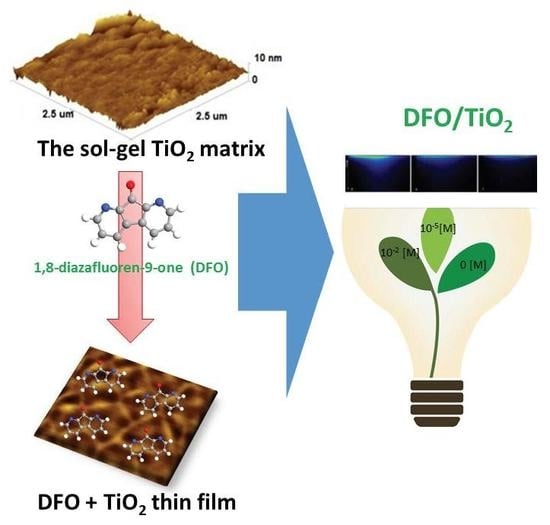
The Luminescence of 1,8-Diazafluoren-9-One/Titanium Dioxide Composite Thin Films for Optical Application
Abstract
:1. Introduction
2. Materials and Methods
Apparatus
3. Results and Discussion
3.1. Morphology of DFO/TiO2 Thin Films
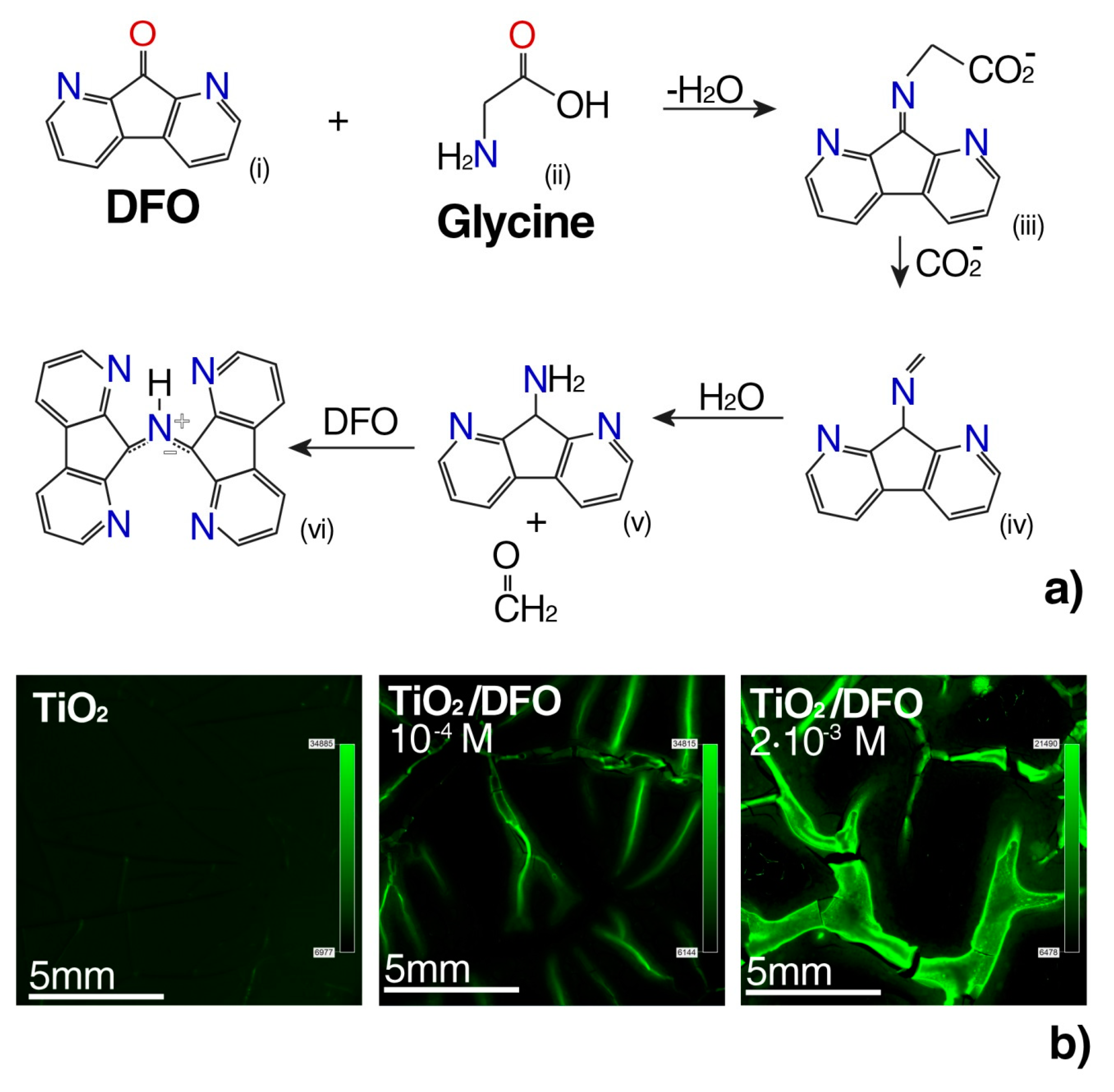
3.2. The Luminescence Properties of the DFO/TiO2 Thin Films
- Emission of monomers and weakly fluorescent TiO2 matrix where both types of emitting species fluoresce in the similar spectral region. The emission close to about 470 nm was present in amorphous film TiO2, although with a small intensity [30]. An increase in the intensity of the monomer fluorescence signal at a small concentration of DFO generally indicates the influence of the TiO2 matrix. However, a decrease in the intensity of the monomer PL signal at a high concentration of DFO shows the creation of fluorescence aggregates, which are more dominant over the TiO2 matrix at long wavelengths.
- DFO is a solvatochromically sensitive probe; therefore, under some changes in the matrix polarity, the effect of spectral shift could be anticipated. However, the matrix remains chemically unmodified for all the samples and the effect of a potential significant polarity change of the whole matrix by only a concentration increase of DFO molecules seems rather unlikely. The location of the fluorescence peak of DFO by comparison to the fluorescence of DFO and other fluorenone-like molecules in liquid solutions [1,31,32] suggests that the TiO2 matrix belongs to relatively nonpolar media. This is also in agreement with other results and discussions performed previously [25,26,33].
3.3. Design of Luminescent Probe Sensitive to the Presence of the Markers of Urogenital Tract Cancer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lewkowicz, A.; Baranowska, K.; Bojarski, P.; Józefowicz, M. Solvent dependent spectroscopic properties of fingerprint reagent-1, 8-Diazafluoren-9-one. J. Mol. Liq. 2019, 285, 754–765. [Google Scholar] [CrossRef]
- Bojarski, P.; Kułak, L.; Grajek, H.; Zurkowska, G.; Kamińska, A.; Kukliński, B.; Bojarski, C. Excitation energy transport and trapping in concentrated solid solutions of flavomononucleotide. Biochim. Biophys. Acta Gen. Subj. 2003, 1619, 201–208. [Google Scholar] [CrossRef]
- Grajek, H.; Zurkowska, G.; Bojarski, P.; Kukliński, B.; Smyk, B.; Drabent, R.; Bojarski, C. Spectroscopic manifestations of flavomononucleotide dimers in polyvinyl alcohol films. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998, 1384, 253–267. [Google Scholar] [CrossRef]
- Rangełowa-Jankowska, S.; Jankowski, D.; Bogdanowicz, R.; Grobelna, B.; Bojarski, P. Surface plasmon-coupled emission of rhodamine 110 aggregates in a silica nanolayer. J. Phys. Chem. Lett. 2012, 3, 3626–3631. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Pandey, R.K.; Prasad, P.N. Organically modified silica nanoparticles co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy. J. Am. Chem. Soc. 2007, 129, 2669–2675. [Google Scholar] [CrossRef] [Green Version]
- Holzwarth, A.R.; Gnebenow, K.; Gnebenow, K.; Schaffner, K. A Photosynthetic Antenna System which Contains a Protein-Free Chromophore Aggregate. Z. für Nat. C 1990, 45, 203–206. [Google Scholar] [CrossRef]
- Starzyk, J.; Gruszecki, M.; Tutaj, K.; Luchowski, R.; Szlazak, R.; Wasko, P.; Grudzinski, W.; Czub, J.; Gruszecki, W.I. Self-association of amphotericin b: Spontaneous formation of molecular structures responsible for the toxic side effects of the antibiotic. J. Phys. Chem. B 2014, 118, 13821–13832. [Google Scholar] [CrossRef]
- Wasko, P.; Luchowski, R.; Tutaj, K.; Grudzinski, W.; Adamkiewicz, P.; Gruszecki, W.I. Toward understanding of toxic side effects of a polyene antibiotic amphotericin B: Fluorescence spectroscopy reveals widespread formation of the specific supramolecular structures of the drug. Mol. Pharm. 2012, 9, 1511–1520. [Google Scholar] [CrossRef]
- Holzwarth, A.R.; Schaffner, K. On the structure of bacteriochlorophyll molecular aggregates in the chlorosomes of green bacteria. A molecular modelling study. Photosynth. Res. 1994, 41, 225–233. [Google Scholar] [CrossRef]
- Gruszecki, W.I. Structure-function relationship of the plant photosynthetic pigment-protein complex LHCII studied with molecular spectroscopy techniques. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 93, pp. 81–93. ISBN 9780124165960. [Google Scholar]
- Janik, E.; Bednarska, J.; Zubik, M.; Sowinski, K.; Luchowski, R.; Grudzinski, W.; Gruszecki, W.I. Is It Beneficial for the Major Photosynthetic Antenna Complex of Plants to Form Trimers? J. Phys. Chem. B 2015, 119, 8501–8508. [Google Scholar] [CrossRef]
- Grajek, H. Review—Flavins as photoreceptors of blue light and their spectroscopic properties. Curr. Top. Biophys. 2012, 34, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Polavarapu, L.; Pérez-Juste, J.; Xu, Q.H.; Liz-Marzán, L.M. Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles. J. Mater. Chem. C 2014, 2, 7460–7476. [Google Scholar] [CrossRef]
- Synak, A.; Grobelna, B.; Raut, S.; Bojarski, P.; Gryczyński, I.; Karczewski, J.; Shtoyko, T. Metal enhanced fluorescence of flavin mononucleotide using new plasmonic platform. Opt. Mater. 2016, 59, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Synak, A.; Szczepańska, E.; Grobelna, B.; Gondek, J.; Mońka, M.; Gryczynski, I.; Bojarski, P. Photophysical properties and detection of Valrubicin on plasmonic platforms. Dye. Pigment. 2019, 163, 623–627. [Google Scholar] [CrossRef]
- Silva, A.H.; Locatelli, C.; Filho, U.P.; Gomes, B.F.; Júnior, R.M.D.C.; De Gois, J.S.; Borges, D.L.G.; Creczynski-Pasa, T.B. Visceral fat increase and signals of inflammation in adipose tissue after administration of titanium dioxide nanoparticles in mice. Toxicol. Ind. Heal. 2016, 33, 147–158. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, J.; Ji, L.; Wang, L.; Hong, F. Respiratory exposure to nano-TiO2 induces pulmonarytoxicity in mice involving reactive free radical-activatedTGF-β/Smad/p38MAPK/Wnt pathways. J. Biomed Mater. Res. 2019, 107, 2567–2575. [Google Scholar] [CrossRef]
- Ferrara, G.; Salvaggio, A.; Pecoraro, R.; Scalisi, E.M.; Presti, A.M.; Impellizzeri, G.; Brundo, M.V. Toxicity assessment of nano-TiO2 in Apis mellifera L., 1758: Histological and immunohistochemical assays. Microsc. Res. Tech. 2020, 83, 332–337. [Google Scholar] [CrossRef]
- Flaccavento, A.; Pecoraro, R.; Scalisi, E.M.; Messina, G.; Salvaggio, A.; Impellizzeri, G.; Lombardo, B.M.; Brundo, M.V. Morphostructural and immunohistochemical study forevaluation of nano-TiO2 toxicity in Armadillo officinalis Duméril,1816 (Crustacea, Isopoda, Oniscidea). Microsc. Res. Tech. 2020, 83, 297–303. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Lewkowicz, A.; Rycewicz, M.; Szczodrowski, K.; Ruello, M.L.; Bogdanowicz, R. Enhanced photocatalytic activity of transparent carbon nanowall/TiO2 heterostructures. Mater. Lett. 2020, 262, 127155. [Google Scholar] [CrossRef]
- El-Shabasy, R.; Yosri, N.; El-Seedi, H.; Shoueir, K.; El-Kemary, M. A green synthetic approach using chili plant supported Ag/Ag2O@P25 heterostructure with enhanced photocatalytic properties under solar irradiation. Optik 2019, 192, 162943. [Google Scholar] [CrossRef]
- Struck-Lewicka, W.; Kordalewska, M.; Bujak, R.; Yumba Mpanga, A.; Markuszewski, M.; Jacyna, J.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M.J. Urine metabolic fingerprinting using LC-MS and GC-MS reveals metabolite changes in prostate cancer: A pilot study. J. Pharm. Biomed. Anal. 2015, 111, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Yumba-Mpanga, A.; Struck-Lewicka, W.; Wawrzyniak, R.; Markuszewski, M.; Roslan, M.; Kaliszan, R.; Markuszewski, M.J. Metabolomic Heterogeneity of Urogenital Tract Cancers Analyzed by Complementary Chromatographic Techniques Coupled with Mass Spectrometry. Curr. Med. Chem. 2019, 26, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Bujak, R.; Daghir, E.; Rybka, J.; Koslinski, P.; Markuszewski, M.J. Metabolomics in urogenital cancer. Bioanalysis 2011, 3, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, A.R.; Bloomer, I. Optical properties of crystalline semiconductors and dielectrics. Phys. Rev. B 1988, 38, 1865–1874. [Google Scholar] [CrossRef]
- Lewkowicz, A.; Bojarski, P.; Synak, A.; Grobelna, B.; Bogdanowicz, R.; Karczewski, J.; Szczodrowski, K.; Behrendt, M. Thickness and structure change of titanium(IV) oxide thin films synthesized by the sol–gel spin coating method. Opt. Mater. 2014, 36, 1739–1744. [Google Scholar] [CrossRef]
- Kubicki, A.A.; Bojarski, P.; Grinberg, M.; Sadownik, M.; Kukliński, B. Time-resolved streak camera system with solid state laser and optical parametric generator in different spectroscopic applications. Opt. Commun. 2006, 263, 275–280. [Google Scholar] [CrossRef]
- Gartner, M.; Parlog, C.; Osiceanu, P. Spectroellipsometric characterization of lanthanide-doped TiO2 films obtained via the sol-gel technique. Thin Solid Film. 1993, 234, 561–565. [Google Scholar] [CrossRef]
- Karasiński, P. Influence of technological parameters on the properties of sol-gel silica films. Opt. Appl. 2005, 35, 117–128. [Google Scholar]
- Buha, J. Photoluminescence study of carbon doped and hydrogen co-doped TiO2 thin films. Thin Solid Film. 2013, 545, 234–240. [Google Scholar] [CrossRef]
- Józefowicz, M.; Heldt, J.R. Preferential solvation of fluorenone and 4-hydroxyfluorenone in binary solvent mixtures. Chem. Phys. 2003, 294, 105–116. [Google Scholar] [CrossRef]
- Józefowicz, M.; Heldt, J.R.; Heldt, J. Solvent effects on electronic transitions of fluorenone and 4-hydroxyfluorenone. Chem. Phys. 2006, 323, 617–621. [Google Scholar] [CrossRef]
- Khataee, A.; Mansoori, G.A. Nanostructured Titanium Dioxide Materials: Properties, Preparation and Applications; World Scientific Publishing: Hackensack, NJ, USA, 2012. [Google Scholar]
- Bojarski, P.; Matczuk, A.; Bojarski, C.; Kawski, A.; Kukliński, B.; Zurkowska, G.; Diehl, H. Fluorescent dimers of rhodamine 6G in concentrated ethylene glycol solution. Chem. Phys. 1996, 210, 485–499. [Google Scholar] [CrossRef]
- Bojarski, P.; Matczuk, A.; Leszek, K.; Bojarski, C. Quantitative analysis of concentration fluorescence quenching in condensed systems. Asian J. Spectrosc. 1999, 3, 1–21. [Google Scholar]
- Grajek, H.; Gryczynski, I.; Bojarski, P.; Gryczynski, Z.; Bharill, S.; Kułak, L. Flavin mononucleotide fluorescence intensity decay in concentrated aqueous solutions. Chem. Phys. Lett. 2007, 439, 151–156. [Google Scholar] [CrossRef]
- Zakharova, G.V.; Kombaev, A.R.; Chibisov, A.K. J aggregation of meso-ethylsubstituted carbocyanine dyes in polymer films. High Energy Chem. 2004, 38, 180–183. [Google Scholar] [CrossRef]
- Bojarski, P.; Jankowicz, A. Excitation energy transport between the ionic forms of rhodamine B in viscous solutions. J. Lumin. 1999, 81, 21–31. [Google Scholar] [CrossRef]
- Vogel, R.; Meredith, P.; Harvey, M.D.; Rubinsztein-Dunlop, H. Absorption and fluorescence spectroscopy of rhodamine 6G in titanium dioxide nanocomposites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 245–249. [Google Scholar] [CrossRef]
- Grigg, R.; Mongkolaussavaratana, T.; Anthony Pounds, C.; Sivagnanam, S. 1,8-diazafluorenone and related compounds. A new reagent for the detection of (α-amino acids and latent fingerprints. Tetrahedron Lett. 1990, 31, 7215–7218. [Google Scholar] [CrossRef]
- Cernei, N.; Heger, Z.; Gumulec, J.; Zitka, O.; Masarik, M.; Babula, P.; Eckschlager, T.; Stiborova, M.; Kizek, R.; Adam, V. Sarcosine as a potential prostate cancer biomarker-a review. Int. J. Mol. Sci. 2013, 14, 13893–13908. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, D. Study of the reaction mechanism of 1,8-diazafluoren-9-one with the amino acid, L-alanine. Forensic Sci. Int. 2000, 109, 87–103. [Google Scholar] [CrossRef]



| Metabolite | FDR p Value | VIP |
|---|---|---|
| Glycine | 1.2 × 10−4 | 2.9 |
| Alanine | 4.0 × 10−2 | 1.1 |
| Acetic acid | 9.3 × 10−11 | 1.7 |
| Hippuric acid | 4.5 × 10−3 | 1.8 |
| Meso-erythritol | 2.1 × 10−9 | 1.5 |
| Threonic acid | 4.9 × 10−8 | 1.6 |
| Butanoic acid | 2.7 × 10−2 | 1.5 |
| Inositol | 8.9 × 10−5 | 1.1 |
| Hydroxytryptophan | 4.4 × 10−5 | 1.4 |
| Methyllinosine | 4.4 × 10−5 | 1.5 |
| Xanthosine | 2.4 × 10−3 | 1.3 |
| Dimethylguanosine | 1.9 × 10−4 | 1.8 |
| Methylguanosine | 2.0 × 10−2 | 1.1 |
| Tryptophan | 2.8 × 10−3 | 1.4 |
| DFO | Energy Bandgap (eV) | Thickness (nm) | Refractive Index @550nm | Porosity, p (%) |
|---|---|---|---|---|
| 10−2 M | 2.62 | 356 | 1.624 | 69.4 |
| 10−5 M | 2.97 | 386 | 1.592 | 71.32 |
| 0 | 2.95 | 300 | 1.514 | 75.85 |
| CDFO [M] | Mean Fluorescence Lifetime (ns) |
|---|---|
| 2 × 10−2 | 0.39 |
| 2 × 10−3 | 0.51 |
| 10−5 | 0.63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewkowicz, A.; Bogdanowicz, R.; Bojarski, P.; Pierpaoli, M.; Gryczyński, I.; Synak, A.; Mońka, M.; Karczewski, J.; Struck-Lewicka, W.; Wawrzyniak, R.; et al. The Luminescence of 1,8-Diazafluoren-9-One/Titanium Dioxide Composite Thin Films for Optical Application. Materials 2020, 13, 3014. https://doi.org/10.3390/ma13133014
Lewkowicz A, Bogdanowicz R, Bojarski P, Pierpaoli M, Gryczyński I, Synak A, Mońka M, Karczewski J, Struck-Lewicka W, Wawrzyniak R, et al. The Luminescence of 1,8-Diazafluoren-9-One/Titanium Dioxide Composite Thin Films for Optical Application. Materials. 2020; 13(13):3014. https://doi.org/10.3390/ma13133014
Chicago/Turabian StyleLewkowicz, Aneta, Robert Bogdanowicz, Piotr Bojarski, Mattia Pierpaoli, Ignacy Gryczyński, Anna Synak, Michał Mońka, Jakub Karczewski, Wiktoria Struck-Lewicka, Renata Wawrzyniak, and et al. 2020. "The Luminescence of 1,8-Diazafluoren-9-One/Titanium Dioxide Composite Thin Films for Optical Application" Materials 13, no. 13: 3014. https://doi.org/10.3390/ma13133014
APA StyleLewkowicz, A., Bogdanowicz, R., Bojarski, P., Pierpaoli, M., Gryczyński, I., Synak, A., Mońka, M., Karczewski, J., Struck-Lewicka, W., Wawrzyniak, R., & Markuszewski, M. J. (2020). The Luminescence of 1,8-Diazafluoren-9-One/Titanium Dioxide Composite Thin Films for Optical Application. Materials, 13(13), 3014. https://doi.org/10.3390/ma13133014