Lanthanum Ferrites-Based Exsolved Perovskites as Fuel-Flexible Anode for Solid Oxide Fuel Cells
Abstract
1. Introduction
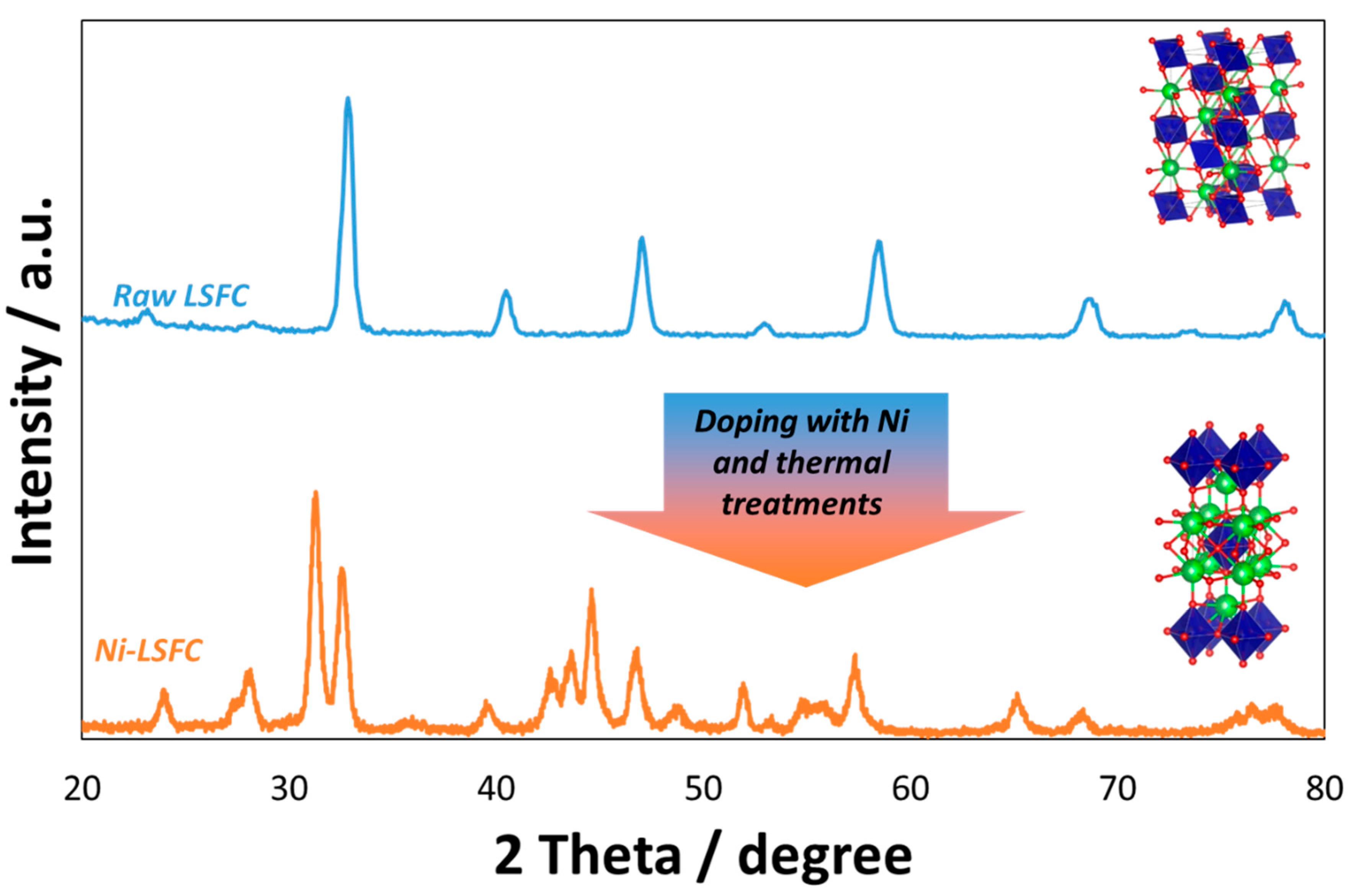
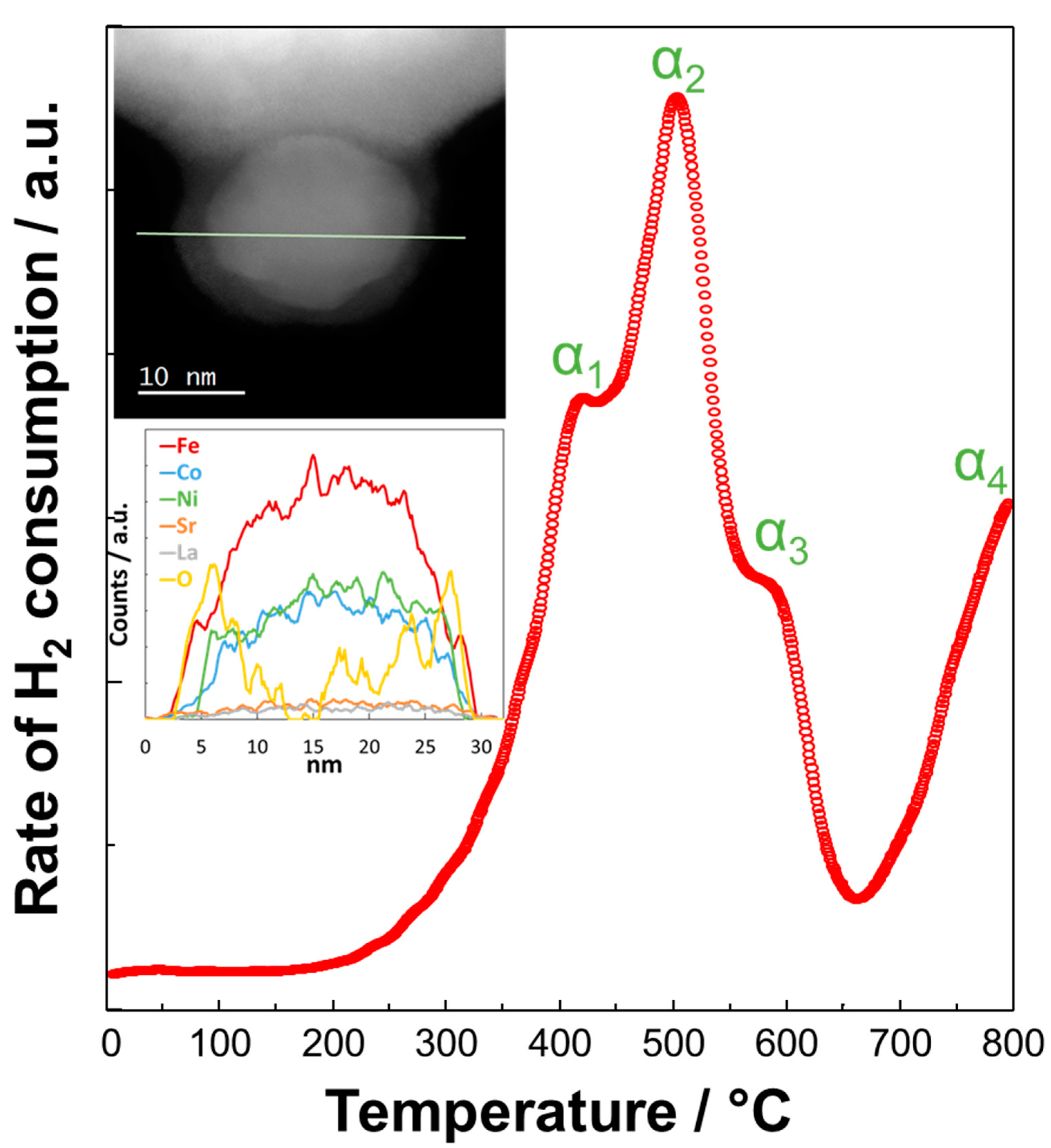
2. Surface Exsolution and Physicochemical Studies
3. Catalytic Studies
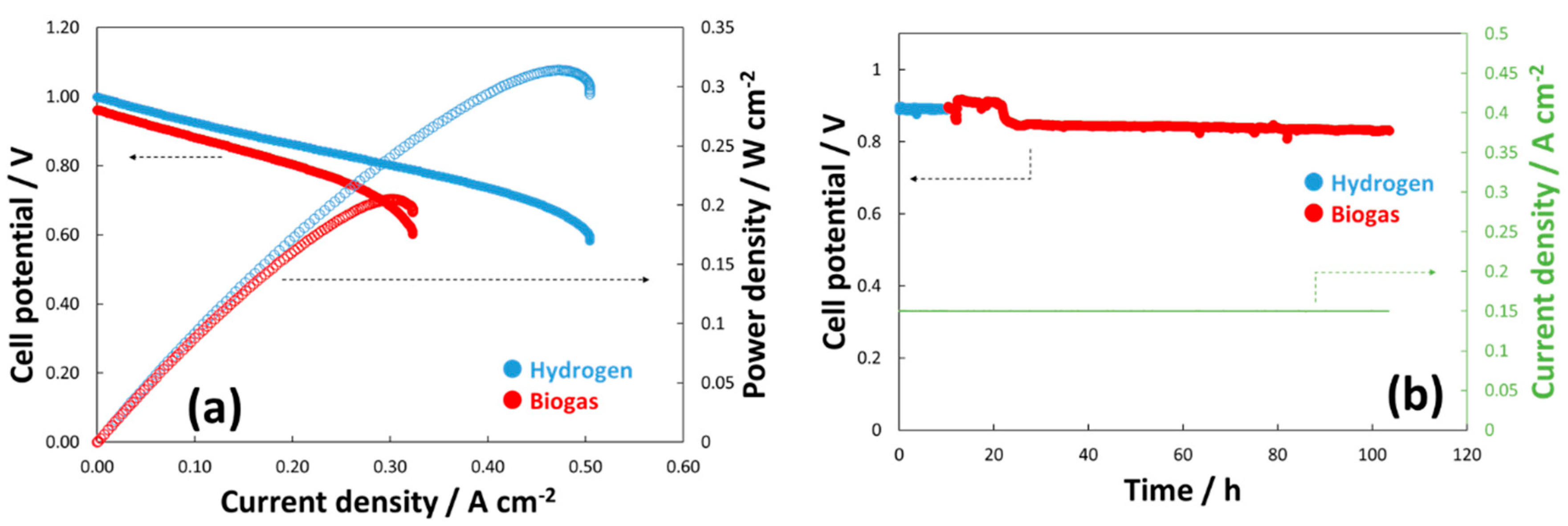
4. Electrochemical Studies
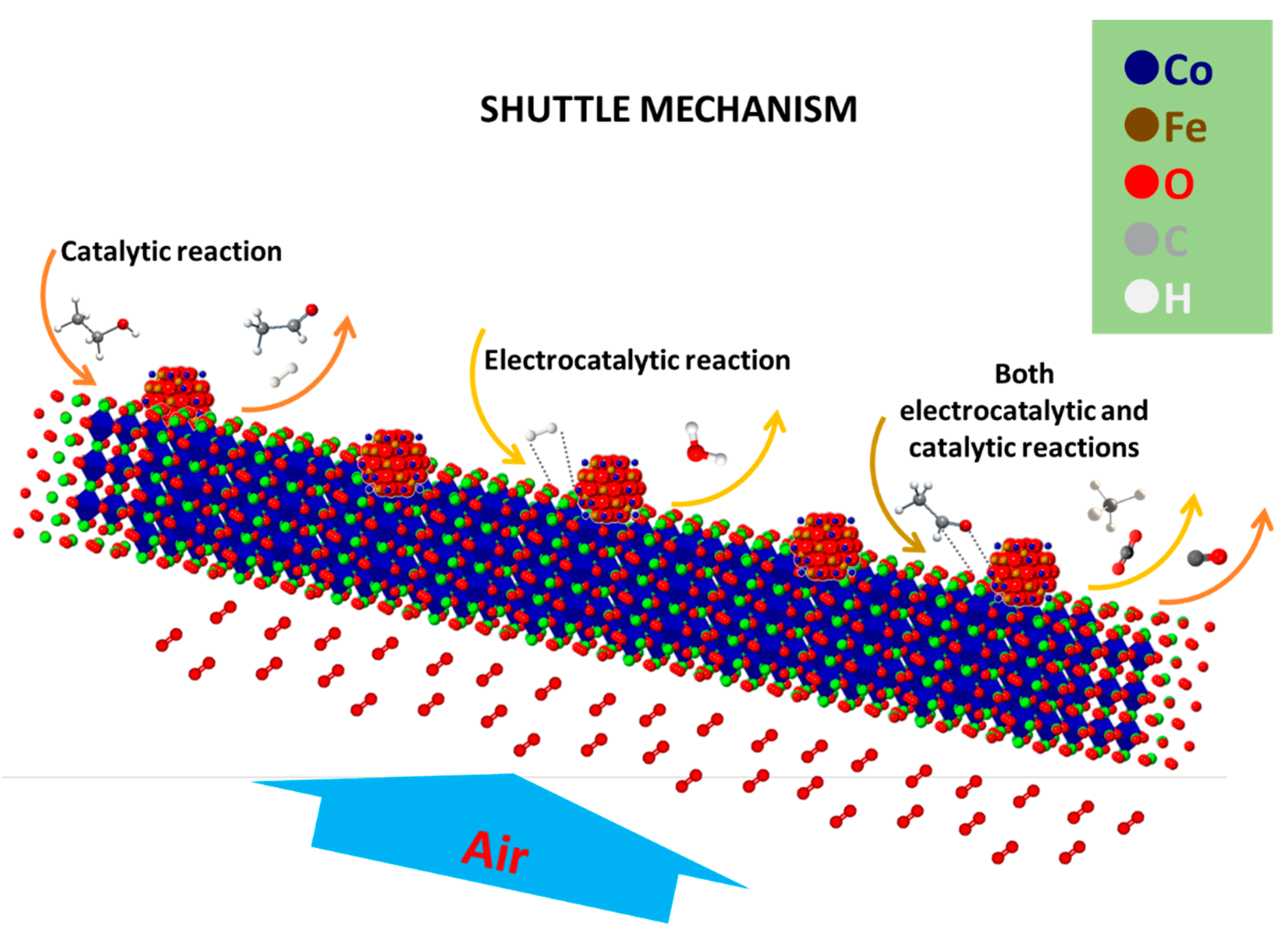
5. Reaction Mechanism
6. Key Insights on Challenges and Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Acronym | Full form |
| ASC | Anode Supporting Cell |
| ASR | Area Specific Resistance |
| ATR | Autothermal Reforming |
| BET | Brunauer–Emmett–Teller surface area analysis |
| BYCO | BaCe0.9Y0.1O3-δ |
| CGO | Gd0.2Ce0.8O2-d |
| CHNS-O | Carbon, Hydrogen, Nitrogen, Sulfur, Oxygen Elemental Analyzer |
| EDX | Energy-dispersive X-ray spectroscopy |
| LSFC | La0.6Sr0.4Fe0.8Co0.2O3 |
| LSGM | La0.8Sr0.2Ga0.8Mg0.2O3 |
| OCV | Open Circuit Voltage |
| POX | Partial Oxidation Reaction |
| SOFC | Solid Oxide Fuel Cells |
| SR | Steam Reforming |
| TPR | Temperature-Programmed Reduction |
| XPS | X-ray Photoelectron Spectroscopy |
| YSZ | Yttria-stabilized Zirconia |
| HR-TEM | High-Resolution Transmission Electron Microscopy |
References
- Dong, Y.; Steinberg, M. Hynol—An economical process for methanol production from biomass and natural gas with reduced CO2 emission. Int. J. Hydrog. Energy 1997, 22, 971–977. [Google Scholar] [CrossRef]
- Gibbs, C.E.; Steel, M.C.F. European opportunities for fuel cell commercialisation. J. Power Sources 1992, 37, 35–43. [Google Scholar] [CrossRef]
- Lee, A.L.; Zabransky, R.F.; Huber, W.J. Internal reforming development for Solid Oxide Fuel Cells. Ind. Eng. Chem. Res. 1990, 29, 766–773. [Google Scholar] [CrossRef]
- Bove, R.; Sammes, N.M. Thermodynamic analysis of SOFC systems using different fuel processors. In Proceedings of the ASME 2004 2nd International Conference on Fuel Cell Science, Engineering and Technology, Rochester, NY, USA, 14–16 June 2004; pp. 461–466. [Google Scholar]
- Steele, B.C.H. Materials for IT-SOFC stacks—35 years R&D: The inevitability of gradualness? Solid State Ion. 2000, 134, 3–20. [Google Scholar] [CrossRef]
- La Rosa, D.; Lo Faro, M.; Monforte, G.; Antonucci, V.; Arico, A.S. Comparison of the electrochemical properties of intermediate temperature solid oxide fuel cells based on protonic and anionic electrolytes. J. Appl. Electrochem. 2009, 39, 477–483. [Google Scholar] [CrossRef]
- Sauvert, A.L.; Fouletier, J. Research trends: Electrochemical properties of new type of IT-SOFC anode material. Fuel Cells Bull. 2002, 2002, 12. [Google Scholar]
- Lo Faro, M.; La Rosa, D.; Antonucci, V.; Arico, A.S. Intermediate temperature solid oxide fuel cell electrolytes. J. Indian Inst. Sci. 2009, 89, 363–380. [Google Scholar]
- Kikuchi, R.; Koashi, N.; Matsui, T.; Eguchi, K.; Norby, T. Novel anode materials for multi-fuel applicable solid oxide fuel cells. J. Alloy. Compd. 2006, 408, 622–627. [Google Scholar] [CrossRef]
- La Rosa, D.; Lo Faro, M.; Monforte, G.; Antonucci, V.; Arico, A.S.; Antonucci, P. Propane conversion over a Ru/CGO catalyst and its application in intermediate temperature solid oxide fuel cells. J. Appl. Electrochem. 2007, 37, 203–208. [Google Scholar]
- La Rosa, D.; Sin, A.; Lo Faro, M.; Monforte, G.; Antonucci, V.; Arico, A.S. Mitigation of carbon deposits formation in intermediate temperature solid oxide fuel cells fed with dry methane by anode doping with barium. J. Power Sources 2009, 193, 160–164. [Google Scholar] [CrossRef]
- Lo Faro, M.; La Rosa, D.; Frontera, P.; Antonucci, P.; Antonucci, V.; Arico, A.S. Propane-fed Solid Oxide Fuel Cell based on a composite Ni-La-CGO anode catalyst. Catal. Lett. 2010, 136, 57–64. [Google Scholar] [CrossRef]
- De Marco, V.; Iannaci, A.; Lo Faro, M.; Sglavo, V.M. Influence of Copper-based anode composition on intermediate temperature Solid Oxide Fuel Cells performance. Fuel Cells 2017, 17, 708–715. [Google Scholar] [CrossRef]
- Escudero, M.J.; Yeste, M.P.; Cauqui, M.A.; Muñoz, M.A. Performance of a direct methane solid oxide fuel cell using nickel-ceria-yttria stabilized zirconia as the anode. Materials 2020, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Gandiglio, M.; Lanzini, A.; Santarelli, M.; Acri, M.; Hakala, T.; Rautanen, M. Results from an industrial size biogas-fed SOFC plant (the DEMOSOFC project). Int. J. Hydrog. Energy 2020, 45, 5449–5464. [Google Scholar] [CrossRef]
- Yokokawa, H.; Suzuki, M.; Yoda, M.; Suto, T.; Tomida, K.; Hiwatashi, K.; Shimazu, M.; Kawakami, A.; Sumi, H.; Ohmori, M.; et al. Achievements of NEDO durability projects on SOFC stacks in the light of physicochemical mechanisms. Fuel Cells 2019, 19, 311–339. [Google Scholar] [CrossRef]
- Santhanam, S.; Ullmer, D.; Wuillemin, Z.; Varkaraki, E.; Beetschen, C.; Antonetti, Y.; Ansar, A. Experimental analysis of a 25 kWe solid oxide fuel cell module for co-generation of hydrogen and power. Ecs Trans. 2019, 91, 159–166. [Google Scholar] [CrossRef]
- McPhail, S.J.; Pumiglia, D.; Laurencin, J.; Hagen, A.; Leon, A.; Van Herle, J.; Vladikova, D.; Montinaro, D.; Piccardo, P.; Polverino, P.; et al. Developing accelerated stress test protocols for solid oxide fuel cells and electrolysers: The European project AD ASTRA. Ecs Trans. 2019, 91, 563–570. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.H.; Xu, Q. A solid oxide fuel cell (SOFC)-based biogas-from-waste generation system for residential buildings in China: A feasibility study. Sustainability 2018, 10, 2395. [Google Scholar] [CrossRef]
- Stoeckl, B.; Subotić, V.; Preininger, M.; Schroettner, H.; Hochenauer, C. SOFC operation with carbon oxides: Experimental analysis of performance and degradation. Electrochim. Acta 2018, 275, 256–264. [Google Scholar] [CrossRef]
- Maraver, D.; Tondi, G.; Goodchild, R. Overview and current status of eu funded actions on bio-fuelled heating and combined heating & power within the energy challenge of Horizon 2020. In Proceedings of the 26th European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–18 May 2018; pp. 1289–1298. [Google Scholar]
- Montinaro, D.; Sglavo, V.M.; Bertoldi, M.; Zandonella, T.; Aricò, A.; Lo Faro, M.; Antonucci, V. Tape casting fabrication and co-sintering of solid oxide “half cells” with a cathode–electrolyte porous interface. Solid State Ion. 2006, 177, 2093–2097. [Google Scholar] [CrossRef]
- Murata, K.; Shimotsu, M. Fabrication and evaluation of electrode-supported planar SOFC. Denki Kagaku 1997, 65, 38–43. [Google Scholar] [CrossRef]
- Wincewicz, K.C.; Cooper, J.S. Taxonomies of SOFC material and manufacturing alternatives. J. Power Sources 2005, 140, 280–296. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, M.; Hong, J.; Kim, H.; Yoon, K.J.; Son, J.W.; Lee, J.H.; Kim, B.K. Constrained sintering in fabrication of solid oxide fuel cells. Materials 2016, 9, 675. [Google Scholar] [CrossRef]
- Singh, D.; Hernández-Pacheco, E.; Hutton, P.N.; Patel, N.; Mann, M.D. Carbon deposition in an SOFC fueled by tar-laden biomass gas: A thermodynamic analysis. J. Power Sources 2005, 142, 194–199. [Google Scholar] [CrossRef]
- Grgicak, C.M.; Green, R.G.; Giorgi, J.B. SOFC anodes for direct oxidation of hydrogen and methane fuels containing H2S. J. Power Sources 2008, 179, 317–328. [Google Scholar] [CrossRef]
- Schluckner, C.; Subotić, V.; Lawlor, V.; Hochenauer, C. Carbon deposition simulation in porous SOFC anodes: A detailed numerical analysis of major carbon precursors. J. Fuel Cell Sci. Technol. 2015, 12, 051007. [Google Scholar] [CrossRef]
- Fernandes, M.D.; Bistritzki, V.; Domingues, R.Z.; Matencio, T.; Rapini, M.; Sinisterra, R.D. Solid oxide fuel cell technology paths: National innovation system contributions from Japan and the United States. Renew. Sustain. Energy Rev. 2020, 127, 109879. [Google Scholar] [CrossRef]
- Lo Faro, M.; Trocino, S.; Zignani, S.C.; Aricò, A.S.; Maggio, G.; Italiano, C.; Fabiano, C.; Pino, L.; Vita, A. Study of a Solid Oxide Fuel Cell fed with n-dodecane reformate. Part I: Endurance test. Int. J. Hydrog. Energy 2016, 41, 5741–5747. [Google Scholar] [CrossRef]
- Lo Faro, M.; Trocino, S.; Zignani, S.C.; Italiano, C.; Vita, A.; Aricò, A.S. Study of a solid oxide fuel cell fed with n-dodecane reformate. Part II: Effect of the reformate composition. Int. J. Hydrog. Energy 2017, 42, 1751–1757. [Google Scholar] [CrossRef]
- Zhan, Z.; Lin, Y.; Bamett, S. Anode catalyst layers for direct hydrocarbon and internal reforming SOFCs. Electrochem. Soc. 2005, 9, 1321–1330. [Google Scholar] [CrossRef]
- Lo Faro, M.; Reis, R.M.; Saglietti, G.G.A.; Sato, A.G.; Ticianelli, E.A.; Zignani, S.C.; Aricò, A.S. Nickel-Copper/Gadolinium-doped Ceria (CGO) composite electrocatalyst as a protective layer for a Solid-Oxide Fuel Cell anode fed with ethanol. ChemElectroChem 2014, 1, 1395–1402. [Google Scholar] [CrossRef]
- Lo Faro, M.; Reis, R.M.; Saglietti, G.G.A.; Zignani, S.C.; Trocino, S.; Frontera, P.; Antonucci, P.L.; Ticianelli, E.A.; Aricò, A.S. Investigation of Ni-based alloy/CGO electro-catalysts as protective layer for a solid oxide fuel cell anode fed with ethanol. J. Appl. Electrochem. 2015, 45, 647–656. [Google Scholar] [CrossRef]
- Lo Faro, M.; Trocino, S.; Zignani, S.C.; Italiano, C.; Reis, R.M.; Ticianelli, E.A.; Aricò, A.S. Nickel–Iron/Gadolinium-doped Ceria (CGO) composite electrocatalyst as a protective layer for a Solid-Oxide Fuel Cell anode fed with biofuels. ChemCatChem 2016, 8, 648–655. [Google Scholar] [CrossRef]
- Li, J.; Croiset, E.; Ricardez-Sandoval, L. Theoretical investigation of the methane cracking reaction pathways on Ni (1 1 1) surface. Chem. Phys. Lett. 2015, 639, 205–210. [Google Scholar] [CrossRef]
- Lo Faro, M.; Frontera, P.; Antonucci, P.; Aricò, A.S. Ni-Cu based catalysts prepared by two different methods and their catalytic activity toward the ATR of methane. Chem. Eng. Res. Des. 2015, 93, 269–277. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. The effect of specific surface area on the activity of nano-scale ceria catalysts for methanol decomposition with and without steam at SOFC operating temperatures. Chem. Eng. Sci. 2006, 61, 2540–2549. [Google Scholar] [CrossRef]
- Ioselevich, A.; Kornyshev, A.A.; Lehnert, W. Statistical geometry of reaction space in porous cermet anodes based on ion-conducting electrolytes patterns of degradation. Solid State Ion. 1999, 124, 221–237. [Google Scholar] [CrossRef]
- Pecho, O.M.; Mai, A.; Münch, B.; Hocker, T.; Flatt, R.J.; Holzer, L. 3D microstructure effects in Ni-YSZ anodes: Influence of TPB lengths on the electrochemical performance. Materials 2015, 8, 7129–7144. [Google Scholar] [CrossRef]
- Crosbie, G.M.; Murray, E.P.; Bauer, D.R.; Kim, H.; Park, S.; Vohs, J.M.; Gorte, R.J. Solid oxide fuel cells for direct oxidation of liquid hydrocarbon fuels in automotive auxiliary power units: Sulfur tolerance and operation on gasoline. SAE Trans. 2002, 111, 832–839. [Google Scholar] [CrossRef]
- Mogensen, M.B. Direct conversion of hydrocarbons in Solid Oxide Fuel Cells: A review. ACS Division of Fuel Chemistry, Preprints. In Proceedings of the 224th ACS National Meeting, Boston, MA, USA, 18–22 August 2002; p. 498. [Google Scholar]
- Sun, Y.F.; Zhou, X.W.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.N.; Zhang, L.Z.; Hua, B.; Li, J.; Li, J.H.; Luo, J.L. An ingenious Ni/Ce co-doped titanate based perovskite as a coking-tolerant anode material for direct hydrocarbon solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 22830–22838. [Google Scholar] [CrossRef]
- Moure, C.; Peña, O. Recent advances in perovskites: Processing and properties. Prog. Solid State Chem. 2015, 43, 123–148. [Google Scholar] [CrossRef]
- Perry, N.H.; Ishihara, T. Roles of bulk and surface chemistry in the oxygen exchange kinetics and related properties of mixed conducting perovskite oxide electrodes. Materials 2016, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Cascos, V.; Alonso, J.A.; Fernández-Díaz, M.T. Novel Mg-doped SrMoO3 Perovskites designed as anode materials for solid oxide fuel cells. Materials 2016, 9, 588. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Høydalsvik, K.; Einarsrud, M.A.; Grande, T. Effect of A-site cation ordering on chemical stability, oxygen stoichiometry and electrical conductivity in layered LaBaCo2O5+δ double perovskite. Materials 2016, 90, 154. [Google Scholar] [CrossRef]
- Yao, Y.-F.Y. The oxidation of hydrocarbons and CO over metal oxides. IV. Perovskite-type oxides. J. Catal. 1975, 36, 266–275. [Google Scholar] [CrossRef]
- Shimizu, T. Partial oxidation of hydrocarbons and oxygenated compounds on perovskite oxides. Catal. Rev. 1992, 34, 355–371. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Yang, X.; Feng, T.; He, T. Resisting coking and sulfur poisoning of double perovskite Sr2TiFe0.5Mo0.5O6–Δ anode material for solid oxide fuel cells. Int. J. Hydrog. Energy 2018, 43, 3280–3290. [Google Scholar] [CrossRef]
- Swartz, S.L. Sulfur tolerant fuel processing catalysts. ACS National Meeting Book of Abstracts. In Proceedings of the 230th ACS National Meeting, Washington, DC, USA, 28 August–1 September 2005; p. 1. [Google Scholar]
- Wang, S.; Jiang, Y.; Zhang, Y.; Li, W.; Yan, J.; Lu, Z. Electrochemical performance of mixed ionic-electronic conducting oxides as anodes for solid oxide fuel cell. Solid State Ion. 1999, 120, 75–84. [Google Scholar] [CrossRef]
- Sammes, N.M.; Ratnaraj, R. High-temperature mechanical properties of La0.7Sr0.3Cr1-yCoyO3 in reducing environments. J. Mater. Sci. 1997, 32, 687–692. [Google Scholar] [CrossRef]
- Xu, S.J.; Thomson, W.J. Stability of La0.6Sr0.4Co0.2Fe0.8O3-δ perovskite membranes in reducing and nonreducing environments. Ind. Eng. Chem. Res. 1998, 37, 1290–1299. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.F.; Luo, J.L. Ce/Ni decorated titanate based perovskite for solid oxide fuel cells. Ecs Trans. 2017, 75, 91–97. [Google Scholar] [CrossRef]
- Lo Faro, M.; La Rosa, D.; Nicotera, I.; Antonucci, V.; Arico, A.S. Electrochemical investigation of a propane-fed solid oxide fuel cell based on a composite Ni-perovskite anode catalyst. Appl. Catal. B-Environ. 2009, 89, 49–57. [Google Scholar] [CrossRef]
- Lo Faro, M.; Minutoli, M.; Monforte, G.; Antonucci, V.; Arico, A.S. Glycerol oxidation in solid oxide fuel cells based on a Ni-perovskite electrocatalyst. Biomass Bioenergy 2011, 35, 1075–1084. [Google Scholar] [CrossRef]
- Lo Faro, M.; Stassi, A.; Antonucci, V.; Modafferi, V.; Frontera, P.; Antonucci, P.; Arico, A.S. Direct utilization of methanol in solid oxide fuel cells: An electrochemical and catalytic study. Int. J. Hydrog. Energy 2011, 36, 9977–9986. [Google Scholar] [CrossRef]
- Lo Faro, M.; Antonucci, V.; Antonucci, P.L.; Aricò, A.S. Fuel flexibility: A key challenge for SOFC technology. Fuel 2012, 102, 554–559. [Google Scholar] [CrossRef]
- Lo Faro, M.; Modafferi, V.; Frontera, P.; Antonucci, P.; Aricò, A.S. Catalytic behavior of Ni-modified perovskite and doped ceria composite catalyst for the conversion of odorized propane to syngas. Fuel Process. Technol. 2013, 113, 28–33. [Google Scholar] [CrossRef]
- Lo Faro, M.; Arico, A.S. Electrochemical behaviour of an all-perovskite-based intermediate temperature solid oxide fuel cell. Int. J. Hydrog. Energy 2013, 38, 14773–14778. [Google Scholar] [CrossRef]
- Lo Faro, M.; Reis, R.M.; Saglietti, G.G.A.; Oliveira, V.L.; Zignani, S.C.; Trocino, S.; Maisano, S.; Ticianelli, E.A.; Hodnik, N.; Ruiz-Zepeda, F.; et al. Solid oxide fuel cells fed with dry ethanol: The effect of a perovskite protective anodic layer containing dispersed Ni-alloy @ FeOx core-shell nanoparticles. Appl. Catal. B Environ. 2018, 220, 98–110. [Google Scholar] [CrossRef]
- Lo Faro, M.; Oliveira, V.L.; Reis, R.M.; Saglietti, G.G.A.; Zignani, S.C.; Trocino, S.; Ticianelli, E.A.; Aricò, A.S. Solid Oxide Fuel Cell fed directly with dry glycerol. Energy Technol. 2019, 7, 45–47. [Google Scholar] [CrossRef]
- Jardiel, T.; Caldes, M.T.; Moser, F.; Hamon, J.; Gauthier, G.; Joubert, O. New SOFC electrode materials: The Ni-substituted LSCM-based compounds (La0.75Sr0.25) (Cr0.5Mn0.5-xNix) O3-δ and (La0.75Sr0.25) (Cr0.5-xNixMn0.5)O3-δ. Solid State Ion. 2010, 181, 894–901. [Google Scholar] [CrossRef]
- Van Den Bossche, M.; McIntosh, S. Pulse reactor studies to assess the potential of La0.75Sr 0.25Cr0.5Mn0.4X0.1O 3-δ (X = Co, Fe, Mn, Ni, V) as direct hydrocarbon solid oxide fuel cell anodes. Chem. Mater. 2010, 22, 5856–5865. [Google Scholar] [CrossRef]
- Lay, E.; Gauthier, G.; Dessemond, L. Preliminary studies of the new Ce-doped La/Sr chromo-manganite series as potential SOFC anode or SOEC cathode materials. Solid State Ion. 2011, 189, 91–99. [Google Scholar] [CrossRef]
- Li, X.; Dai, L.; He, Z.; Meng, W.; Li, Y.; Wang, L. In situ exsolution of PdO nanoparticles from non-stoichiometric LaFePd0.05O3+δ electrode for impedancemetric NO2 sensor. Sens. Actuators B Chem. 2019, 298, 126827. [Google Scholar] [CrossRef]
- Hou, N.; Yao, T.; Li, P.; Yao, X.; Gan, T.; Fan, L.; Wang, J.; Zhi, X.; Zhao, Y.; Li, Y. A-site ordered double perovskite with in situ exsolved core-shell nanoparticles as anode for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2019, 11, 6995–7005. [Google Scholar] [CrossRef]
- Lo Faro, M.; La Rosa, D.; Nicotera, I.; Antonucci, V.; Aricò, A.S. Electrochemical behaviour of propane-fed solid oxide fuel cells based on low Ni content anode catalysts. Electrochim. Acta 2009, 54, 5280–5285. [Google Scholar] [CrossRef]
- Vecino-Mantilla, S.; Quintero, E.; Fonseca, C.; Gauthier, G.H.; Gauthier-Maradei, P. Catalytic steam reforming of natural gas over a new Ni exsolved Ruddlesden-Popper manganite in SOFC anode conditions. ChemCatChem 2020, 12, 1453–1466. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Swinnea, J.S.; Steinfink, H.; Reiff, W.M.; Lightfoot, P.; Pei, S.; Jorgensen, J.D. Ruddlesden-Popper phases An+1MnO3n+1. Structures and properties. In Proceedings of the International Conference on the Chemistry of Electronic Ceramic Materials, Jackson, WY, USA, 17–22 August 1990; pp. 301–306. [Google Scholar]
- Lee, D.; Lee, H.N. Controlling oxygen mobility in ruddlesden-popper oxides. Materials 2017, 10, 368. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Zhang, Z.; Sedach, P.A.; Siu, M.K.J.; Trudel, S.; Berlinguette, C.P. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 2013, 340, 60–63. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Water oxidation catalysis: Electrocatalytic response to metal stoichiometry in amorphous metal oxide films containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 2013, 135, 11580–11586. [Google Scholar] [CrossRef]
- Tang, C.-W.; Wang, C.-B.; Chien, S.-H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Tiernan, M.J.; Barnes, P.A.; Parkes, G.M.B. Reduction of Iron oxide catalysts: The Investigation of kinetic parameters using rate perturbation and linear heating thermoanalytical techniques. J. Phys. Chem. B 2001, 105, 220–228. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Marrero-Jerez, J.; Larrondo, S.; Rodríguez-Castellón, E.; Núñez, P. TPR, XRD and XPS characterisation of ceria-based materials synthesized by freeze-drying precursor method. Ceram. Int. 2014, 40, 6807–6814. [Google Scholar] [CrossRef]
- Steele, B.C.H. Oxygen transport and exchange in oxide ceramics. J. Power Sources 1994, 49, 1–14. [Google Scholar] [CrossRef]
- Liu, D.J.; Krumpelt, M. Activity and structure of perovskites as diesel-reforming catalysts for solid oxide fuel cell. Int. J. Appl. Ceram. Technol. 2005, 2, 301–307. [Google Scholar] [CrossRef]
- Aguiar, P.; Lapeña-Rey, N.; Chadwick, D.; Kershenbaum, L. Improving catalyst structures and reactor configurations for autothermal reaction systems: Application to solid oxide fuel cells. Chem. Eng. Sci. 2001, 56, 651–658. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Irvine, J.T.S. LSCM based SOFC a suitable system for direct propane operation. In Proceedings of the 1st European Fuel Cell Technology and Applications Conference 2005–Book of Abstracts, Rome, Italy, 14–16 December 2005; p. 150. [Google Scholar]
- Cheekatamarla, P.K.; Finnerty, C.M.; Cai, J. Internal reforming of hydrocarbon fuels in tubular solid oxide fuel cells. Int. J. Hydrog. Energy 2008, 33, 1853–1858. [Google Scholar] [CrossRef]
- Douvartzides, S.L.; Coutelieris, F.A.; Tsiakaras, P.E. Effect of reforming on the overall efficiency of a solid oxide fuel-cell based power plant system fed by methane. Int. J. Exergy 2004, 1, 179–188. [Google Scholar] [CrossRef]
- Lo Faro, M.; Vita, A.; Pino, L.; Aricò, A.S. Performance evaluation of a solid oxide fuel cell coupled to an external biogas tri-reforming process. Fuel Process. Technol. 2013, 115, 238–245. [Google Scholar] [CrossRef]
- Manenti, F.; Pelosato, R.; Vallevi, P.; Leon-Garzon, A.R.; Dotelli, G.; Vita, A.; Faro, M.L.; Maggio, G.; Pino, L.; Arico, A.S. Biogas-fed solid oxide fuel cell (SOFC) coupled to tri-reforming process: Modelling and simulation. Int. J. Hydrog. Energy 2015, 40, 14640–14650. [Google Scholar] [CrossRef]
- Al-Qattan, A.M.; Chmielewski, D.J. Distributed feed design for SOFCs with internal reforming. J. Electrochem. Soc. 2004, 151, A1891. [Google Scholar] [CrossRef]
- Lim, L.T.; Chadwick, D.; Kershenbaum, L. Achieving autothermal operation in internally reformed solid oxide fuel cells: Simulation studies. Ind. Eng. Chem. Res. 2005, 44, 9609–9618. [Google Scholar] [CrossRef]
- Georges, S.; Parrour, G.; Henault, M.; Fouletier, J. Gradual internal reforming of methane: A demonstration. Solid State Ion. 2006, 177, 2109–2112. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Corigliano, O.; Lo Faro, M.; Frontera, P.; Antonucci, P.; Zignani, S.C.; Trocino, S.; Mirandola, F.A.; Aricò, A.S.; Fragiacomo, P. Thermoelectric characterization of an intermediate temperature solid oxide fuel cell system directly fed by dry biogas. Energy Convers. Manag. 2016, 127, 90–102. [Google Scholar] [CrossRef]
- Leah, R.; Bone, A.; Selcuk, A.; Corcoran, D.; Lankin, M.; Dehaney-Steven, Z.; Selby, M.; Whalen, P. Development of highly robust, volume-manufacturable metal-supported SOFCs for operation below 600 °C. ECS Trans. 2011, 35, 351. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen ion conductors. Mater. Today 2003, 6, 30–37. [Google Scholar] [CrossRef]
- Lo Faro, M.; Arico, A.S. Ceramic membranes for intermediate temperature solid oxide fuel cells (SOFCs): State of the art and perspectives. In Membranes for Clean and Renewable Power Applications; Gugliuzza, A., Basile, A., Eds.; Woodhead Publ Ltd.: Cambridge, UK, 2014; pp. 237–265. [Google Scholar] [CrossRef]
- Coors, W.G. Protonic ceramic steam-permeable membranes. Solid State Ion. 2007, 178, 481–485. [Google Scholar] [CrossRef]
- Oishi, M.; Akoshima, S.; Yashiro, K.; Sato, K.; Mizusaki, J.; Kawada, T. Defect structure analysis of B-site doped perovskite-type proton conducting oxide BaCeO3. Part 2: The electrical conductivity and diffusion coefficient of BaCe0.9Y0.1O3-δ. Solid State Ion. 2008, 179, 2240–2247. [Google Scholar] [CrossRef]
- Ivanova, M.; Ricote, S.; Baumann, S.; Meulenberg, W.A.; Tietz, F.; Serra, J.M.; Richter, H. Ceramic materials for energy and environmental applications: Functionalizing of properties by tailored compositions. In Doping: Properties, Mechanisms and Applications; Nova Science Pub Inc.: New York, NY, USA, 2013; pp. 221–276. [Google Scholar]
- Giannici, F.; Longo, A.; Deganello, F.; Balerna, A.; Arico, A.S.; Martorana, A. Local environment of Barium, Cerium and Yttrium in BaCe1-xYxO3-δ ceramic protonic conductors. Solid State Ion. 2007, 178, 587–591. [Google Scholar] [CrossRef]
- Shamsi, A.; Zahir, K. Oxidative-coupling of methane over Perovskite-type oxides and correlation of TMAX for oxygen desorption with C2 selectivity. Prepr. Symp. 1989, 34, 544. [Google Scholar] [CrossRef]
- Mai, A.; Haanappel, V.A.C.; Uhlenbruck, S.; Tietz, F.; Stöver, D. Ferrite-based perovskites as cathode materials for anode-supported solid oxide fuel cells: Part I. Variation of composition. Solid State Ion. 2005, 176, 1341–1350. [Google Scholar] [CrossRef]
- Liu, J.; Co, A.C.; Paulson, S.; Birss, V.I. Oxygen reduction at sol-gel derived La0.8Sr0.2Co0.8Fe0.2O3 cathodes. Solid State Ion. 2006, 177, 377–387. [Google Scholar] [CrossRef]
- Dias, J.A.; Andrade, M.A.S.J.; Santos, H.L.S.; Morelli, M.R.; Mascaro, L.H. Lanthanum-Based Perovskites for Catalytic Oxygen Evolution Reaction. ChemElectroChem 2020. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, B.; Zhang, Y.; Lü, Z.; Wang, Z.; Huang, X.; Cao, Z.; Jiang, W.; Li, Y. Investigation on a novel composite solid oxide fuel cell anode with La0.6Sr0.4Co0.2Fe0.8O3-δ derived phases. Electrochim. Acta 2015, 160, 89–93. [Google Scholar] [CrossRef]
- Benson, S.J.; Waller, D.; Kilner, J.A. Degradation of La0.6Sr0.4Fe0.8Co0.2O3-δ in carbon dioxide and water atmospheres. J. Electrochem. Soc. 1999, 146, 1305. [Google Scholar] [CrossRef]
- Kim, H.; Lu, C.; Worrell, W.L.; Vohs, J.M.; Gorte, R.J. Cu-Ni cermet anodes for direct oxidation of methane in solid-oxide fuel cells. J. Electrochem. Soc. 2002, 149, A247. [Google Scholar] [CrossRef]
- An, W.; Gatewood, D.; Dunlap, B.; Turner, C.H. Catalytic activity of bimetallic nickel alloys for solid-oxide fuel cell anode reactions from density-functional theory. J. Power Sources 2011, 196, 4724–4728. [Google Scholar] [CrossRef]
- Nabae, Y.; Yamanaka, I.; Hatano, M.; Otsuka, K. Catalytic behavior of Pd-Ni/composite anode for direct oxidation of methane in SOFCs. J. Electrochem. Soc. 2006, 153, A140. [Google Scholar] [CrossRef]
- Lo Faro, M.; Trocino, S.; Zignani, S.C.; Reis, R.M.; Monforte, G.; Ticianelli, E.A.; Aricò, A.S. Ni-based Alloys as Protective Layer for a Conventional Solid Oxide Fuel Cell Fed with Biofuels. ECS Trans 2015, 68, 2653–2658. [Google Scholar] [CrossRef]


| H2 % | CO % | CO2 % | CH4 % | C2H4 % | C3H8 % | Others % | Selectivity to Syngas % | ||
|---|---|---|---|---|---|---|---|---|---|
| SR-800 °C | Glycerol (S/C = 0.2) [57] | 29.45 | 28.39 | 32.90 | 7.50 | 1.76 | - | - | - |
| SR-800 °C | Glycerol (S/C = 2) [57] | 80.79 | 5.60 | 2.36 | 5.82 | 5.43 | - | - | - |
| ATR-800 °C | Methane (S/C = 2.5; O/C = 0.5) [59] | 5.95 | 2.19 | 1.62 | 85.68 | - | - | - | - |
| ATR-800 °C | Methanol (S/C = 2.5; O/C = 0.5) [58,59] | 67.44 | 13.73 | 17.71 | 0.56 | - | - | - | 87.42 |
| ATR-800 °C | Propane (S/C = 2.5; O/C = 0.5) [59,60] | 66.59 | 17.40 | 4.94 | 6.98 | 2.35 | 0.97 | 0.77 | - |
| ATR-800 °C | Glycerol (S/C = 2.5; O/C = 0.5) [59] | 31.10 | 29.62 | 29.01 | 9.56 | - | - | - | - |
| SR-800 °C | Methanol (S/C = 2.5) [58] | - | - | - | - | - | - | - | 81.70 |
| POX-800 °C | Methanol (O/C = 0.5) [58] | - | - | - | - | - | - | - | 92.02 |
| SR-800 °C | Propane (S/C = 2.5) [60] | 64.59 | 15.04 | 9.34 | 6.75 | 1.99 | 1.48 | 0.81 | - |
| POX-800 °C | Propane (O/C = 0.5) [60] | 43.73 | 29.20 | 0.68 | 13.03 | 10.06 | 1.68 | 1.62 | - |
| ATR-800 °C | Propane (S/C = 2.5; O/C = 0.5) + 20 ppm H2S [60] | 22.38 | 8.73 | 18.76 | 15.48 | 28.57 | 6.18 | - | - |
| ATR-800 °C | Propane (S/C = 2.5; O/C = 0.5) + 40 ppm H2S [60] | 23.97 | 10.29 | 18.07 | 16.28 | 25.02 | 6.37 | - | - |
| ATR-800 °C | Propane (S/C = 2.5; O/C = 0.5) + 60 ppm H2S [60] | 14.41 | 10.62 | 19.75 | 15.91 | 26.38 | 10.14 | 2.79 | - |
| ATR-800 °C | Propane (S/C = 2.5; O/C = 0.5) + 80 ppm H2S [60] | 13.25 | 8.76 | 19.60 | 16.55 | 29.06 | 12.74 | 0.04 | - |
| Type of Cell/Electrolyte | Maximum Power Density/mW cm−2 | Series Resistance /Ω cm−2 | Total Resistance /Ω cm−2 | Maximum Durability Demonstrated/h | Average Decay during the Life Time Test/A h−1 | |
|---|---|---|---|---|---|---|
| Methane [59] | CGO (250 μm) | 37 @ 0.22 V | 0.51 @ 0.5 V | 2.78 @ 0.5 V | 15 | 0 |
| Syngas [58] | CGO (250 μm) | 346 @ 0.44 V | 0.24 @ 0.765 V | 0.29 @ 0.765 V | 17 [59] | 0 |
| Methanol [58] | CGO (250 μm) | 358 @ 0.47 V | 0.26 @ 0.75 V | 0.33 @ 0.75 V | 18 [59] | 4 10−3 |
| Ethanol [62] | Elcogen | 648 @ 0.60 V | 0.18 @ 0.7 V | 0.46 @ 0.7 V | 400 | 1.5 10−4 |
| Propane [56] | CGO (250 μm) | 288 @ 0.51 V | 0.25 @ 0.5 V | 0.33 @ 0.5 V | 780 | 1.1 10−4 |
| Propane [61] | LSGM (300 μm) | 328 @ 0.43 V | 0.32 @ 0.7 V | 0.92 @ 0.7 V | 15 | 5 10−4 |
| Glycerol [57] | CGO (250 μm) | 320 @ 0.46 V | 0.30 @ 0.5 V | 0.66 @ 0.5 V | 19 [59] | 0 |
| Glycerol [63] | Elcogen | 864 @ 0.62 V | 0.12 @ 0.7 V | 0.25 @ 0.7 V | 157 | 1 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Faro, M.; Campagna Zignani, S.; Aricò, A.S. Lanthanum Ferrites-Based Exsolved Perovskites as Fuel-Flexible Anode for Solid Oxide Fuel Cells. Materials 2020, 13, 3231. https://doi.org/10.3390/ma13143231
Lo Faro M, Campagna Zignani S, Aricò AS. Lanthanum Ferrites-Based Exsolved Perovskites as Fuel-Flexible Anode for Solid Oxide Fuel Cells. Materials. 2020; 13(14):3231. https://doi.org/10.3390/ma13143231
Chicago/Turabian StyleLo Faro, Massimiliano, Sabrina Campagna Zignani, and Antonino Salvatore Aricò. 2020. "Lanthanum Ferrites-Based Exsolved Perovskites as Fuel-Flexible Anode for Solid Oxide Fuel Cells" Materials 13, no. 14: 3231. https://doi.org/10.3390/ma13143231
APA StyleLo Faro, M., Campagna Zignani, S., & Aricò, A. S. (2020). Lanthanum Ferrites-Based Exsolved Perovskites as Fuel-Flexible Anode for Solid Oxide Fuel Cells. Materials, 13(14), 3231. https://doi.org/10.3390/ma13143231







