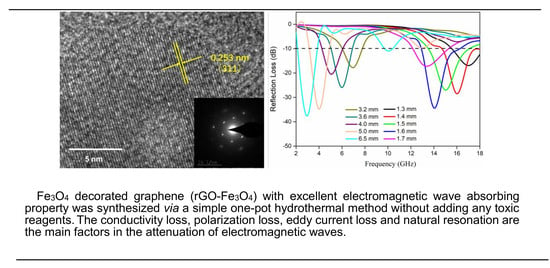
One-Pot Hydrothermal Preparation of Fe3O4 Decorated Graphene for Microwave Absorption
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of rGO-Fe3O4
2.3. Characterization
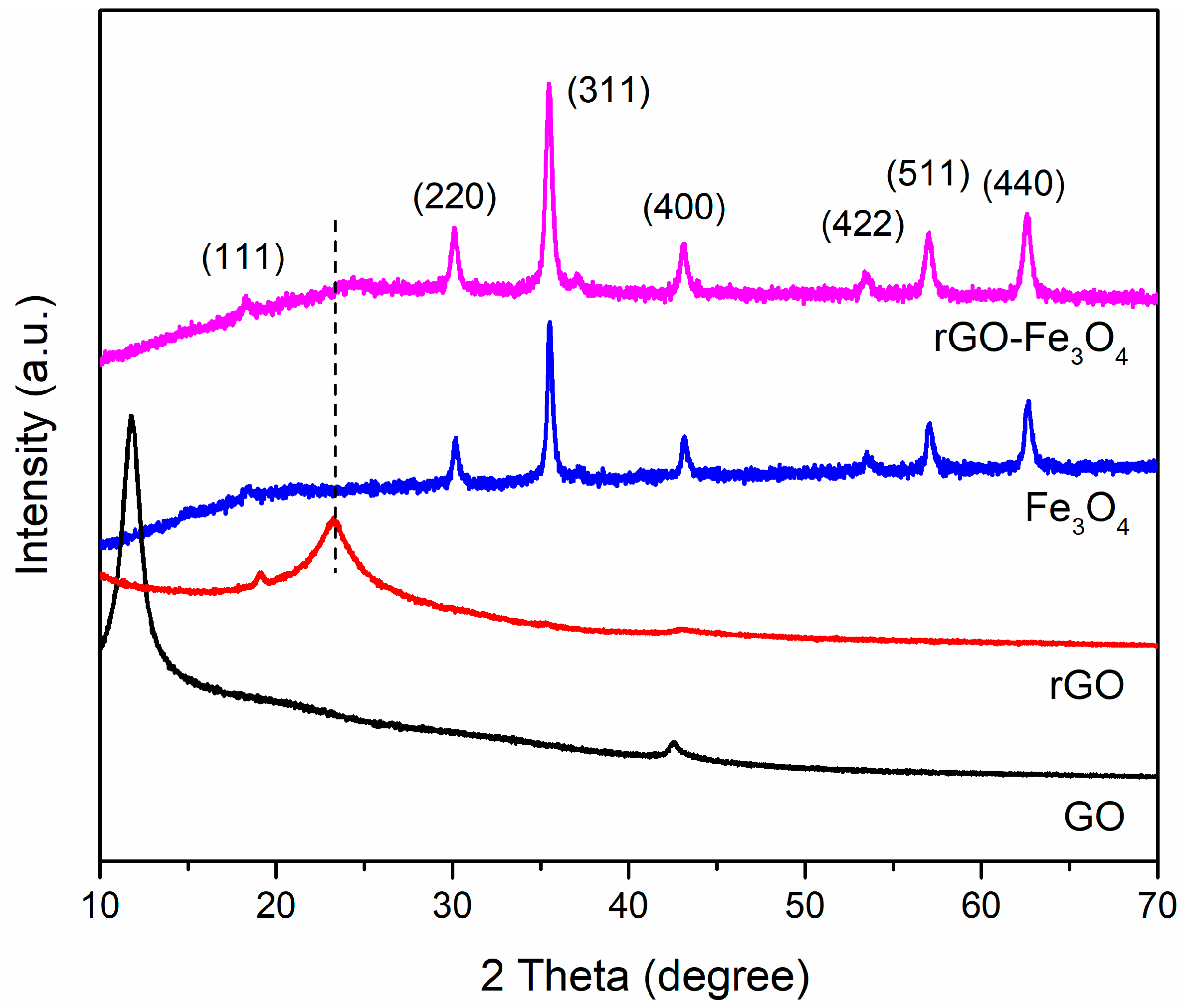
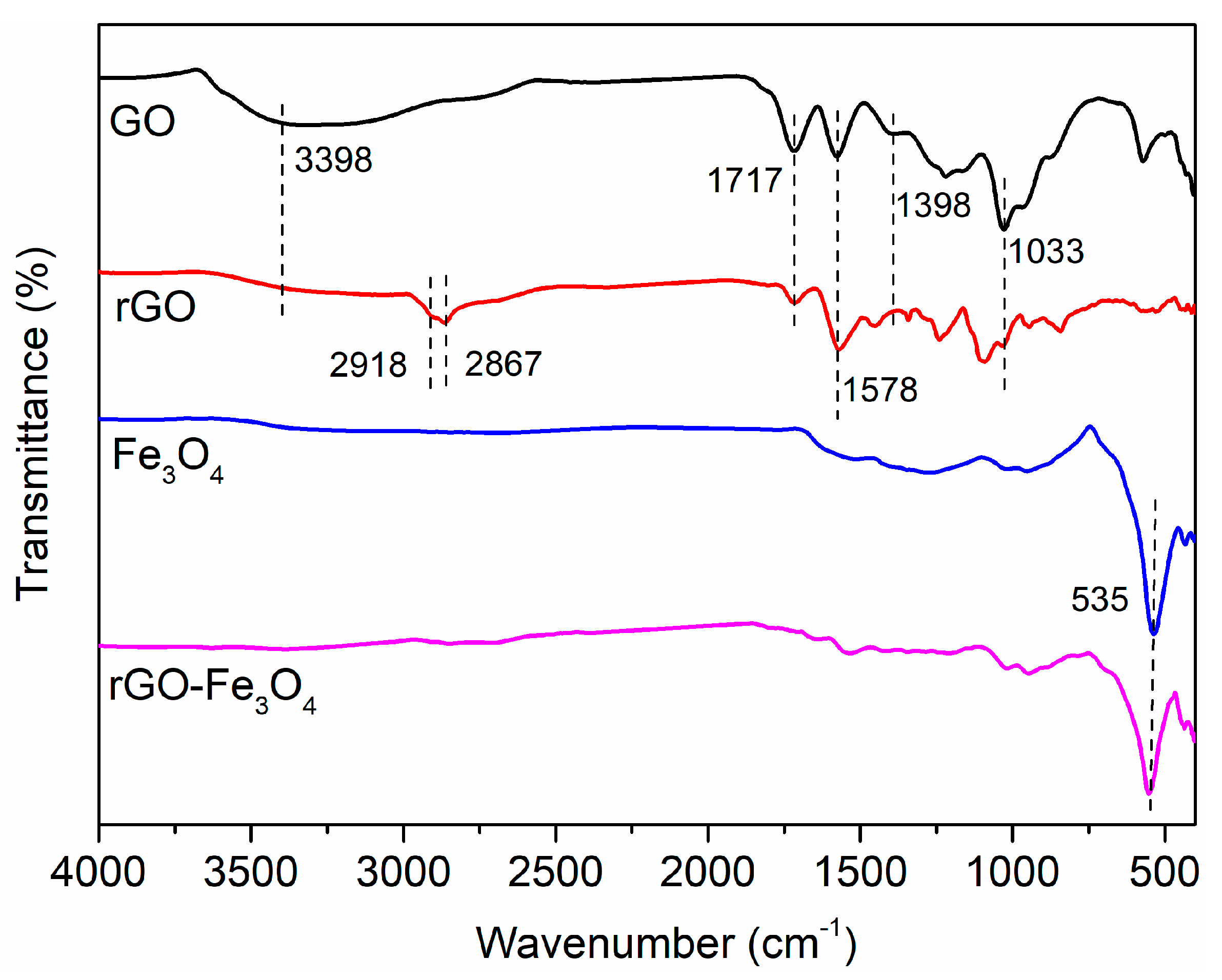
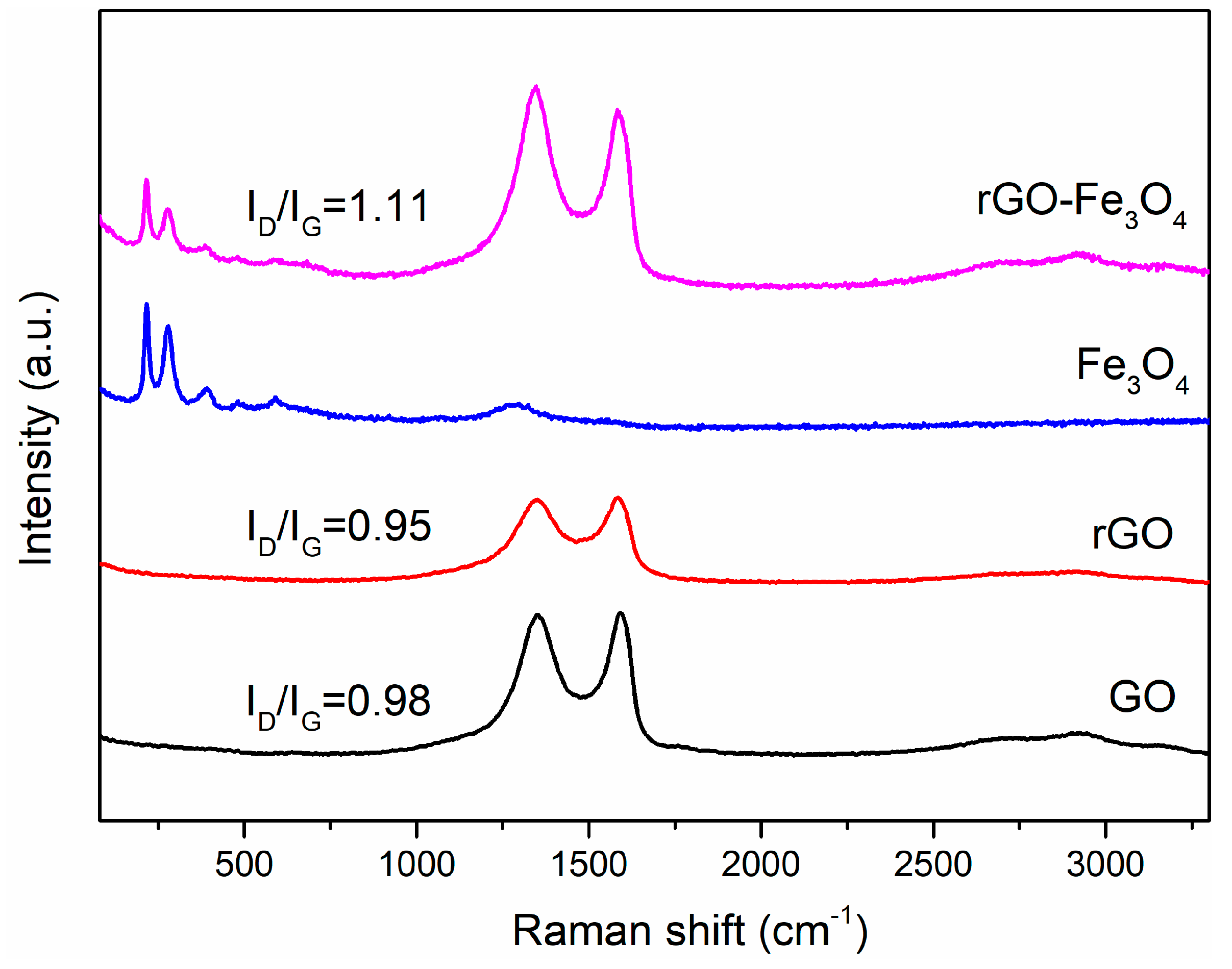
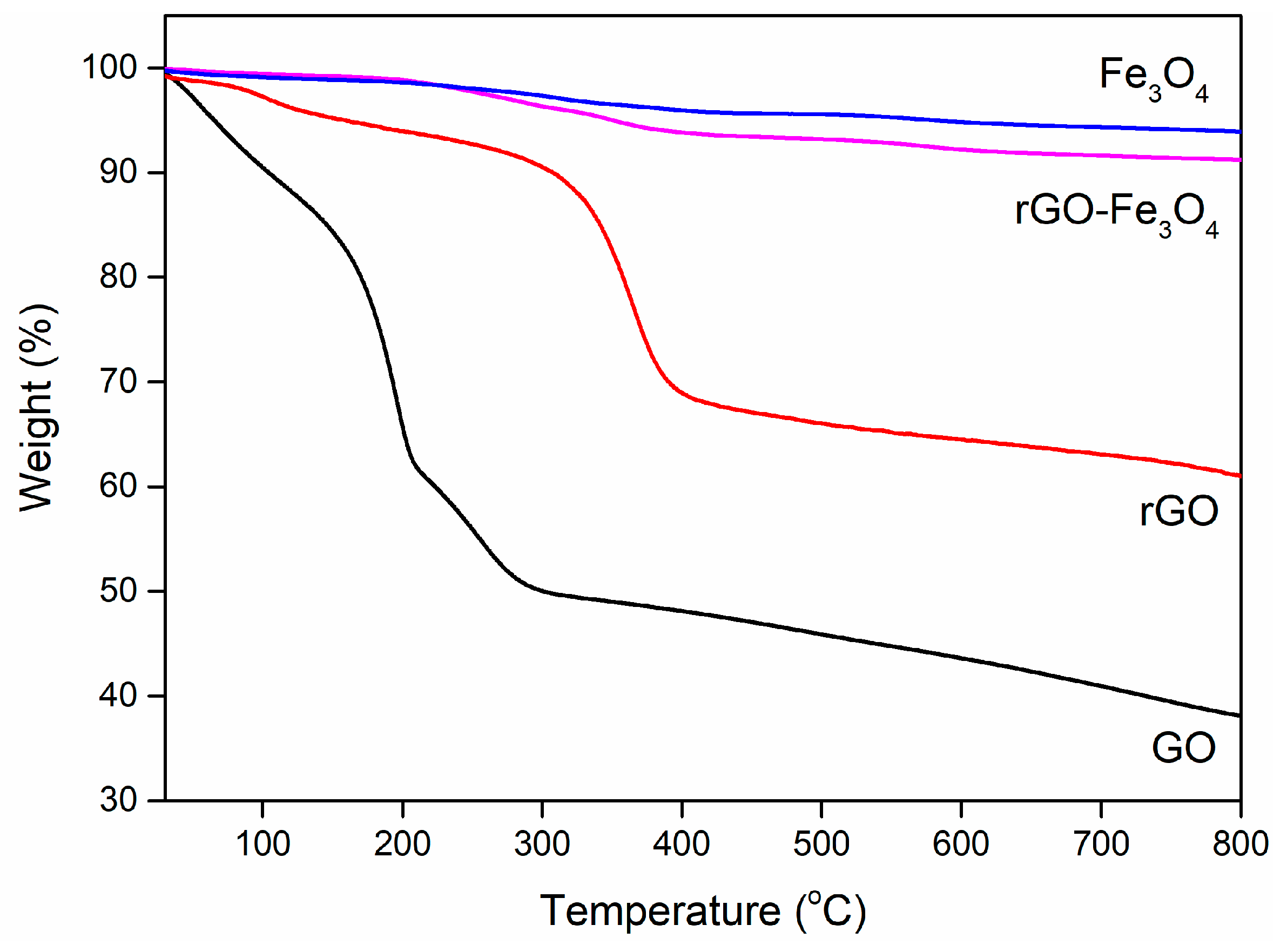
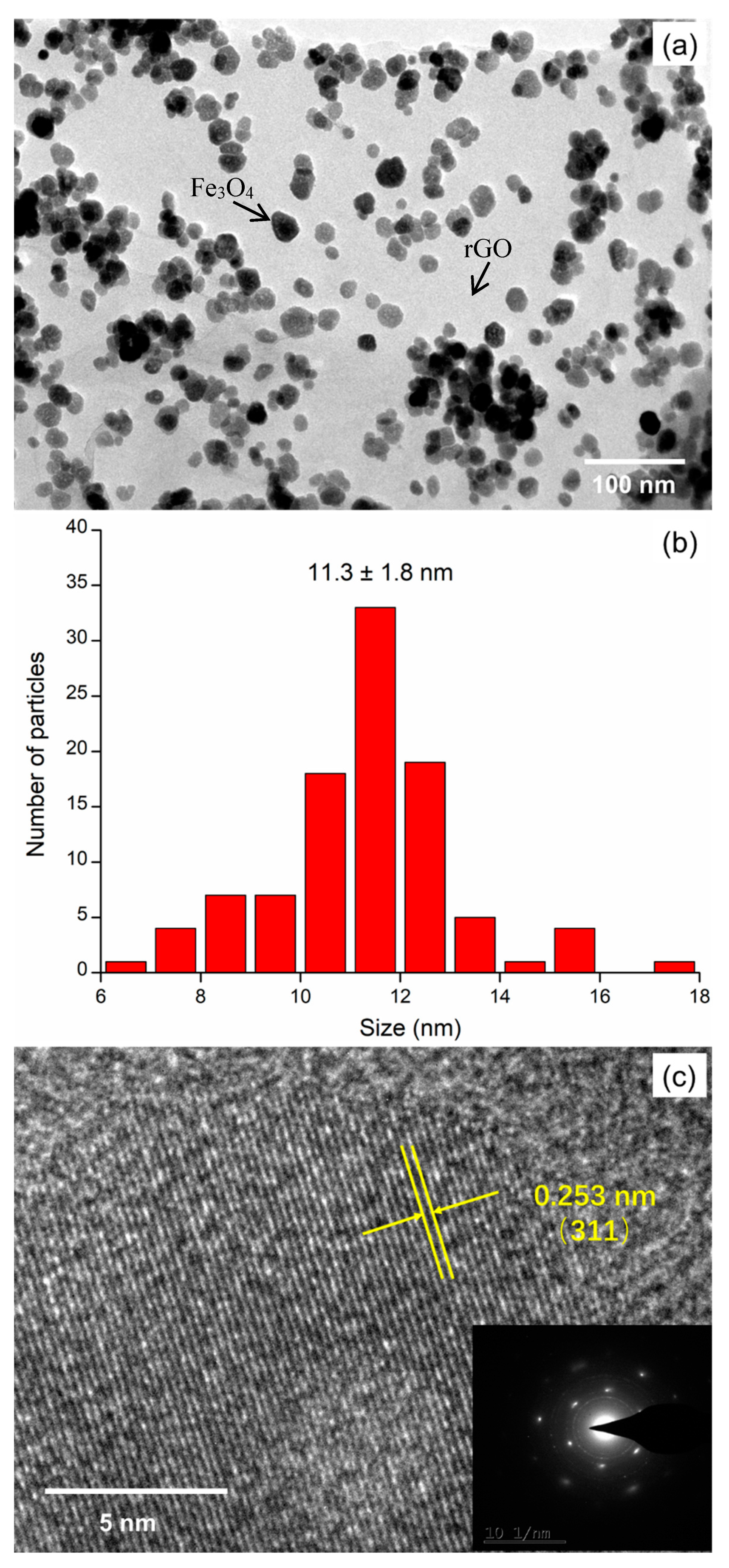
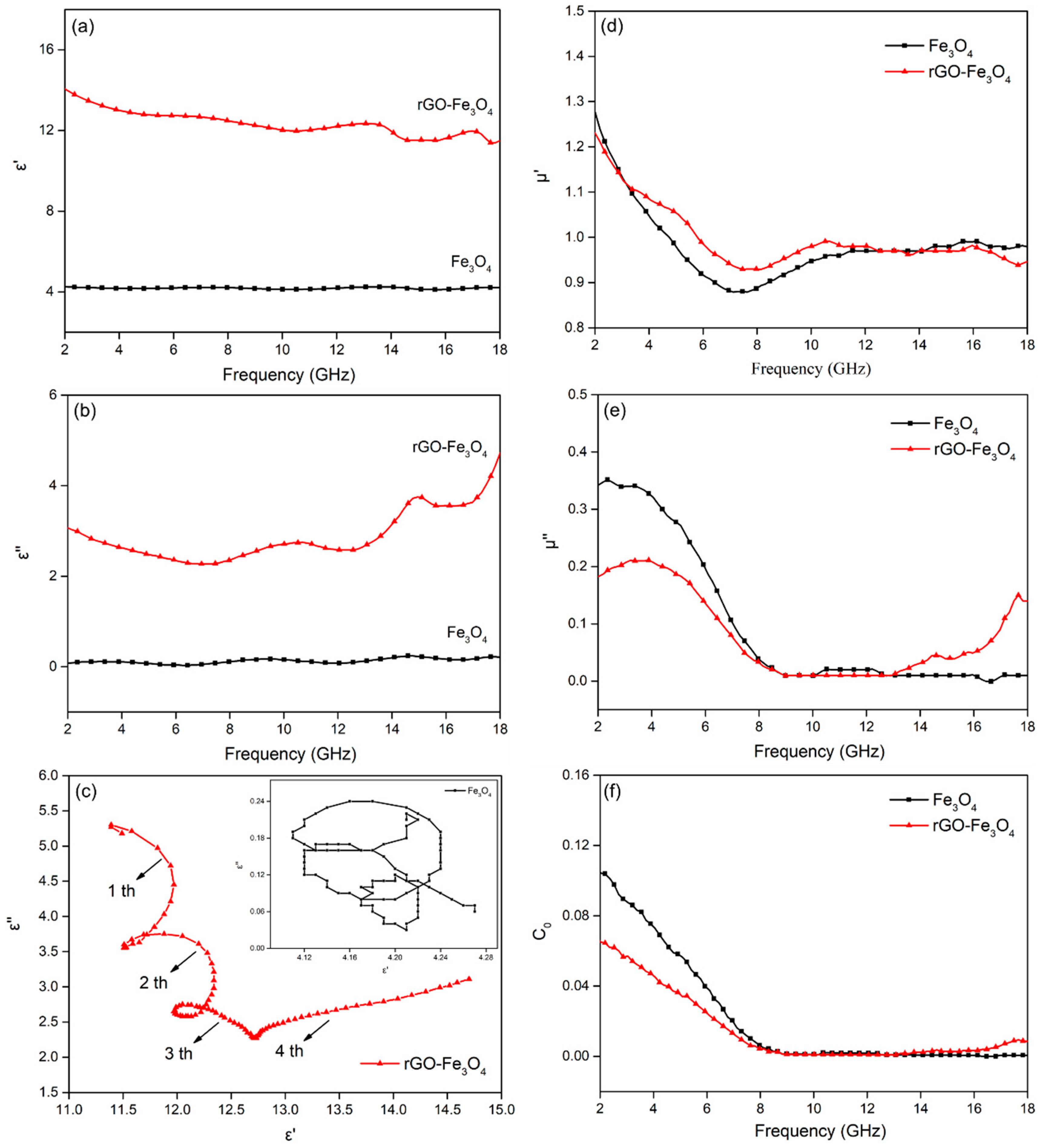
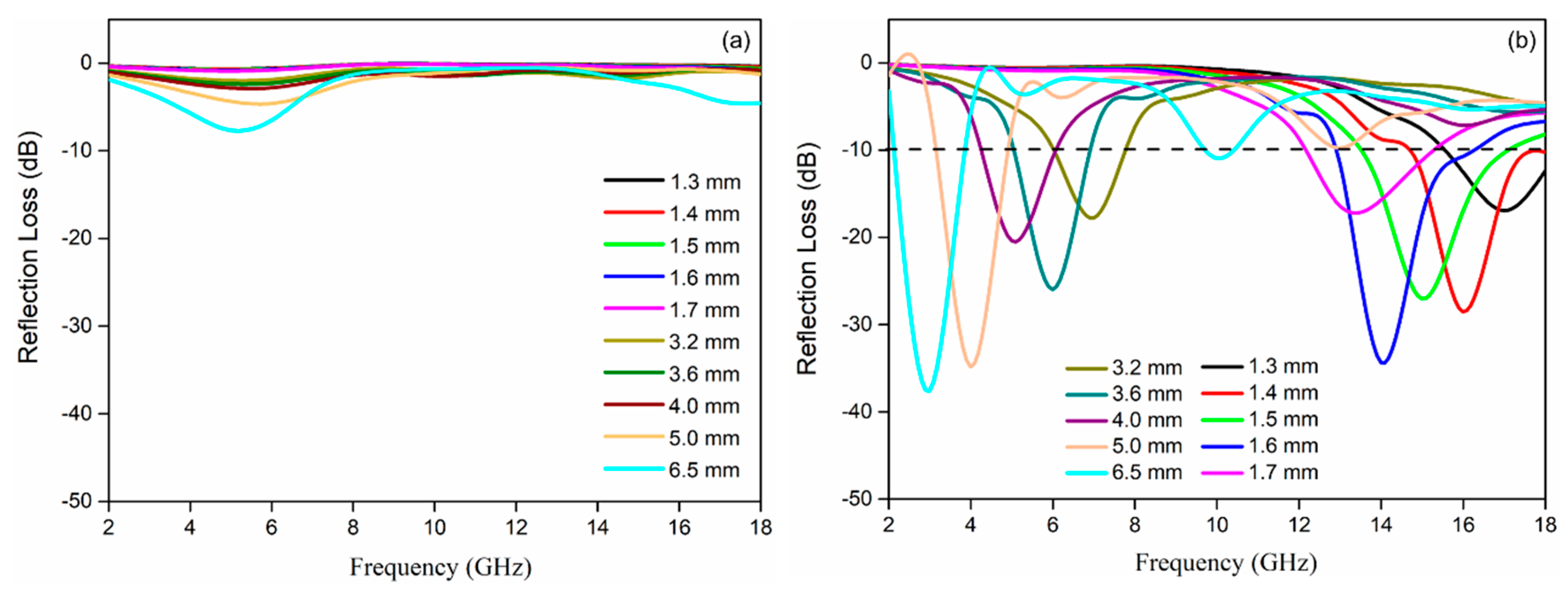
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, J.; Li, Z.B.; Jia, Y.S.; Yang, B.; Liu, S.J.; Zhao, X.; Li, L.W.; Zuo, L. Significant high-frequency electromagnetic wave absorption performance of Ni2+x Mn1-x Ga alloys. J. Mater. Sci. 2018, 53, 11779–11790. [Google Scholar] [CrossRef]
- Kang, S.; Qiao, S.Y.; Hu, Z.M.; Yu, J.R.; Wang, Y.; Zhu, J. Interfacial polymerized reduced graphene oxide covalently grafted polyaniline nanocomposites for high-performance electromagnetic wave absorber. J. Mater. Sci. 2019, 54, 6410–6424. [Google Scholar] [CrossRef]
- Yan, P.; Miao, J.; Cao, J.; Zhang, H.; Wang, C.P.; Xie, A.J.; Shen, Y.H. Facile synthesis and excellent electromagnetic wave absorption properties of flower-like porous RGO/PANI/Cu2O nanocomposites. J. Mater. Sci. 2017, 52, 13078–13090. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, X.; Chen, Y.; Liu, Y.J.; Yang, R.; Sui, G.X. Hierarchically structured cellulose aerogels with interconnected MXene networks and their enhanced microwave absorption properties. J. Mater. Chem C 2018, 6, 8679–8687. [Google Scholar] [CrossRef]
- Wang, H.; Xie, G.Z.; Xie, N.Y.; Ye, L.J.; Chen, J.W.; Chen, J. Electromagnetic and absorbing properties of the composites based on iron, cobalt, B and rare earth Nd. J. Mater. Sci-Mater. El. 2019, 30, 401–405. [Google Scholar] [CrossRef]
- Xiong, Y.; Luo, H.; Nie, Y.; Chen, F.; Dai, W.Y.; Wang, X.; Cheng, Y.Z.; Gong, R.Z. Synergistic effect of silica coated porous rodlike nickel ferrite and multiwalled carbon nanotube with improved electromagnetic wave absorption performance. J. Alloy. Compd. 2019, 802, 364–372. [Google Scholar] [CrossRef]
- Chen, Y.J.; Cao, M.S.; Tian, Q.; Wang, T.H.; Zhu, J. A novel preparation and surface decorated approach for α-Fe nanoparticles by chemical vapor–liquid reaction at low temperature. Mater. Lett. 2004, 58, 1481–1484. [Google Scholar] [CrossRef]
- Zhao, T.K.; Jin, W.B.; Ji, X.L.; Yan, H.B.; Jiang, Y.T.; Dong, Y.; Yang, Y.L.; Dang, A.L.; Li, H.; Li, T.H.; et al. Synthesis of sandwich microstructured expanded graphite/barium ferrite connected with carbon nanotube composite and its electromagnetic wave absorbing properties. J. Alloy. Compd. 2017, 712, 59–68. [Google Scholar] [CrossRef]
- Duan, Y.L.; Li, Y.; Wang, D.E.; Wang, R.Q.; Wang, Y.L.; Hou, L.Q.; Yan, X.Y.; Li, Q.; Yang, W.; Li, Y.F. Transverse size effect on electromagnetic wave absorption performance of exfoliated thin-layered flake graphite. Carbon 2019, 153, 682–690. [Google Scholar] [CrossRef]
- Zhang, J.B.; Shu, R.W.; Guo, C.L.; Sun, R.R.; Chen, Y.N.; Yuan, J. Fabrication of nickel ferrite microspheres decorated multi-walled carbon nanotubes hybrid composites with enhanced electromagnetic wave absorption properties. J. Alloy. Compd. 2019, 784, 422–430. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhang, S.; Yan, F.; Li, C.Y.; Zhu, C.L.; Zhang, X.T.; Chen, Y.J. Three-Dimensional Hierarchical MoS2 Nanosheets/Ultralong N-Doped Carbon Nanotubes as High-Performance Electromagnetic Wave Absorbing Material. ACS Appl. Mater. Inter. 2018, 10, 14108–14115. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, D.W.; Chen, P.; Yu, Q.; Xiong, X.H.; Chu, H.R.; Wang, Q. 3D graphene-Ni microspheres with excellent microwave absorption and corrosion resistance properties. J. Mater. Sci-Mater. El. 2018, 29, 2421–2433. [Google Scholar] [CrossRef]
- Xiong, L.L.; Yu, M.; Liu, J.H.; Li, S.M.; Xue, B. Preparation and evaluation of the microwave absorption properties of template-free graphene foam-supported Ni nanoparticles. RSC Adv. 2017, 7, 14733–14741. [Google Scholar] [CrossRef]
- Liu, P.B.; Huang, Y. Synthesis of reduced graphene oxide-conducting polymers-Co3O4 composites and their excellent microwave absorption properties. RSC Adv. 2013, 3, 19033–19039. [Google Scholar] [CrossRef]
- Guan, X.H.; Kuang, J.M.; Yang, L.; Lu, M.; Wang, G.S. Membrane-Solvothermal Synthesis of Cobalt Ferrite/Reduced Graphene Oxide Nanocomposites and Their Photocatalytic and Electromagnetic Wave Absorption Properties. Chemistryselect 2019, 4, 9516–9522. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, C.Y.; Su, T.; Zhong, B. One-step hydrothermal synthesis of Ni-Fe-P/graphene nanosheet composites with excellent electromagnetic wave absorption properties. RSC Adv. 2019, 9, 5570–5581. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, C.Y.; Zhong, B. Optimization of electromagnetic wave absorbing properties for Ni-Co-P/GNs by controlling the content ratio of Ni to Co. J. Magn Magn Mater. 2020, 495. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, L.; Liao, Q.L.; Zhang, G.J.; Liu, S.; Zhang, Y. Electromagnetic wave absorption in reduced graphene oxide functionalized with Fe3O4/Fe nanorings. Nano Res. 2016, 9, 2018–2025. [Google Scholar] [CrossRef]
- Haga, K.; Sugimoto, S.; Kagotani, T.; Inomata, K. Electromagnetic wave absorption properties of Co-Ti substituted Ba M-type ferrite produced by a modified chemical coprecipitation. Mater. Trans. 2004, 45, 2606–2609. [Google Scholar] [CrossRef]
- Xie, J.L.; Han, M.G.; Chen, L.A.; Kuang, R.X.; Deng, L.J. Microwave-absorbing properties of NiCoZn spinel ferrites. J. Magn Magn Mater. 2007, 314, 37–42. [Google Scholar] [CrossRef]
- Hameed, A.S.; Reddy, M.V.; Chowdari, B.V.R.; Vittal, J.J. Preparation of rGO-wrapped magnetite nanocomposites and their energy storage properties. RSC Adv. 2014, 4, 64142–64150. [Google Scholar] [CrossRef]
- Liang, T.; Wang, H.W.; Xu, D.M.; Liao, K.; Wang, R.; He, B.B.; Gong, Y.S.; Yan, C.J. High-energy flexible quasi-solid-state lithium-ion capacitors enabled by a freestanding rGO-encapsulated Fe3O4 nanocube anode and a holey rGO film cathode. Nanoscale 2018, 10, 17814–17823. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Ahmad, I.; Israr, M. Graphene/Fe3O4 nanocomposite: Interplay between photo-Fenton type reaction, and carbon purity for the removal of methyl orange. Ceram. Int. 2018, 44, 2643–2648. [Google Scholar] [CrossRef]
- Yan, L.; Pu, Z.J.; Xu, M.Z.; Wei, R.B.; Liu, X.B. Fabrication and Electromagnetic Properties of Conjugated NH2-CuPc@Fe3O4. J. Electron. Mater. 2017, 46, 5608–5618. [Google Scholar] [CrossRef]
- Wang, X.X.; Yu, J.H.; Dong, H.Z.; Yu, M.X.; Zhang, B.Q.; Wang, W.; Dong, L.F. Synthesis of nanostructured MnO2, SnO2, and Co3O4: Graphene composites with enhanced microwave absorption properties. Appl. Phys. A-Mater. 2015, 119, 1483–1490. [Google Scholar] [CrossRef]
- Zhou, N.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Shi, Z. Solvothermal synthesis of three-dimensional, Fe2O3 NPs-embedded CNT/N-doped graphene composites with excellent microwave absorption performance. RSC Adv. 2017, 7, 45156–45169. [Google Scholar] [CrossRef]
- Wang, Y.P.; Peng, Z.; Jiang, W. Size-controllable synthesis of Fe3O4 nanospheres decorated graphene for electromagnetic wave absorber. J. Mater. Sci. Mater. El. 2016, 27, 6010–6019. [Google Scholar] [CrossRef]
- Che, R.C.; Peng, L.-M.; Duan, X.F.; Chen, Q.; Liang, X.L. Microwave Absorption Enhancement and Complex Permittivity and Permeability of Fe Encapsulated within Carbon Nanotubes. Adv. Mater. 2004, 16, 401–405. [Google Scholar] [CrossRef]
- Shu, X.F.; Ren, H.D.; Jiang, Y.; Zhou, J.; Wang, Y.Q.; Wang, Y.F.; Liu, Y.; Oh, W.C. Enhanced electromagnetic wave absorption performance of silane coupling agent KH550@Fe3O4 hollow nanospheres/graphene composites. J. Mater. Chem. C 2020, 8, 2913–2926. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Quan, B.; Liu, W.; Liang, X.H.; Ji, G.B.; Du, Y.W. A facile one-pot strategy for fabrication of carbon-based microwave absorbers: Effects on annealing and paraffin content. Dalton T 2017, 46, 9097–9102. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Ma, H.L.; Cao, K.; Wang, L.C.; Zeng, X.M.; Zhang, X.Q.; He, L.H.; Liu, P.G.; Wang, Z.Y.; Zhai, M.L. Gamma Irradiation-Induced Preparation of Graphene-Ni Nanocomposites with Efficient Electromagnetic Wave Absorption. Materials 2018, 11, 2145. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Ye, Z.M.; Liu, W.X.; Liu, Z.F.; Chen, J. The effect of GO loading on electromagnetic wave absorption properties of Fe3O4/reduced graphene oxide hybrids. Ceram. Int 2017, 43, 13146–13153. [Google Scholar] [CrossRef]
- Zhang, K.L.; Lv, W.X.; Chen, J.; Ge, H.Y.; Chu, C.X.; Tang, D.B. Synthesis of RGO/AC/Fe3O4 composite having 3D hierarchically porous morphology for high effective electromagnetic wave absorption. Compos. Part. B-Eng. 2019, 169, 1–8. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Chen, X.; Zhang, Y.; Que, X.; Liu, P.; Zhang, X.; Ma, H.-L.; Zhai, M. One-Pot Hydrothermal Preparation of Fe3O4 Decorated Graphene for Microwave Absorption. Materials 2020, 13, 3065. https://doi.org/10.3390/ma13143065
Du Z, Chen X, Zhang Y, Que X, Liu P, Zhang X, Ma H-L, Zhai M. One-Pot Hydrothermal Preparation of Fe3O4 Decorated Graphene for Microwave Absorption. Materials. 2020; 13(14):3065. https://doi.org/10.3390/ma13143065
Chicago/Turabian StyleDu, Zhonghe, Xibang Chen, Youwei Zhang, Xueyan Que, Pinggui Liu, Xiuqin Zhang, Hui-Ling Ma, and Maolin Zhai. 2020. "One-Pot Hydrothermal Preparation of Fe3O4 Decorated Graphene for Microwave Absorption" Materials 13, no. 14: 3065. https://doi.org/10.3390/ma13143065
APA StyleDu, Z., Chen, X., Zhang, Y., Que, X., Liu, P., Zhang, X., Ma, H.-L., & Zhai, M. (2020). One-Pot Hydrothermal Preparation of Fe3O4 Decorated Graphene for Microwave Absorption. Materials, 13(14), 3065. https://doi.org/10.3390/ma13143065