Why Polyurethanes Have Been Used in the Manufacture and Design of Cardiovascular Devices: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Design
2.2. Definition of the Research Question
2.3. Eligibility Criteria
2.4. Information Sources, Search Strategy, and Study Selection
3. Results
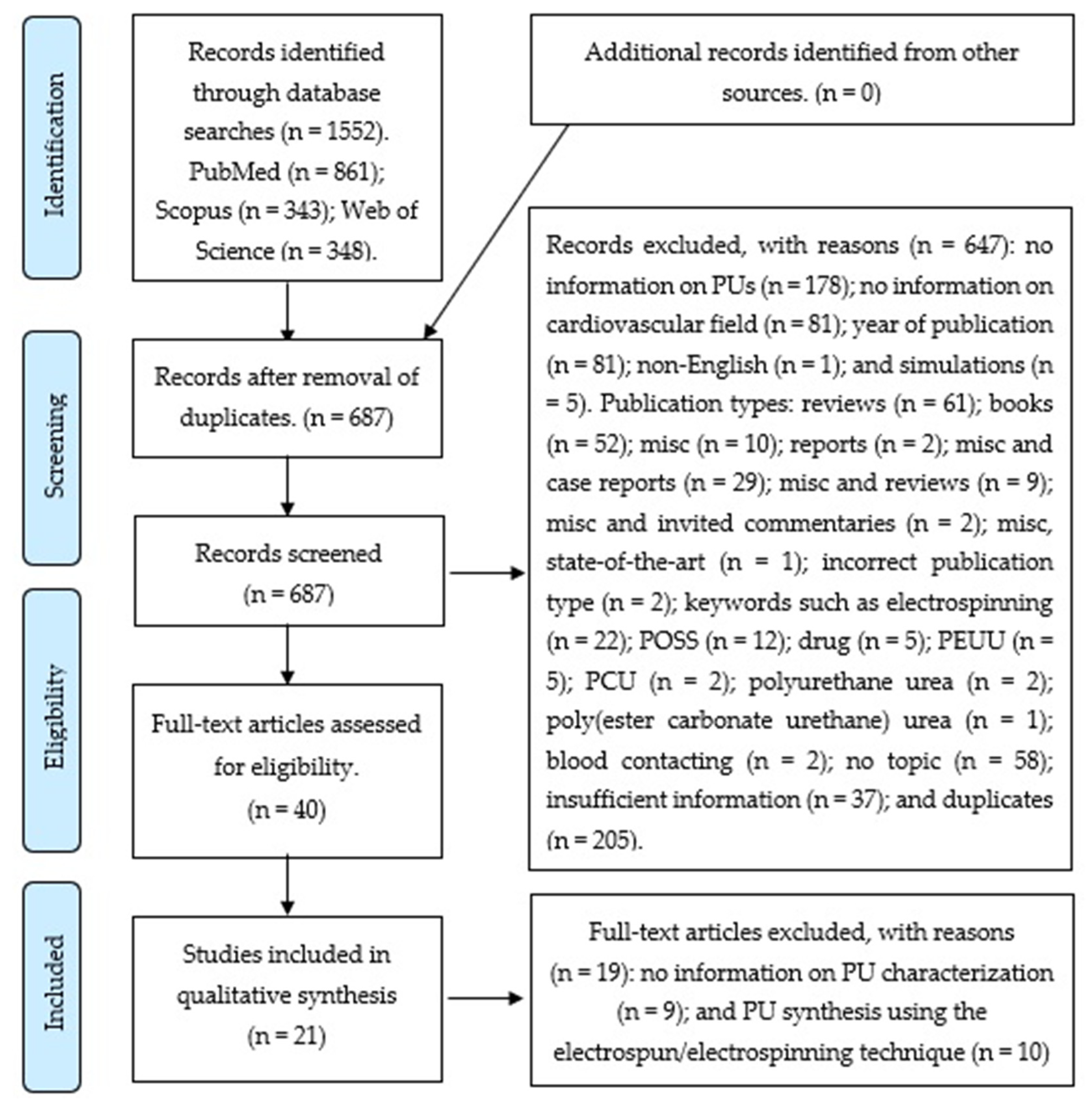
3.1. Search Results
3.2. Characteristics and Results of Included Studies
4. Discussion
4.1. PU Modification in Terms of Chemical Composition
4.1.1. Silicone
4.1.2. PCL
4.1.3. CO
4.1.4. Nanomaterial Carbon Dot–Silver Nitrate
4.2. PU Modification in Terms of Surface Functionalization
4.2.1. Surface Functionalization-Modified PU to Promote EC Adhesion and Proliferation
4.2.2. Use of Surface Functionalization-Modified PU to Enhance Biocompatibility, Bioactivity, Biodegradation Resistance, and Electrical Conductivity
5. Conclusions
- For stent design, it is important that the selected material displays self-expandable and shape memory behavior, which must be maintained at temperatures similar to those encountered in the human body. In addition, this expansion must be carried out as quickly as possible to prevent the migration of the stent during surgery. Modified PUs, such as those with added PCL or carbon dot–silver nanohybrid, show high modulus and tensile strength with low elongation and biocompatibility. Furthermore, these maintain self-expandable and shape memory behaviors. All these properties permit us to propose the use of these PUs as potential materials for stent implants.
- Among cardiovascular devices, heart patches and heart valves require the use of materials with appropriate properties such as strength and an elastomeric mechanical behavior to tolerate the contractile cardiac tissue and support its regeneration. For the design of heart patches and heart valves, it is important to create a structure that is similar to the muscle tissue. PUs are appropriate materials for cardiac applications because their biocompatibility and elastomeric behavior enable them to resist the cyclic heart stresses without deformation or failure. The PU structure can be modified to create anisotropic microstructures that may mimic the heart tissue function of different pore sizes. This could promote cell colonization, cell migration, nutrient supply, and vascularization.
- In general, for blood-contacting devices, the interactions between the material and blood generate cell responses that could favor the formation of thrombi. PUs provide a surface that can be modified to reduce non-specific protein adsorption and promote endothelial cell attachment and proliferation. This can enhance the biocompatibility and hemocompatibility. This implies that the fabrication of blood-contacting devices with modified PUs could decrease the numbers of repeated operations and deaths in cardiovascular surgery [3].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAK | alanine-alanine-lysine |
| ADP | adenosine diphosphate |
| AT | antithrombin |
| BD | 1,4-butane diol |
| BDI | 1,4-diisocyanatobutane |
| BDO | 1,4-butanediol |
| BOECs | blood outgrowth endothelial cells |
| Chol | cholesterol |
| CO | castor oil |
| Coll | collagen |
| CS | chitosan |
| DBP | di-tert-butylphenol |
| DBTDL | di-butyltindilaurate |
| DLA | direct laser ablation technique |
| D-PHI | elastomeric degradable/polar/hydrophobic/ionic |
| DS | dextran sulfate |
| ECFCs | endothelial colony forming cells |
| ECM | extracellular matrix |
| ECs | endothelial cells |
| Fn | fibronectin |
| GFs | growth factors |
| hBMSCs | human bone marrow mesenchymal stem cells |
| HDI | 1,6-diisocyanatohexane |
| HMEC | human microvascular endothelial cells |
| HPF | human plasma fibrinogen |
| HS | hard segment |
| HUVEC | human umbilical vein endothelial cell |
| IPDI | isophorone diisocyanate |
| LBL | layer-by-layer |
| MDI | 4,4′-methylenediphenyl diisocyanate |
| MDMs | monocyte-derived macrophages |
| MI | myocardial infarction |
| MISC | miscellaneous |
| PCL | polycaprolactone |
| PCU | poly(carbonate urethane) |
| PDMS | poly(dimethyl siloxane) |
| PEAP | polymeric endoaortic paving. |
| PEG | poly (ethylene glycol) |
| PEMs | polyelectrolyte multilayers |
| PEO | polyethylene oxide |
| PEUU | poly(etherurethane urea) |
| PLAL | pulsed laser ablation in liquid |
| PN | poly (ethylene glycol) bis(amine) |
| PO | poly (ethylene glycol) diglycidyl ether |
| POSS | polyhedral oligomeric silesquioxanes |
| PTFE | polytetrafluoroethylene |
| PTMEG | poly (oxytetramethylene) glycol |
| PTMO | poly (tetramethylene oxide) |
| PU | polyurethane |
| ROS | reactive oxygen species |
| SD | Sprague-Dawley |
| SMAs | shape memory alloys |
| SMCs | smooth muscle cells |
| SPEU | semi-microporous segmented polyurethane |
| TDI | 2, 4-2, 6-toluene diisocyanate |
| TE | tissue engineering |
| THP-1 | a human cell line |
| TIPS | thermally-induced phase separation |
| TPU | thermoplastic polyurethane |
| WBPU | biodegradable waterborne polyurethane |
References
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4398-6313-8. [Google Scholar]
- Cortella, L.R.X.; Cestari, I.A.; Guenther, D.; Lasagni, A.F.; Cestari, I.N. Endothelial cell responses to castor oil-based polyurethane substrates functionalized by direct laser ablation. Biomed. Mater. 2017, 12, 065010. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.; Schwenke, A.; Wagener, P.; Franzka, S.; Laszlo Sajti, C.; Pflaum, M.; Wiegmann, B.; Haverich, A.; Barcikowski, S. Dose-dependent surface endothelialization and biocompatibility of polyurethane noble metal nanocomposites. J. Biomed. Mater. Res. Part A 2014, 102, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Hulley, S.; Cummings, S.; Browner, W.; Grady, D.; Newman, T. Designing Clinical Research: Fourth Edition; Lippincott Williams & Wilkins, a Wolters Kluwer Business: Philadelphia, PA, USA, 2013; ISBN 978-1-60831-804-9. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Stachelek, S.J.; Alferiev, I.; Fulmer, J.; Ischiropouios, H.; Levy, R.J. Biological stability of polyurethane modified with covalent attachment of di-tert-butyl-phenol. J. Biomed. Mater. Res. Part A 2007, 82, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Trigwell, S.; De, S.; Sharma, R.; Mazumder, M.K.; Mehta, J.L. Structural evaluation of radially expandable cardiovascular stents encased in a polyurethane film. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006. [Google Scholar] [CrossRef]
- Stachelek, S.J.; Alferiev, I.; Connolly, J.M.; Sacks, M.; Hebbel, R.P.; Bianco, R.; Levy, R.J. Cholesterol-modified polyurethane valve cusps demonstrate blood outgrowth endothelial cell adhesion post-seeding in vitro and in vivo. Ann. Thorac. Surg. 2006. [Google Scholar] [CrossRef]
- Ashton, J.H.; Mertz, J.A.M.; Harper, J.L.; Slepian, M.J.; Mills, J.L.; McGrath, D.V.; Vande Geest, J.P. Polymeric endoaortic paving: Mechanical, thermoforming, and degradation properties of polycaprolactone/polyurethane blends for cardiovascular applications. Acta Biomater. 2011, 7, 287–294. [Google Scholar] [CrossRef]
- Silvestri, A.; Serafini, P.M.; Sartori, S.; Ferrando, P.; Boccafoschi, F.; Milione, S.; Conzatti, L.; Ciardelli, G. Polyurethane-based biomaterials for shape-adjustable cardiovascular devices. J. Appl. Polym. Sci. 2011, 122, 3661–3671. [Google Scholar] [CrossRef]
- Silvestri, A.; Sartori, S.; Boffito, M.; Mattu, C.; Di Rienzo, A.M.; Boccafoschi, F.; Ciardelli, G. Biomimetic myocardial patches fabricated with poly(ɛ-caprolactone) and polyethylene glycol-based polyurethanes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1002–1013. [Google Scholar] [CrossRef]
- Vozzi, F.; Logrand, F.; Cabiati, M.; Cicione, C.; Boffito, M.; Carmagnola, I.; Vitale, N.; Gori, M.; Brancaccio, M.; Del Ry, S.; et al. Biomimetic engineering of the cardiac tissue through processing, functionalization, and biological characterization of polyesterurethanes. Biomed. Mater. 2018, 13, 055006. [Google Scholar] [CrossRef] [PubMed]
- Raut, P.W.; Shitole, A.A.; Khandwekar, A.; Sharma, N. Engineering biomimetic polyurethane using polyethylene glycol and gelatin for blood-contacting applications. J. Mater. Sci. 2019, 54, 10457–10472. [Google Scholar] [CrossRef]
- Duarah, R.; Singh, Y.P.; Gupta, P.; Mandal, B.B.; Karak, N. High performance bio-based hyperbranched polyurethane/carbon dot-silver nanocomposite: A rapid self-expandable stent. Biofabrication 2016, 8, 045013. [Google Scholar] [CrossRef] [PubMed]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Azami, M.; Faghihi, F. Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application. Mater. Sci. Eng. C 2014, 44, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Ajili, S.H.; Ebrahimi, N.G.; Soleimani, M. Polyurethane/polycaprolactane blend with shape memory effect as a proposed material for cardiovascular implants. Acta Biomater. 2009, 5, 1519–1530. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Evans, M.D.M.; Vashi, A.V.; Gunatillake, P. Graphene/polyurethane composites: Fabrication and evaluation of electrical conductivity, mechanical properties and cell viability. RSC Adv. 2015, 5, 98762–98772. [Google Scholar] [CrossRef]
- Sharifpoor, S.; Simmons, C.A.; Labow, R.S.; Santerre, J.P. A study of vascular smooth muscle cell function under cyclic mechanical loading in a polyurethane scaffold with optimized porosity. Acta Biomater. 2010, 6, 4218–4228. [Google Scholar] [CrossRef]
- Arévalo, F.; Uscategui, Y.L.; Diaz, L.; Cobo, M.; Valero, M.F. Effect of the incorporation of chitosan on the physico-chemical, mechanical properties and biological activity on a mixture of polycaprolactone and polyurethanes obtained from castor oil. J. Biomater. Appl. 2016, 31, 708–720. [Google Scholar] [CrossRef]
- Cho, E.H.; Yang, Y.I.; Mun, C.W.; Kim, J.K. Tissue-engineered semi-microporous segmented polyetherurethane vascular prostheses. J. Biomater. Sci. Polym. Ed. 2005, 16, 775–790. [Google Scholar] [CrossRef]
- Yu, D.-G.; Lin, W.-C.; Lin, C.-H.; Yeh, Y.-H.; Yang, M.-C. Construction of antithrombogenic polyelectrolyte multilayer on thermoplastic polyurethane via layer-by-layer self-assembly technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 105–113. [Google Scholar] [CrossRef]
- Sgarioto, M.; Adhikari, R.; Gunatillake, P.A.; Moore, T.; Malherbe, F.; Nagel, M.-D.; Patterson, J. Properties and in vitro evaluation of high modulus biodegradable polyurethanes for applications in cardiovascular stents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Sun, F.; Xie, X.; Wu, X.; Zhang, Z.; Guidoin, R.; Fu, Q.; Zhong, Y.; Zhao, C. Prenatal developmental safety of functional polyurethanes for cardiovascular implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, F.; Wang, Z.; Li, J.; Tan, H.; Ding, M.; Fu, Q. Fabrication and characterization of waterborne biodegradable polyurethanes 3-dimensional porous scaffolds for vascular tissue engineering. J. Biomater. Sci. Polym. Ed. 2010, 21, 1637–1652. [Google Scholar] [CrossRef] [PubMed]
- Alperin, C.; Zandstra, P.W.; Woodhouse, K.A. Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications. Biomaterials 2005, 26, 7377–7386. [Google Scholar] [CrossRef] [PubMed]
- Grenier, S.; Sandig, M.; Mequanint, K. Polyurethane biomaterials for fabricating 3D porous scaffolds and supporting vascular cells. J. Biomed. Mater. Res. Part A 2007, 82, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.E.M.; Lehner, A.; Hollweck, T.; Haas, U.; Fano, C.; Fehrenbach, D.; Kozlik-Feldmann, R.; Wintermantel, E.; Eissner, G.; Hagl, C.; et al. In vitro biological and mechanical evaluation of various scaffold materials for myocardial tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 958–966. [Google Scholar] [CrossRef]
- Briganti, E.; Losi, P.; Raffi, A.; Scoccianti, M.; Munaò, A.; Soldani, G. Silicone based polyurethane materials: A promising biocompatible elastomeric formulation for cardiovascular applications. J. Mater. Sci. Mater. Med. 2006, 17, 259–266. [Google Scholar] [CrossRef]
- Thierfelder, N.; Koenig, F.; Bombien, R.; Fano, C.; Reichart, B.; Wintermantel, E.; Schmitz, C.; Akra, B. In vitro comparison of novel polyurethane aortic valves and homografts after seeding and conditioning. ASAIO J. 2013. [Google Scholar] [CrossRef]
- Bergmeister, H.; Seyidova, N.; Schreiber, C.; Strobl, M.; Grasl, C.; Walter, I.; Messner, B.; Baudis, S.; Fröhlich, S.; Marchetti-Deschmann, M.; et al. Biodegradable, thermoplastic polyurethane grafts for small diameter vascular replacements. Acta Biomater. 2015, 11, 104–113. [Google Scholar] [CrossRef]
- Hu, Z.J.; Li, Z.L.; Hu, L.Y.; He, W.; Liu, R.M.; Qin, Y.S.; Wang, S.M. The in vivo performance of small-caliber nanofibrous polyurethane vascular grafts. BMC Cardiovasc. Disord. 2012, 12, 115. [Google Scholar] [CrossRef]
- Lehle, K.; Stock, M.; Schmid, T.; Schopka, S.; Straub, R.H.; Schmid, C. Cell-type specific evaluation of biocompatibility of commercially available polyurethanes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xue, Y.; Sun, J. Indirect induction of endothelial cell injury by PU- or PTFE-mediated activation of monocytes. J. Biomater. Sci. Polym. Ed. 2010, 21, 1783–1797. [Google Scholar] [CrossRef]
- Ye, S.H.; Hong, Y.; Sakaguchi, H.; Shankarraman, V.; Luketich, S.K.; DAmore, A.; Wagner, W.R. Nonthrombogenic, biodegradable elastomeric polyurethanes with variable sulfobetaine content. ACS Appl. Mater. Interfaces 2014, 6, 22796–22806. [Google Scholar] [CrossRef]
- Bezuidenhout, D.; Davies, N.; Black, M.; Schmidt, C.; Oosthuysen, A.; Zilla, P. Covalent surface heparinization potentiates porous polyurethane scaffold vascularization. J. Biomater. Appl. 2010, 24, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, X.; Sun, J. PU/PTFE-stimulated monocyte-derived soluble factors induced inflammatory activation in endothelial cells. Toxicol. Vitr. 2010, 24, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Boffito, M.; Di Meglio, F.; Mozetic, P.; Giannitelli, S.M.; Carmagnola, I.; Castaldo, C.; Nurzynska, D.; Sacco, A.M.; Miraglia, R.; Montagnani, S.; et al. Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLoS ONE 2018, 13, e199896. [Google Scholar] [CrossRef]
- Klement, P.; Du, Y.J.; Berry, L.R.; Tressel, P.; Chan, A.K.C. Chronic performance of polyurethane catheters covalently coated with ATH complex: A rabbit jugular vein model. Biomaterials 2006, 27, 5107–5117. [Google Scholar] [CrossRef]
- Davoudi, P.; Assadpour, S.; Derakhshan, M.A.; Ai, J.; Solouk, A.; Ghanbari, H. Biomimetic modification of polyurethane-based nanofibrous vascular grafts: A promising approach towards stable endothelial lining. Mater. Sci. Eng. C 2017, 80, 213–221. [Google Scholar] [CrossRef]
- Spiller, D.; Mirtelli, C.; Losi, P.; Briganti, E.; Sbrana, S.; Counoupas, C.; Kull, S.; Tonlorenzi, S.; Soldani, G. In vitro evaluation of the PEtU-PDMS material immunocompatibility: The influence of surface topography and PDMS content. J. Mater. Sci. Mater. Med. 2009, 20, 2511–2520. [Google Scholar] [CrossRef]
- Du, Y.J.; Klement, P.; Berry, L.R.; Tressel, P.; Chan, A.K.C. In vivo rabbit acute model tests of polyurethane catheters coated with a novel antithrombin-heparin covalent complex. Thromb. Haemost. 2005, 94, 366–372. [Google Scholar] [CrossRef]
- Thampi, S.; Nandkumar, A.M.; Muthuvijayan, V.; Parameswaran, R. Differential Adhesive and Bioactive Properties of the Polymeric Surface Coated with Graphene Oxide Thin Film. ACS Appl. Mater. Interfaces 2017, 9, 4498–4508. [Google Scholar] [CrossRef] [PubMed]
| Author | Year of Publication | Geographic Setting | PU/Chemical Composition | Type of Study (in vitro, in vivo)/Cells | Field of Application | Main Results (Extracted and/or Adapted) |
|---|---|---|---|---|---|---|
| Trigwell et al. [8] | 2005 | USA | ChronoFlex® AR PU/polytetrafluoroethylene (PTFE) | x 1 | Cardiovascular stents | A method of encasing cardiovascular stents with an expandable PU coating was developed to provide a smooth homogeneous inner wall that allows for a confluent growth of endothelial cells. The PU film covered the metal wire stent structure, thereby minimizing biocorrosion of the metal (stainless steel or nitinol) and providing a homogeneous surface. The stent structure covered with a film of less than 25 µm could display sufficient corrosion resistance and flexibility without producing excess stress in the structure. |
| Cho et al. [21] | 2005 | South Korea | Poly (oxytetramethylene) glycol (PTMEG)/ 1,4-butane diol (BD)/4,4′-methylenediphenyl diisocyanate (MDI) + Collagen (Coll) | In vitro. HMEC | Vascular prostheses | The semi-microporous segmented polyurethane (SPEU) used in this work showed properties that promote endothelial cell attachment and proliferation. The endothelial cells were attached to the SPEU semi-pores, which resulted in less platelet adhesion. The authors recommend collagen as a coating. However, a collagen coating on the SPEU surface affected the endothelial cell attachment, as well as platelet attachment. It is not recommended for use if factors such as blood coagulation and prosthesis patency are important. |
| Yu et al. [22] | 2006 | Taiwan | Thermoplastic polyurethane (TPU)/chitosan (CS)/dextran sulfate (DS) | In vitro. L-929 fibroblast | Antithrombo-genic coating for application in hemodialysis or cardiovascular devices. | In this work, PEM of CS/DS (CS as a positively charged agent, and DS as a negatively-charged and an antiadhesive agent) were deposited onto the aminolyzed TPU film surface by the LBL self-assembly technique. The authors note that the deposition of over four bilayers with DS, as the outermost layer could improve the hydrophilicity of the TPU film, suppress the protein adsorption and platelet adhesion, and prolong the blood coagulation time. |
| Stachelek et al. [9] | 2006 | USA | Tecothane™/cholesterol (Chol) | In vitro, in vivo. Sheep blood outgrowth endothelial cells (BOECs) | Heart valve | PU-Chol has been presented as an option for BOECs for applications such as PU heart valve leaflet implants. This work indicated that PU-Chol has significantly better BOEC adhesion properties than unmodified PU under simulated and in vivo heart valve shear force conditions. |
| Stachelek et al. [7] | 2007 | USA | Tecothane™/di-tert-butylphenol (DBP) | In vitro, in vivo. THP-1 cells | Heart valve leaflets and PU artificial heart devices. | This work showed that the covalent modification of PUs such as Tecothane by using DBP conferred biodegradation resistance in vivo and that this biodegradation is dependent on the DBP dose. It is important to note that the modification with DBP could be effective in trapping oxygen radicals that are released from adherent MDMs that interact with PUs. |
| Ajili et al. [17] | 2009 | Iran | Polyesterurethane (MDI/1,4-butanediol (BDO)/polycaprolactone (PCL) | In vitro. human bone marrow mesenchymal stem cells (hBMSCs) | Cardiovascular stents | In this work, authors observed that shape memory materials have been proposed for cardiovascular stents owing to their self-expansion capability. This capability is important for polymeric stent deployment at temperatures near the body temperature. To work on this capability, the investigators used crystallinity-induced shape memory effect to incorporate elastic memory in a stent. They used PU/PCL blends as materials for shape memory stents. The PU/PCL blend compositions and crystallization conditions were modified. The PU/PCL (70/30) blend showed remarkable biocompatibility, which was indicated by the adhesion and proliferation of bone marrow mesenchymal stem cells compared with the other blends. Furthermore, this blend is a potential material for use in stent implantx. |
| Sharifpoor et al. [19] | 2010 | Canada | D-PHI. 2 porogen (sodium bicarbonate (salt) and poly(ethylene glycol) (PEG)) | In vitro. A10 smooth muscle cells (SMCs) -thoracic aorta of embryonic rats. | Vascular tissue engineering applications | In this study, the authors used double porogen (PEG–salt) to optimize the pore interconnectivity and to increase the porosity in the PU without compromising on the scaffold mechanical integrity. The materials were tested under dynamic mechanical stretching to mimic the biomechanical conditions. The use of PEG–salt porogens was effective in improving the pore interconnectivity through the production of micropores in the range of 1 to −5 µm, and in increasing the total scaffold porosity by enabling the addition of more salt within the monomer–porogen mixture. |
| Ashton et al. [10] | 2010 | USA | PCL/Tecoflex® SG-80A | x | Cardiovascular applications. Polymeric endoaortic paving | In this work, PCL/PU blends were used as paving materials for PEAP. The authors noted that the blends’ stiffness was similar to that of aortic tissue, depends on the PCL content, and may be affected by thermoforming and degradation. The PEAP, consisting of a PCL/PU blend, may be effective for developing highly advanced endoaortic therapy. |
| Silvestri et al. [11] | 2011 | Italy | Poly(dimethyl siloxane)—poly (tetramethylene oxide) (PDMS–PTMO) -based PU. Clay as a filler | In vitro. NIH 3T3 mouse fibroblast | Cardiovascular devices | In this work, the authors modified the PU chemical composition and used clay as a filler. They described that these modifications enable the determination of an appropriate formulation for biostable cardiovascular devices. Among the chemical compositions, the authors used a nonaromatic diisocyanate (HDI). This enables the development of mechanical properties close to those of the native mitralic tissue and prevents the production of highly toxic aromatic diamines. The authors indicated that a higher percentage of PTMO in the soft segment improved the mechanical performances of PUs. That is, it increased the Young’s modulus, the stress at break, and the maximum strain. The Young’s modulus values at 37 °C were included in the required range (6 MPa in the circumferential (parallel to the annulus) direction and 2 MPa in the longitudinal (perpendicular to the annulus) direction) for annuloplastic applications. |
| Jiang et al. [25] | 2012 | People’s Republic of China–Canada | Biodegradable waterborne polyurethane (WBPU): isophorone diisocyanate (IPDI) /BDO/PEG/PCL/L-lysine | In vitro. HUVECs | Soft tissue engineering | In this study, the freeze-drying technique was used to fabricate 3-D interconnected porous scaffolds using a non-toxic, waterborne, biodegradable PU emulsion. The goal was to prepare scaffolds with appropriate pore diameter, pore diameter distribution, and porosity for use in soft tissue engineering. The authors observed that the relatively smaller pore diameter, narrower pore diameter distribution, and lower porosity were more advantageous for the scaffold endothelialization. |
| Silvestri et al. [12] | 2013 | Italy | PEG/PCL/1,4-diisocyanatobutane (BDI). L-Lysine Ethyl Ester and AAK as chain extenders. | In vitro. Rat heart cell line (H9C2 cardiomyoblasts) | Heart patches for myocardial function restoration and cardiac tissue regeneration after an MI | The authors used TIPS technique to prepare scaffolds with an analogous structure of the striated myocardial tissue for myocardial applications. The authors noted that the material was similar to the streaked muscle tissue because of the presence of the anisotropic microstructure. Thereby, the so called “structural biomimicry” was achieved, which is a requirement for the application of biomaterials in TE. The different pore sizes can promote different processes: large pores favor cell colonization, cell migration, and nutrient supply; meanwhile, small pores can promote vascularization. |
| Hess et al. [3] | 2013 | Germany | Elastogran/Au-Pt | In vitro. ECFCs | Blood-contacting medical devices | The authors applied the PLAL technique to generate TPU–noble metal nanocomposites with different concentrations of Pt or Au nanoparticles between 0 and 1 wt%. The presence of metal nanocomposites in TPU improved the biocompatibility and cell adhesion. The authors relate this effect to the hydrophilic and negatively-charged surface. Results showed that ECFCs seeded onto the nanocomposites remained in a nonthrombogenic and noninflammatory state. Thereby, the material can potentially decrease the number of reoperations and deaths in the field of cardiovascular medicine. |
| Baheiraei et al. [16] | 2014 | Iran | PEG/PCL/IPDI/aniline | In vitro. L-929 HUVECs | Cardiac tissue engineering | The authors worked on materials for TE. They studied the low conductivity of the patch in cardiac TE because this characteristic could limit the patch’s capability to couple transplanted cells electrically to the local host myocardium. This study recommends the use of oligoaniline as an electroactive conductive polymer. These materials were non-toxic, supported cell proliferation and attachment, and combined with antioxidant properties. |
| Sgarioto et al. [23] | 2014 | Australia, France | NovoSorb™. extracellular matrix (ECM) as Coll and fibronectin (Fn) | In vitro. HUVECs | Cardiovascular stents | The authors studied materials for application in cardiovascular stents. Specifically, they worked with biodegradable PUs with different HS percentages. The main results revealed that the PUs showed high modulus and tensile strength with low elongation, which are key characteristic for fabricating vascular stents. The tensile strength reduced significantly upon gamma sterilization in the case of PUs with a low content of HSs. Meanwhile, the strength was maintained for the materials with high HS contents. |
| Gu et al. [24] | 2015 | People’s Republic of China–Canada | Polycarbonate urethanes with poly (ethylene glycol) diglycidyl ether (PEG-PO) or poly (ethylene glycol) bis(amine) (PEG-PN) | In vivo. Sprague-Dawley (SD) rats | Coatings for cardiovascular devices | The authors aimed to study the toxicity development in cardiovascular implants. The materials synthesized included hydrophilic PEG side-chains attached to the HS. The authors noted that these chains increase the hydrophilicity of the macromolecules and modify the hydrogen bonds among the HS, both of which favor hydrolytic degradation. The results did not show any maternal and fetal toxicity. The use of this material in cardiovascular applications was proposed based on this observation. |
| Kaur et al. [18] | 2015 | Australia | Graphene/ElastEon™ composite films | In vitro. L-929 cells | Biomedical applications | The authors used three methods (solution mixing, melt processing, and in situ methods) to synthesize conductive composites of a siloxane PU and graphene for potential use in biomedical applications. The results showed that the solution mixing method yielded composites with the highest electrical conductivity and that it is suitable for preparing composites with better mechanical properties. The authors also noted that the materials were not cytotoxic to living cells in vitro and are potentially useful in biomedical applications. |
| Arevalo et al. [20] | 2016 | Colombia | Castor oil/PCL/IPDI/CS | In vitro. L-929 and 3T3 cells | Biomedical applications related to soft and cardiovascular tissues | The authors aimed to study materials for potential biomedical applications. They synthesized PU from castor oil, PCL, and IPDI, using CS as the additive. The results showed that the presence of CS in materials enhances the ultimate tensile strength and does not affect the strain at fracture in PUs with 5% w/w of PCL and CS in the range of 0–2% w/w. The authors noted that PUs had mechanical properties similar to those of the aorta and skin. |
| Duarah et al. [15] | 2016 | India | PCL/2, 4-2, 6-toluene diisocyanate (TDI)/silver nitrate (AgNO3) /soluble starch | In vitro. SMCs. endothelial cells (ECs). | Cardiovascular stents | The goal of this work was to synthesize materials for rapid self-expandable stents for possible endoscopic surgeries. The authors used a one-pot single step technique to obtain a carbon dot–silver nanohybrid. The results showed that it was possible to obtain a material with self-expandable and shape memory behaviors, as well as enhanced thermal and mechanical properties. |
| Cortella et al. [2] | 2017 | Brazil–Germany | CO/MDI | In vitro. iHUVEC | Cardiovascular devices | In this work, the authors recommended the use of the DLA technique for producing patterned topographies in the micrometer range. The results show that the topographical patterns produced by the DLA technique on cellular processes may contribute to the development and maintenance of a functional endothelium on target surfaces by the functionalization of their blood-contacting surfaces. |
| Vozzi et al. [13] | 2018 | Italy | PCL/BDI/L-lysine ethyl ester | In vitro. Cardiomyocytes | Cardiac tissue engineering | The authors aimed to design a material that mimics cardiac tissue properties. They used the TIPS technique to fabricate scaffolds, which were functionalized with fibronectin. The results highlighted the feasibility of fabricating PU scaffolds with porous-aligned structures and mechanical properties consistent with those of the myocardial tissue. |
| Rau et al. [14] | 2019 | India | Elastollan/PEG/gelatin as a surface modifier | In vitro. HUVECs | Blood-contacting devices. | The authors recommended PU modification with PEG for blood-contacting applications. They observed that the PU modification reduced non-specific protein adsorption and promoted endothelial cell attachment and proliferation. They also emphasized that the PU surfaces modified with PEG and gelatin enhanced the hydrophilicity. This yielded enhanced biocompatibility and hemocompatibility. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navas-Gómez, K.; Valero, M.F. Why Polyurethanes Have Been Used in the Manufacture and Design of Cardiovascular Devices: A Systematic Review. Materials 2020, 13, 3250. https://doi.org/10.3390/ma13153250
Navas-Gómez K, Valero MF. Why Polyurethanes Have Been Used in the Manufacture and Design of Cardiovascular Devices: A Systematic Review. Materials. 2020; 13(15):3250. https://doi.org/10.3390/ma13153250
Chicago/Turabian StyleNavas-Gómez, Kelly, and Manuel F. Valero. 2020. "Why Polyurethanes Have Been Used in the Manufacture and Design of Cardiovascular Devices: A Systematic Review" Materials 13, no. 15: 3250. https://doi.org/10.3390/ma13153250
APA StyleNavas-Gómez, K., & Valero, M. F. (2020). Why Polyurethanes Have Been Used in the Manufacture and Design of Cardiovascular Devices: A Systematic Review. Materials, 13(15), 3250. https://doi.org/10.3390/ma13153250





