Design and Fabrication of New High Entropy Alloys for Evaluating Titanium Replacements in Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
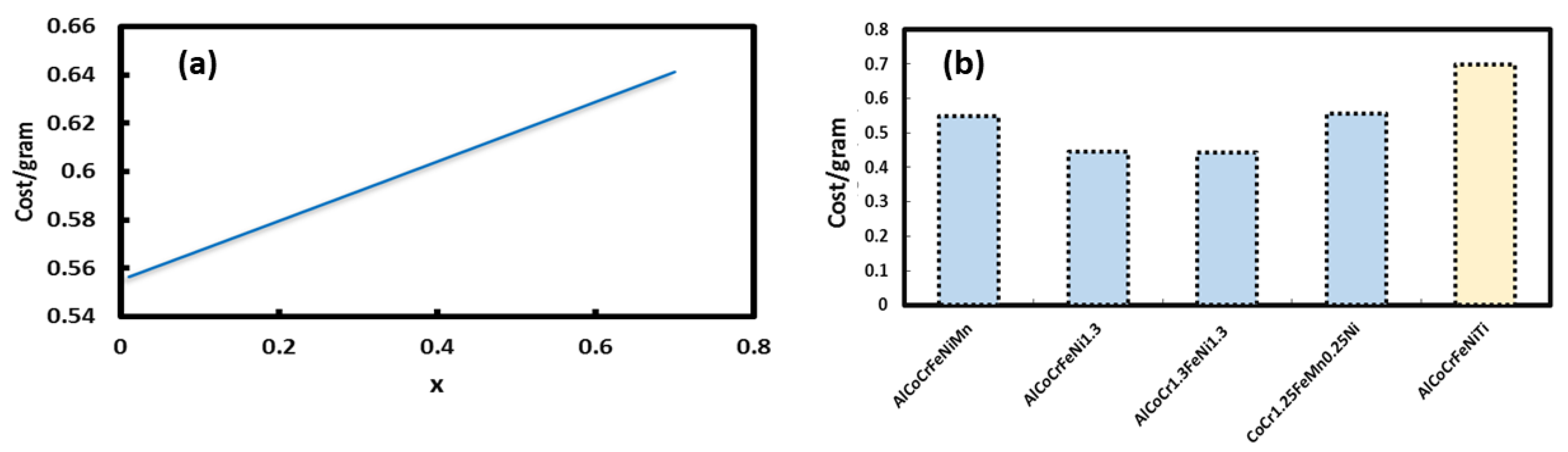
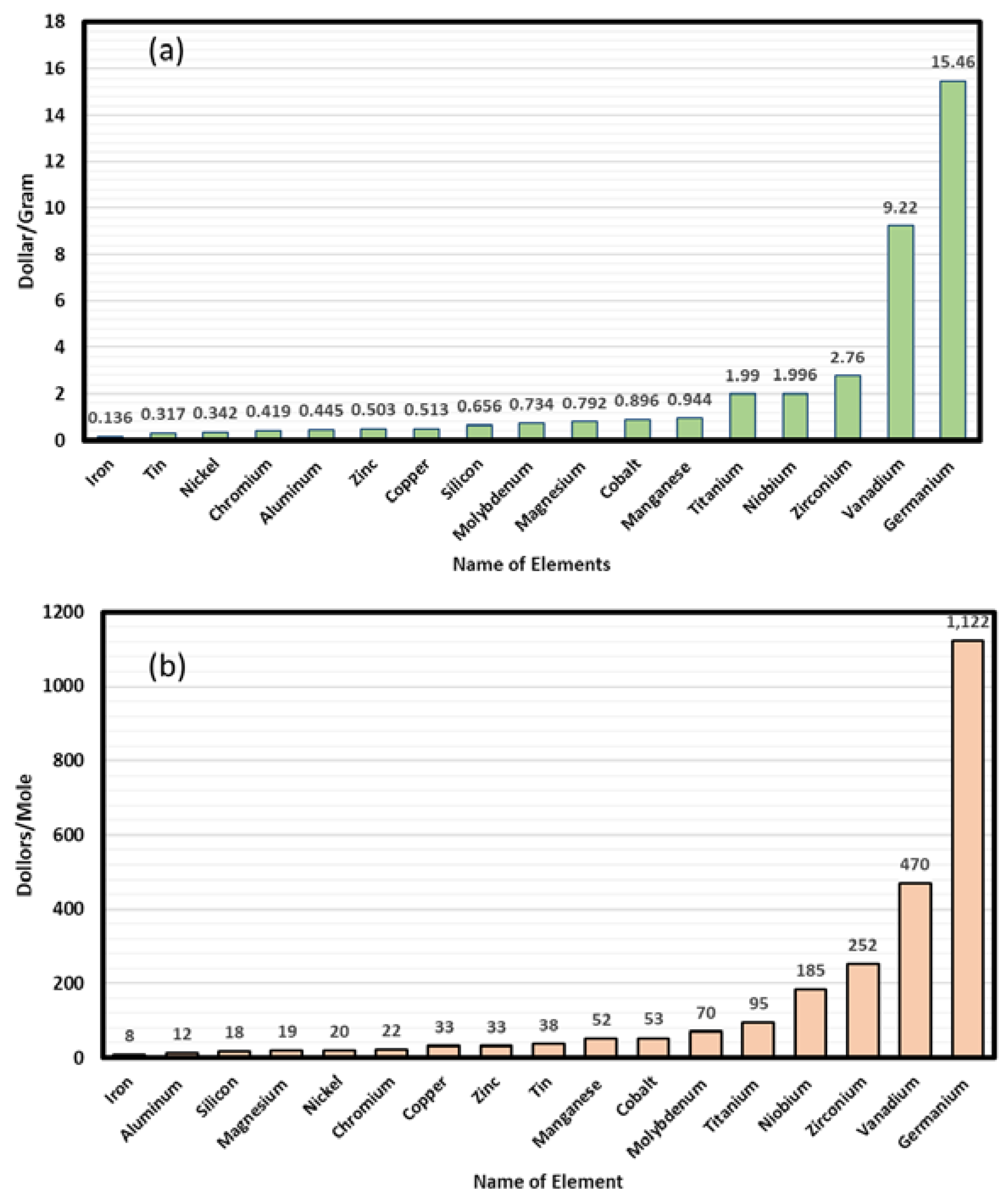
2.1. Cost Analysis of Selected HEAs
- Cost of the metal powder (size <100 micrometer, usually atomized mill product)
- Cost of labor (metal mixture preparation efforts for selective laser melting)
- Cost of laser melting (equipment cost, hourly charges; laser power used in melting)
- Cost of post-deposition sample preparation steps (removal, polishing etc.)
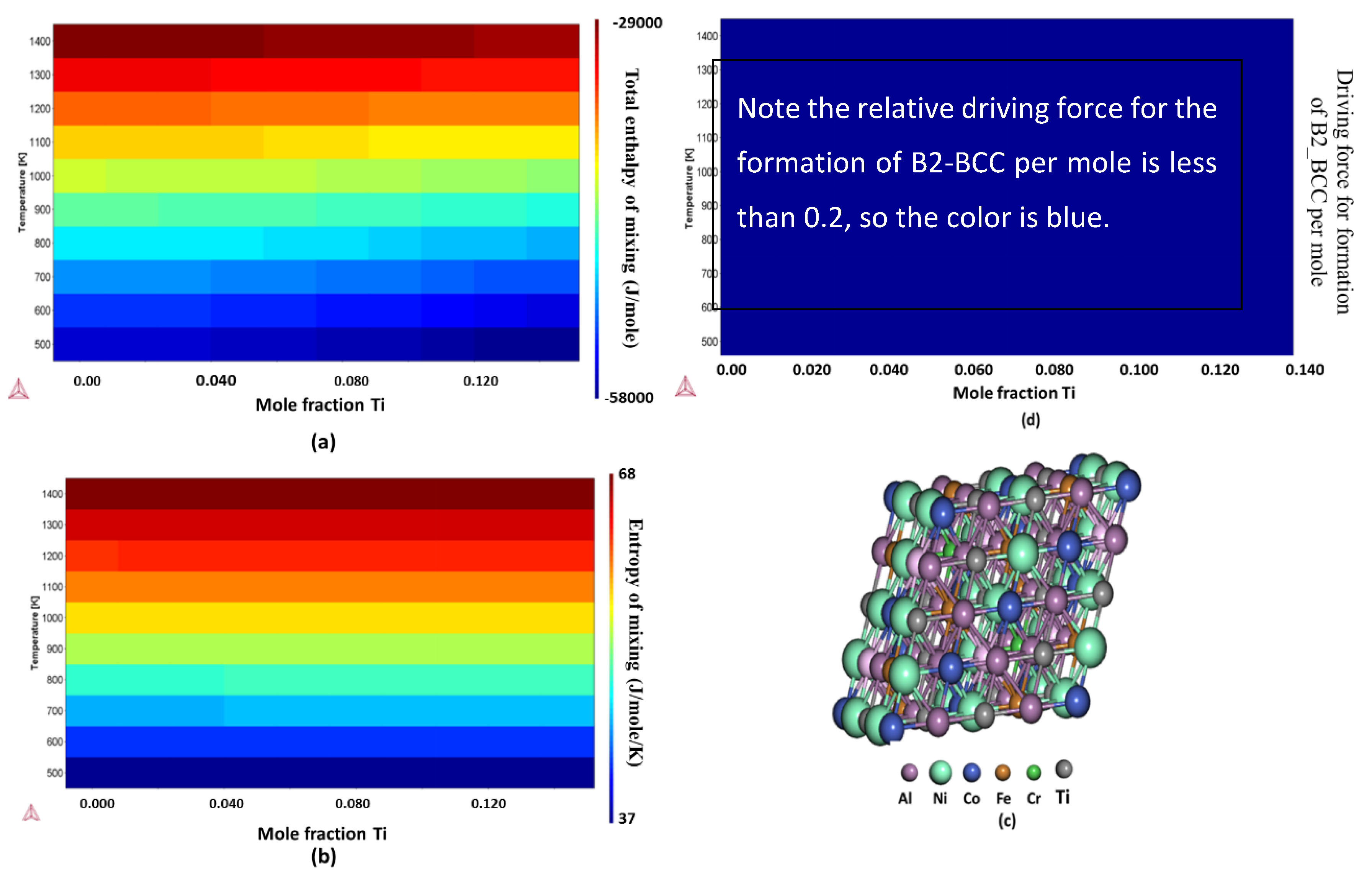
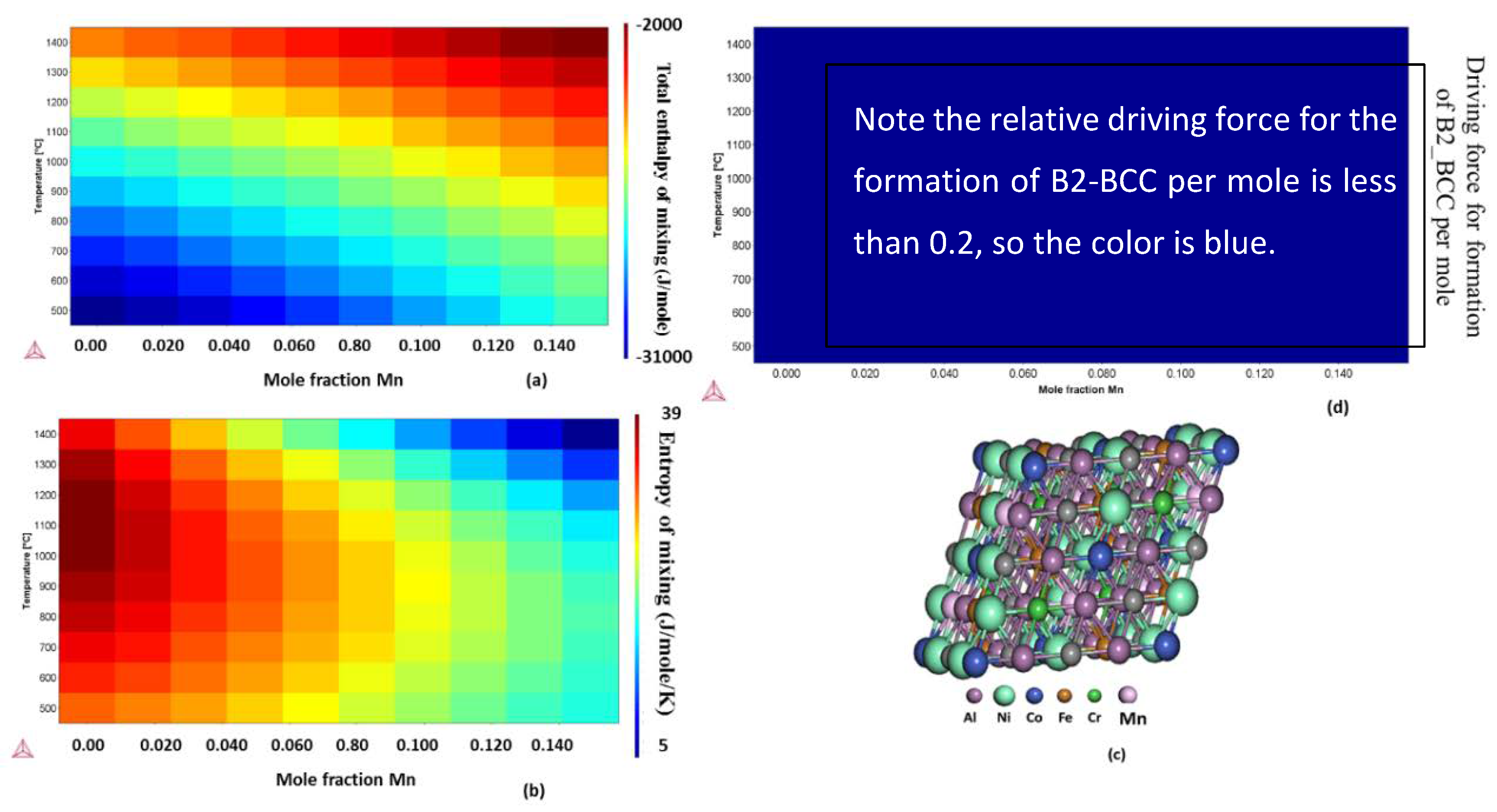
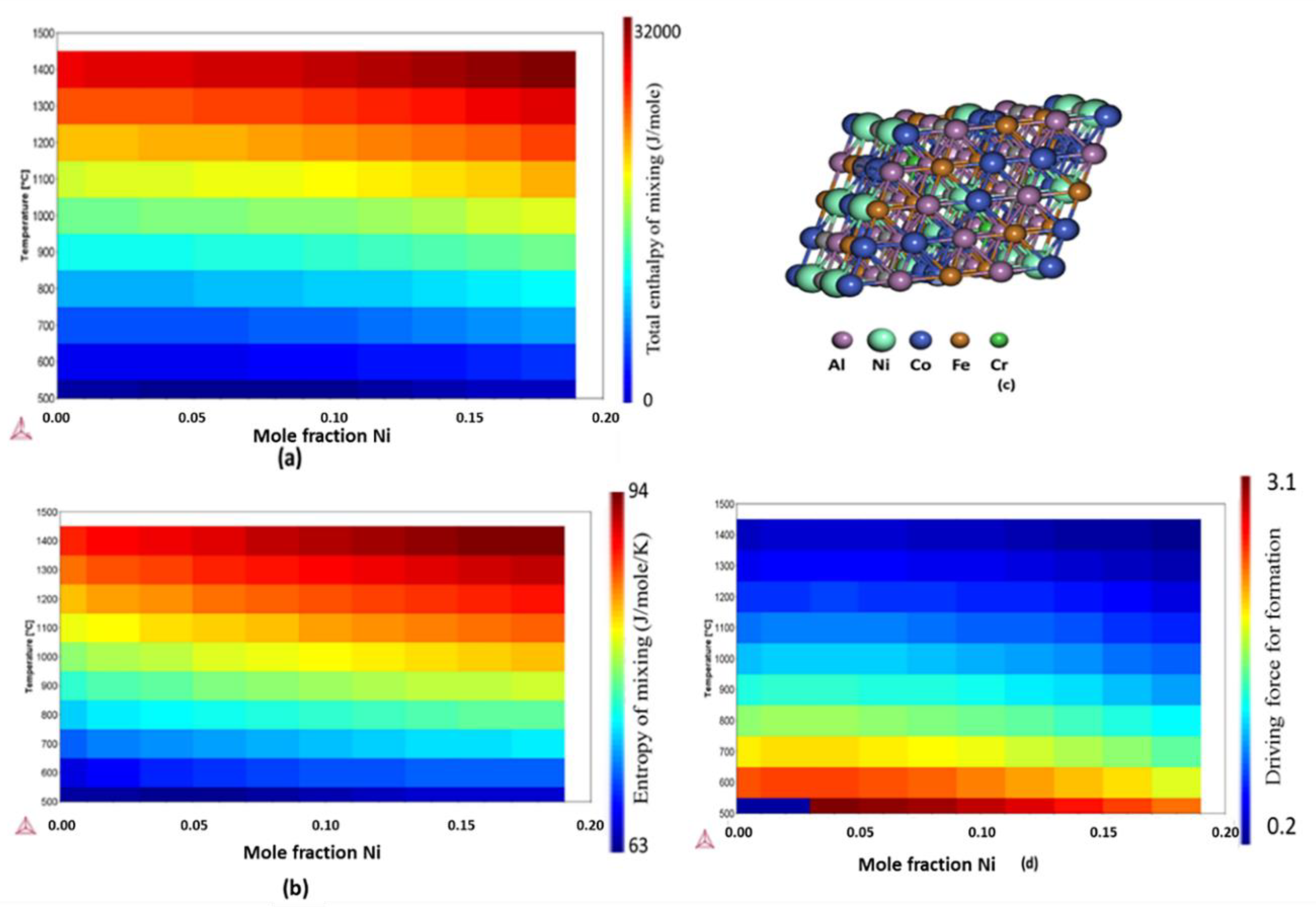
2.2. Free Energy, Enthalpy, and Entropy Calculations
2.3. Calculations of Elastic Constants
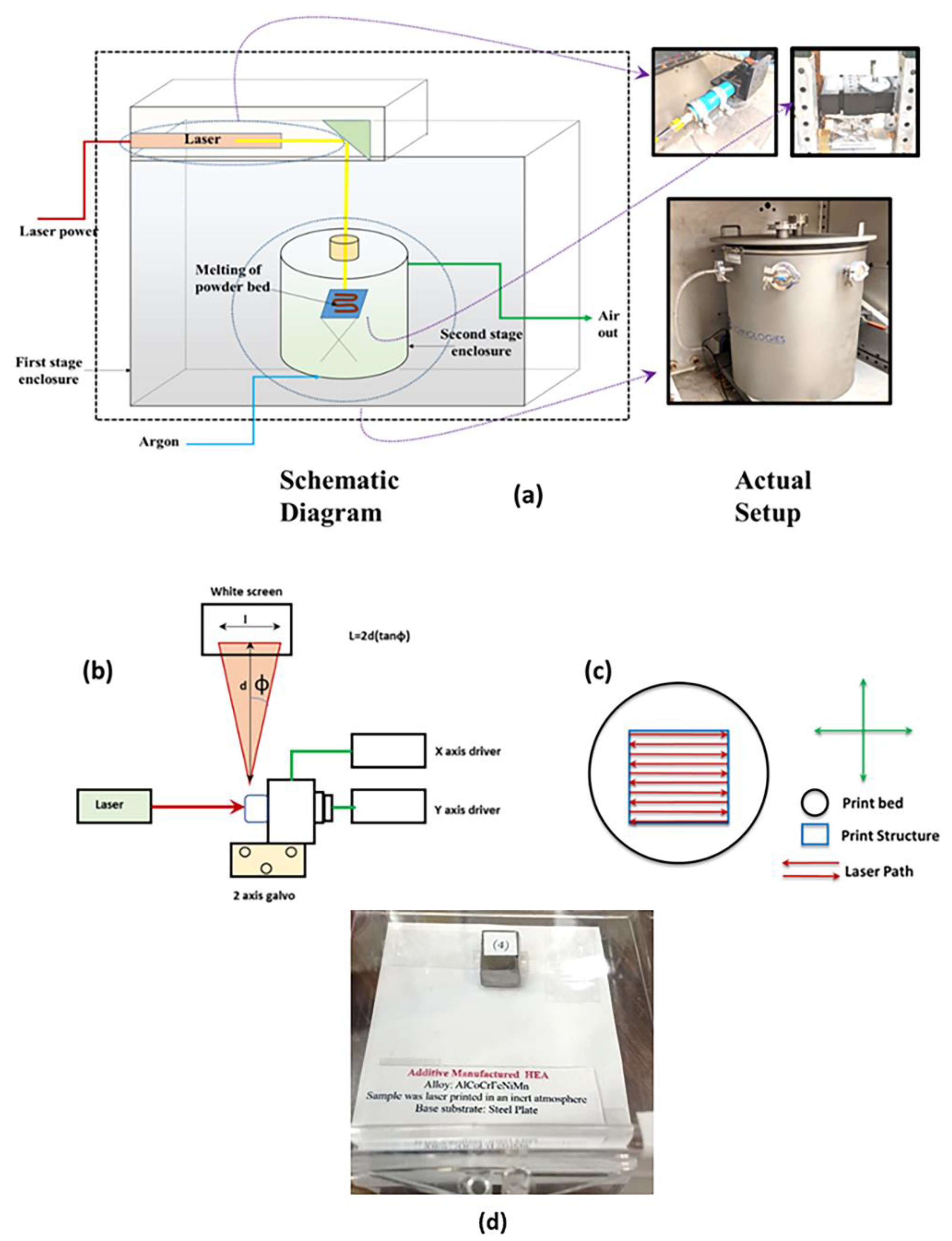
2.4. Laser Components
2.5. Galvanometer Setup and Detail of Phidgets Hub for Laser-PBF Processing Control
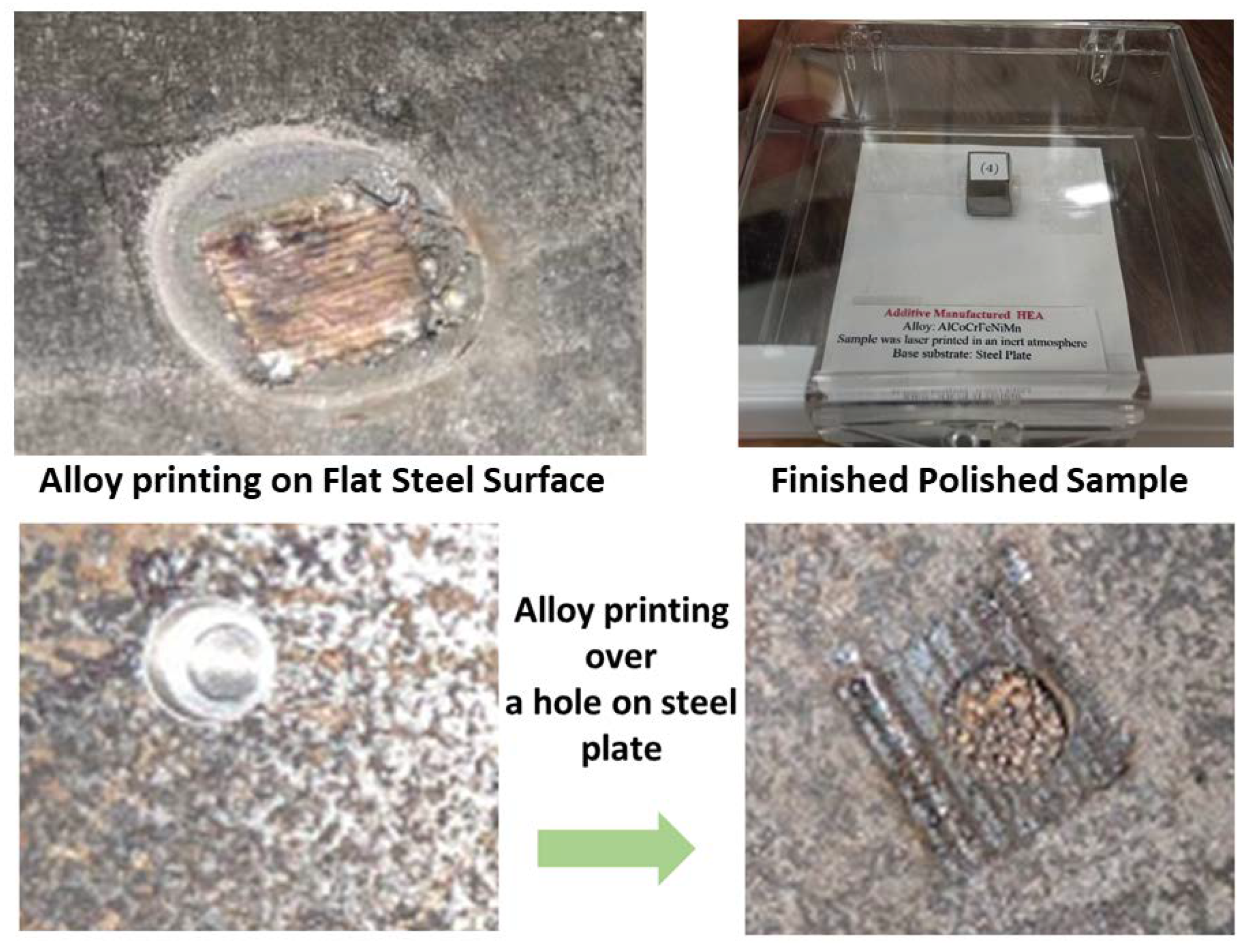
2.6. Optimization of Parameters
2.7. Details about Closed Atmosphere Tests
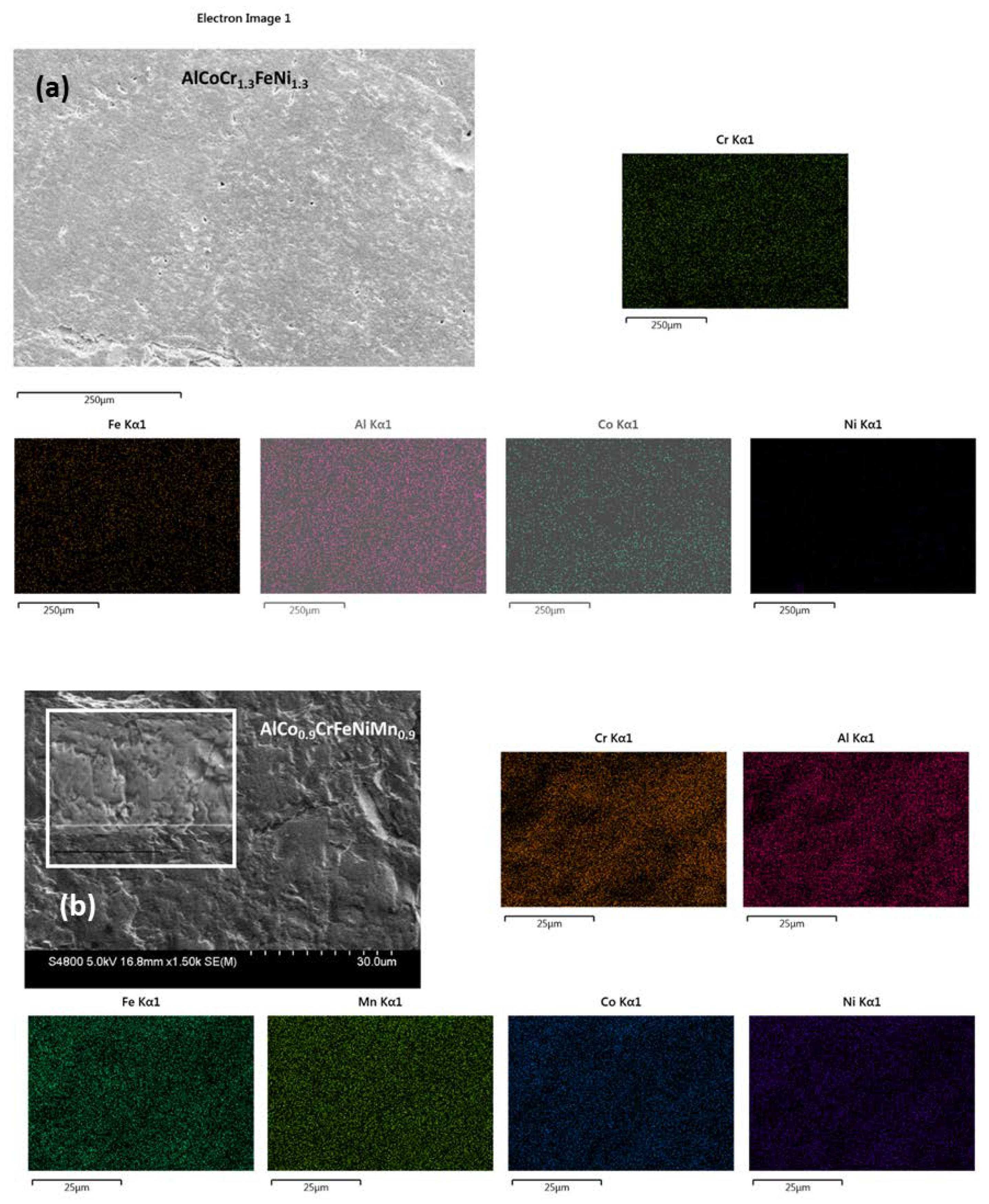
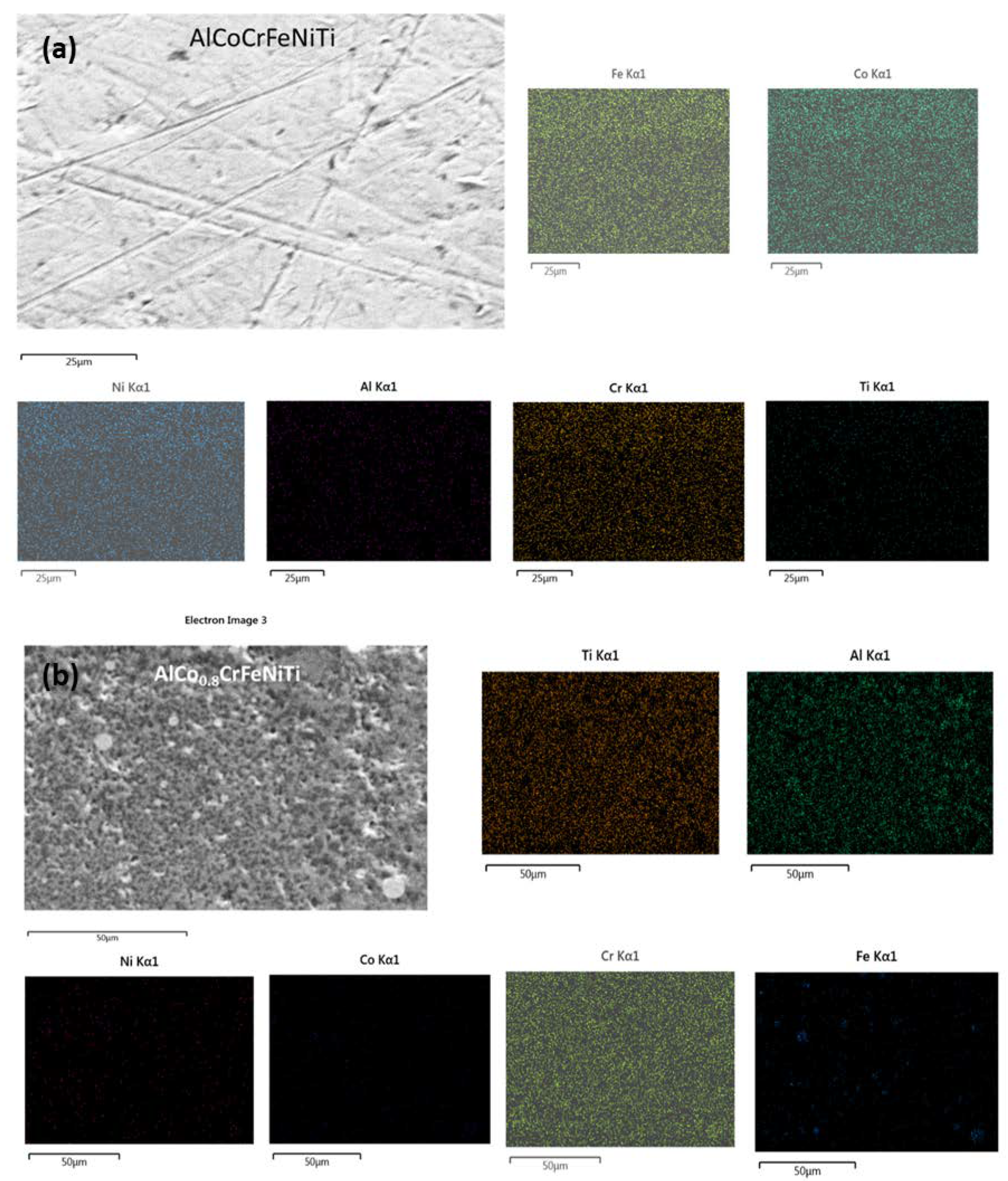
2.8. Morphology, Elemental Characterizations, and Electron Backscatter Diffraction
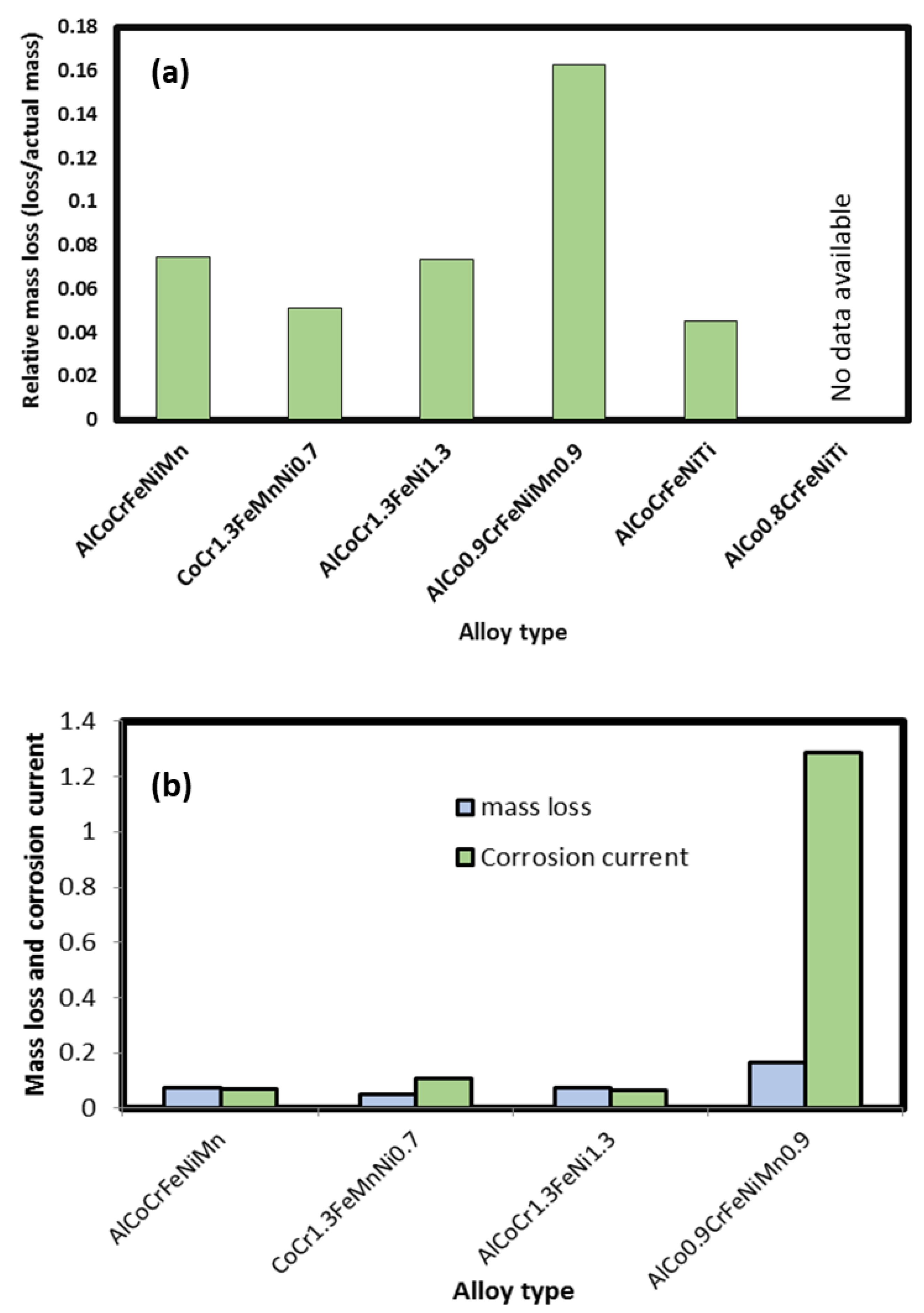
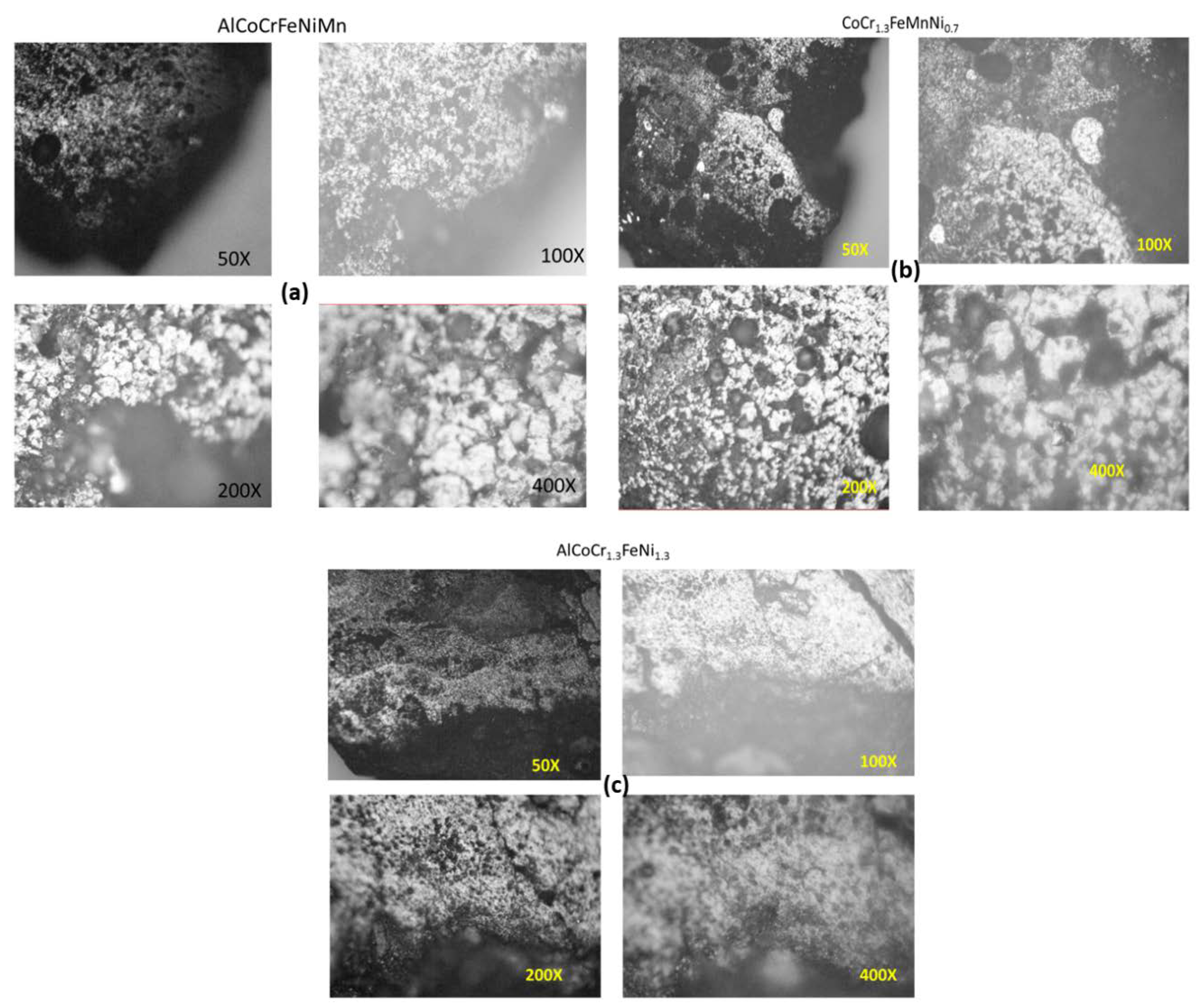
2.9. Corrosion Behavior of HEAs
Accelerated Immersion Tests
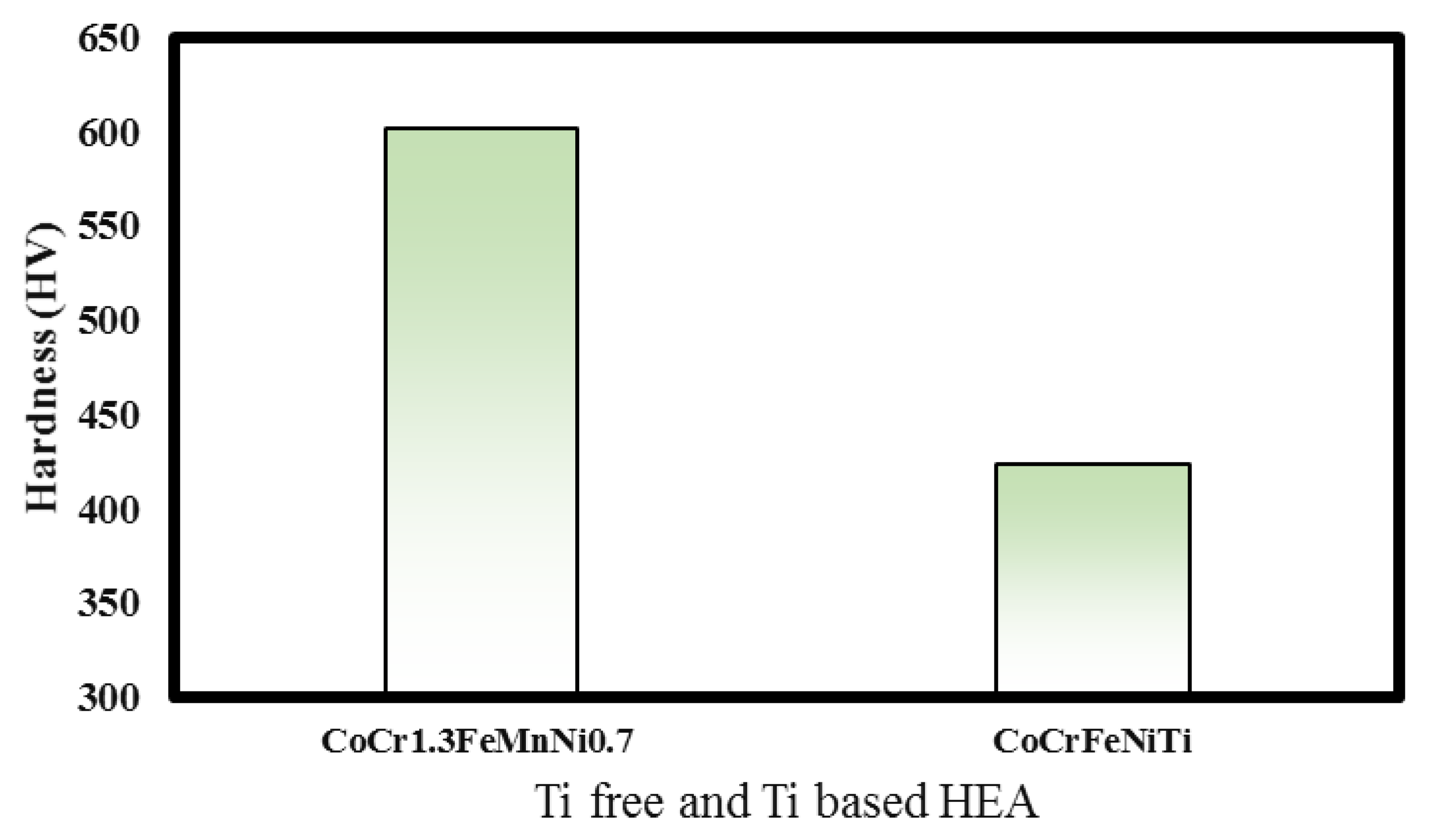
2.10. Hardness Tests
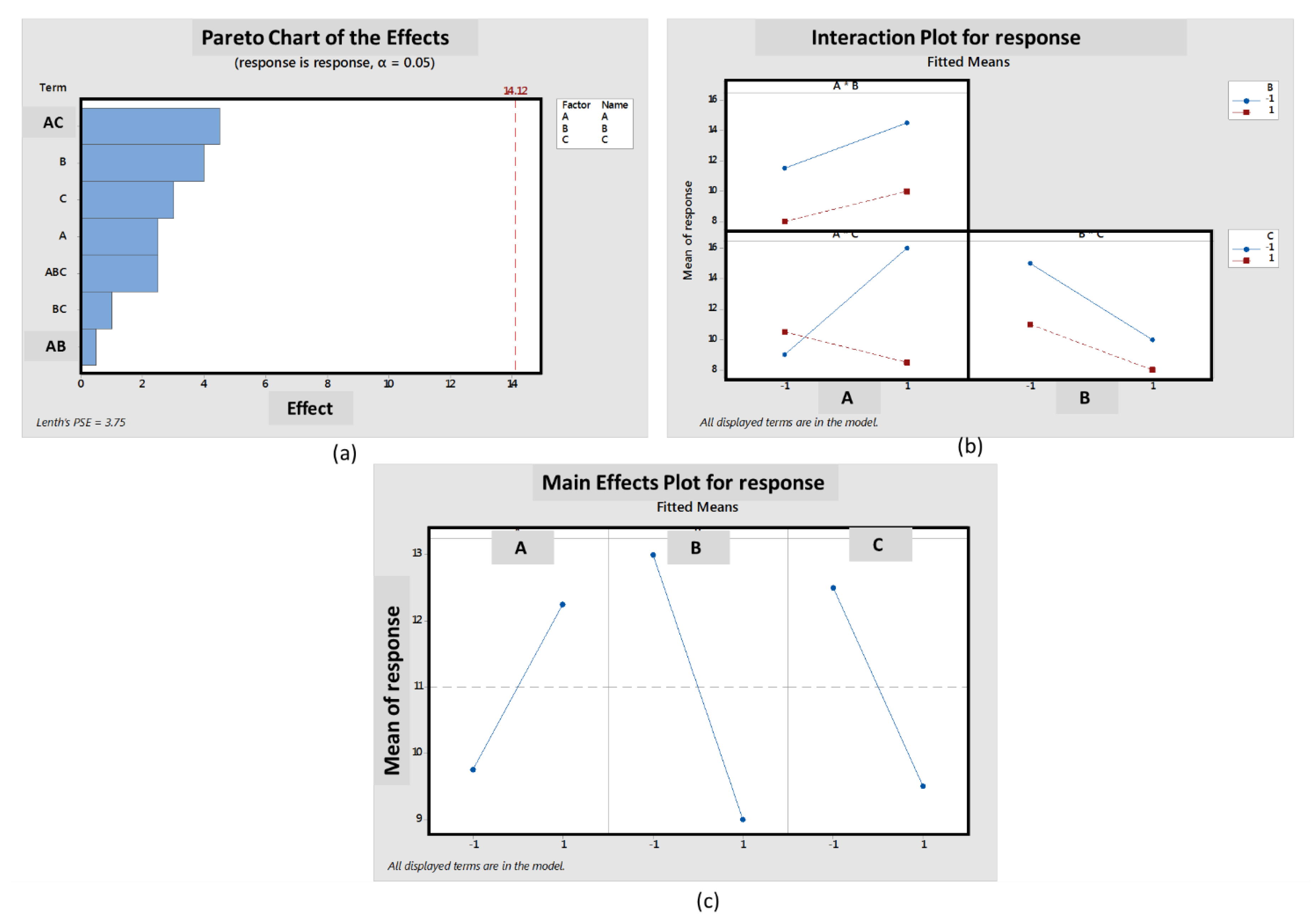
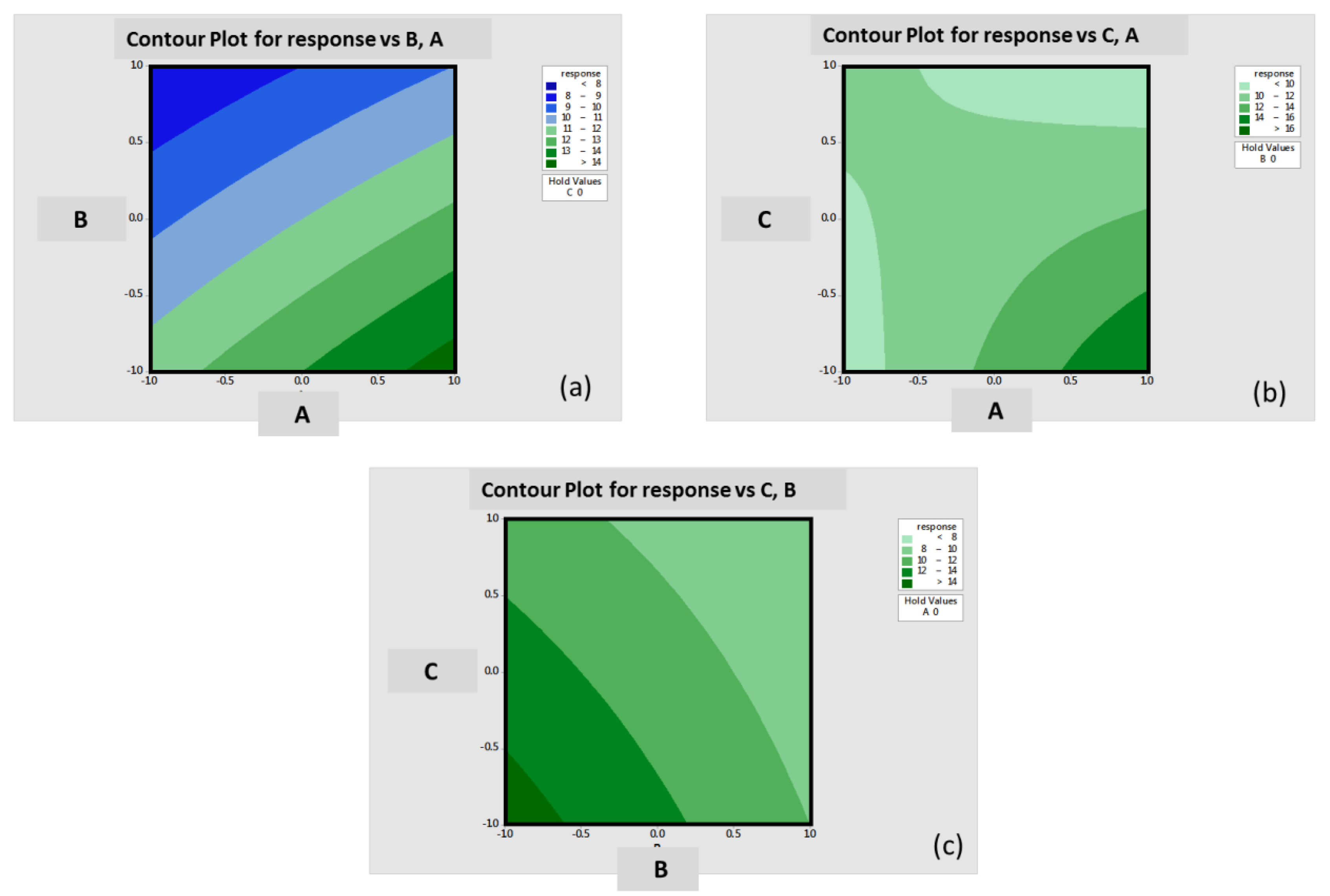
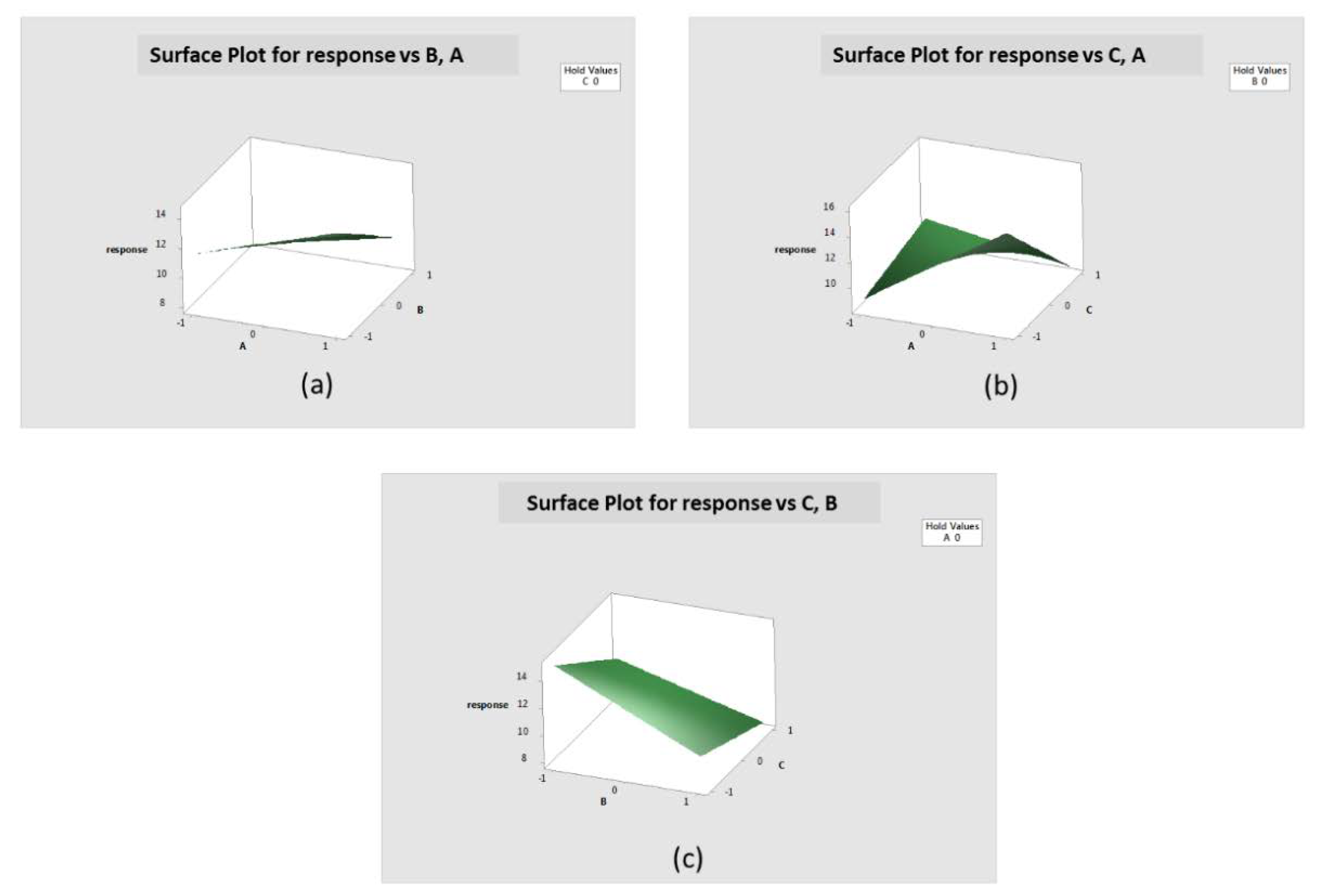
2.11 Factorial Design of Experiments Testing
3. Results and Discussion
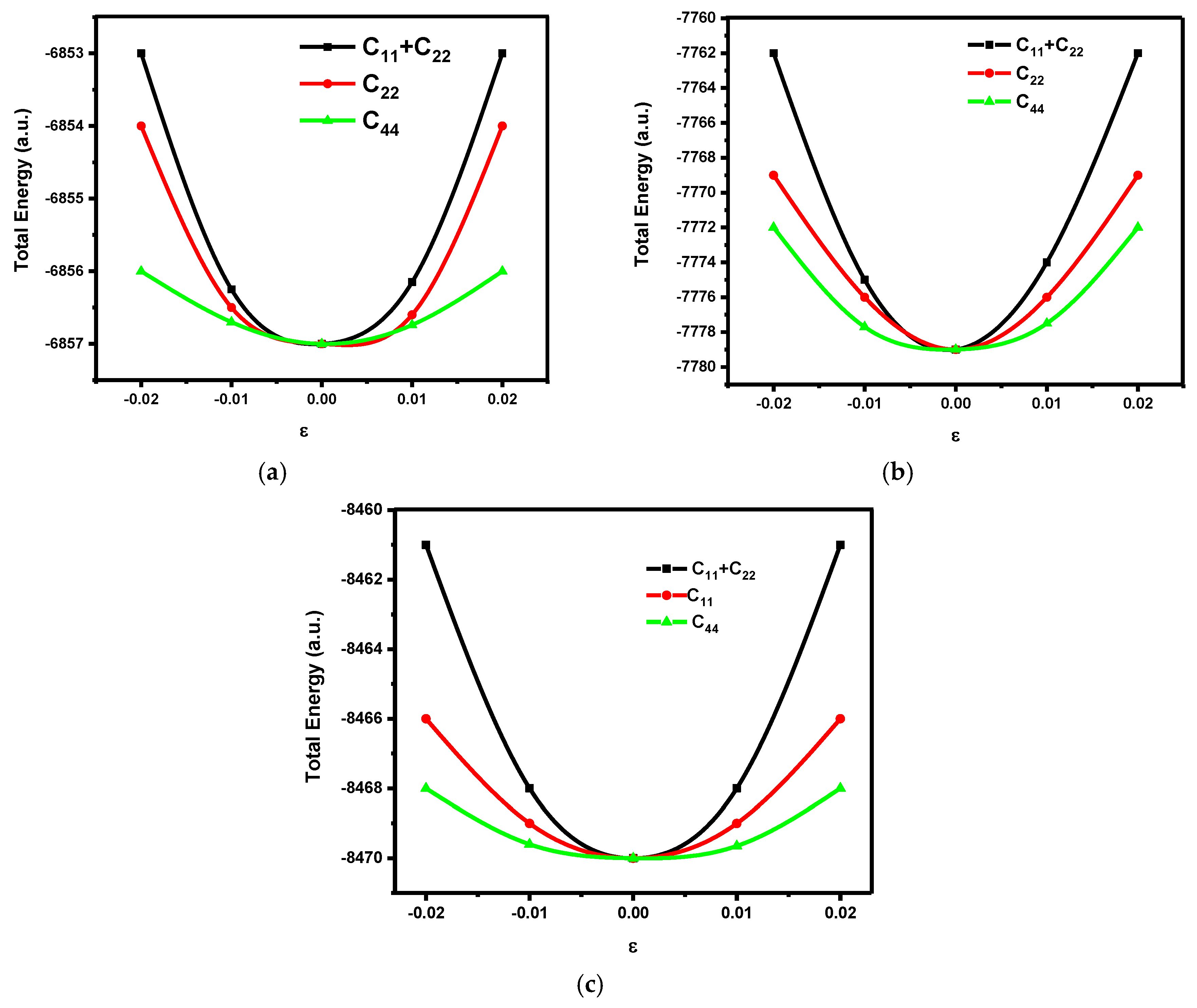
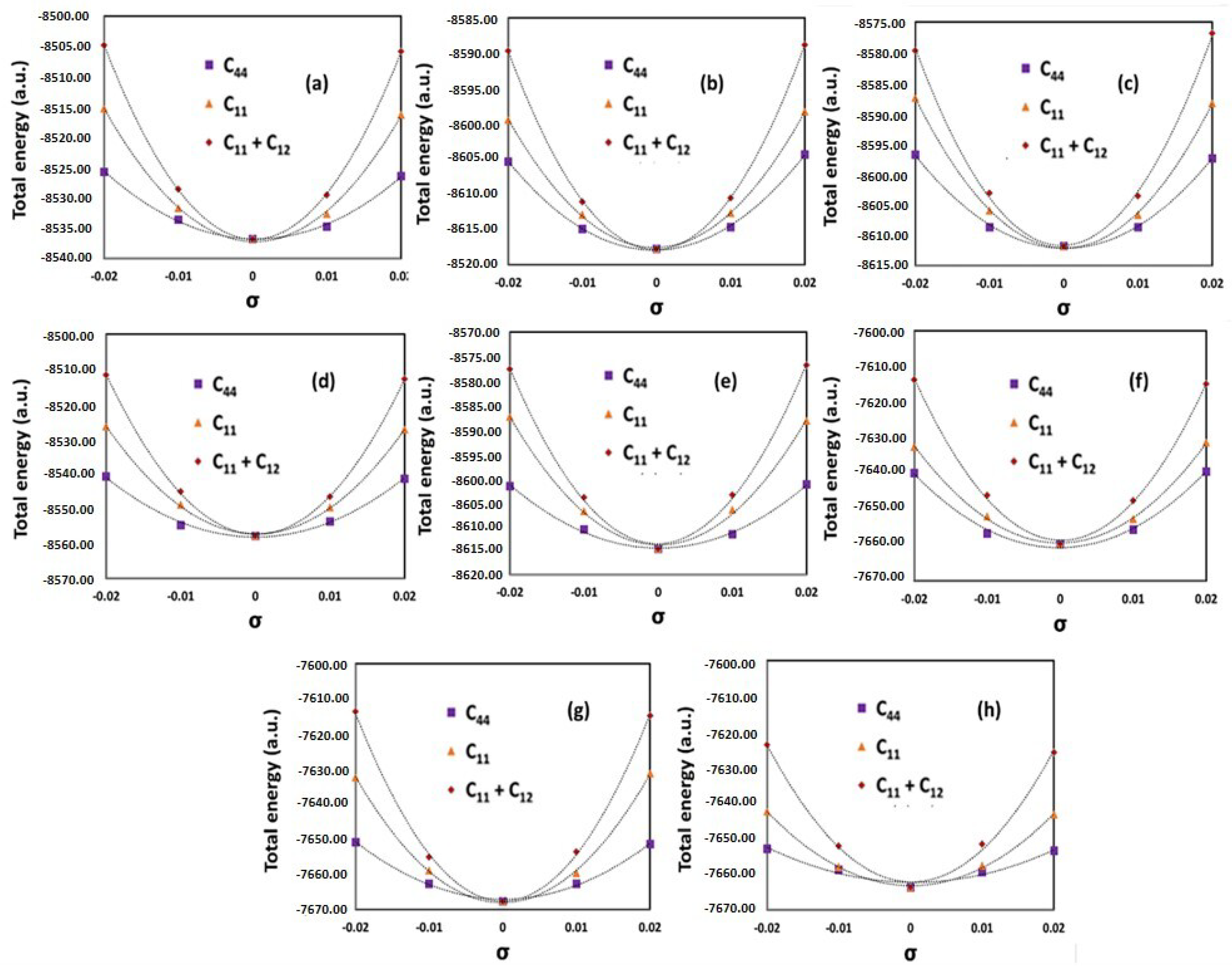
3.1. Elastic Properties
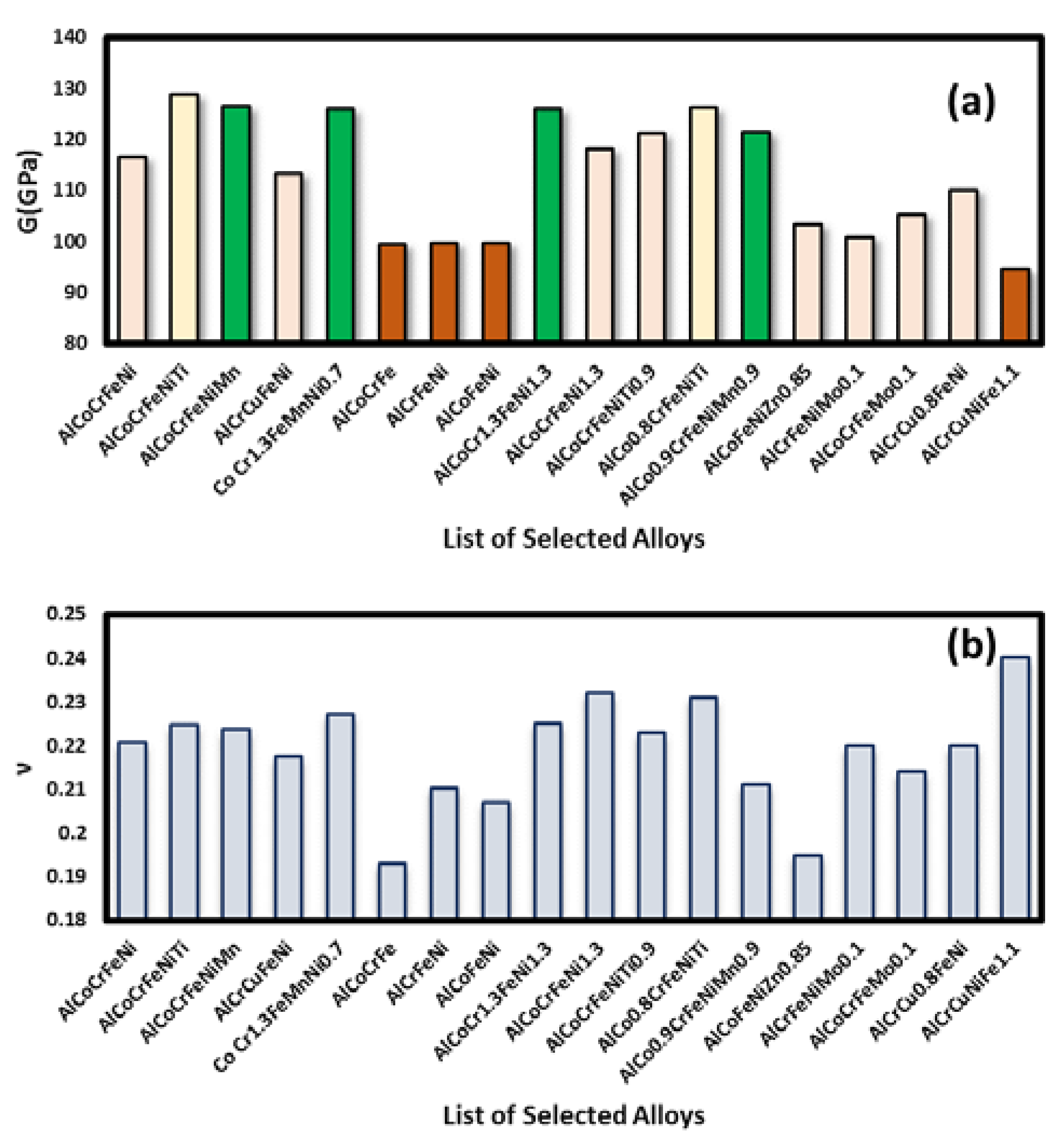
3.2. Analysis of Elastic Properties and Shortlisting of HEAs
3.3. Density Calculations for Short-Listed Alloys
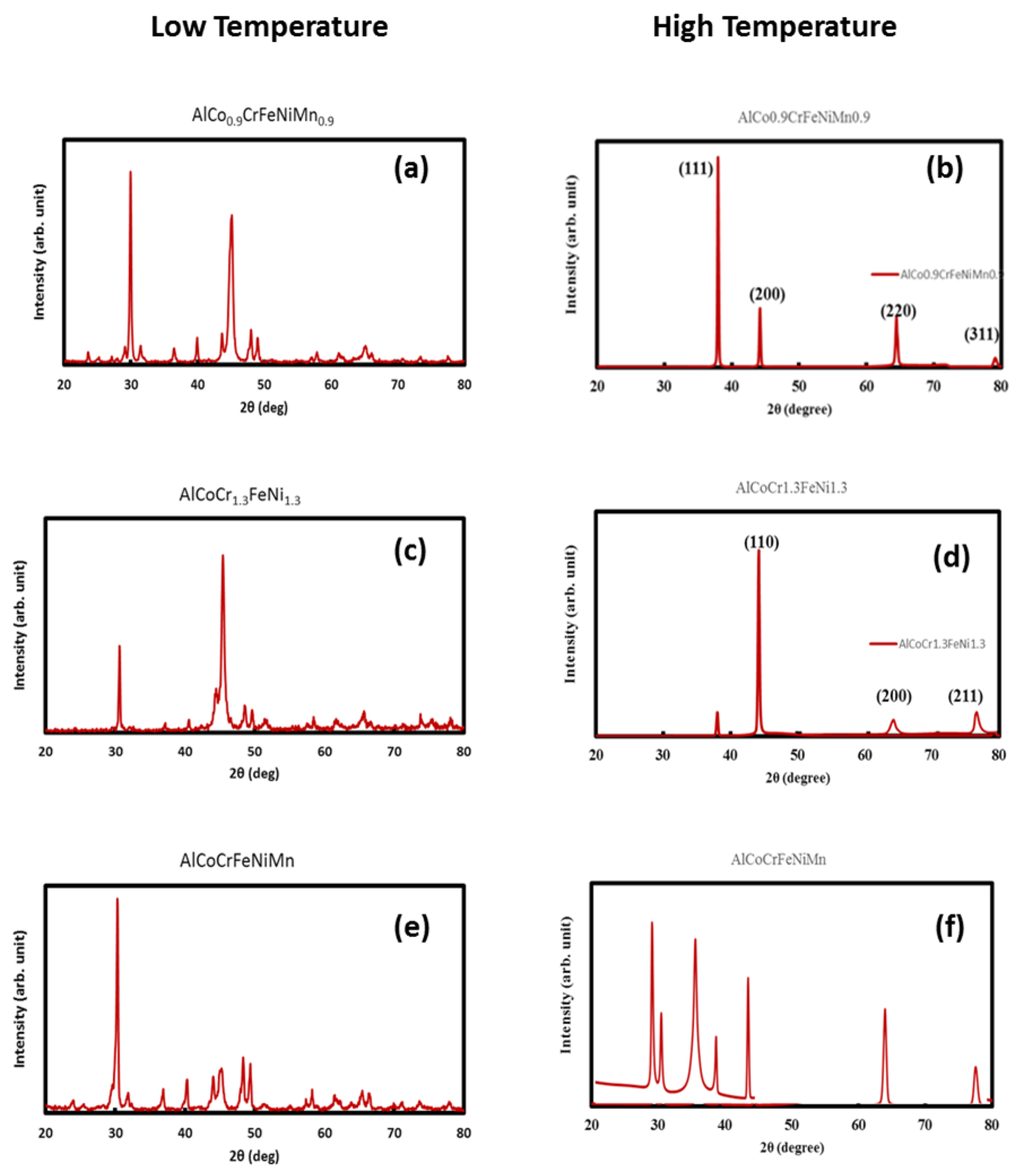
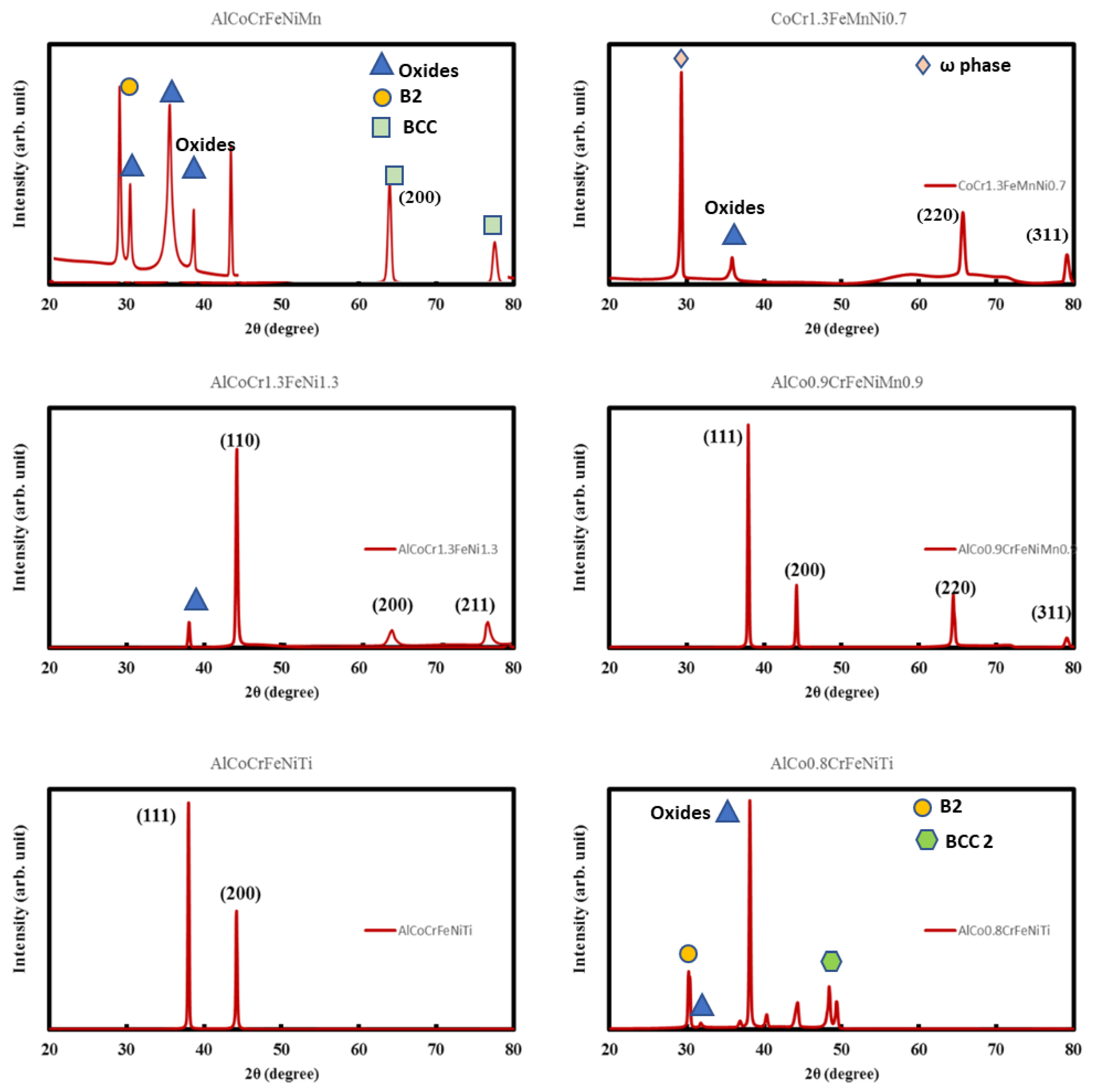
3.4. X-ray Diffraction Tests
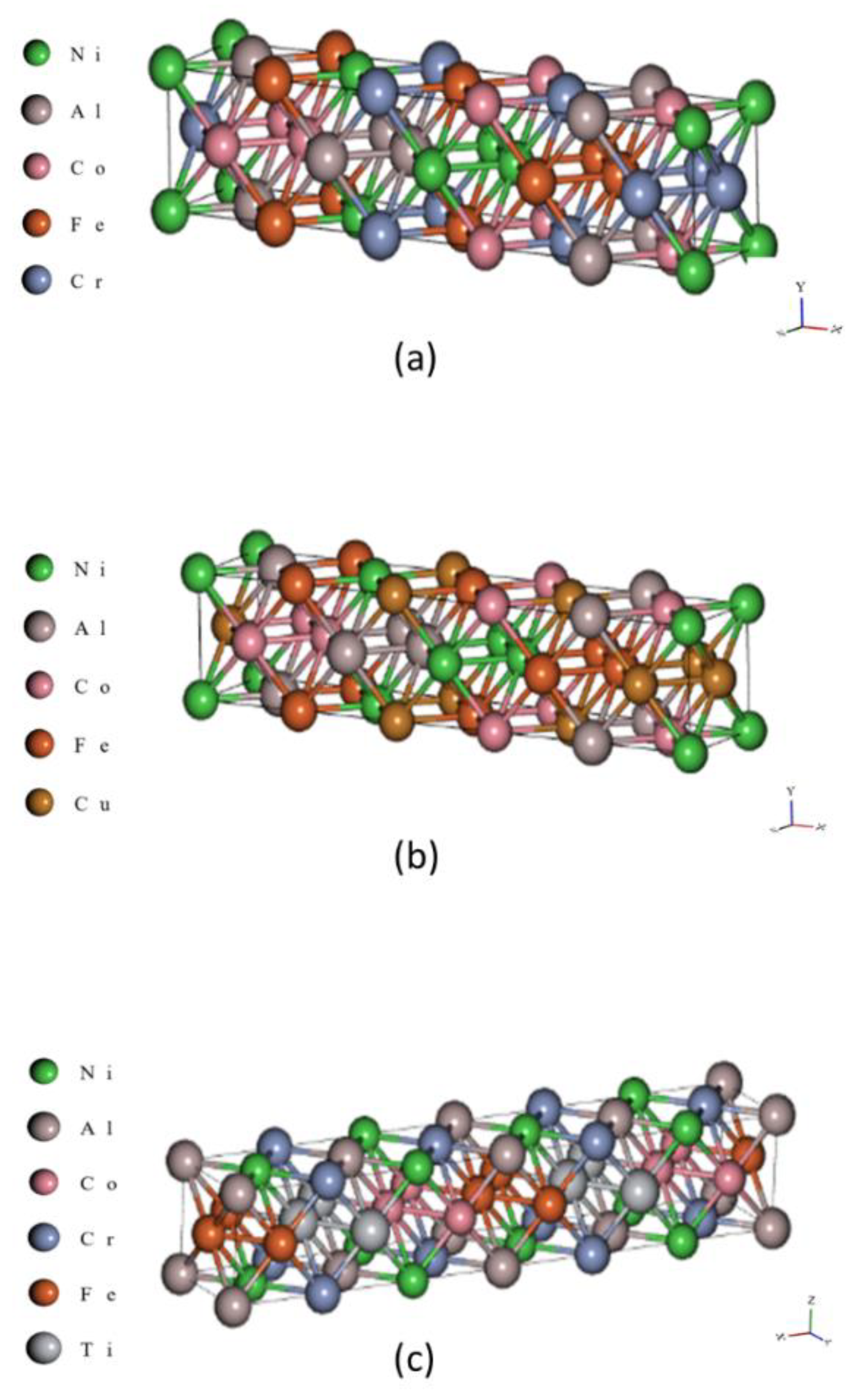
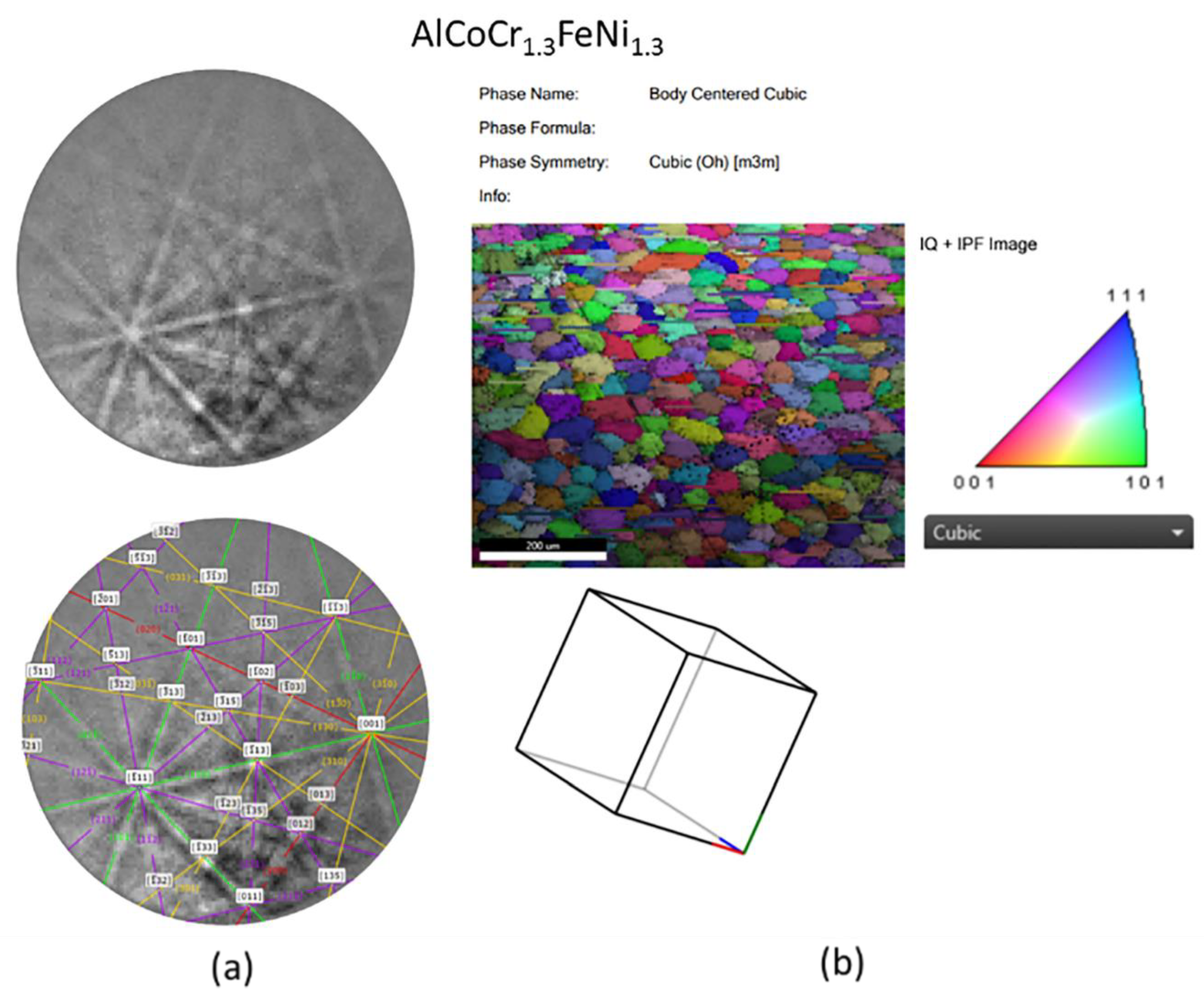
3.5. SEM/EBSD
3.6. Potentiodynamic Scans and EIS
3.7. Hardness Tests
3.8. Factorial Design of Experiments Laser Printing Test Matrix
3.9. Effect of Temperature (X-ray Diffraction Tests on Arc Melted Samples)
3.10. Reduced Elastic Constants for HEAs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
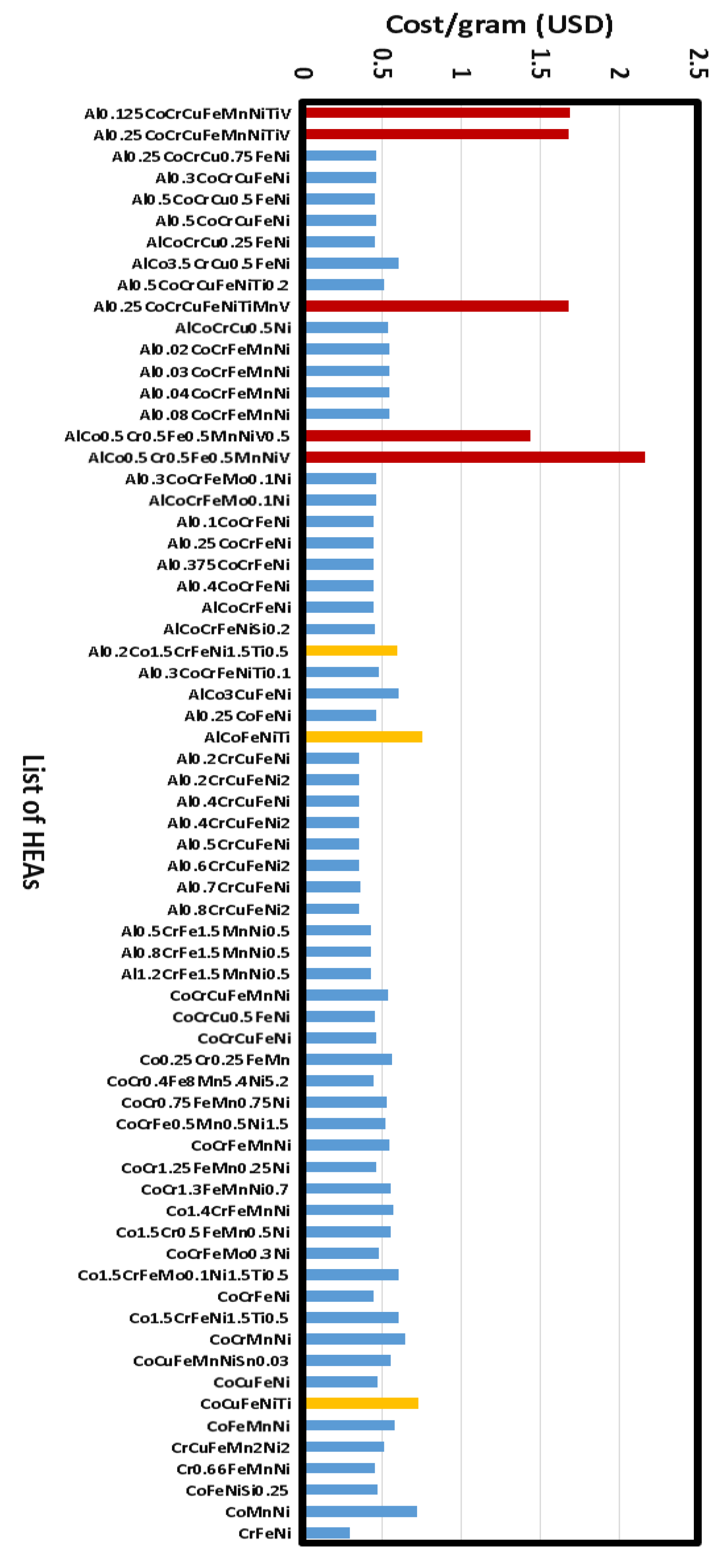





| Sr. no | High Entropy Alloy Composition | Sr. no | High Entropy Alloy Composition |
|---|---|---|---|
| 1 | Al0.125CoCrCuFeMnNiTiV | 41 | Al1.2CrFe1.5MnNi0.5 |
| 2 | Al0.25CoCrCuFeMnNiTiV | 42 | CoCrCuFeMnNi |
| 3 | Al0.25CoCrCu0.75FeNi | 43 | CoCrCu0.5FeNi |
| 4 | Al0.3CoCrCuFeNi | 44 | CoCrCuFeNi |
| 5 | Al0.5CoCrCu0.5FeNi | 45 | Co0.25Cr0.25FeMn |
| 6 | Al0.5CoCrCuFeNi | 46 | CoCr0.4Fe8Mn5.4Ni5.2 |
| 7 | AlCoCrCu0.25FeNi | 47 | CoCr0.75FeMn0.75Ni |
| 8 | AlCo3.5CrCu0.5FeNi | 48 | CoCrFe0.5Mn0.5Ni1.5 |
| 9 | Al0.5CoCrCuFeNiTi0.2 | 49 | CoCrFeMnNi |
| 10 | Al0.25CoCrCuFeNiTiMnV | 50 | CoCr1.25FeMn0.25Ni |
| 11 | AlCoCrCu0.5Ni | 51 | CoCr1.3FeMnNi0.7 |
| 12 | Al0.02CoCrFeMnNi | 52 | Co1.4CrFeMnNi |
| 13 | Al0.03CoCrFeMnNi | 53 | Co1.5Cr0.5FeMn0.5Ni |
| 14 | Al0.04CoCrFeMnNi | 54 | CoCrFeMo0.3Ni |
| 15 | Al0.08CoCrFeMnNi | 55 | Co1.5CrFeMo0.1Ni1.5Ti0.5 |
| 16 | AlCo0.5Cr0.5Fe0.5MnNiV0.5 | 56 | CoCrFeNi |
| 17 | AlCo0.5Cr0.5Fe0.5MnNiV | 57 | Co1.5CrFeNi1.5Ti0.5 |
| 18 | Al0.3CoCrFeMo0.1Ni | 58 | CoCrMnNi |
| 19 | AlCoCrFeMo0.1Ni | 59 | CoCuFeMnNiSn0.03 |
| 20 | Al0.1CoCrFeNi | 60 | CoCuFeNi |
| 21 | Al0.25CoCrFeNi | 61 | CoCuFeNiTi |
| 22 | Al0.375CoCrFeNi | 62 | CoFeMnNi |
| 23 | Al0.4CoCrFeNi | 63 | CrCuFeMn2Ni2 |
| 24 | AlCoCrFeNi | 64 | Cr0.66FeMnNi |
| 25 | AlCoCrFeNiSi0.2 | 65 | CoFeNiSi0.25 |
| 26 | Al0.2Co1.5CrFeNi1.5Ti0.5 | 66 | CoMnNi |
| 27 | Al0.3CoCrFeNiTi0.1 | 67 | CrFeNi |
| 28 | AlCo3CuFeNi | – | – |
| 29 | Al0.25CoFeNi | – | – |
| 30 | AlCoFeNiTi | – | – |
| 31 | Al0.2CrCuFeNi | – | – |
| 32 | Al0.2CrCuFeNi2 | – | – |
| 33 | Al0.4CrCuFeNi | – | – |
| 34 | Al0.4CrCuFeNi2 | – | – |
| 35 | Al0.5CrCuFeNi | – | – |
| 36 | Al0.6CrCuFeNi2 | – | – |
| 37 | Al0.7CrCuFeNi | – | – |
| 38 | Al0.8CrCuFeNi2 | – | – |
| 39 | Al0.5CrFe1.5MnNi0.5 | – | – |
| 40 | Al0.8CrFe1.5MnNi0.5 | – | – |
| Run | A | B | C | AB | AC | BC | ABC | Observation |
|---|---|---|---|---|---|---|---|---|
| 1 | − | − | − | + | + | + | − | Y1 |
| 2 | + | − | − | − | − | + | + | Y2 |
| 3 | − | + | − | − | + | − | + | Y3 |
| 4 | + | + | − | + | − | − | + | Y4 |
| 5 | − | − | + | + | − | − | + | Y5 |
| 6 | + | − | + | − | + | − | − | Y6 |
| 7 | − | + | + | − | − | + | − | Y7 |
| 8 | + | + | + | + | + | + | + | Y8 |
| High Entropy Alloy Composition | C11(GPa) | C12(GPa) | C44(GPa) | E(GPa) | B(GPa) | G(GPa) | B/G | ν | a0(Å) |
|---|---|---|---|---|---|---|---|---|---|
| AlCoCrFeNi | 214.82 | 135.38 | 167.59 | 284.31 | 169.74 | 116.442 | 1.45 | 0.2208 | 3.567 |
| AlCoCrFeNiTi | 253.69 | 157.39 | 182.67 | 315.67 | 191.23 | 128.862 | 1.48 | 0.2248 | 3.781 |
| AlCoCrFeNiMn | 248.71 | 153.61 | 179.14 | 309.60 | 186.77 | 126.504 | 1.47 | 0.2237 | 3.763 |
| AlCrCuFeNi | 210.37 | 126.94 | 161.09 | 275.99 | 162.87 | 113.34 | 1.43 | 0.2175 | 3.579 |
| CoCr1.3FeMnNi0.7 | 251.97 | 155.83 | 177.91 | 309.19 | 188.93 | 125.974 | 1.49 | 0.2272 | 3.774 |
| AlCoCrFe | 168.94 | 84.96 | 137.93 | 237.53 | 128.96 | 99.554 | 1.29 | 0.1930 | 3.462 |
| AlCrFeNi | 176.58 | 97.89 | 139.79 | 241.11 | 138.69 | 99.612 | 1.39 | 0.2102 | 3.458 |
| AlCoFeNi | 173.71 | 92.68 | 138.95 | 240.37 | 136.74 | 99.576 | 1.37 | 0.2070 | 3.467 |
| AlCoCr1.3FeNi1.3 | 262.82 | 151.73 | 172.98 | 308.79 | 187.38 | 126.01 | 1.49 | 0.225 | 4.091 |
| AlCoCrFeNi1.3 | 237.83 | 134.97 | 162.67 | 291.22 | 181.23 | 118.17 | 1.53 | 0.232 | 3.994 |
| AlCoCrFeNiTi0.9 | 257.99 | 144.58 | 164.14 | 296.51 | 178.77 | 121.17 | 1.47 | 0.223 | 4.203 |
| AlCo0.8CrFeNiTi | 260.96 | 118.74 | 162.96 | 310.85 | 192.87 | 126.22 | 1.53 | 0.231 | 4.116 |
| AlCo0.9CrFeNiMn0.9 | 242.66 | 142.68 | 168.93 | 294.03 | 169.85 | 121.35 | 1.4 | 0.211 | 4.118 |
| AlCoFeNiZn0.85 | 181.83 | 102.75 | 145.89 | 247.01 | 134.98 | 103.35 | 1.31 | 0.195 | 3.962 |
| AlCrFeNiMo0.1 | 185.58 | 112.89 | 143.96 | 246.27 | 146.69 | 100.91 | 1.45 | 0.220 | 3.467 |
| AlCoCrFeMo0.1 | 188.71 | 117.68 | 151.79 | 255.55 | 148.74 | 105.28 | 1.41 | 0.214 | 3.463 |
| AlCrCu0.8FeNi | 201.2 | 131.1 | 160.2 | 267.84 | 158.1 | 110.14 | 1.44 | 0.22 | 3.98 |
| AlCrCuNiFe1.1 | 170.1 | 95.7 | 133.4 | 235.2 | 147.7 | 94.66 | 1.56 | 0.24 | 3.65 |
| Alloy | Density (g/cm3) |
|---|---|
| AlCoCrFeNiMn | 6.79 |
| CoCr1.3FeMnNi0.7 | 7.86 |
| AlCoCrFeNi1.3 | 6.84 |
| AlCoCr1.3FeNi1.3 | 6.86275 |
| AlCoCrFeNiTi | 6.22 |
| AlCo0.9CrFeNiMn0.9 | 6.75 |
| Test ID | Run | Power Ratio A | Scan Speed Ratio B | Thickness C | (Approximate % of Powder Printed) |
|---|---|---|---|---|---|
| P25Speed003T2 | 1 | − | − | − | 10 |
| P30Speed003T2 | 2 | + | − | − | 20 |
| P25Speed005T2 | 3 | − | + | − | 8 |
| P30Speed005T2 | 4 | + | + | − | 12 |
| P25Speed003T4 | 5 | − | − | + | 13 |
| P30Speed003T4 | 6 | + | − | + | 9 |
| P25Speed005T4 | 7 | − | + | + | 8 |
| P30Speed005T4 | 8 | + | + | + | 8 |
References
- Macdonald, B.E.; Fu, Z.; Zheng, B.; Chen, W.; Lin, Y.; Chen, F.; Zhang, L.; Ivanisenko, J.; Zhou, Y.; Hahn, H.; et al. Recent Progress in High Entropy Alloy Research. JOM 2017, 69, 2024–2031. [Google Scholar] [CrossRef]
- Miracle, D.; Miller, J.D.; Senkov, O.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and Development of High Entropy Alloys for Structural Applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Joseph, J.; Jarvis, T.; Wu, X.; Stanford, N.; Hodgson, P.; Fabijanic, D. Comparative study of the microstructures and mechanical properties of direct laser fabricated and arc-melted Al x CoCrFeNi high entropy alloys. Mater. Sci. Eng. A 2015, 633, 184–193. [Google Scholar] [CrossRef]
- Chen, T.-K.; Shun, T.; Yeh, J.; Wong, M. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coatings Technol. 2004, 188, 193–200. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Met. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Qiu, X.-W. Microstructure and properties of AlCrFeNiCoCu high entropy alloy prepared by powder metallurgy. J. Alloys Compd. 2013, 555, 246–249. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Ma, J.; Lu, Z.; Zhao, Y. Compositional gradient films constructed by sputtering in a multicomponent Ti–Al–(Cr, Fe, Ni) system. J. Mater. Res. 2018, 33, 3330–3338. [Google Scholar] [CrossRef] [Green Version]
- Xing, Q.; Ma, J.; Wang, C.; Zhang, Y. High throughput screening solar-thermal conversion films in a pseudo-binary (Cr, Fe, V)-(Ta, W) system. ACS Comb. Sci. 2018, 20, 602–610. [Google Scholar] [CrossRef]
- Brif, Y.; Thomas, M.; Todd, I. The use of high-entropy alloys in additive manufacturing. Scr. Mater. 2015, 99, 93–96. [Google Scholar] [CrossRef]
- Ocelík, V.; Janssen, N.; Smith, S.N.; De Hosson, J.T.M. Additive Manufacturing of High-Entropy Alloys by Laser Processing. JOM 2016, 68, 1810–1818. [Google Scholar] [CrossRef] [Green Version]
- Holshouser, C.; Newell, C.; Palas, S.; Love, L.J.; Kunc, V.; Lind, R.F.; Lloyd, P.D.; Rowe, J.C.; Blue, C.A.; Duty, C.E.; et al. Out of bounds additive manufacturing. Adv. Mater. Process. 2013, 171, 15–17. [Google Scholar]
- Joshi, P.C.; Dehoff, R.R.; Duty, C.E.; Peter, W.H.; Ott, R.D.; Love, L.J.; Blue, C.A. Direct digital additive manufacturing technologies: Path towards hybrid integration. In Proceedings of the 2012 Future of Instrumentation International Workshop (FIIW), Gatlinburg, TN, USA, 8–9 October 2012; pp. 1–4. [Google Scholar]
- Li, X. Additive Manufacturing of Advanced Multi-Component Alloys: Bulk Metallic Glasses and High Entropy Alloys. Adv. Eng. Mater. 2017, 20, 1700874. [Google Scholar] [CrossRef]
- Li, R.; Niu, P.; Yuan, T.; Cao, P.; Chen, C.; Zhou, K. Selective laser melting of an equiatomic CoCrFeMnNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical property. J. Alloys Compd. 2018, 746, 125–134. [Google Scholar] [CrossRef]
- Tan, J.H.K.; Sing, S.L.; Yeong, W.Y. Microstructure modelling for metallic additive manufacturing: A review. Virtual Phys. Prototyp. 2019, 15, 87–105. [Google Scholar] [CrossRef]
- Gatsos, T.; Elsayed, K.A.; Zhai, Y.; Lados, D.A. Review on Computational Modeling of Process–Microstructure–Property Relationships in Metal Additive Manufacturing. JOM 2019, 72, 403–419. [Google Scholar] [CrossRef]
- Dutta, B.; Froes, F. (Sam) The Additive Manufacturing (AM) of titanium alloys. Met. Powder Rep. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.S.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro-arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Bahrami, A.; Delgado, A.; Onofre, C.; Muhl, S.; Rodil, S.E. Structure, mechanical properties and corrosion resistance of amorphous Ti-Cr-O coatings. Surf. Coatings Technol. 2019, 374, 690–699. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. npj Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miracle, D.; Senkov, O. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Otero-De-La-Roza, A.; Abbasi-Pérez, D.; Luaña, V. Gibbs2: A new version of the quasiharmonic model code. II. Models for solid-state thermodynamics, features and implementation. Comput. Phys. Commun. 2011, 182, 2232–2248. [Google Scholar] [CrossRef]
- Landau, L. EM Lifshitz Statistical Physics. Course Theor. Phys. 1980, 5, 396–400. [Google Scholar]
- Zheng, S.-M.; Feng, W.-Q.; Wang, S.Q. Elastic properties of high entropy alloys by MaxEnt approach. Comput. Mater. Sci. 2018, 142, 332–337. [Google Scholar] [CrossRef]
- Sarswat, P.K.; Sarkar, S.; Murali, A.; Huang, W.; Tan, W.; Free, M.L. Additive manufactured new hybrid high entropy alloys derived from the AlCoFeNiSmTiVZr system. Appl. Surf. Sci. 2019, 476, 242–258. [Google Scholar] [CrossRef]
- Murali, A.; Sohn, H.Y. Photocatalytic Properties of Plasma-Synthesized Aluminum-Doped Zinc Oxide Nanopowder. J. Nanosci. Nanotechnol. 2019, 19, 4377–4386. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarswat, P.K.; Free, M.L. Metal oxides and novel metallates coated stable engineered steel for corrosion resistance applications. Appl. Surf. Sci. 2018, 456, 328–341. [Google Scholar] [CrossRef]
- Murali, A.; Sarswat, P.; Sohn, H.Y. Enhanced photocatalytic activity and photocurrent properties of plasma-synthesized indium-doped zinc oxide nanopowder. Mater. Today Chem. 2019, 11, 60–68. [Google Scholar] [CrossRef]
- Murali, A.; Lan, Y.P.; Sarswat, P.K.; Free, M.L. Synthesis of CeO2/Reduced Graphene Oxide Nanocomposite for Electrochemical Determination of Ascorbic Acid and Dopamine and for Photocatalytic Applications. Mater. Today Chem. 2019, 12, 222–232. [Google Scholar] [CrossRef]
- Murali, A.; Sarswat, P.K.; Free, M.L. Minimizing electron-hole pair recombination through band-gap engineering in novel ZnO-CeO2-rGO ternary nanocomposite for photoelectrochemical and photocatalytic applications. Environ. Sci. Pollut. Res. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sarswat, P.K.; Free, M.L. Frequency and atomic mass based selective electrochemical recovery of rare earth metals and isotopes. Electrochim. Acta 2016, 219, 435–446. [Google Scholar] [CrossRef]
- Sarswat, P.K.; Sarkar, S.; Bhattacharyya, D.; Cho, J.; Free, M.L. Dopants induced structural and optical anomalies of anisotropic edges of black phosphorous thin films and crystals. Ceram. Int. 2016, 42, 13113–13127. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarswat, P.K.; Yi, G.; Free, M.L. Modifying the band-structure and properties of zirconium telluride using phosphorus addition. Vacuum 2017, 146, 554–561. [Google Scholar] [CrossRef]
- Niu, P.; Li, R.; Yuan, T.; Zhu, S.; Chen, C.; Wang, M.; Huang, L. Microstructures and properties of an equimolar AlCoCrFeNi high entropy alloy printed by selective laser melting. Intermetallics 2019, 104, 24–32. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, A.; Sarswat, P.K.; Perez, J.P.L.; Free, M.L. Synergetic effect of surface plasmon resonance and schottky junction in Ag-AgX-ZnO-rGO (X= Cl & Br) nanocomposite for enhanced visible-light driven photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124684. [Google Scholar] [CrossRef]
- Fan, L.; Liu, L.; Cao, M.; Yu, Z.; Li, Y.; Chen, M.; Wang, F. Corrosion Behavior of Pure Ti under a Solid NaCl Deposit in a Wet Oxygen Flow at 600 °C. Metals 2016, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Ramires, I.; Guastaldi, A. Electrochemical study of the corrosion of Ti-Pd and Ti-6Al-4V electrodes in sodium chloride solutions. Biomecánica 2001, 9, 61–65. [Google Scholar]
- Gorsse, S.; Nguyen, M.; Senkov, O.; Miracle, D. Database on the mechanical properties of high entropy alloys and complex concentrated alloys. Data Brief 2018, 21, 2664–2678. [Google Scholar] [CrossRef]
- Hooper, P.A. Melt pool temperature and cooling rates in laser powder bed fusion. Addit. Manuf. 2018, 22, 548–559. [Google Scholar] [CrossRef]
- Criales, L.E.; Özel, T. Temperature profile and melt depth in laser powder bed fusion of Ti-6Al-4V titanium alloy. Prog. Addit. Manuf. 2017, 2, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Fashu, S.; Lototskyy, M.V.; Davids, M.W.; Pickering, L.; Linkov, V.; Tai, S.; Renheng, T.; Fangming, X.; Fursikov, P.V.; Tarasov, B.P. A review on crucibles for induction melting of titanium alloys. Mater. Des. 2020, 186, 108295. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Okulov, I.; Wendrock, H.; Bӧnisch, M.; Volegov, A.; Calin, M.; Eckert, J.; Dargusch, M. Nanoindentation and wear properties of Ti and Ti-TiB composite materials produced by selective laser melting. Mater. Sci. Eng. A 2017, 688, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yuan, T.; Li, R.; Li, L. Two ways of evaluating the wear property of Ti-13Nb-13Zr fabricated by selective laser melting. Mater. Lett. 2019, 242, 9–12. [Google Scholar] [CrossRef]

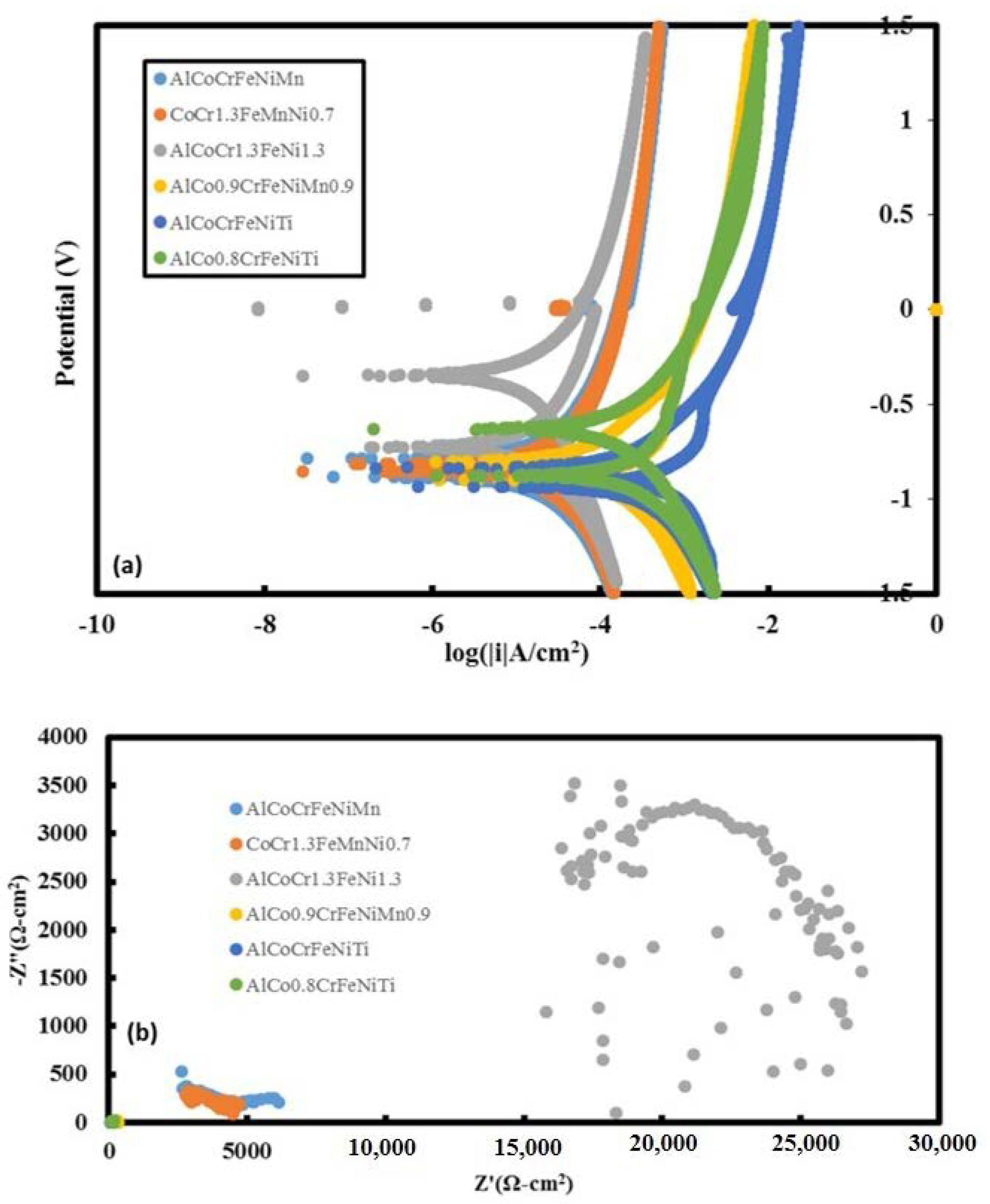
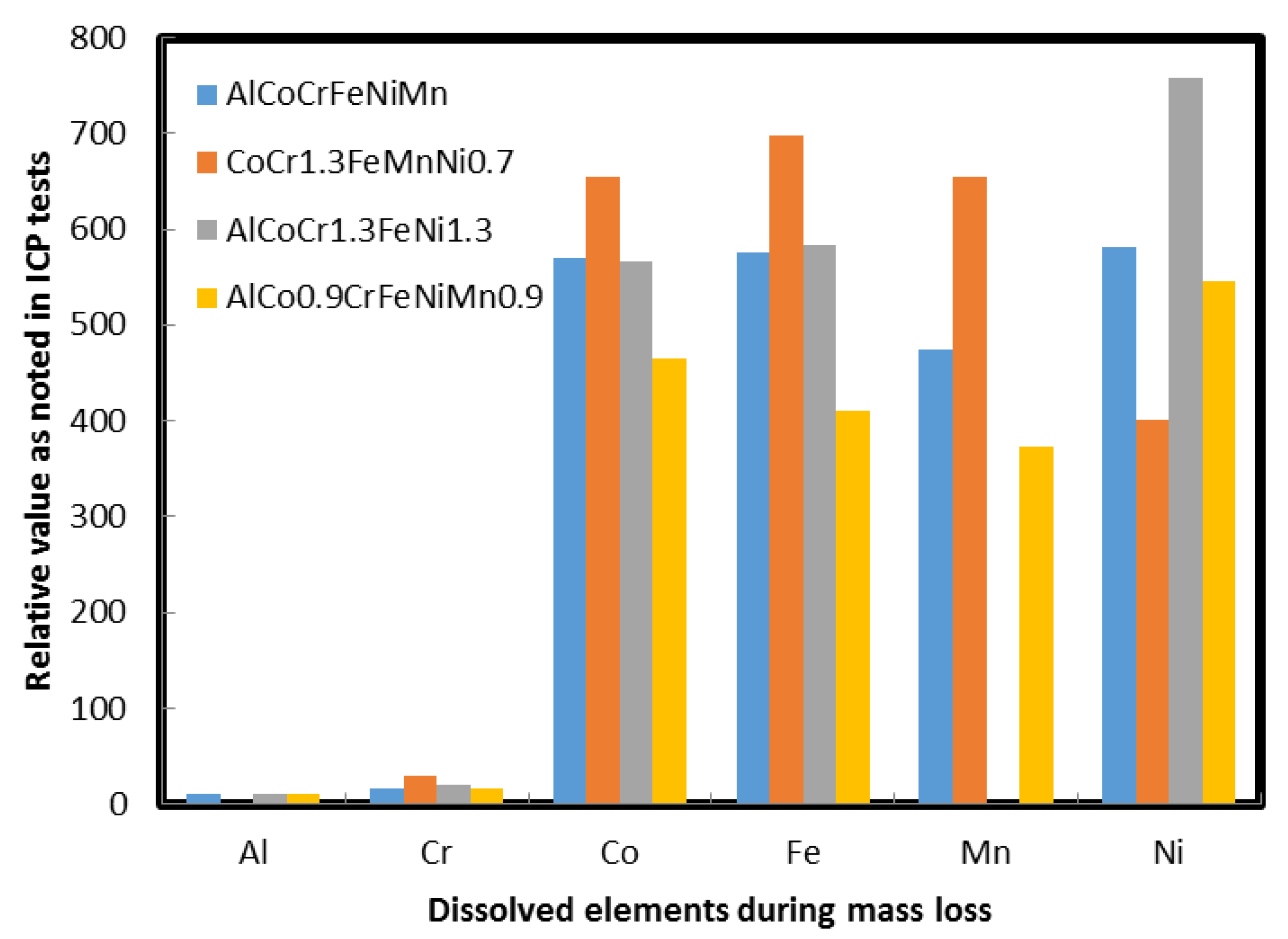
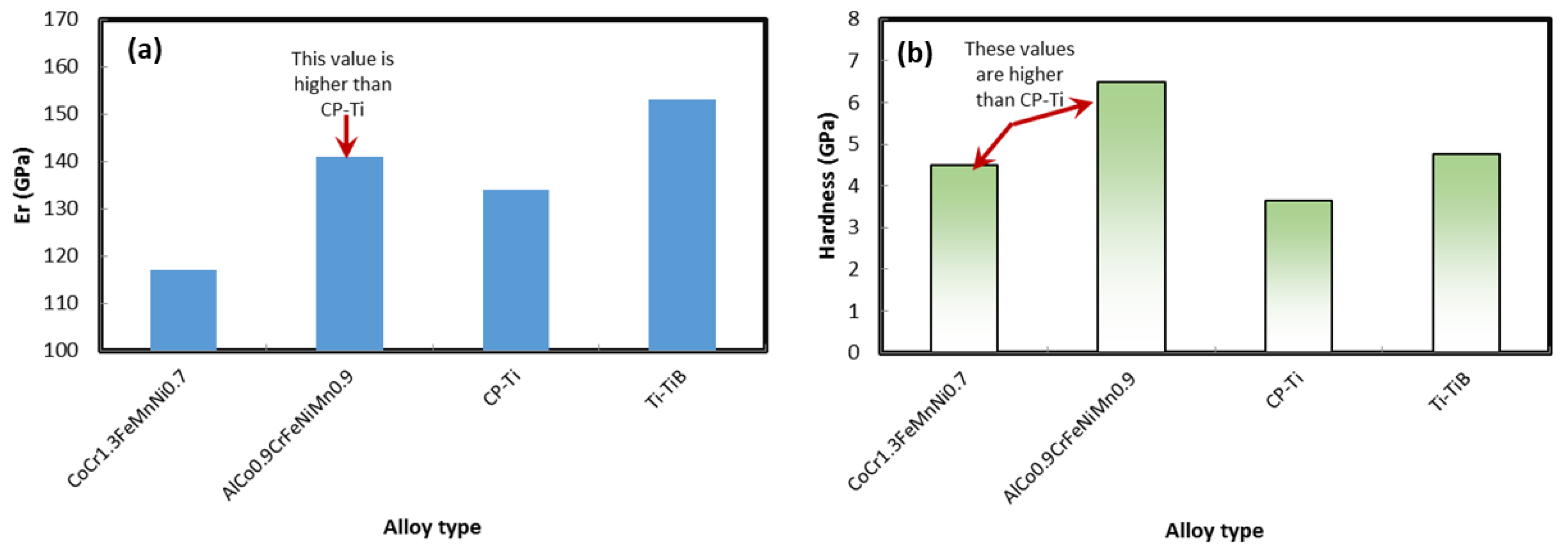
| Additive Manufactured Alloy Stoichiometry | Ecorr (V) | Log Icorr (A/cm2) |
|---|---|---|
| AlCoCrFeNiMn | −0.8838 | −5.15 |
| CoCr1.3FeMnNi0.7 | −0.866 | −4.97 |
| AlCoCr1.3FeNi1.3 | −0.724 | −5.19 |
| AlCo0.9CrFeNiMn0.9 | −0.903 | −3.89 |
| AlCoCrFeNiTi | −0.933 | −3.64 |
| AlCo0.8CrFeNiTi | −0.874 | −3.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarswat, P.; Smith, T.; Sarkar, S.; Murali, A.; Free, M. Design and Fabrication of New High Entropy Alloys for Evaluating Titanium Replacements in Additive Manufacturing. Materials 2020, 13, 3001. https://doi.org/10.3390/ma13133001
Sarswat P, Smith T, Sarkar S, Murali A, Free M. Design and Fabrication of New High Entropy Alloys for Evaluating Titanium Replacements in Additive Manufacturing. Materials. 2020; 13(13):3001. https://doi.org/10.3390/ma13133001
Chicago/Turabian StyleSarswat, Prashant, Taylor Smith, Sayan Sarkar, Arun Murali, and Michael Free. 2020. "Design and Fabrication of New High Entropy Alloys for Evaluating Titanium Replacements in Additive Manufacturing" Materials 13, no. 13: 3001. https://doi.org/10.3390/ma13133001
APA StyleSarswat, P., Smith, T., Sarkar, S., Murali, A., & Free, M. (2020). Design and Fabrication of New High Entropy Alloys for Evaluating Titanium Replacements in Additive Manufacturing. Materials, 13(13), 3001. https://doi.org/10.3390/ma13133001








