A Facile Synthesis Process and Evaluations of α-Calcium Sulfate Hemihydrate for Bone Substitute
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dehydration Process into Hemihydrate in Production
2.2. Hydration Process into Dihydrate in Usage
2.3. In Vitro Preliminary Assessment in SBF
2.4. Material Characterization
2.5. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
2.6. Statistical Analysis
3. Results and Discussion
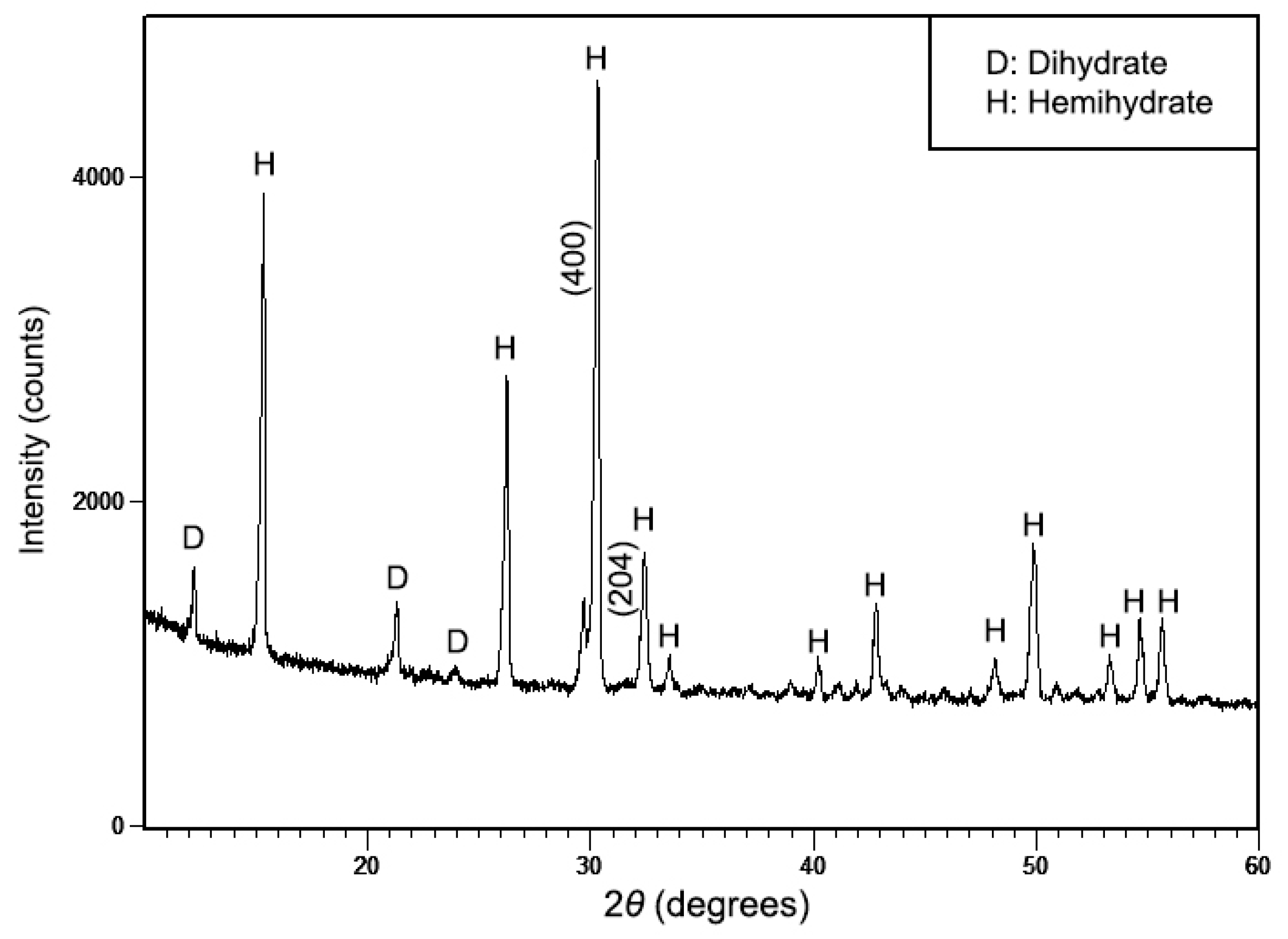
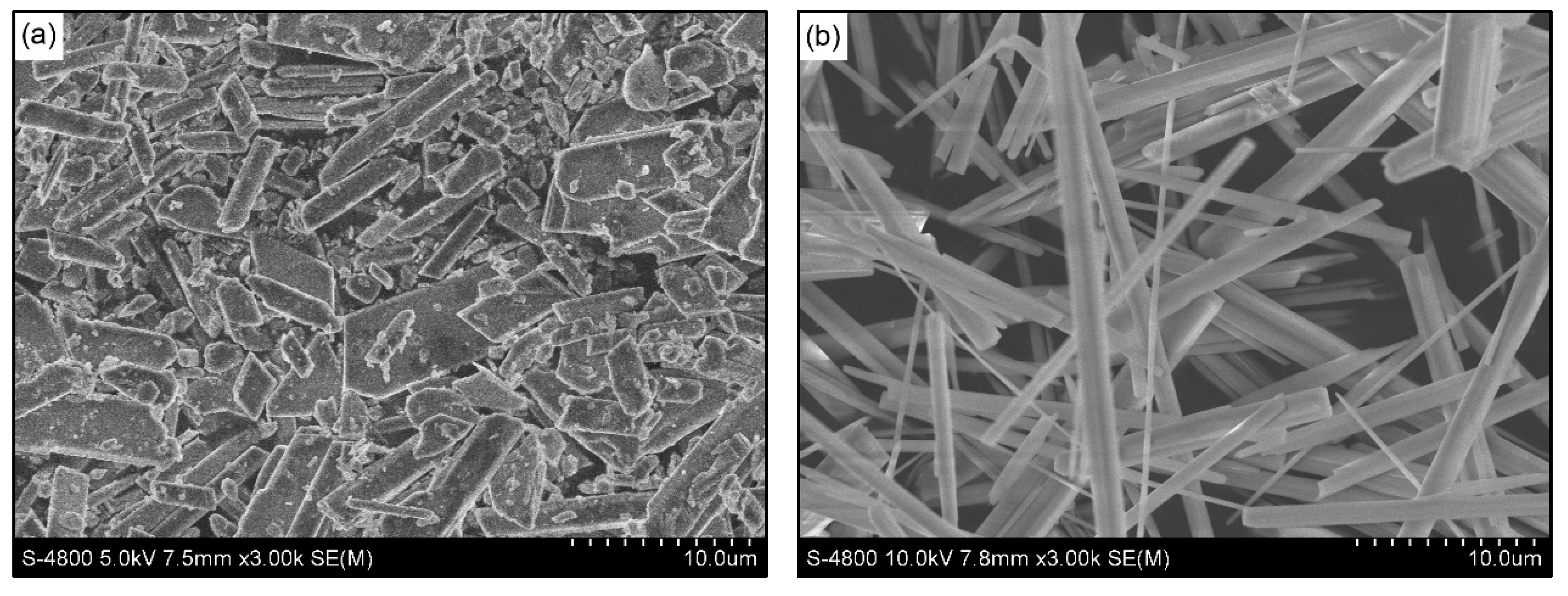
3.1. Characterization of the Synthesized Powder
3.2. In Vitro Studies of Cement Samples in SBF
3.2.1. Bioactivity Assessment
3.2.2. Degradation and pH Alteration of Cement Samples
3.3. Cytotoxicity Tests of the Synthesized Powder
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- González-Vázquez, A.; Planell, J.A.; Engel, E. Extracellular calcium and CaSR drive osteoinduction in mesenchymal stromal cells. Acta Biomater. 2014, 10, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Bryant, S.J.; Ahn, J.; Hankenson, K.D. Bone Regeneration. In Translational Regenerative Medicine; Atala, A., Allickson, J., Eds.; Academic Press: San Diego, CA, USA, 2015; Chapter 24; pp. 313–333. [Google Scholar]
- Laurell, L.; Gottlow, J. Guided tissue regeneration update. Int. Dent. J. 1998, 48, 386–398. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Strocchi, R.; Orsini, G.; Iezzi, G.; Scarano, A.; Rubini, C.; Pecora, G.; Piattelli, A. Bone Regeneration With Calcium Sulfate: Evidence for Increased Angiogenesis in Rabbits. J. Oral Implantol. 2002, 28, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.V.; Puleo, D.A. Calcium sulfate: Properties and clinical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Artese, L.; Piattelli, A.; Carinci, F.; Mancino, C.; Iezzi, G. Hemostasis Control in Endodontic Surgery: A Comparative Study of Calcium Sulfate Versus Gauzes and Versus Ferric Sulfate. J. Endod. 2012, 38, 20–23. [Google Scholar] [CrossRef]
- Scarano, A.; Carinci, F.; Cimorelli, E.; Quaranta, M.; Piattelli, A. Application of Calcium Sulfate in Surgical-Orthodontic Treatment of Impacted Teeth: A New Procedure to Control Hemostasis. J. Oral Maxillofac. Surg. 2010, 68, 964–968. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Calcium sulphate hemihydrate hydration leading to gypsum crystallization. Prog. Cryst. Growth Charact. Matter. 2007, 53, 57–77. [Google Scholar] [CrossRef]
- Odler, I. Calcium sulfate based binders. In Modern Concrete Technology Series: Special Inorganic Cements; CRC Press: New York, NY, USA, 2003; pp. 205–215. [Google Scholar]
- Driessche, A.E.S.V.; Stawski, T.M.; Benning, L.G.; Kellermeier, M. Calcium Sulfate Precipitation Throughout Its Phase Diagram. In New Perspectives on Mineral Nucleation and Growth; Springer: Cham, Switzerland, 2017; pp. 227–256. [Google Scholar]
- Coetzee, A.S. Regeneration of bone in the presence of calcium sulfate. Arch. Otolaryngol. 1980, 106, 405–409. [Google Scholar] [CrossRef]
- Lee, G.H.; Khoury, J.G.; Bell, J.E.; Buckwalter, J.A. Adverse reactions to OsteoSet bone graft substitute. Iowa Orthop. J. 2002, 22, 35–38. [Google Scholar] [PubMed]
- Hughes, E.; Yanni, T.; Jamshidi, P.; Grover, L.M. Inorganic cements for biomedical application: Calcium phosphate, calcium sulphate and calcium silicate. Adv. Appl. Ceram. 2014, 114, 65–76. [Google Scholar] [CrossRef]
- Ling, Y.; Demopoulos, G.P. Preparation of α-Calcium Sulfate Hemihydrate by Reaction of Sulfuric Acid with Lime. Ind. Eng. Chem. Res. 2005, 44, 715–724. [Google Scholar] [CrossRef]
- Combe, E.C.; Smith, D.C. Studies on the preparation of calcium sulphate hemihydrate by an autoclave process. J. Appl. Chem. 1968, 18, 307–312. [Google Scholar] [CrossRef]
- Kong, B.; Guan, B.; Yates, M.Z.; Wu, Z. Control of α-Calcium Sulfate Hemihydrate Morphology Using Reverse Microemulsions. Langmiur 2012, 28, 14137–14142. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, M.K.; Lee, H. Surfactant-Free Synthesis of CaSO4 Nanorod/Nanowire by Electrochemical Deposition. J. Electrochem. Soc. 2010, 157, k43–k46. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhu, Y.J.; Ma, M.G. Microwave-Assisted preparation of calcium sulfate nanowires. Mater. Lett. 2008, 62, 4552–4554. [Google Scholar] [CrossRef]
- American Society of Testing and Materials. ASTM F2224–03: Standard Specification for High Purity Calcium Sulfate Hemihydrate or Dihydrate for Surgical Implants. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Singh, M.; Rai, M. Autoclaved gypsum plaster from selenite and by-product phosphogypsum. J. Chem. Tech. Biotechnol. 1988, 43, 1–12. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. 2003, 65, 188–195. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Pecheva, E. Characterization of Biomaterials and Improved Techniques for Better Imaging. In Study of Biocompatible and Biological Materials: Can They be Influenced by External Factors? Materials Research Forum LLC: Millersville, PA, USA, 2017; pp. 14–53. [Google Scholar]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. Using a synthetic body fluid (SBF) solution of 27 mM HCO3− to make bone substitutes more osteointegrative. Mater. Sci. Eng. C 2008, 28, 129–140. [Google Scholar] [CrossRef]
- Husak, Y.; Solodovnyk, O.; Yanovska, A.; Kozik, Y.; Liubchak, I.; Ivchenko, V.; Mishchenko, O.; Zinchenko, Y.; Kuznetsov, V.; Pogorielov, M. Degradation and In Vivo Response of Hydroxyapatite-Coated Mg Alloy. Coatings 2018, 8, 375. [Google Scholar] [CrossRef] [Green Version]
- Mandal, P.K.; Mandal, T.K. Anion water in gypsum (CaSO4·2H2O) and hemihydrate (CaSO4·1/2H2O). Cem. Concr. Res. 2002, 32, 313–316. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, Q.; Jia, C.; Zhang, S.; Wu, Z.; Guan, B. Controlled synthesis of monodisperse α-calcium sulfate hemihydrate nanoellipsoids with a porous structure. Phys. Chem. Chem. Phys. 2015, 17, 11509–11515. [Google Scholar] [CrossRef] [PubMed]
- Mistry, S.; Roy, S.; Maitra, N.J.; Kundu, B.; Chanda, A.; Datta, S.; Joy, M. A novel, multi-barrier, drug eluting calcium sulfate/biphasic calcium phosphate biodegradable composite bone cement for treatment of experimental MRSA osteomyelitis in rabbit model. J. Control. Release 2016, 239, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiong, C.; Zhang, D.; Zhang, L. In vitro bioactivity evaluation of α-calcium sulphate hemihydrate and bioactive glass composites for their potential use in bone regeneration. Bull. Mater. Sci. 2018, 41, 59. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Liu, H.; Liu, X.; Lian, X.; Guo, Z.; Jiang, H.J.; Cui, F.Z. Improved workability of injectable calcium sulfate bone cement by regulation of self-setting properties. Mater. Sci. Eng. C 2013, 33, 1048–1053. [Google Scholar] [CrossRef]
- Huan, Z.; Chang, J. Self-Setting properties and in vitro bioactivity of calcium sulfate hemihydrate-tricalcium silicate composite bone cements. Acta Biomater. 2007, 3, 952–960. [Google Scholar] [CrossRef]
- Anbalagana, G.; Mukundakumarib, S.; Murugesana, K.S.; Gunasekaran, S. Infrared, optical absorption, and EPR spectroscopic studies on natural gypsum. Vib. Spectrosc. 2009, 50, 226–230. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, S.; Yu, B.; Yan, Y.; Li, H.; Wei, J.; Su, J. In vitro degradability, bioactivity and primary cell responses to bone cements containing mesoporous magnesium-calcium silicate and calcium sulfate for bone regeneration. J. R. Soc. Interface 2015, 12, 20150779. [Google Scholar] [CrossRef]
- Hsu, H.J.; Waris, R.A.; Ruslin, M.; Lin, Y.H.; Chen, C.S.; Ou, K.L. An innovative a-calcium sulfate hemihydrate bioceramic as a potential bone graft substitute. J. Am. Ceram. Soc. 2018, 101, 419–427. [Google Scholar] [CrossRef]
- Strohfeldt, K.A. Alkaline Earth Metals. In Essentials of Inorganic Chemistry: For Students of Pharmacy, Pharmaceutical Sciences and Medicinal Chemistry; John Wiley & Sons: Chichester, WS, UK, 2015; pp. 49–65. [Google Scholar]
- Starr, C.; Taggart, R.; Evers, C.; Starr, L. Principles of Cellular Life. In Biology: The Unity and Diversity of Life, 13th ed.; Cengage Learning: Boston, MA, USA, 2012; pp. 82–89. [Google Scholar]
- Ferreira, C.; Pinto, I.S.S.; Soares, E.V.; Soares, H.M.V.M. (Un)suitability of the use of pH buffers in biological, biochemical and environmental studies and its interaction with metal ions—A review. RSC Adv. 2015, 5, 30989–31003. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.; Alk, D.; Sandbank, J.; Farber, R.; Halperin, N. Inflammatory reactions associated with a calcium sulfate bone substitute. Ann. Transplant. 1999, 4, 91–97. [Google Scholar] [PubMed]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef] [Green Version]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, Fracture and Bone Repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Olkowski, R.; Kaszczewski, P.; Czechowska, J.; Siek, D.; Pijocha, D.; Zima, A.; Ślósarczyk, A.; Lewandowska-Szumieł, M. Cytocompatibility of the Selected Calcium Phosphate Based Bone Cements: Comparative Study in Human Cell Culture. J. Mater. Sci. Mater. Med. 2015, 26, 270. [Google Scholar] [CrossRef] [Green Version]
- Velard, F.; Braux, J.; Amedee, J.; Laquerriere, P. Inflammatory Cell Response to Calcium Phosphate Biomaterial Particles: An Overview. Acta Biomater. 2013, 9, 4956–4963. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Vasquez-Lee, M.; Fulzele, K.S.; Smith, D.H.; Branch, K.D.; Christian, S.I.; Williams, D.L. Biocompatibility: Testing. In Concise Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2017; Volume 1, pp. 129–138. [Google Scholar]
- Scarcello, E.; Lambremont, A.; Vanbever, R.; Jacques, P.J.; Lison, D. Mind your assays: Misleading cytotoxicity with the WST-1 assay in the presence of manganese. PLoS ONE 2020, 15, e0231634. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, N.T.N.; Le, N.T.T.; Nguyen, Q.L.; Pham, T.L.-B.; Nguyen-Le, M.-T.; Nguyen, D.H. A Facile Synthesis Process and Evaluations of α-Calcium Sulfate Hemihydrate for Bone Substitute. Materials 2020, 13, 3099. https://doi.org/10.3390/ma13143099
Le NTN, Le NTT, Nguyen QL, Pham TL-B, Nguyen-Le M-T, Nguyen DH. A Facile Synthesis Process and Evaluations of α-Calcium Sulfate Hemihydrate for Bone Substitute. Materials. 2020; 13(14):3099. https://doi.org/10.3390/ma13143099
Chicago/Turabian StyleLe, Nhi Thao Ngoc, Ngoc Thuy Trang Le, Quang Lam Nguyen, Truc Le-Buu Pham, Minh-Tri Nguyen-Le, and Dai Hai Nguyen. 2020. "A Facile Synthesis Process and Evaluations of α-Calcium Sulfate Hemihydrate for Bone Substitute" Materials 13, no. 14: 3099. https://doi.org/10.3390/ma13143099
APA StyleLe, N. T. N., Le, N. T. T., Nguyen, Q. L., Pham, T. L.-B., Nguyen-Le, M.-T., & Nguyen, D. H. (2020). A Facile Synthesis Process and Evaluations of α-Calcium Sulfate Hemihydrate for Bone Substitute. Materials, 13(14), 3099. https://doi.org/10.3390/ma13143099




