Compositional Dependence of Pore Structure, Strengthand Freezing-Thawing Resistance of Metakaolin-Based Geopolymers
Abstract
1. Introduction
2. Experiments
2.1. Materials and Specimen Preparation
2.2. Testing Methods
2.2.1. Strength
2.2.2. Freezing-Thawing
2.2.3. Pore Structure
2.2.4. Micro Morphology
3. Materials’ Properties and Pore Structure
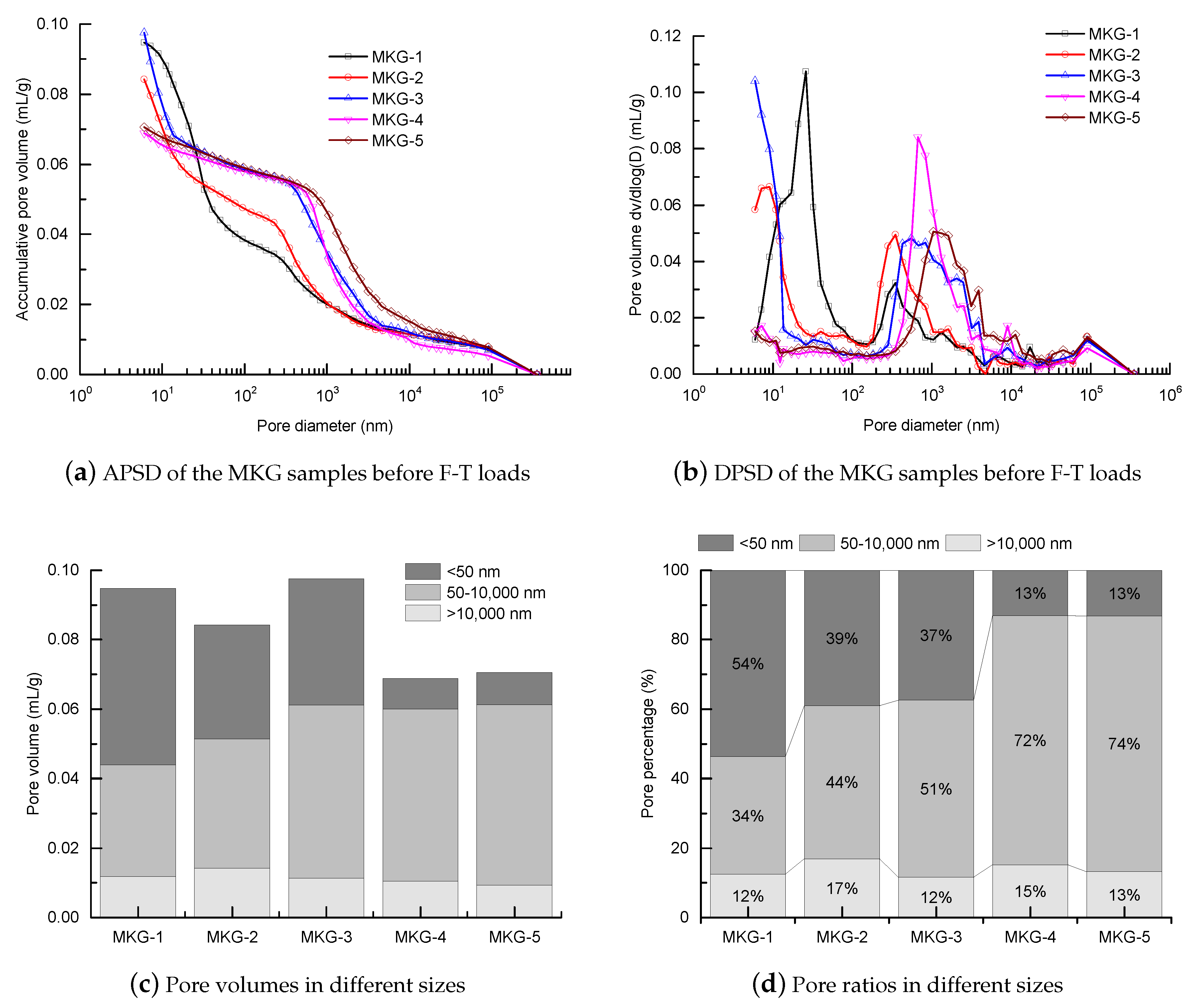
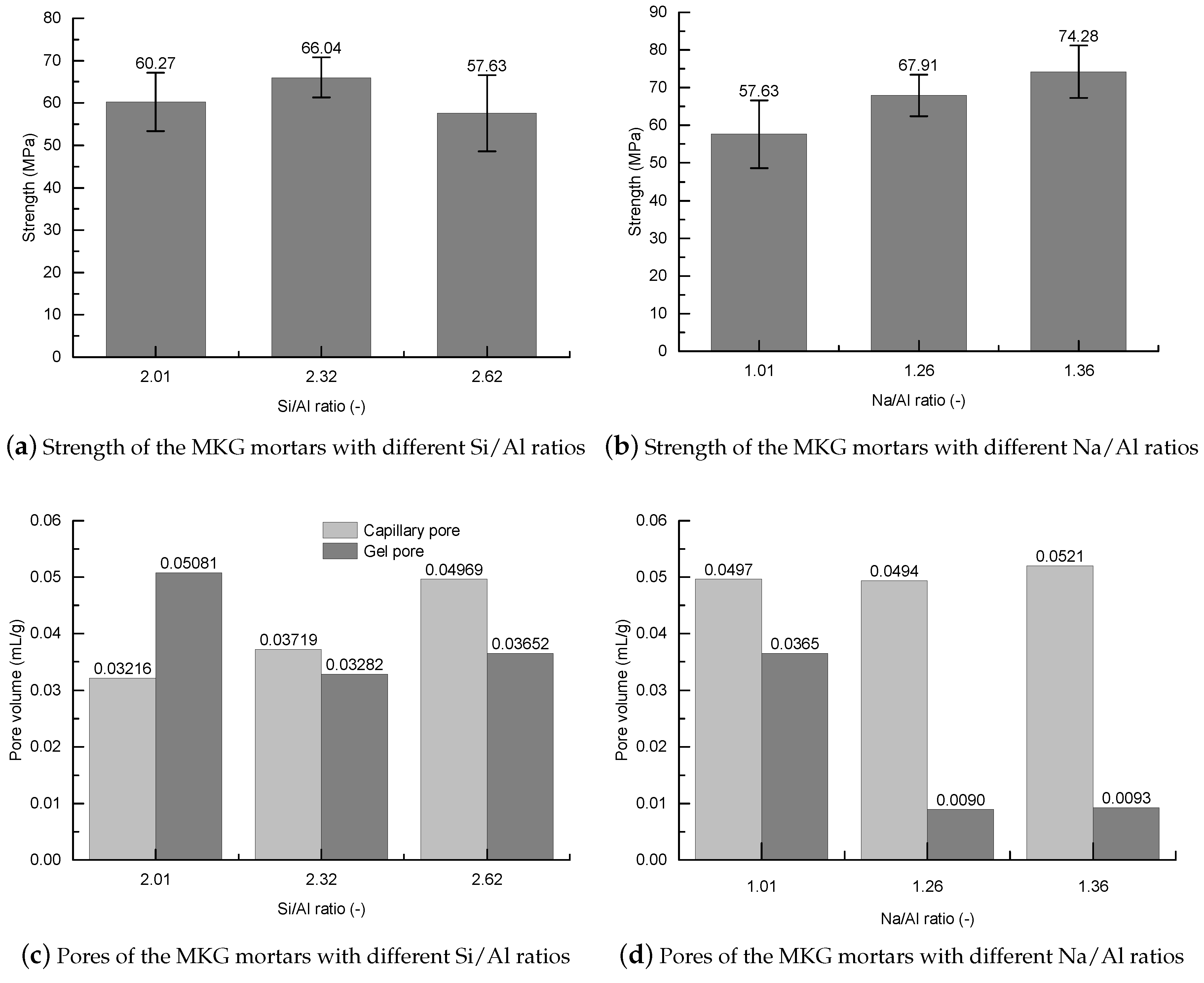
3.1. Pore Structure
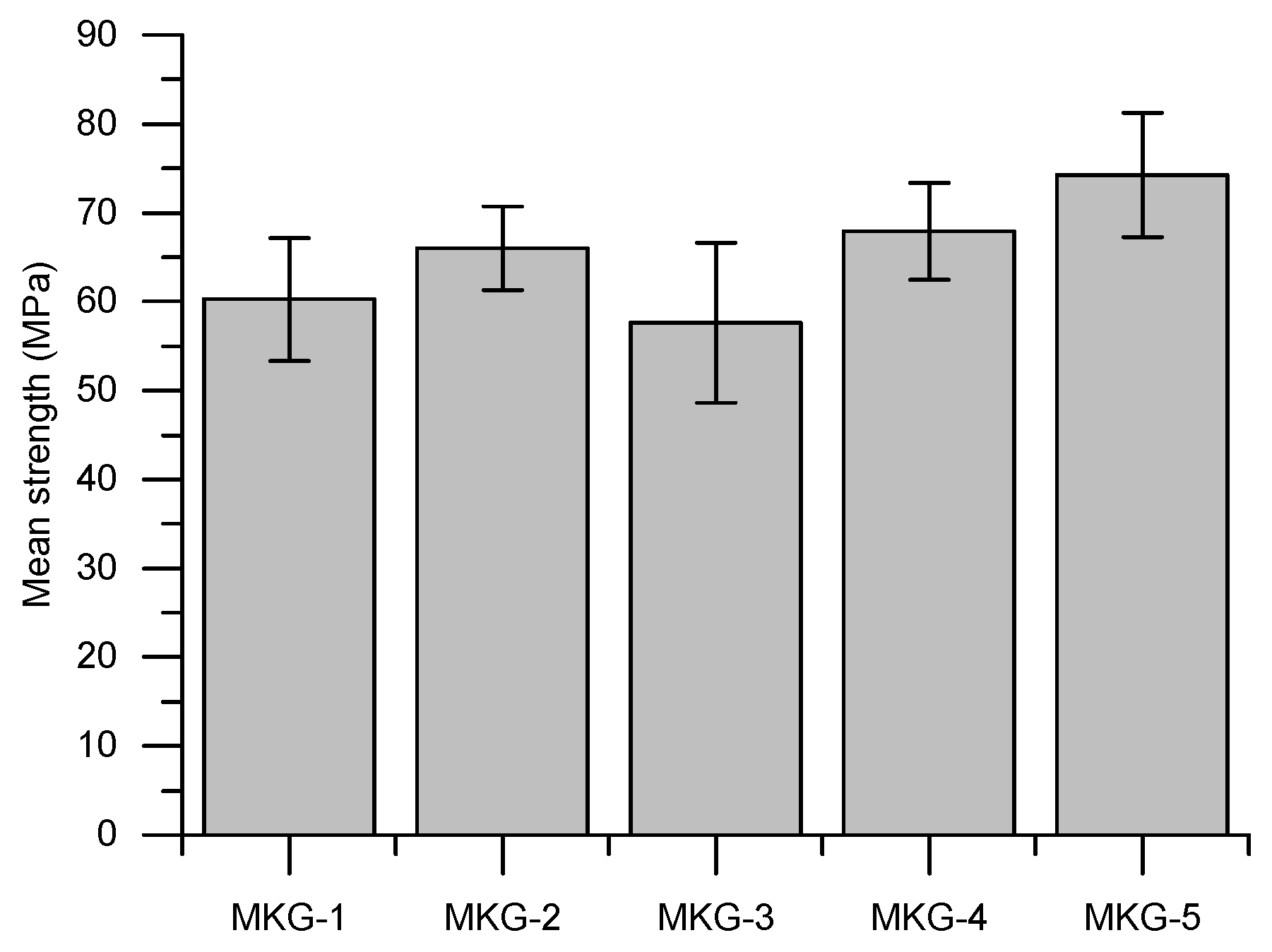
3.2. Strength
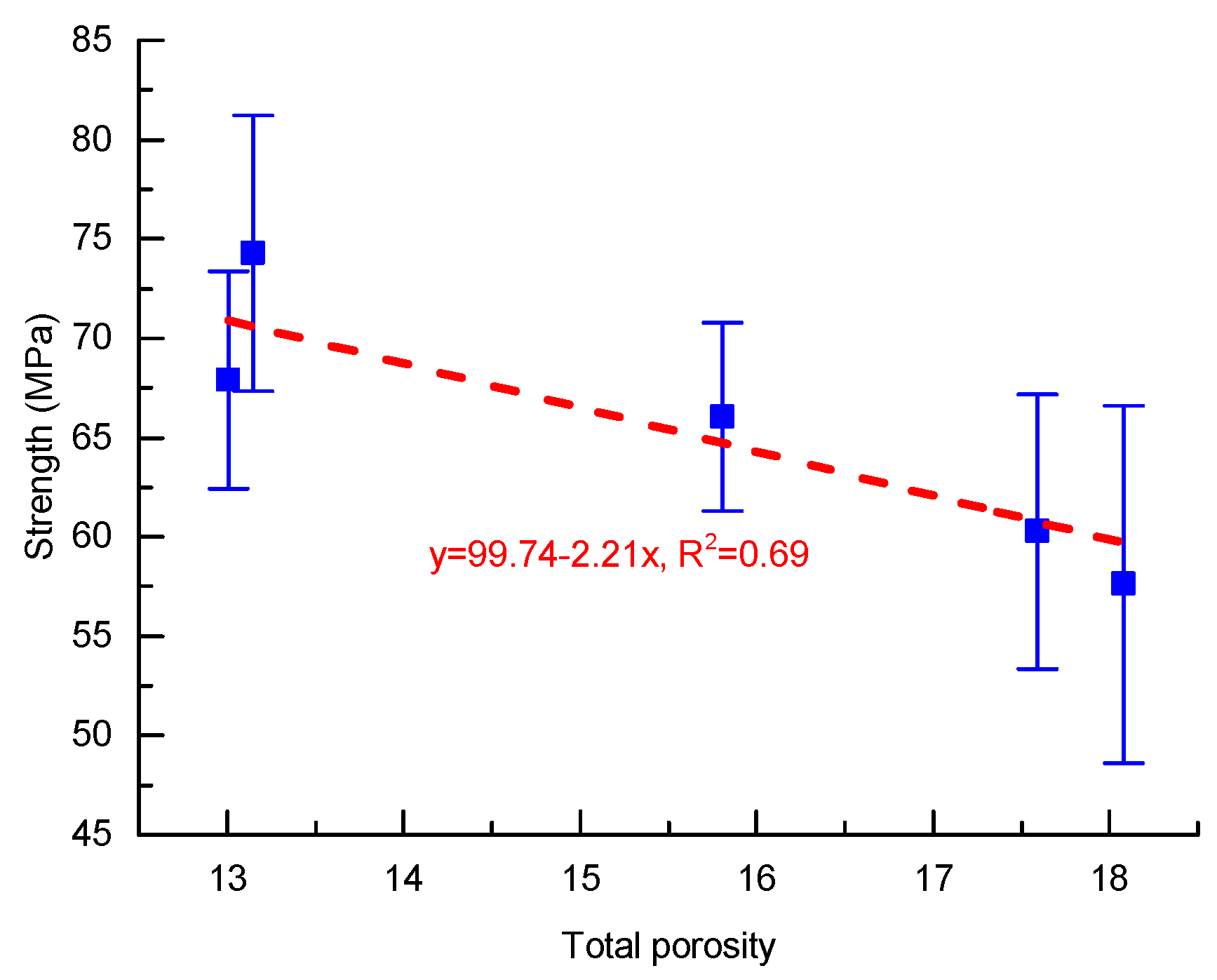
3.3. Roles of the Materials’ Composition
4. Freezing-Thawing Damages
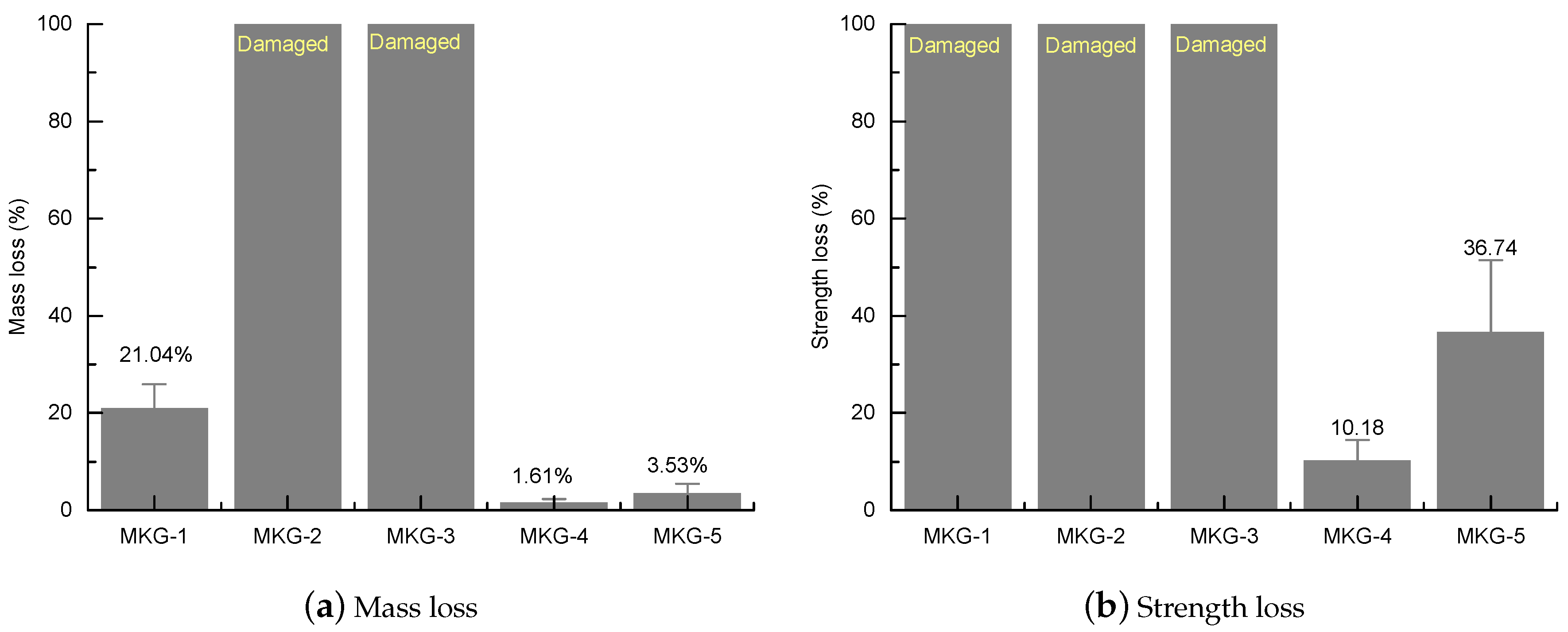
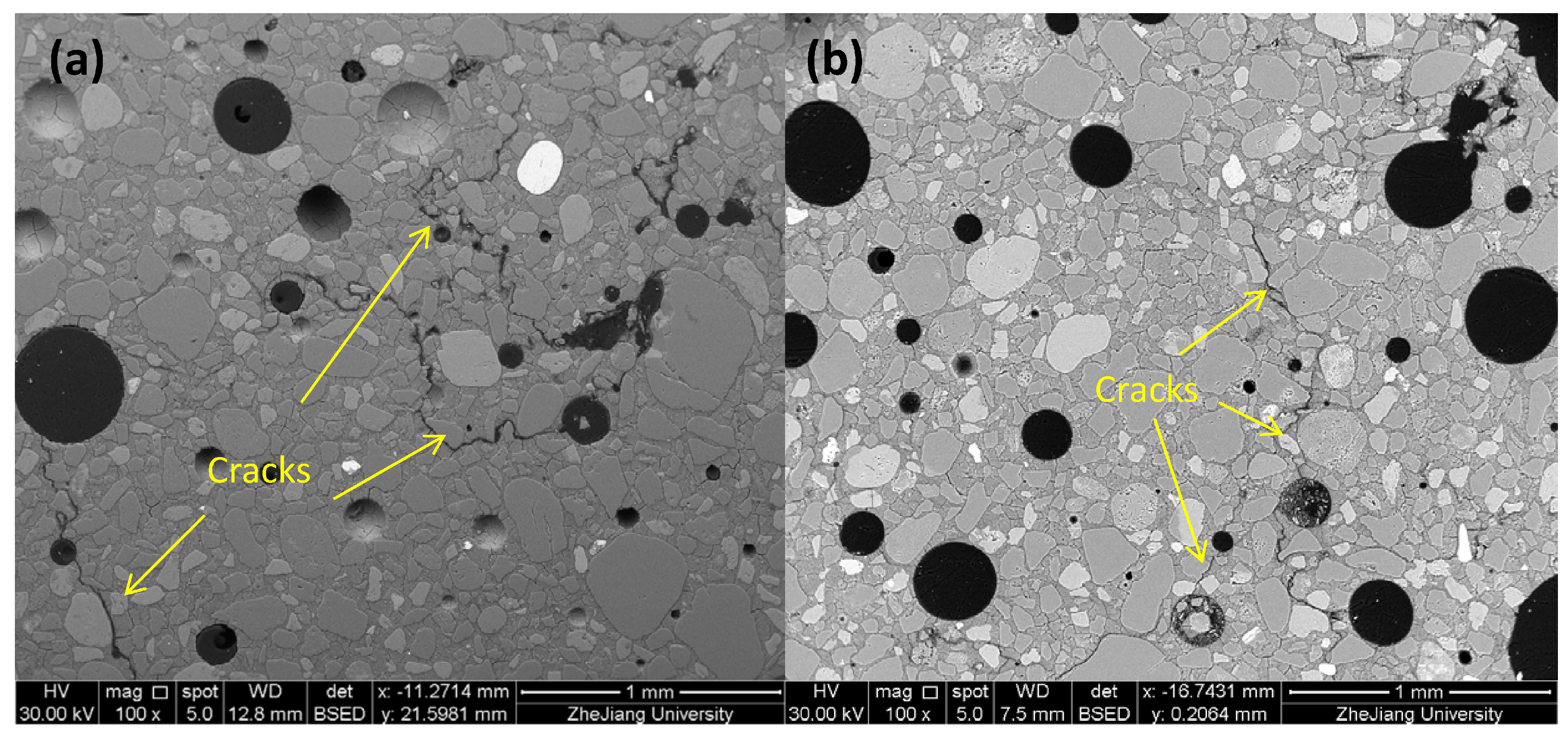
4.1. Morphology, Mass Loss, and Strength Loss
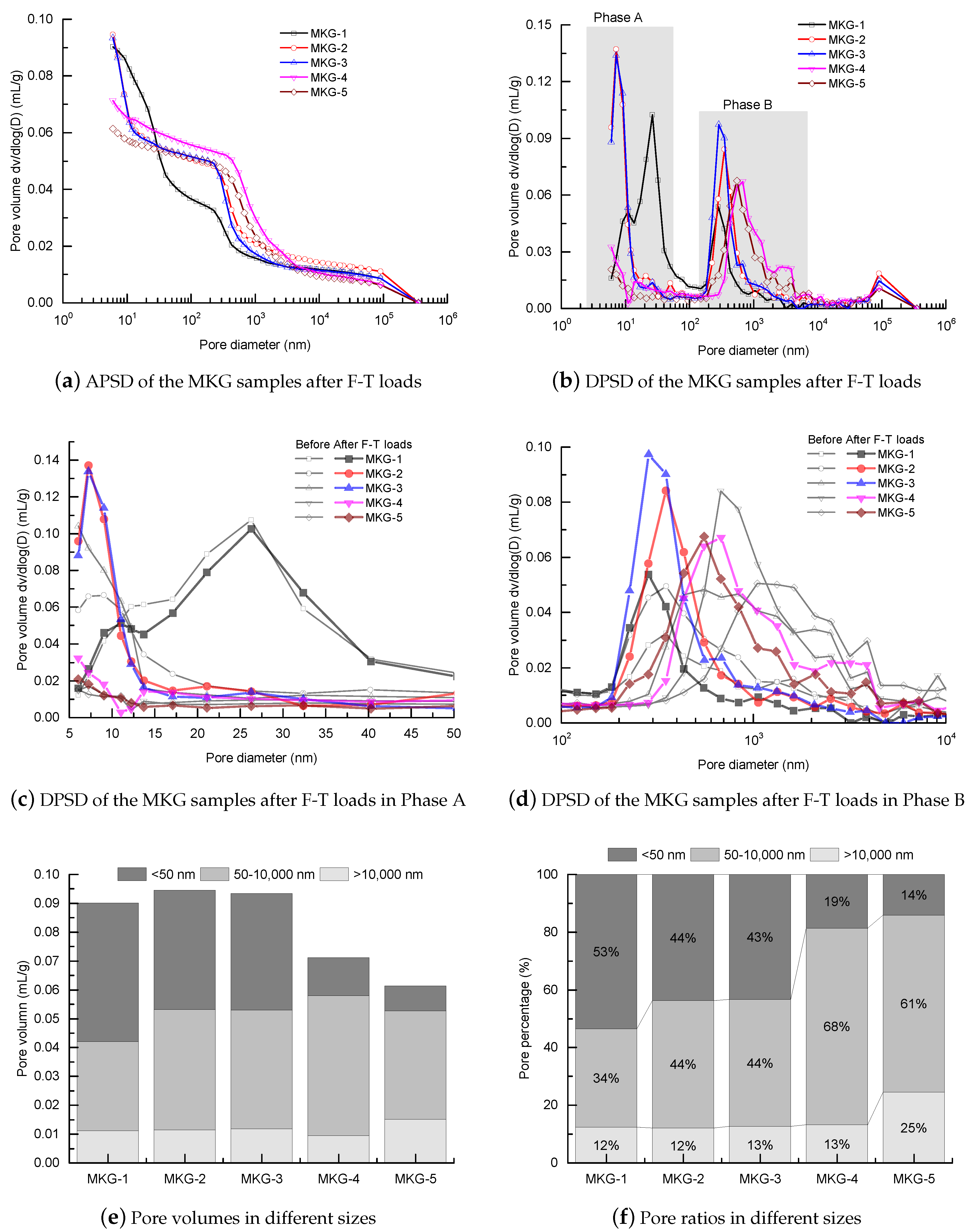
4.2. Pore Structure Alterations
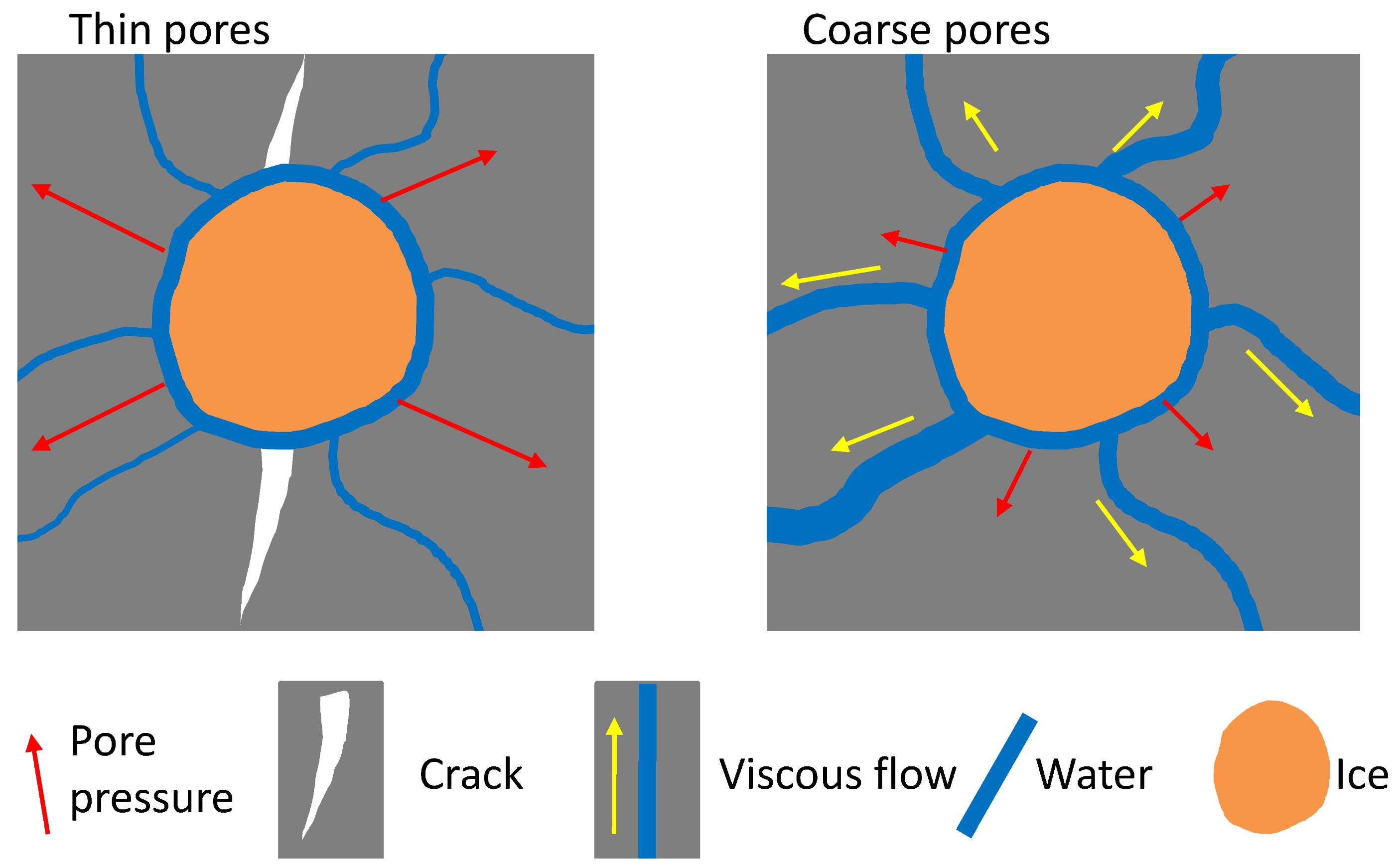
4.3. Further Discussion: Permeability Associated Pressure Relaxation
5. Conclusions
- The MKG mortars with different Si/Al ratios and Na/Al ratios showed different MIP pore structures. Except the first mercury rises, which were identical to the surface fillings of the mercury under low pressures showing negligible volume changes, both the capillary pores and gel pores were greatly impacted by the Si/Al ratio and the Na/Al ratio. Increasing both the Si/Al ratio and the Na/Al ratio decreased the gel pores, but promoted the capillary pores.
- All the MKG mortars showed relatively high strengths. The strength decreased with increasing the total porosity, which roughly followed a linear plot.
- The MKG mortars showed different F-T resistances: MKG-4 > MKG-5 > MKG-1 > MKG-2 = MKG-3. The MKG mortars (MKG-1, MKG-2, and MKG-3) at Na/Al ratios lower than 1.26 all showed serious F-T damages. Increasing the Na/Al ratio promoted the F-T resistance. F-T loads also caused obvious cracking of MKG-4 and MKG-5.
- MIP tests showed that the pore structures were refined after F-T loads, which was probably caused by the continual curing of the materials. Materials with finer pore structures showed worse F-T resistances owing to the slower pore pressure relaxation rates.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monteiro, P.J.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Kastiukas, G.; Ruan, S.; Liang, S.; Zhou, X. Development of precast geopolymer concrete via oven and microwave radiation curing with an environmental assessment. J. Clean. Prod. 2020, 255, 120290. [Google Scholar] [CrossRef]
- Panda, B.; Ruan, S.; Unluer, C.; Tan, M. nvestigation of the properties of alkali-activated slag mixes involving the use of nanoclay and nucleation seeds for 3D printing. Compos. Part B-Eng. 2020, 186, 107826. [Google Scholar] [CrossRef]
- Panda, B.; Ruan, S.; Unluer, C.; Tan, M. Improving the 3D printability of high volume fly ash mixtures via the use of nano attapulgite clay. Compos. Part B-Eng. 2019, 165, 75–83. [Google Scholar] [CrossRef]
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/ sustainable concrete. J. Clean. Prod. 2020, 263, 121477. [Google Scholar] [CrossRef]
- El-Gamal, S.M.A.; Selim, F.A. Utilization of some industrial wastes for eco-friendly cement production. Sustain. Mater. Technol. 2017, 12, 9–17. [Google Scholar] [CrossRef]
- Bassani, M.; Tefa, L.; Coppola, B.; Palmero, P. Alkali-activation of aggregate fines from construction and demolition waste: Valorisation in view of road pavement subbase applications. J. Clean. Prod. 2019, 234, 71–84. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Rowles, M.; Connor, B.O. Chemical optimization of compressive strength of aluminosilicate geopolymer synthesized by sodium activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Kohout, J.; Koutnik, P. Effect of Filler Type on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Materials 2020, 13, 2395. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shuai, Q.; Li, H.; Ding, Q.; Gu, Y.; Cheng, C.; Xu, Z. Fabrication and fireproofing performance of the coal fly ash-metakaolin-based geopolymer foams. Materials 2020, 13, 1750. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gao, K.; Zhang, P. Experimental and statistical study on mechanical characteristics of geopolymer concrete. Materials 2020, 13, 1651. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Chianese, E.; Ferone, C.; Ricciotti, L.; Roviello, V.; Cioffi, R.; Tarallo, O. Hybrid geopolymeric foams for the removal of metallic ions from aqueous waste solutions. Materials 2019, 12, 4091. [Google Scholar] [CrossRef]
- White, C.E.; Page, K.; Henson, N.J.; Provis, J.L. In situ synchrotron X-ray pair distribution function analysis of the early stages of gel formation in metakaolin-based geopolymers. Appl. Clay Sci. 2013, 73, 17–25. [Google Scholar] [CrossRef]
- Lahoti, M.; Narang, P.; Tan, K.H.; Yang, E. Mix design factors and strength prediction of metakaolin- based geopolymer. Ceram. Int. 2017, 43, 11433–11441. [Google Scholar] [CrossRef]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, e00205. [Google Scholar] [CrossRef]
- Yan, D.; Chen, S.; Zeng, Q.; Xu, S.; Li, H. Correlating the elastic properties of metakaolin-based geopolymer with its composition. Mater. Des. 2016, 95, 306–318. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Sun, P.J.; Wu, H.C. Chemical and freeze-thaw resistance of fly ash-based inorganic mortars. Fuel 2013, 111, 740–745. [Google Scholar] [CrossRef]
- Topcu, I.B.; Toprak, M.U.; Uygunoglu, T. Durability and microstructure characteristics of alkali activated coal bottom ash geopolymer cement. J. Clean. Prod. 2014, 111, 211–217. [Google Scholar]
- Zhao, R.; Yuan, Y.; Cheng, Z.; Wen, T.; Li, J.; Li, F.; Ma, Z.J. Freeze-thaw resistance of Class F fly ash-based geopolymer concrete. Constr. Build. Mater. 2019, 222, 474–483. [Google Scholar] [CrossRef]
- Bocca, P.; Grazzini, A. Mechanical properties and freeze-thaw durability of strengthening mortars. J. Mater. Civ. Eng. 2013, 25, 274–280. [Google Scholar] [CrossRef]
- Carpinteri, A.; Grazzini, A.; Lacidogna, G.; Manuello, A. Durability evaluation of reinforced masonry by fatigue tests and acoustic emission technique. Struct. Control Health Monit. 2014, 21, 950–961. [Google Scholar] [CrossRef]
- Saez del Bosque, I.F.; Van den Heede, P.; De Belie, N.; Sanchez de Rojas, M.I.; Medina, C. Freeze-thaw resistance of concrete containing mixed aggregate and construction and demolition waste-additioned cement in water and de-icing salts. Constr. Build. Mater. 2020, 259, 119772. [Google Scholar] [CrossRef]
- Huang, S.; Ye, Y.; Cui, X.; Cheng, A.; Liu, G. Theoretical and experimental study of the frost heaving characteristics of the saturated sandstone under low temperature. Cold Reg. Sci. Technol. 2020, 74, 103036. [Google Scholar] [CrossRef]
- Zeng, Q.; Fen-Chong, T.; Li, K. Freezing behavior of cement pastes saturated with NaCl solution. Constr. Build. Mater. 2014, 59, 99–110. [Google Scholar] [CrossRef]
- Zeng, Q.; Fen-Chong, T.; Dangla, P.; Li, K. A study of freezing behavior of cementitious materials by poromechanical approach. Int. J. Solids Struct. 2011, 48, 3267–3273. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-Chong, T. Elastic behavior of saturated porous materials under undrained freezing. Acta Mech. Sin. 2013, 29, 827–835. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-Chong, T. Heterogeneous nucleation of ice from supercooled NaCl solution confined in porous cement paste. J. Cryst. Growth 2015, 409, 1–9. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K. Quasi-Liquid Layer on Ice and Its Effect on the Confined Freezing of Porous Materials. Crystals 2019, 9, 250. [Google Scholar] [CrossRef]
- Engemann, S.; Reichert, H.; Dosch, H.; Bilgram, J.; Honkimaki, V.; Snigirev, A. Interfacial melting of ice in contact with SiO2. Phys. Rev. Lett. 2004, 92, 205701. [Google Scholar] [CrossRef] [PubMed]
- Jahnert, S.; Vaca Chavez, F.; Schaumann, G.E.; Schreiber, A.; Schonhoff, M.; Findenegg, G.H. Melting and freezing of water in cylindrical silica nanopores. Phys. Chem. Chem. Phys. 2008, 10, 6039–6051. [Google Scholar] [CrossRef] [PubMed]
- Test Code for Hydraulic Concrete; DL/T 5150-2017; The Electric Power Ministry of the People’s Republic of China: Beijing, China, 2017.
- Leóny León, C.A. New perspectives in mercury porosimetry. Adv. Colloid Interface Sci. 1998, 76–77, 341–372. [Google Scholar] [CrossRef]
- Washburn, E.W. Note on a method of determining the distribution of pore sizes in a porous material. Proc. Natl. Acad. Sci. USA 1921, 7, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, X.; Yang, P.; Wang, J.; Zhou, C. Tracing mercury entrapment in porous cement paste after mercury intrusion test by X-ray computed tomography and implications for pore structure characterization. Mater. Charact. 2019, 151, 203–215. [Google Scholar] [CrossRef]
- Katz, A.J.; Thompson, A.H. Quantitative prediction of permeability in porous rock. Phys. Rev. B 1986, 34, 8179. [Google Scholar] [CrossRef]
- Zhang, Z.; Scherer, G.W. Evaluation of drying methods by nitrogen adsorption. Cem. Concr. Res. 2019, 120, 13–26. [Google Scholar] [CrossRef]
- Diamond, S. Mercury porosimetry: An inappropriate method for the measurement of pore size distributions in cement-based materials. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Hasselman, D.P.H.; Fulrath, R.M. Effect of small fraction of spherical porosity on elastic moduli of glass. J. Am. Ceram Soc. 1963, 47, 52–53. [Google Scholar] [CrossRef]
- Shi, C. Strength, pore structure and permeability of alkali-activated slag mortars. Cem. Concr. Res. 1996, 26, 1789–1799. [Google Scholar] [CrossRef]
- Akono, A.T.; Koric, S.; Kriven, W.M. Influence of pore structure on the strength behavior of particle- and fiber-reinforced metakaolin-based geopolymer composites. Cem. Concr. Compos. 2019, 104, 103361. [Google Scholar] [CrossRef]
- Balczar, I.; Korim, T.; Dobradi, A. Correlation of strength to apparent porosity of geopolymers—Understanding through variations of setting time. Constr. Build. Mater. 2015, 93, 983–988. [Google Scholar] [CrossRef]
- Aygormeza, Y.; Canpolat, O.; Al-mashhadani, M.M. Assessment of geopolymer composites durability at one year age. J. Build. Eng. 2020, 32, 101453. [Google Scholar] [CrossRef]
- Aygormeza, Y.; Canpolat, O.; Al-mashhadani, M.M.; Uysal, M. Elevated temperature, freezing-thawing and wetting-drying effects on polypropylene fiber reinforced metakaolin based geopolymer composites. Constr. Build. Mater. 2020, 235, 117502. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, G.; Htet, K.W.; Kwon, M.; Liu, C.; Xu, Y.; Tao, M. Freeze-thaw durability of red mud slurry-class F fly ash-based geopolymer: Effect of curing conditions. Constr. Build. Mater. 2019, 215, 381–390. [Google Scholar] [CrossRef]
- Sun, Z.H.; Scherer, G.W. Effect of air voids on salt scaling and internal freezing. Cem. Concr. Res. 2010, 40, 260–270. [Google Scholar] [CrossRef]
- Zhang, D.; Weinbaum, S.; Cowin, S.C. Estimates of the peak pressures in bone pore water. J. Biomech. Eng. 1998, 120, 697–703. [Google Scholar] [CrossRef]
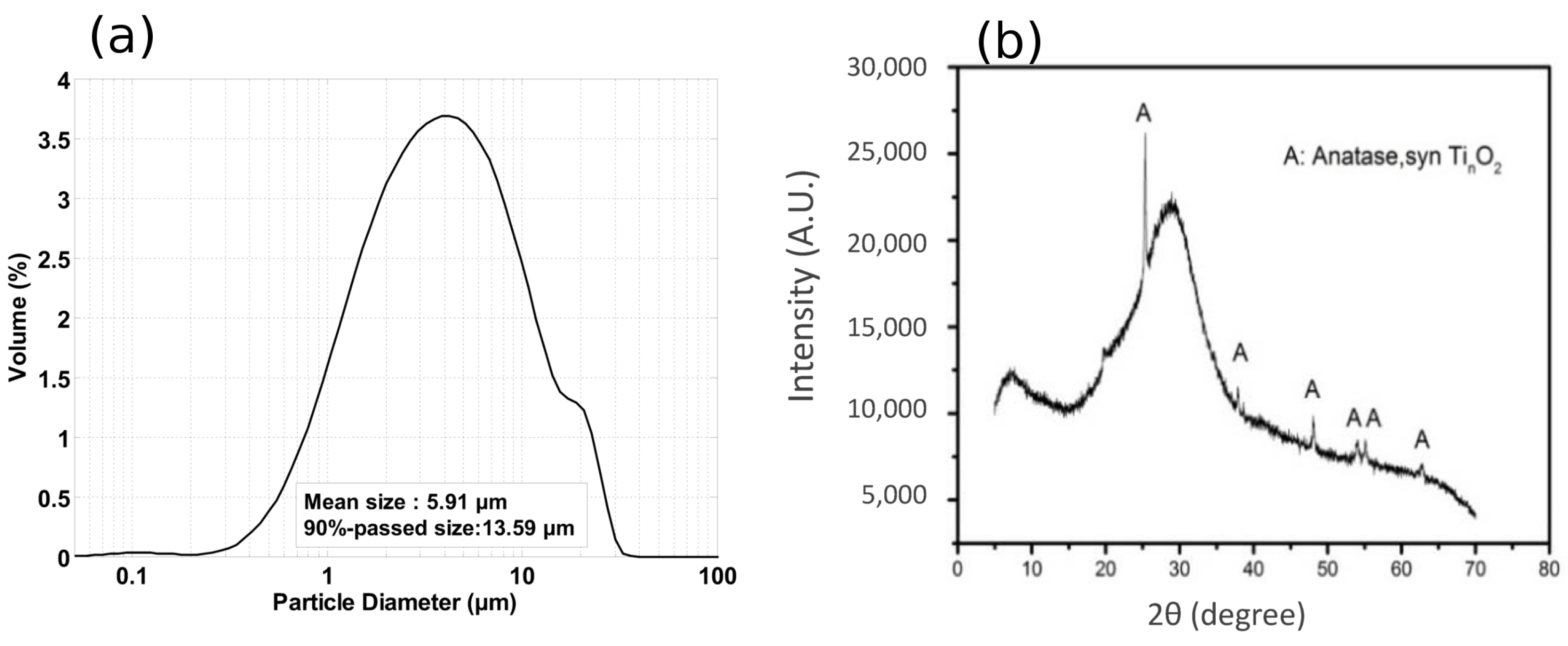

| Composition | |||||||
|---|---|---|---|---|---|---|---|
| Mass content (%) | 57.47 | 39.81 | 1.79 | 0.43 | 0.27 | 0.21 | 0.04 |
| Mix ID | Metakaolin | Water Glass | NaOH | Water | Si/Al | Na/Al |
|---|---|---|---|---|---|---|
| MKG-1 | 1016 | 640 | 193 | 410 | 2.01 | 1.01 |
| MKG-2 | 936 | 993 | 138 | 197 | 2.32 | 1.01 |
| MKG-3 | 868 | 1290 | 84 | 17 | 2.62 | 1.01 |
| MKG-4 | 842 | 1251 | 136 | 31 | 2.62 | 1.26 |
| MKG-5 | 832 | 1233 | 156 | 38 | 2.62 | 1.36 |
| Sample | Total Porosity (%) | Average Pore Size (nm) | Specific Surface Area () | Threshold Pore Size (nm) |
|---|---|---|---|---|
| MKG-1 | 17.59 | 32.0 | 11.83 | 350.1 |
| MKG-2 | 15.81 | 27.1 | 12.43 | 350.1 |
| MKG-3 | 18.08 | 25.2 | 15.46 | 553.7 |
| MKG-4 | 13.01 | 84.5 | 3.25 | 675.9 |
| MKG-5 | 13.15 | 89.9 | 3.14 | 1049.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, D.; Xie, L.; Qian, X.; Ruan, S.; Zeng, Q. Compositional Dependence of Pore Structure, Strengthand Freezing-Thawing Resistance of Metakaolin-Based Geopolymers. Materials 2020, 13, 2973. https://doi.org/10.3390/ma13132973
Yan D, Xie L, Qian X, Ruan S, Zeng Q. Compositional Dependence of Pore Structure, Strengthand Freezing-Thawing Resistance of Metakaolin-Based Geopolymers. Materials. 2020; 13(13):2973. https://doi.org/10.3390/ma13132973
Chicago/Turabian StyleYan, Dongming, Lingjun Xie, Xiaoqian Qian, Shaoqin Ruan, and Qiang Zeng. 2020. "Compositional Dependence of Pore Structure, Strengthand Freezing-Thawing Resistance of Metakaolin-Based Geopolymers" Materials 13, no. 13: 2973. https://doi.org/10.3390/ma13132973
APA StyleYan, D., Xie, L., Qian, X., Ruan, S., & Zeng, Q. (2020). Compositional Dependence of Pore Structure, Strengthand Freezing-Thawing Resistance of Metakaolin-Based Geopolymers. Materials, 13(13), 2973. https://doi.org/10.3390/ma13132973









