Selective Adsorption, Reduction, and Separation of Au(III) from Aqueous Solution with Amine-Type Non-Woven Fabric Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
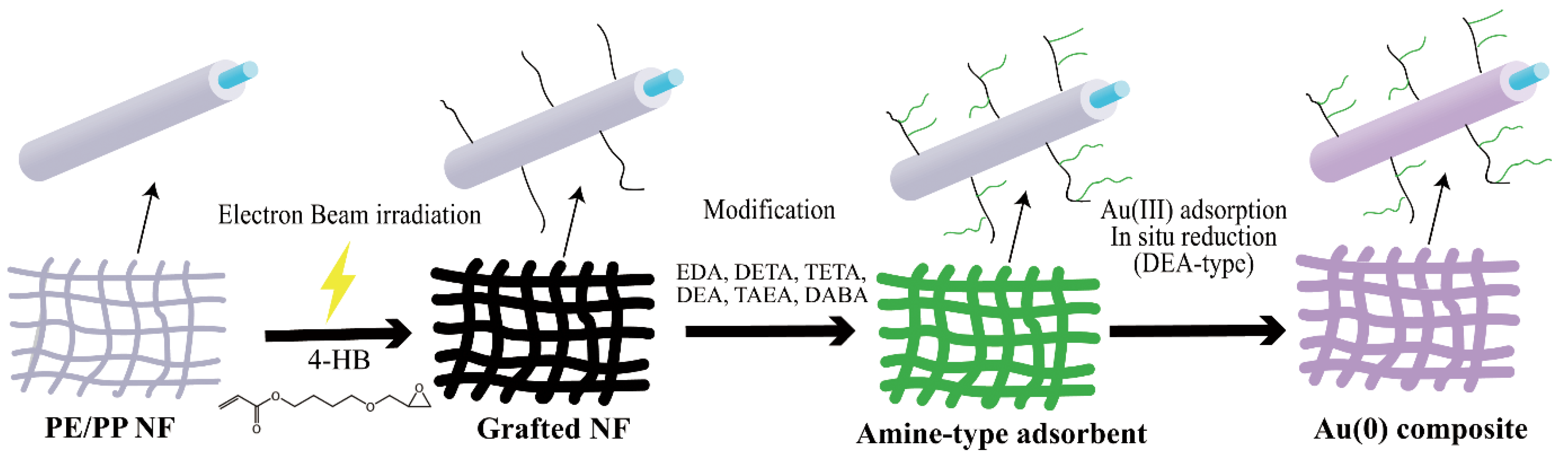
2.2. Preparation of Amine-Type Adsorbent
2.3. Characterization
2.4. Adsorption and Elution, Column and Batch Mode
3. Results and Discussion
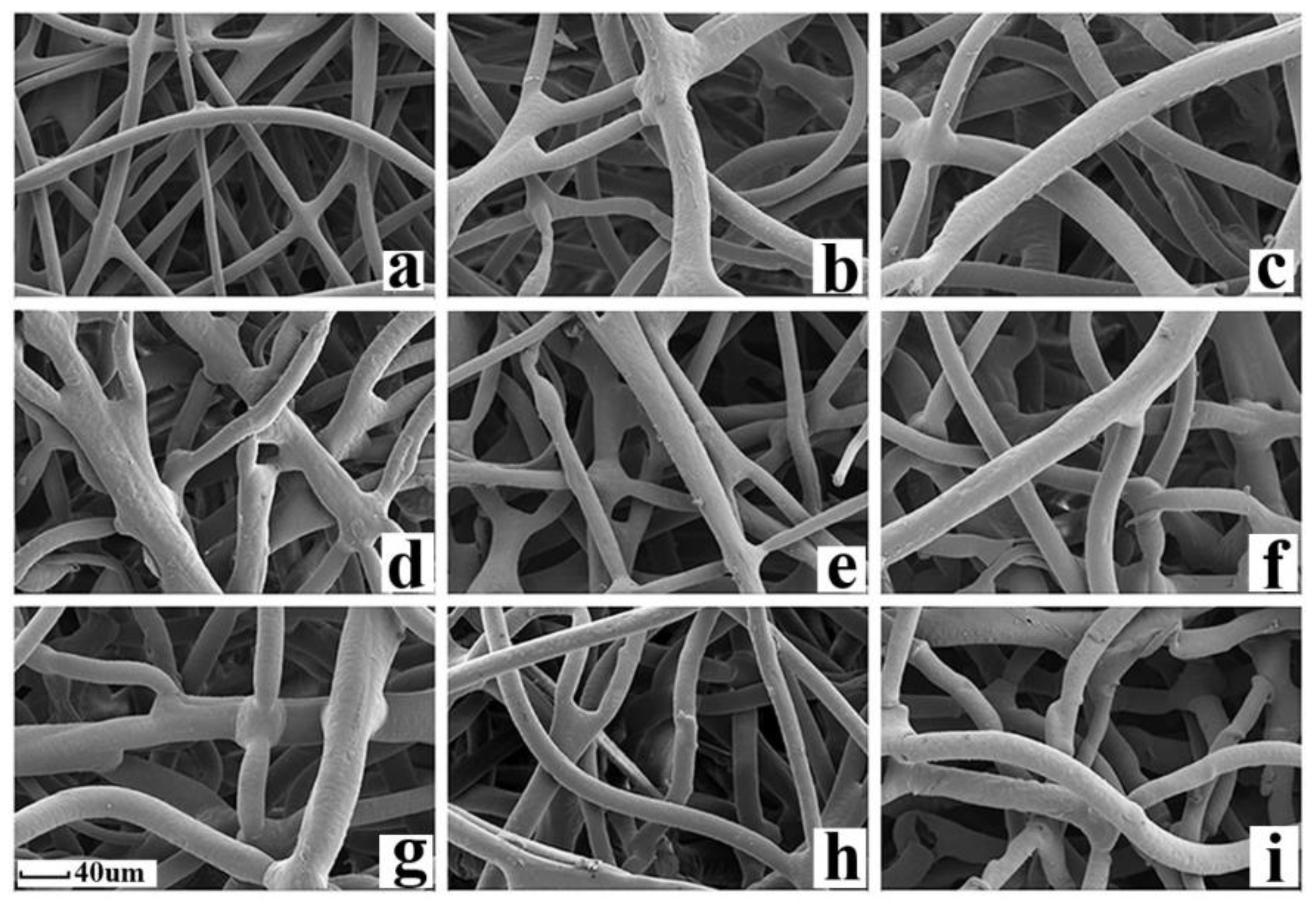
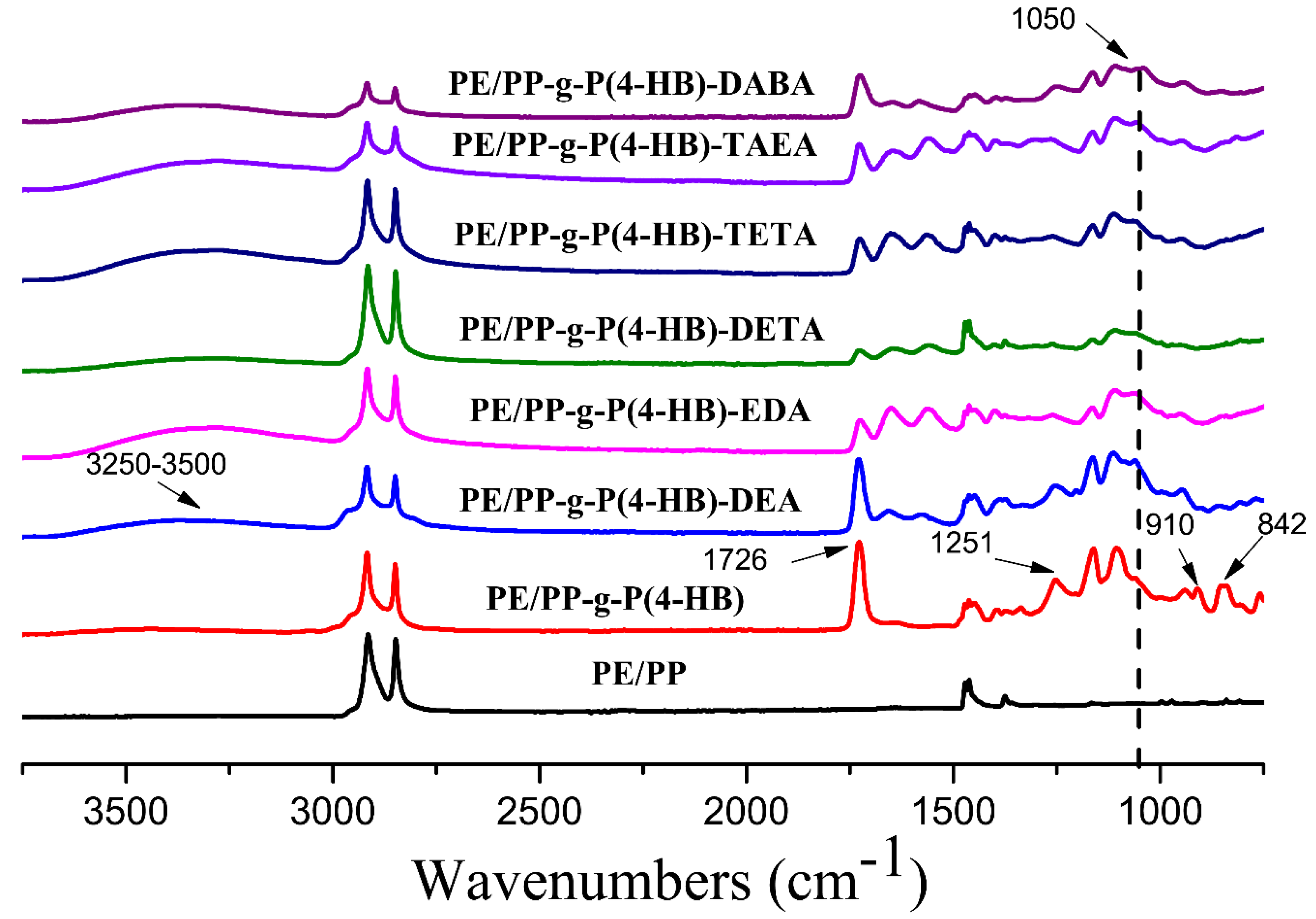
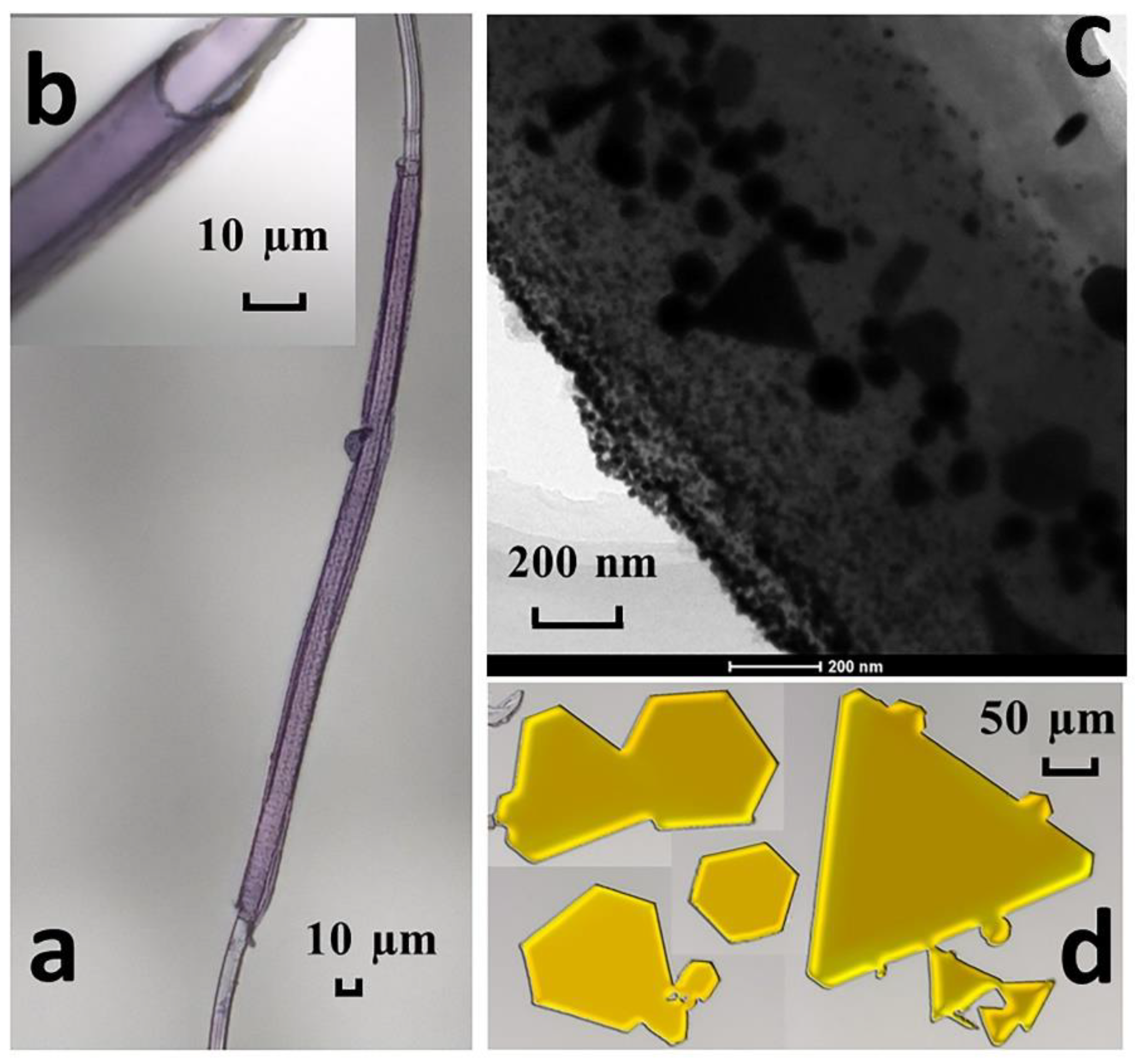
3.1. Characterization of the Adsorbent
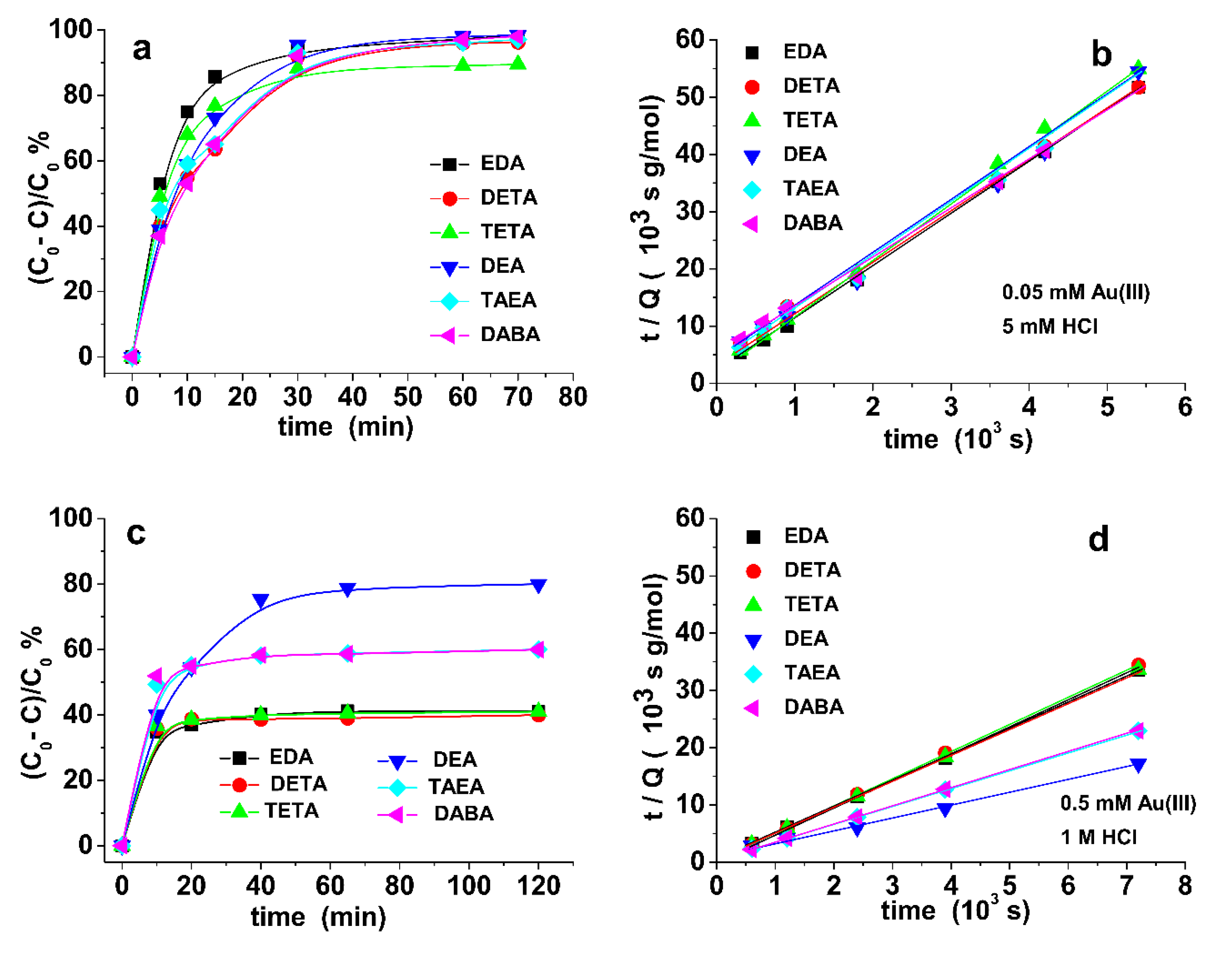
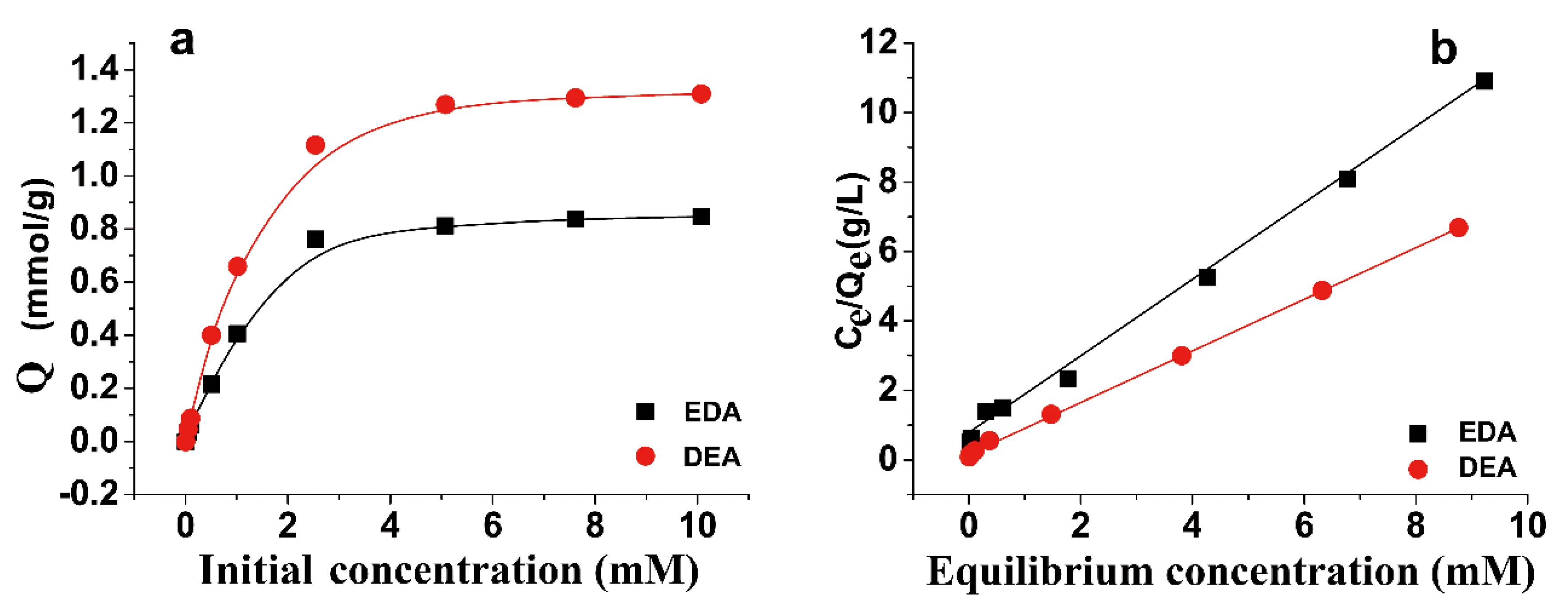
3.2. Sorption Kinetics and Sorption Isotherm of Au(III)
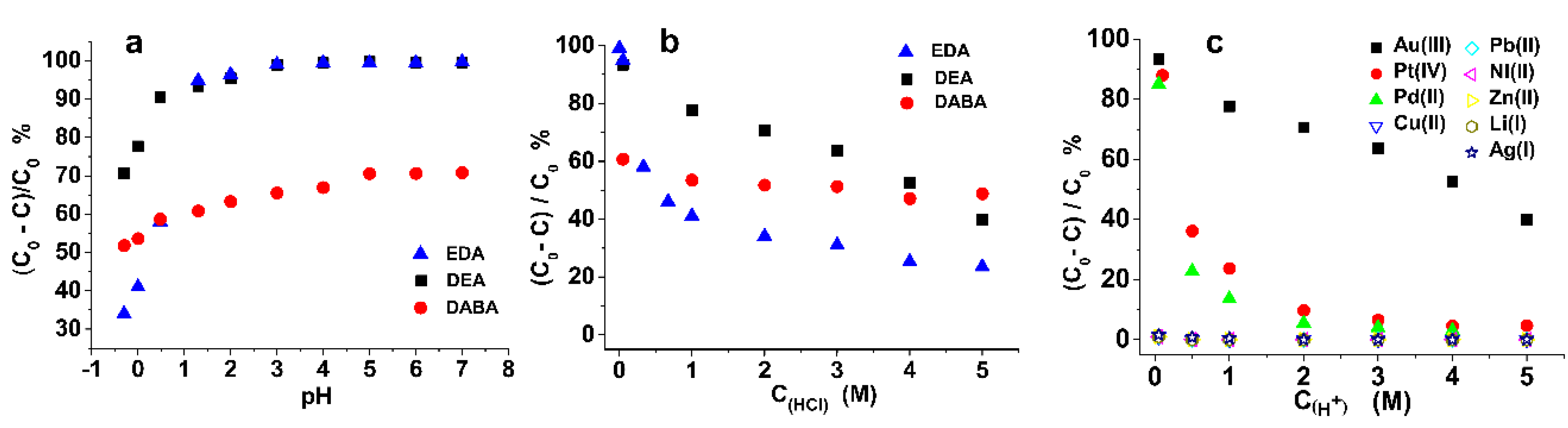
3.3. Effect of HCl Concentration on the Adsorption Test
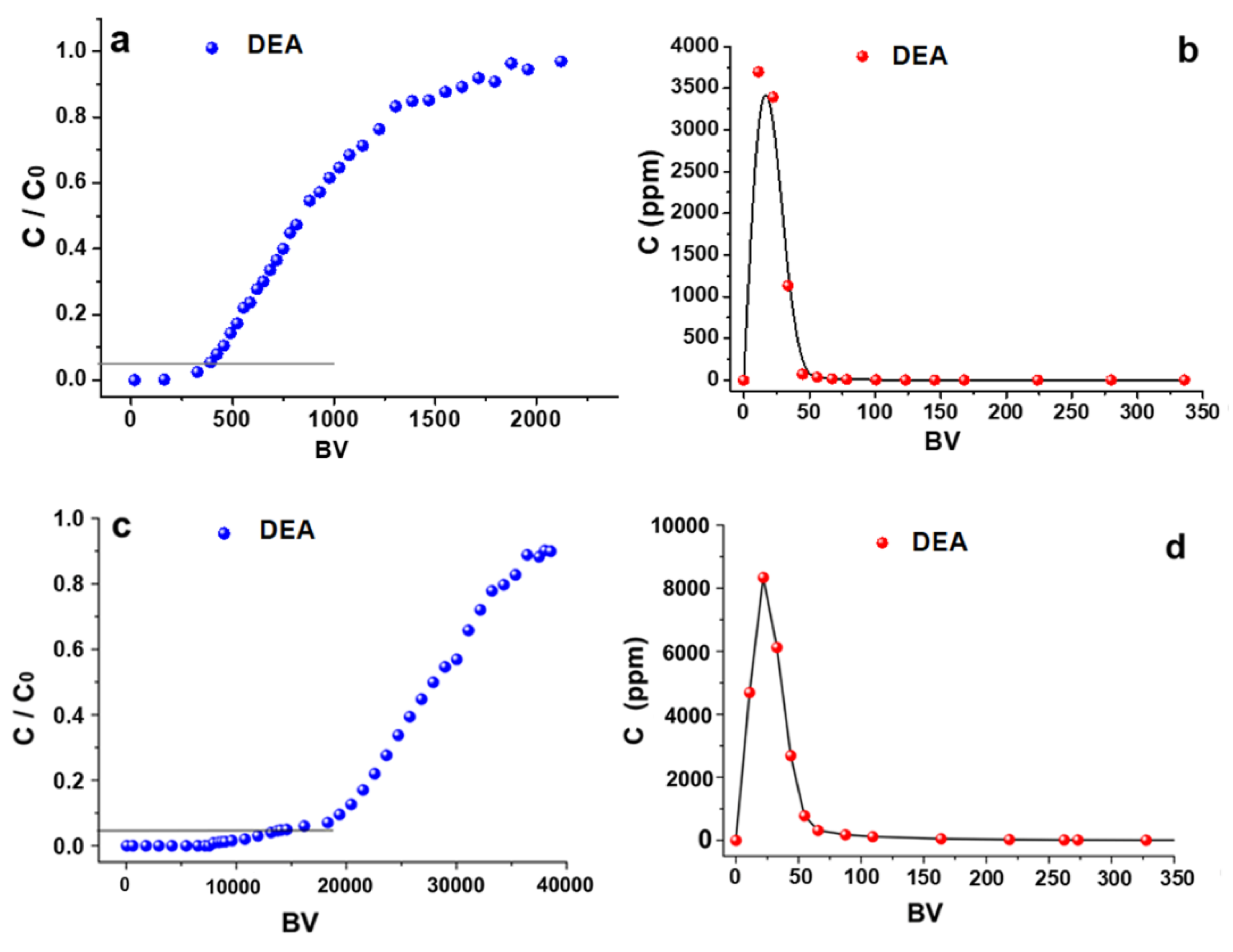
3.4. Column Mode Adsorption and Separation of Au(III)
3.5. Reduction of Au(III)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yen, C.H.; Lien, H.L.; Chung, J.S.; Yeh, H.D. Adsorption of precious metals in water by dendrimer modified magnetic nanoparticles. J. Hazard. Mater. 2017, 322, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bhandare, A.; Argekar, A. Separation and recovery of platinum and rhodium by supported liquid membranes using bis (2-ethylhexyl) phosphoric acid (HDEHP) as a mobile carrier. J. Membr. Sci. 2002, 201, 233–237. [Google Scholar] [CrossRef]
- Tauetsile, P.; Oraby, E.; Eksteen, J. Activated carbon adsorption of gold from cyanide-starved glycine solutions containing copper. Part 1: Isotherms. Sep. Purif. Technol. 2019, 211, 594–601. [Google Scholar] [CrossRef]
- Tauetsile, P.J.; Oraby, E.A.; Eksteen, J.J. Adsorption behaviour of copper and gold Glycinates in alkaline media onto activated carbon. Part 2: Kinetics. Hydrometallurgy 2018, 178, 195–201. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, Y.; Li, Q. An eco-friendly and efficient process of low potential thiosulfate leaching-resin adsorption recovery for extracting gold from a roasted gold concentrate. J. Clean. Prod. 2019, 229, 387–398. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, S. Adsorption behavior of Au (III) and Pd (II) on persimmon tannin functionalized viscose fiber and the mechanism. Int. J. Biol. Macromol. 2020, 152, 1242–1251. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Dong, Z.; Yang, Y.; Li, Q.; Liu, X.; Jiang, T. Eco-friendly and economical gold extraction by nickel catalyzed ammoniacal thiosulfate leaching-resin adsorption recovery. J. Clean. Prod. 2019, 233, 1475–1485. [Google Scholar] [CrossRef]
- Chang, Z.; Li, F.; Qi, X.; Jiang, B.; Kou, J.; Sun, C. Selective and efficient adsorption of Au (III) in aqueous solution by Zr-based metal-organic frameworks (MOFs): An unconventional way for gold recycling. J. Hazard. Mater. 2020, 391, 122175. [Google Scholar] [CrossRef]
- Morcali, M.H.; Zeytuncu, B.; Akman, S.; Yucel, O. Sorption of Gold from Electronic Waste Solutions by A Commercial Sorbent. Chem. Eng. Commun. 2014, 201, 1041–1053. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Adhikari, C.R.; Parajuli, D.; Kawakita, H.; Inoue, K.; Ohto, K.; Harada, H. Dimethylamine-Modified Waste Paper for the Recovery of Precious Metals. Environ. Sci. Technol. 2008, 42, 5486–5491. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xu, W.; Mo, X.; Li, H.; Zhou, S.; Zhang, P.; Tang, K. Efficient adsorption toward precious metal from aqueous solution by zeolitic imidazolate framework-8. Adsorption 2018, 24, 733–744. [Google Scholar] [CrossRef]
- Ciesielczyk, F.; Bartczak, P.; Jesionowski, T. Removal of cadmium (II) and lead (II) ions from model aqueous solutions using sol–gel-derived inorganic oxide adsorbent. Adsorption 2016, 22, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lam, K.F.; Mak, S.F.; Yeung, K.L. Precious metal recovery by selective adsorption using biosorbents. J. Hazard. Mater. 2011, 186, 902–910. [Google Scholar] [CrossRef]
- Linnikov, O.D.; Krasil’nikov, V.N.; Gyrdasova, O.I.; Rodina, I.V.; Baklanova, I.V.; Tyutyunnik, A.P.; Dyachkova, T.V.; Suntsov, A.Y.; Sauerzopf, F.; Marchenkov, V.V. Precursor synthesis of maghemite and its adsorption properties with respect to bivalent copper ions. Adsorption 2018, 24, 629–636. [Google Scholar] [CrossRef]
- Roslan, N.A.; Suah, F.B.M.; Mohamed, N. The use of an electrogenerative process as a greener method for recovery of gold (III) from the E-waste. Sep. Purif. Technol. 2017, 182, 1–8. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Yan, W.; Chen, L.; Mahadevan, G.; Zhao, F. Enhanced bioleaching efficiency of metals from E-wastes driven by biochar. J. Hazard. Mater. 2016, 320, 393–400. [Google Scholar] [CrossRef]
- Cui, J.L.; Luo, C.L.; Tang, C.W.Y.; Chan, T.S.; Li, X.D. Speciation and leaching of trace metal contaminants from e-waste contaminated soils. J. Hazard. Mater. 2017, 329, 150–158. [Google Scholar] [CrossRef]
- Seyedhakimi, A.; Bastami, S.; Ghassa, S.; Razavi, H.; Chehreh Chelgani, S.C. Exploring relationships between various activations of granular activated carbon on silver and gold adsorption: A kinetic and equilibrium study. Sep. Sci. Technol. 2019, 54, 1710–1721. [Google Scholar] [CrossRef]
- Chen, Y.; Zi, F.; Hu, X.; Yang, P.; Ma, Y.; Cheng, H.; Wang, Q.; Qin, X.; Liu, Y.; Chen, S.J.S.; et al. The use of new modified activated carbon in thiosulfate solution: A green gold recovery technology. Sep. Purif. Technol. 2020, 230, 115834. [Google Scholar] [CrossRef]
- Xue, X.-Y.; Li, H.-F.; Wang, Y.; An, F.-Q.; Hu, T.-P.; Gao, J.-F. Effective recovery of AuCl4-using thiosemicarbazide and thiocarbohydrazide functionalized D301 resin. Desalin. Water Treat. 2019, 150, 301–309. [Google Scholar] [CrossRef]
- Fan, R.; Min, H.; Hong, X.; Yi, Q.; Liu, W.; Zhang, Q.; Luo, Z. Plant tannin immobilized Fe3O4@SiO2 microspheres: A novel and green magnetic bio-sorbent with superior adsorption capacities for gold and palladium. J. Hazard. Mater. 2019, 364, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Morita, K.; Hoshina, H.; Seko, N.J.M.S. Synthesis of amine-type adsorbents with emulsion graft polymerization of 4-hydroxybutyl acrylate glycidylether. Mater. Sci. Appl. 2011, 2, 776. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ao, J.; Yang, X.; Ling, C.; Zhang, B.; Wang, Z.; Yu, M.; Shen, R.; Ma, H.; Li, J. Green and efficient synthesis of an adsorbent fiber by preirradiation-induced grafting of PDMAEMA and its Au (III) adsorption and reduction performance. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Rizki, I.N.; Tanaka, Y.; Okibe, N. Thiourea bioleaching for gold recycling from e-waste. Waste Manag. 2019, 84, 158–165. [Google Scholar] [CrossRef]
- Ma, H.; Chi, H.; Wu, J.; Wang, M.; Li, J.; Hoshina, H.; Saiki, S.; Seko, N. A Novel Avenue to Gold Nanostructured Microtubes Using Functionalized Fiber as the Ligand, the Reductant, and the Template. ACS Appl. Mater. Interfaces 2013, 5, 8761–8765. [Google Scholar] [CrossRef]
- Ma, H.; Yao, S.; Li, J.; Cao, C.; Wang, M. A mild method of amine-type adsorbents syntheses with emulsion graft polymerization of glycidyl methacrylate on polyethylene non-woven fabric by pre-irradiation. Radiat. Phys. Chem. 2012, 81, 1393–1397. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Yang, C.; Geng, Q.; Fan, H.-L.; Wang, B.-J.; Wu, M.-M.; Tian, Z. Adsorption of hydrogen sulfide by amine-functionalized metal organic framework (MOF-199): An experimental and simulation study. Appl. Surf. Sci. 2019, 497, 143815. [Google Scholar] [CrossRef]
- Li, R.; Ma, H.; Xing, Z.; Wu, G. Synergistic effects of different co-monomers on the uranium adsorption performance of amidoximated polyethylene nonwoven fabric in natural seawater. J. Radioanal. Nucl. Chem. 2018, 315, 111–117. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Wang, W.; Yu, G.; Deng, S. Adsorptive recovery of Au(III) from aqueous solution using crosslinked polyethyleneimine resins. Chemosphere 2020, 241, 125122. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Han, J.; Xu, X.; Qi, S.; Ma, L.; Wang, Z.; Zhang, L.; Li, Q.; Xu, L.; Ma, H. Enhanced Performance in Uranium Extraction by Quaternary Ammonium-Functionalized Amidoxime-Based Fibers. Ind. Eng. Chem. Res. 2020, 59, 5828–5837. [Google Scholar] [CrossRef]
- Liu, F.; Peng, G.; Li, T.; Yu, G.; Deng, S. Au (III) adsorption and reduction to gold particles on cost-effective tannin acid immobilized dialdehyde corn starch. Chem. Eng. J. 2019, 370, 228–236. [Google Scholar] [CrossRef]
- Liu, D.; Deng, S.; Vakili, M.; Du, R.; Tao, L.; Sun, J.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Fast and high adsorption of Ni (II) on vermiculite-based nanoscale hydrated zirconium oxides. Chem. Eng. J. 2019, 360, 1150–1157. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ramesh, A.; Maki, T.; Hasegawa, H.; Ueda, K. Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. J. Hazard. Mater. 2007, 146, 39–50. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Zhang, Q.; Li, C.; Bao, C.; Liu, X.; Xiao, P. Adsorption of Au (III), Pd (II), and Pt (IV) from Aqueous Solution onto Graphene Oxide. J. Chem. Eng. Data 2013, 58, 209–216. [Google Scholar] [CrossRef]
- Elshehy, E.A.; El-Safty, S.A.; Shenashen, M.A.; Khairy, M. Design and evaluation of optical mesocaptor for the detection/recovery of Au (III) from an urban mine. Sens. Actuators B Chem. 2014, 203, 363–374. [Google Scholar] [CrossRef]
- Regalbuto, J.R.; Navada, A.; Shadid, S.; Bricker, M.L.; Chen, Q. An Experimental Verification of the Physical Nature of Pt Adsorption onto Alumina. J. Catal. 1999, 184, 335–348. [Google Scholar] [CrossRef]
- Morisada, S.; Kim, Y.-H.; Yakuwa, S.; Ogata, T.; Nakano, Y. Improved adsorption and separation of palladium (II) and platinum (IV) in strong hydrochloric acid solutions using thiocyanate-retaining tannin gel. J. Appl. Polym. Sci. 2012, 126, E478–E483. [Google Scholar] [CrossRef]
- Wei, W.; Reddy, D.H.K.; Bediako, J.K.; Yun, Y.-S. Aliquat-336-impregnated alginate capsule as a green sorbent for selective recovery of gold from metal mixtures. Chem. Eng. J. 2016, 289, 413–422. [Google Scholar] [CrossRef]
- Ma, H.; Hoshina, H.; Seko, N. Crosslinking induced by irradiation raises selectivity of polymeric adsorbent. J. Appl. Polym. Sci. 2013, 128, 4253–4260. [Google Scholar] [CrossRef]
- Feng, C.; Gu, L.; Yang, D.; Hu, J.; Lu, G.; Huang, X. Size-controllable gold nanoparticles stabilized by PDEAEMA-based double hydrophilic graft copolymer. Polymer 2009, 50, 3990–3996. [Google Scholar] [CrossRef]
- Parajuli, D.; Hirota, K.; Inoue, K. Trimethylamine-Modified Lignophenol for the Recovery of Precious Metals. Ind. Eng. Chem. Res. 2009, 48, 10163–10168. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhan, J.; Chai, Y.; Yang, C.; Xu, L.; Zhuang, W.; Jing, B. Synthesis of Gold Nano- and Microplates in Hexagonal Liquid Crystals. J. Phys. Chem. B 2005, 109, 3189–3194. [Google Scholar] [CrossRef]
- Vigderman, L.; Khanal, B.P.; Zubarev, E.R. Functional Gold Nanorods: Synthesis, Self-Assembly, and Sensing Applications. Adv. Mater. 2012, 24, 4811–4841. [Google Scholar] [CrossRef]
- Devan, R.S.; Patil, R.A.; Lin, J.H.; Ma, Y.R. One-dimensional metal-oxide nanostructures: Recent developments in synthesis, characterization, and applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Hu, D.; Huang, Y.; Liu, H.; Wang, H.; Wang, S.; Shen, M.; Zhu, M.; Shi, X. The assembly of dendrimer-stabilized gold nanoparticles onto electrospun polymer nanofibers for catalytic applications. J. Mater. Chem. A 2014, 2, 2323–2332. [Google Scholar] [CrossRef]
- Milligan, W.; Morriss, R.H. Morphology of Colloidal Gold—A Comparative Study. J. Am. Chem. Soc. 1964, 86, 3461–3467. [Google Scholar] [CrossRef]




| Organic Amine | Structure | Type of Ammonium | Functional Group Density (mmol/g) |
|---|---|---|---|
| EDA | 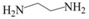 | Secondary | 2.9 |
| DETA | 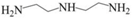 | Secondary, Tertiary | 1.7 |
| TETA |  | Secondary, Tertiary | 1.6 |
| DEA | 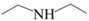 | Tertiary | 2.2 |
| TAEA | 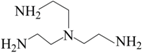 | Secondary, Tertiary, Quaternary | 1.5 |
| DABA |  | Secondary, Tertiary | 0.2 |
| Solution | Adsorbent | ||||||
|---|---|---|---|---|---|---|---|
| Au(III) (mM) | HCl (M) | EDA | DETA | TETA | DEA | TAEA | DABA |
| k2 (kg mol−1 s−1) | |||||||
| 0.05 | 5 × 10−3 | 0.03055 | 0.02454 | 0.02845 | 0.02419 | 0.01387 | 0.01313 |
| 0.5 | 1 | 0.05903 | 0.05885 | 0.05321 | 0.00357 | 0.02324 | 0.02615 |
| Sample | Langmuir | ||
|---|---|---|---|
| Qmax (mmol/g) | KL (L/mmol) | R2 | |
| DEA | 1.335 | 4.179 | 0.999 |
| EDA | 0.909 | 1.663 | 0.998 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Xu, X.; Ao, J.; Ma, L.; Ye, F.; Wang, Z.; Xu, L.; Zhao, X.; Ma, H. Selective Adsorption, Reduction, and Separation of Au(III) from Aqueous Solution with Amine-Type Non-Woven Fabric Adsorbents. Materials 2020, 13, 2958. https://doi.org/10.3390/ma13132958
Huang C, Xu X, Ao J, Ma L, Ye F, Wang Z, Xu L, Zhao X, Ma H. Selective Adsorption, Reduction, and Separation of Au(III) from Aqueous Solution with Amine-Type Non-Woven Fabric Adsorbents. Materials. 2020; 13(13):2958. https://doi.org/10.3390/ma13132958
Chicago/Turabian StyleHuang, Chen, Xiao Xu, Junxuan Ao, Lin Ma, Feng Ye, Ziqiang Wang, Lu Xu, Xiaoyan Zhao, and Hongjuan Ma. 2020. "Selective Adsorption, Reduction, and Separation of Au(III) from Aqueous Solution with Amine-Type Non-Woven Fabric Adsorbents" Materials 13, no. 13: 2958. https://doi.org/10.3390/ma13132958




