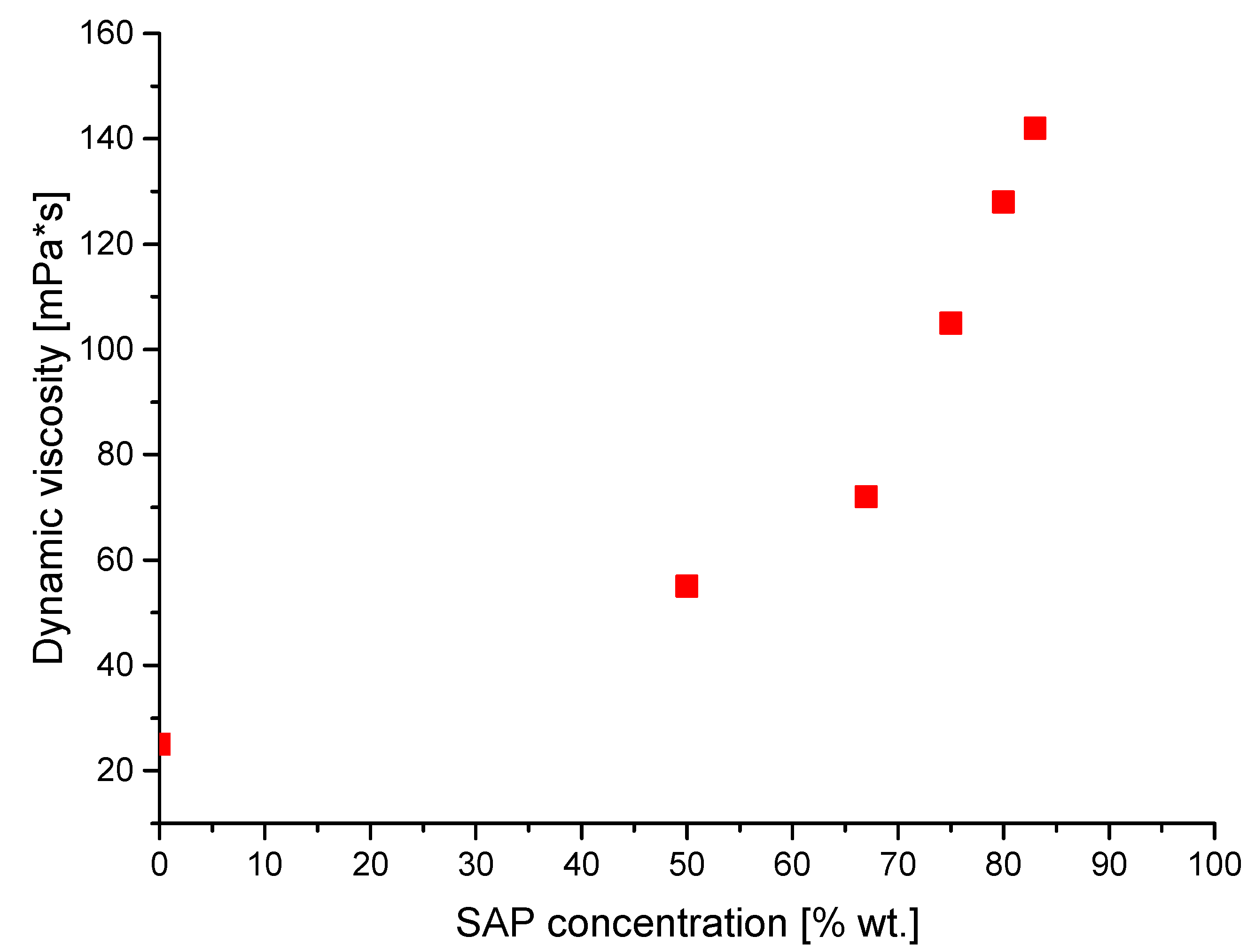
3.7. Investigations on the Dynamic Viscosity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buscaglia, V.; Randall, C.A. Size and scaling effects in barium titanate. An overview. J. Eur. Ceram. Soc. 2020, 40, 3744–3758. [Google Scholar] [CrossRef]
- Barber, P.; Balasubramanian, S.; Anguchamy, Y.; Gong, S.; Wibowo, A.; Gao, H.; Ploehn, H.J.; Loye, H.C. Polymer Composite and Nanocomposite Dielectric Materials for Pulse Power Energy Storage. Materials 2009, 2, 1697–1733. [Google Scholar] [CrossRef]
- Reynolds, G.J.; Kratzer, M.; Dubs, M.; Felzer, H.; Mamazza, R. Electrical Properties of Thin-Film Capacitors Fabricated Using High Temperature Sputtered Modified Barium titanate. Materials 2012, 5, 644–660. [Google Scholar] [CrossRef]
- Wodecka-Duś, B.; Adamczyk-Habrajska, M.; Goryczka, T.; Bochenek, D. Chemical and Physical Properties of the BLT4 of Ultra Capacitors—A Suitable Material for Ultracapacitors. Materials 2020, 13, 659. [Google Scholar] [CrossRef]
- Wu, Y.; Isakov, D.; Grant, P.S. Fabrication of Composite Filaments with High Dielectric Permittivity for Fused Deposition 3D Printing. Materials 2017, 10, 1218. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Zhao, D.; Ge, S.S. 3D printing of piezoelectric barium tinatate with high density form milled powders. J. Eur. Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Ziegmann, A.; Schubert, D.W. Influence of the particle size and the filing degree of barium titanate filled silicone elastomers used as potential dielectric elastomers on the mechanical properties and the crosslinking density. Mater. Today Commun. 2018, 14, 90–98. [Google Scholar] [CrossRef]
- Uttam, R.; Yadav, N.; Kumar, S.; Dhar, R. Strengthening of columnar hexagonal phase of a room temperature discotic liquid crystalline material by using ferroelectric barium titanate nanoparticles. J. Mol. Liq. 2019, 294, 111609. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Barczak, M.; Pearsall, F.; O’Brien, S.; Bandosz, T.J. Composite porous carbon textile with deposited barium titanate nanospheres as wearable protection medium against toxic vapors. Chem. Eng. J. 2020, 384, 123280. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, C.; Deng, H.; Fu, Q. Ni(OH)2 as a novel shell layer material for core-shell dielectric filler based on barium titanate and their dielectric polymer composites in P(VDF-HFP) matrix. Comp. Sci. Tech. 2020, 108274. [Google Scholar] [CrossRef]
- Jaglarz, J.; Duraj, R.; Szopa, P.; Cisowski, J.; Czternastek, H. Investigations of white standards by means of bidirectional reflection distribution function and integrating sphere methods. Opt. Appl. 2006, 36, 97–103. [Google Scholar]
- Gu, L.; Li, T.; Xu, Y.; Sun, C.; Yang, Z.; Zhu, D.; Chen, D. Effects of the particle size of BaTiO3 fillers on fabrication and dielectric properties of BaTiO3/Polymer/Al films for capacitor energy-storage application. Materials 2019, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, S.; Kanwal, F.; Atiq, S.; Moussa, M.; Azhar, U.; Imran, M.; Losic, D. Advancing dielectric and ferroelectric properties of piezoelectric polymers by combining graphene and ferroelectric ceramic additives for energy storage applications. Materials 2018, 11, 1553. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Wu, R.-R.; Lee, D.-J. Room-temperature vulcanized silicone rubber/barium titanate-based high-performance nanocomposite for energy harvesting. Mater. Today Chem. 2020, 16, 100232. [Google Scholar] [CrossRef]
- Binhayeeniyi, N.; Sukwisute, P.; Nawae, S.; Muensit, N. Energy conversion capacity of barium zirconate titanate. Materials 2020, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, C.; Wu, Z.; Hu, L.; Zhang, W.; Zhao, K. Fabrication and in vitro biological properties of piezoelectric bioceramics for bone regeneration. Sci. Rep. 2017, 7, 43360. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, L.; Shao, C.; Zhang, F.; Zhou, K.; Cao, J.; Zhang, D. Improved osteoblasts growth on osteomimetic hydroxyapatite/BaTiO3 composites with aligned lamellar porous structure. Mater. Sci. Eng. C Mat. Biol. App. 2016, 1, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Baxter, F.R.; Turner, I.G.; Bowen, C.R.; Gittings, J.P.; Chaudhuri, J.B. An in vitro study of electrically active hydroxyapatite-barium titanate ceramics using saos-2 cells. J. Mater. Sci. Mater. Med. 2009, 20, 1697–1708. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Zhou, K.; Zhang, D. Aligned porous barium titanate/hydroxyapatite composites with high piezoelectric coefficients for bone tissue engineering. Mat. Sci. Eng. C Mater. Biol. Appl. 2014, 1, 143–149. [Google Scholar] [CrossRef]
- Koju, N.; Sikder, P.; Galhre, B.; Bhaduri, S.B. Smart Injectable Self-Setting Monetite Based Bioceramics for Orthopedic Applications. Materials 2018, 11, 1258. [Google Scholar] [CrossRef]
- Polley, C.; Distler, T.; Detsch, R.; Lund, H.; Springer, A.; Boccaccini, A.R.; Seitz, H. 3D Printing of Piezoelectric Barium Titanate-Hydroxyapatite Scaffolds with Interconnected Porosity for Bone Tissue Engineering. Materials 2020, 13, 1773. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Basu, B.; Balani, K.; Guo, R.; Bhalla, A.S. Dielectric and pyroelectric properties of HAp-BaTiO3 composites. Ferroelectrics 2011, 423, 63–76. [Google Scholar] [CrossRef]
- Jiao, H.; Zhao, K.; Ma, L.; Tang, Y.; Liu, X.; Bian, T. Preparation and characterization of BaTiO3/HA nanocomposite materials by hydrothermal synthesis. J. Alloys Compd. 2017, 693, 221–225. [Google Scholar] [CrossRef]
- Jelinek, M.; Vanek, P.; Tolde, Z.; Buixaderas, E.; Kocourek, T.; Studnicka, V.; Drahokoupil, J.; Petzelt, J.; Remsa, J.; Tyunina, M. PLD preparet bioactive BaTiO3 films on TiNb implants. Mater. Sci. Eng. C 2017, 70, 334–339. [Google Scholar] [CrossRef]
- Stojanovic, B.D.; Simoes, A.Z.; Paiva-Santos, C.O.; Jovalekic, C.; Mitic, V.V.; Varela, J.A. Mechanochemical synthesis of barium titanate. J. Eur. Ceram. Soc. 2005, 25, 1985–1989. [Google Scholar] [CrossRef]
- Lazarevic, Z.; Romcevic, N.; Vijatovic, M.; Paunovic, N.; Romcevic, M.; Stojanovic, B.; Dohcevic-Mitrovic, Z. Characterization of barium titanate ceramic powders by raman spectroscopy. Acta Phys. Pol. A 2009, 115, 808–810. [Google Scholar] [CrossRef]
- Sydorchuk, V.; Khalameida, S.; Zazhigalov, V. Mechanochemical synthesis of barium titanate and its photocatalytic properties. Ann. Univ. Mariae Curie-Skłodowska 2009, 64, 159–168. [Google Scholar] [CrossRef]
- Ohara, S.; Kondo, A.; Shimoda, H.; Sato, K.; Abe, H.; Naito, M. Rapid mechanochemical synthesis of fine barium titanate nanoparticles. Mater. Lett. 2008, 62, 2957–2959. [Google Scholar] [CrossRef]
- Tripathy, S.S.; Raichur, A. Dispersibility of barium tsitanate suspension in the presence of polyelectrolytes: A review. J. Dispers. Sci. Technol. 2008, 29, 230–239. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, D.; Button, T.W. Preparation of concentrated barium titanate suspensions incorporating nano-sized powders. J. Eur. Ceram. Soc. 2010, 30, 171–176. [Google Scholar] [CrossRef]
- Blanco Lopez, M.C.; Rand, B.; Riley, F.L. Polymeric Stabilization of Aqueous Suspensions of Barium Titanate. Part II: Effect of Polyelectrolyte Concentration. J. Eur. Ceram. Soc. 2000, 20, 1587–1594. [Google Scholar]
- Blanco Lopez, M.C.; Rand, B.; Riley, F.L. Polymeric Stabilization of Aqueous Suspensions of Barium Titanate. Part I: Effect of pH. J. Eur. Ceram. Soc. 2000, 20, 1579–1586. [Google Scholar] [CrossRef]
- Dulian, P.; Bąk, W.; Wieczorek-Ciurowa, K.; Kajtoch, C. Dielectric properties of vanadium doped barium titanate synthesized via high-energy ball milling. Mater. Sci. Pol. 2014, 32, 257–263. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Piątkowski, M.; Dulian, P.; Bialik-Wąs, K.; Bogdał, D.; Wieczorek-Ciurowa, K.; Wzorek, Z.; Sobczak-Kupiec, A. A novel class of dispersants for colloidal processing of hydroxyapatite. Czas. Tech. Chem. 2011, 108, 225–228. [Google Scholar]
- Tyliszczak, B.; Gaca, K.Z.; Sobczak-Kupiec, A.; Dulian, P. Mechanochemical synthesis and investigations of calcium titanate powders and their acrylic dispersions. J. Eur. Ceram. Soc. 2014, 34, 2259–2264. [Google Scholar] [CrossRef]
- Sun, D.; Jin, X.; Liu, H.; Zhu, J.; Zhu, Y.; Zhu, Y. Investigation on FTIR spectrum of barium titanate ceramics doped with alkali ions. Ferroelectrics 2007, 355, 145–148. [Google Scholar] [CrossRef]
- Ashiri, R. Detailed FT-IR spectroscopy characterization and thermal analysis of synthesis of barium titanate nanoscale particles through a newly developed process. Vib. Spectrosc. 2013, 66, 24–29. [Google Scholar] [CrossRef]
- Beaudrouet, E.; Vivet, A.; Lejeune, M.; Santerne, C.; Rossignol, F.; Dossou-Yovo, C.; Mougenot, M.; Noguera, R. Stability of aqueous barium titanate suspensions for MLCC inkjet printing. J. Am. Ceram. Soc. 2014, 97, 1248–1255. [Google Scholar] [CrossRef]
- Chiang, C.W.; Jean, J.H. Effects of barium dissolution of dispersing aqueous barium titanate suspensions. Mater. Chem. Phys. 2003, 80, 647–655. [Google Scholar] [CrossRef]




| BaTiO3 [g] | Distilled Water [mL] | SAP [g] | SAP [wt.%] |
|---|---|---|---|
| 1.0 | 100 | 0 | 0 |
| 1.00 | 50 | ||
| 2.00 | 67 | ||
| 3.00 | 75 | ||
| 4.00 | 80 | ||
| 5.00 | 83 |
| Compound | Specific Surface Area, m2/g |
|---|---|
| BaTiO3 | 25.275 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Dulian, P.; Bogucki, R.; Miernik, K.; Sobczak-Kupiec, A.; Tyliszczak, B. Mechanochemical Synthesis of BaTiO3 Powders and Evaluation of Their Acrylic Dispersions. Materials 2020, 13, 3275. https://doi.org/10.3390/ma13153275
Kudłacik-Kramarczyk S, Drabczyk A, Głąb M, Dulian P, Bogucki R, Miernik K, Sobczak-Kupiec A, Tyliszczak B. Mechanochemical Synthesis of BaTiO3 Powders and Evaluation of Their Acrylic Dispersions. Materials. 2020; 13(15):3275. https://doi.org/10.3390/ma13153275
Chicago/Turabian StyleKudłacik-Kramarczyk, Sonia, Anna Drabczyk, Magdalena Głąb, Piotr Dulian, Rafał Bogucki, Krzysztof Miernik, Agnieszka Sobczak-Kupiec, and Bożena Tyliszczak. 2020. "Mechanochemical Synthesis of BaTiO3 Powders and Evaluation of Their Acrylic Dispersions" Materials 13, no. 15: 3275. https://doi.org/10.3390/ma13153275
APA StyleKudłacik-Kramarczyk, S., Drabczyk, A., Głąb, M., Dulian, P., Bogucki, R., Miernik, K., Sobczak-Kupiec, A., & Tyliszczak, B. (2020). Mechanochemical Synthesis of BaTiO3 Powders and Evaluation of Their Acrylic Dispersions. Materials, 13(15), 3275. https://doi.org/10.3390/ma13153275









