Cellulose-Multiwall Carbon Nanotube Fiber Actuator Behavior in Aqueous and Organic Electrolyte
Abstract
:1. Introduction
2. Experimental
2.1. Materials
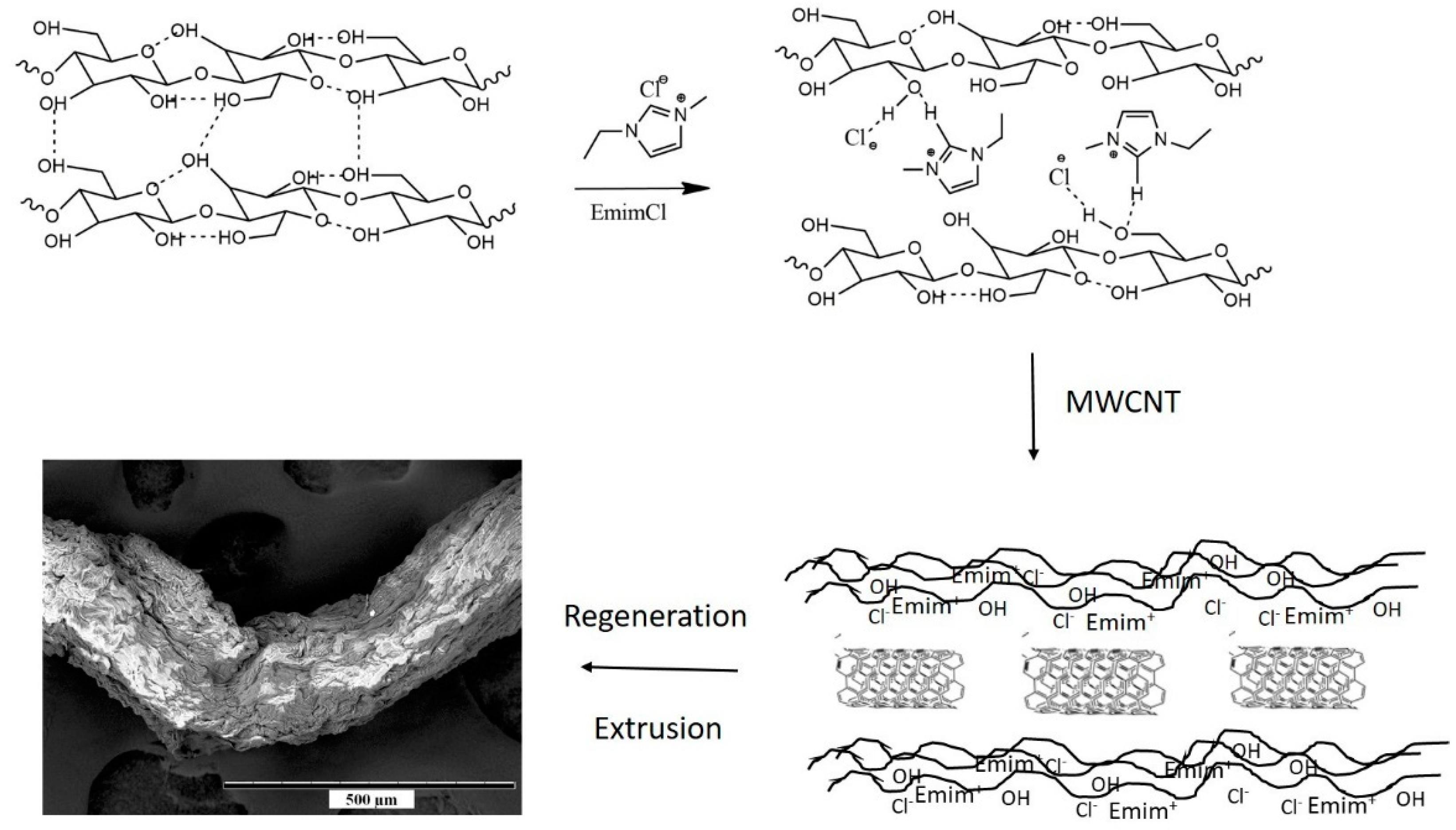
2.2. Formation of Cell-CNT Composite Fibers
2.3. Linear Actuation Measurements of Cell-CNT Composite Fibers
2.4. Material Characterization
3. Results and Discussion
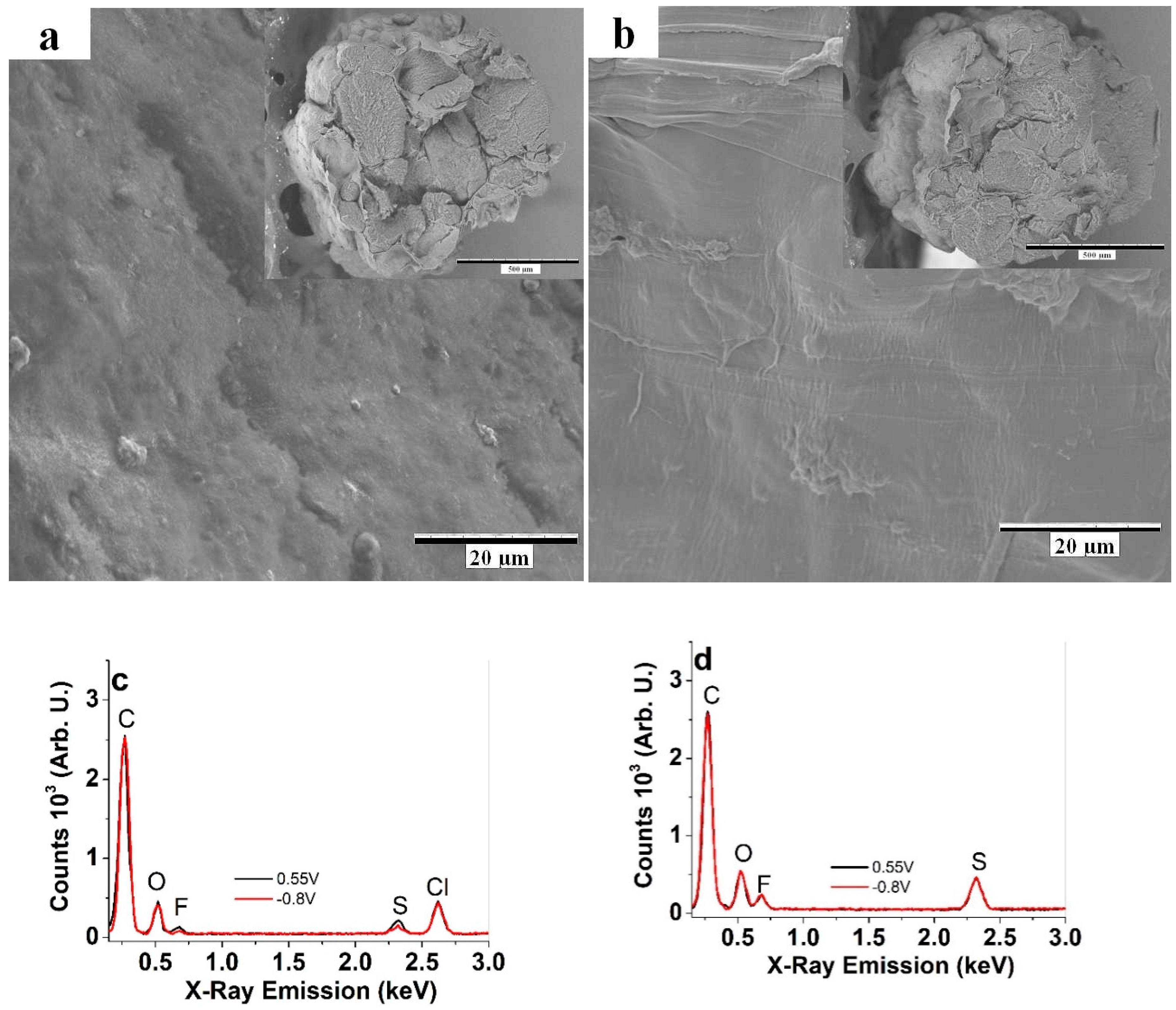
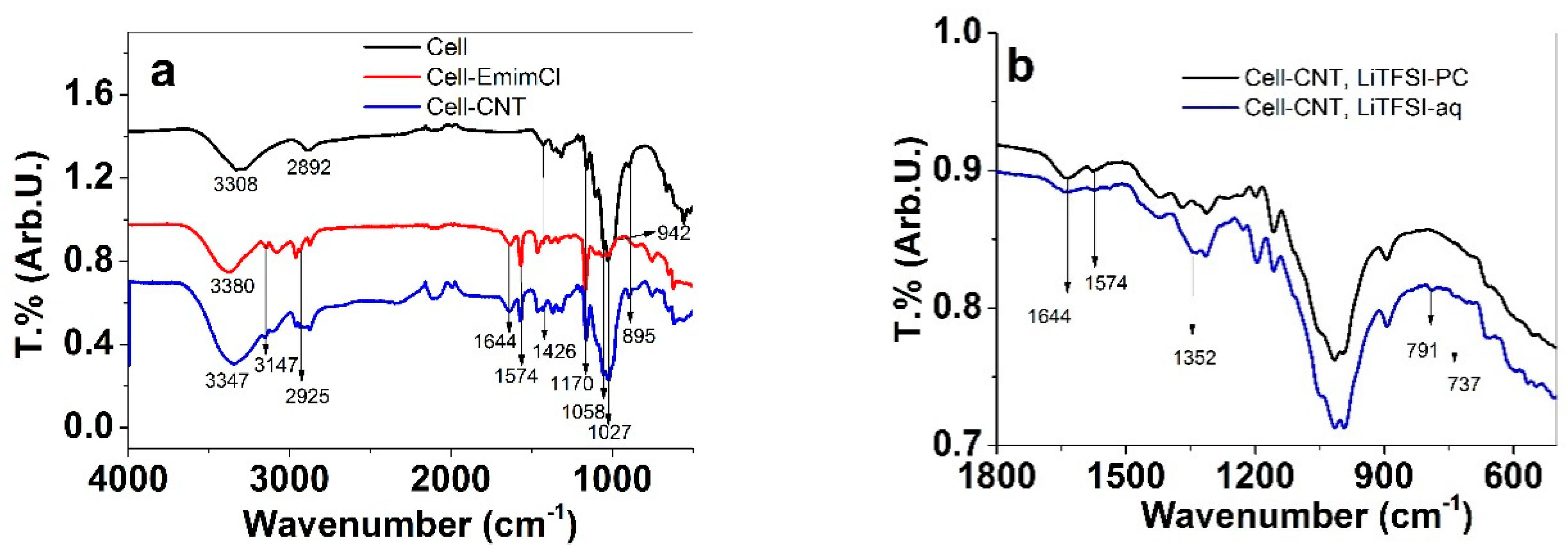
3.1. Characterization of Cell-CNT Fibers
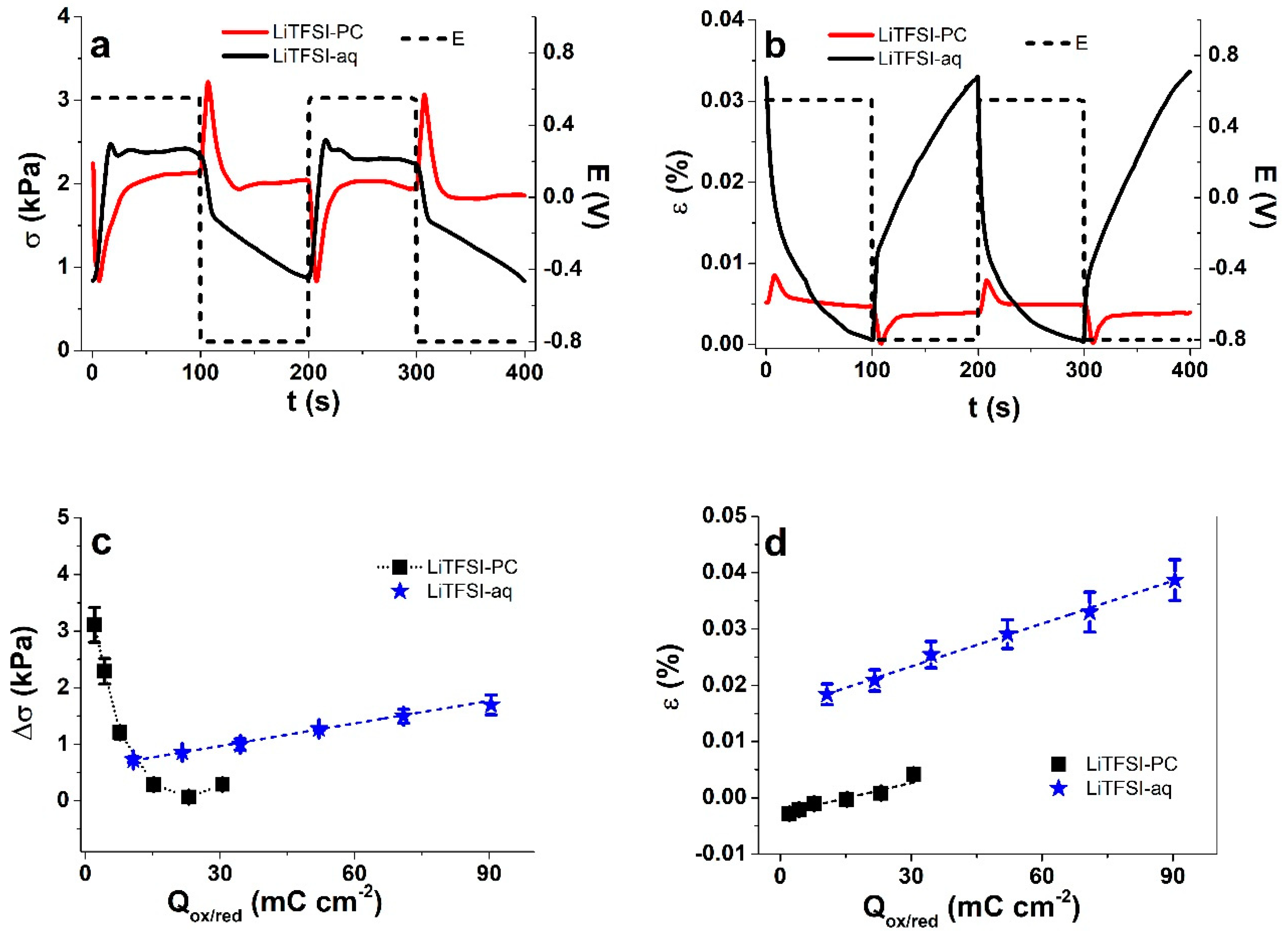
3.2. Linear Actuation of Cell-CNT Fibers
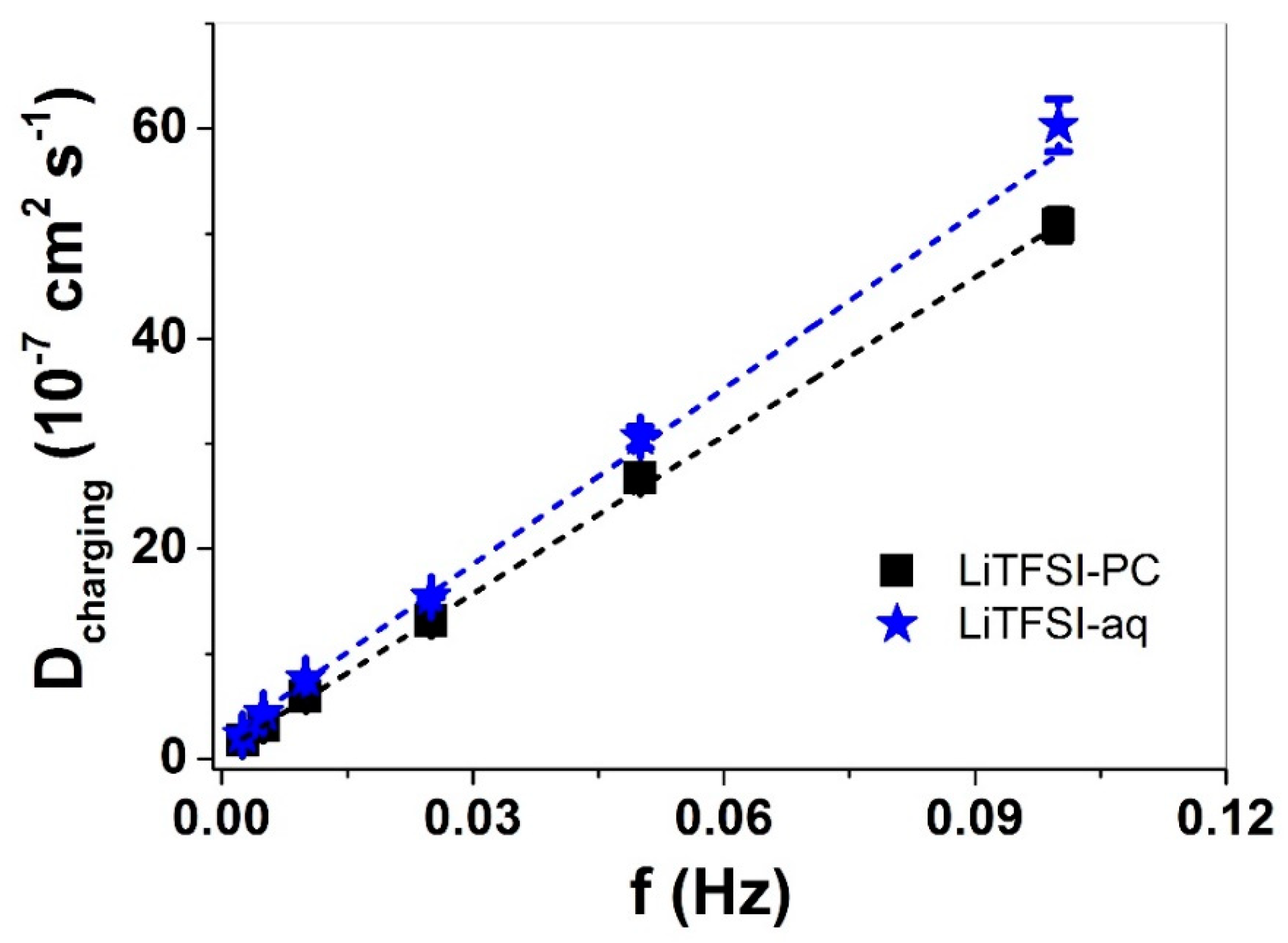
3.2.1. Cyclic Voltammetry
3.2.2. Square Wave Potential Step Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dutta, S.; Kim, J.; Ide, Y.; Ho Kim, J.; Hossain, M.S.A.; Bando, Y.; Yamauchi, Y.; Wu, K.C.W. 3D network of cellulose-based energy storage devices and related emerging applications. Mater. Horizons 2017, 4, 522–545. [Google Scholar] [CrossRef] [Green Version]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417–424. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Xu, A.; Wang, J.; Wang, H. Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem. 2010, 12, 268–275. [Google Scholar] [CrossRef]
- Elhi, F.; Aid, T.; Koel, M. Ionic liquids as solvents for making composite materials from cellulose. Proc. Est. Acad. Sci. 2016, 65, 255–266. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Da Silva, V.C.; Possidonio, S.; Casarano, R.; Arêas, E.P.G.; Gimenes, P. Microwave-assisted derivatization of cellulose, 2—The surprising effect of the structure of ionic liquids on the dissolution and acylation of the biopolymer. Macromol. Chem. Phys. 2011, 212, 2541–2550. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Su, Y.; Wang, D.W.; Li, F.; Du, J.; Cheng, H.M. Graphene-cellulose paper flexible supercapacitors. Adv. Energy Mater. 2011, 1, 917–922. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Kim, J. Multifunctional Smart Biopolymer Composites as Actuators. In Biopolymer Composites in Electronics; Elsevier Inc.: Incheon, Korea, 2017; pp. 311–331. ISBN 9780081009741. [Google Scholar]
- Zhang, S.; Zhang, F.; Pan, Y.; Jin, L.; Liu, B.; Mao, Y.; Jintian, H. Multiwall-carbon-nanotube/cellulose composite fibers with enhanced mechanical and electrical properties by cellulose grafting. RSC Adv. 2018, 8, 5678–5684. [Google Scholar] [CrossRef] [Green Version]
- Lv, P.; Feng, Q.; Wang, Q.; Li, G.; Li, D.; Wei, Q. Biosynthesis of bacterial cellulose/carboxylic multi-walled carbon nanotubes for enzymatic biofuel cell application. Materials (Basel) 2016, 9, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siljander, S.; Keinänen, P.; Ivanova, A.; Lehmonen, J.; Tuukkanen, S.; Kanerva, M.; Björkqvist, T. Conductive cellulose based foam formed 3D shapes-from innovation to designed prototype. Materials (Basel) 2019, 12, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, W.; Sun, J.; Memon, J.; Wang, C.; Geng, J.; Huang, Y. Scalable preparation of three-dimensional porous structures of reduced graphene oxide/cellulose composites and their application in supercapacitors. Carbon N. Y. 2013, 62, 501–509. [Google Scholar] [CrossRef]
- Richardson, M.J.; Johnston, J.H.; Borrmann, T. Electronic properties of intrinsically conducting polymer-cellulose based composites. Curr. Appl. Phys. 2006, 6, 462–465. [Google Scholar] [CrossRef]
- Shi, Z.; Phillips, G.O.; Yang, G. Nanocellulose electroconductive composites. Nanoscale 2013, 5, 3194–3201. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.; Zhang, D.; Song, W. High performance, flexible and renewable nano-biocomposite artificial muscle based on mesoporous cellulose/ ionic liquid electrolyte membrane. Sensors Actuators B Chem. 2019, 283, 579–589. [Google Scholar] [CrossRef]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers (Basel) 2019, 11, 1054. [Google Scholar] [CrossRef] [Green Version]
- Otero, T.F.; Martinez, J.G. Activation energy for polypyrrole oxidation: Film thickness influence. J. Solid State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Kim, S.S.; Ohno, H. Design of polar ionic liquids to solubilize cellulose without heating. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; American Chemical Society: Tokyo, Japan, 2010; pp. 55–66. [Google Scholar]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Gupta, K.M.; Hu, Z.; Jiang, J. Cellulose regeneration from a cellulose/ionic liquid mixture: The role of anti-solvents. RSC Adv. 2013, 3, 12794–12801. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhang, S. The Application of Ionic Liquids in Dissolution and Separation of Lignocellulose. In Clean Energy Systems and Experiences; Intech: Rijeka, Croatia, 2010; pp. 71–84. [Google Scholar]
- Yang, L.; Sun, Z.; Li, F.; Du, S.; Song, W. Performance enhancement of cellulose based biocomposite ionic actuator by doping with MWCNT. Appl. Phys. A 2019, 125, 1–15. [Google Scholar] [CrossRef]
- Yassin, F.A.; El, F.Y.; Ahmed, H.S.; Mohamed, L.K.; Shaban, S.A.; Elfadaly, A.K. Highly effective ionic liquids for biodiesel production from waste vegetable oils. Egypt. J. Pet. 2015, 24, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Kam, W.; Liew, C.; Lim, J.Y.; Ramesh, S. Electrical, structural, and thermal studies of antimony trioxide-doped poly (acrylic acid)-based composite polymer electrolytes. Ionics (Kiel) 2014, 20, 665–674. [Google Scholar] [CrossRef]
- Plaado, M.; Kaasik, F.; Valner, R.; Lust, E.; Saar, R.; Saal, K.; Peikolainen, A.; Aabloo, A.; Kiefer, R. Electrochemical actuation of multiwall carbon nanotube fiber with embedded carbide-derived carbon particles. Carbon N. Y. 2015, 94, 911–918. [Google Scholar] [CrossRef]
- Hughes, M.; Spinks, G.M. Multiwalled Carbon Nanotube Actuators. Adv. Mater. 2005, 17, 443–446. [Google Scholar] [CrossRef]
- Baughman, R.H.; Cui, C.; Zakhidov, A.A.; Iqbal, Z.; Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Mazzoldi, A.; De Rossi, D.; Rinzler, A.G.; et al. Carbon nanotube actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Mäder, E.; Liu, J. Unique water sensors based on carbon nanotube-cellulose composites. Sensors Actuators B Chem. 2013, 185, 225–230. [Google Scholar] [CrossRef]
- Qi, H.; Liu, J.; Pionteck, J.; Pötschke, P.; Mäder, E. Carbon nanotube-cellulose composite aerogels for vapour sensing. Sensors Actuators B Chem. 2015, 213, 20–26. [Google Scholar] [CrossRef]
- Torop, J.; Sugino, T.; Asaka, K.; Jänes, A.; Lust, E.; Aabloo, A. Nanoporous carbide-derived carbon based actuators modified with gold foil: Prospect for fast response and low voltage applications. Sensors Actuators B Chem. 2012, 161, 629–634. [Google Scholar] [CrossRef]
- Vunder, V.; Punning, A.; Aabloo, A. Mechanical interpretation of back-relaxation of ionic electroactive polymer actuators. Smart Mater. Struct. 2012, 21, 115023. [Google Scholar] [CrossRef]
- Kim, O.; Kim, S.J.; Park, M.J. Low-voltage-driven soft actuators. Chem. Commun. 2018, 54, 4895–4904. [Google Scholar] [CrossRef] [Green Version]
- Sahputra, I.H.; Alexiadis, A.; Adams, M.J. Effects of Moisture on the Mechanical Properties of Microcrystalline Cellulose and the Mobility of the Water Molecules as Studied by the Hybrid Molecular Mechanics–Molecular Dynamics Simulation Method. J. Polym. Sci. Part. B Polym. Phys. 2019, 57, 454–464. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.G.; Richter, K.; Persson, N.K.; Jager, E.W.H. Investigation of electrically conducting yarns for use in textile actuators. Smart Mater. Struct. 2018, 27. [Google Scholar] [CrossRef]
- Qi, H.; Schulz, B.; Vad, T.; Liu, J.; Mäder, E.; Seide, G.; Gries, T. Novel Carbon Nanotube/Cellulose Composite Fibers As Multifunctional Materials. ACS Appl. Mater. Interfaces 2015, 7, 22404–22412. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhi, F.; Peikolainen, A.-L.; Kiefer, R.; Tamm, T. Cellulose-Multiwall Carbon Nanotube Fiber Actuator Behavior in Aqueous and Organic Electrolyte. Materials 2020, 13, 3213. https://doi.org/10.3390/ma13143213
Elhi F, Peikolainen A-L, Kiefer R, Tamm T. Cellulose-Multiwall Carbon Nanotube Fiber Actuator Behavior in Aqueous and Organic Electrolyte. Materials. 2020; 13(14):3213. https://doi.org/10.3390/ma13143213
Chicago/Turabian StyleElhi, Fred, Anna-Liisa Peikolainen, Rudolf Kiefer, and Tarmo Tamm. 2020. "Cellulose-Multiwall Carbon Nanotube Fiber Actuator Behavior in Aqueous and Organic Electrolyte" Materials 13, no. 14: 3213. https://doi.org/10.3390/ma13143213
APA StyleElhi, F., Peikolainen, A.-L., Kiefer, R., & Tamm, T. (2020). Cellulose-Multiwall Carbon Nanotube Fiber Actuator Behavior in Aqueous and Organic Electrolyte. Materials, 13(14), 3213. https://doi.org/10.3390/ma13143213






