Microflow Nanoprecipitation of Positively Charged Gastroresistant Polymer Nanoparticles of Eudragit® RS100: A Study of Fluid Dynamics and Chemical Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Viscosity Measurements
2.3. Synthesis of Eudragit® RS100 Nanoparticles by Conventional Batch Method
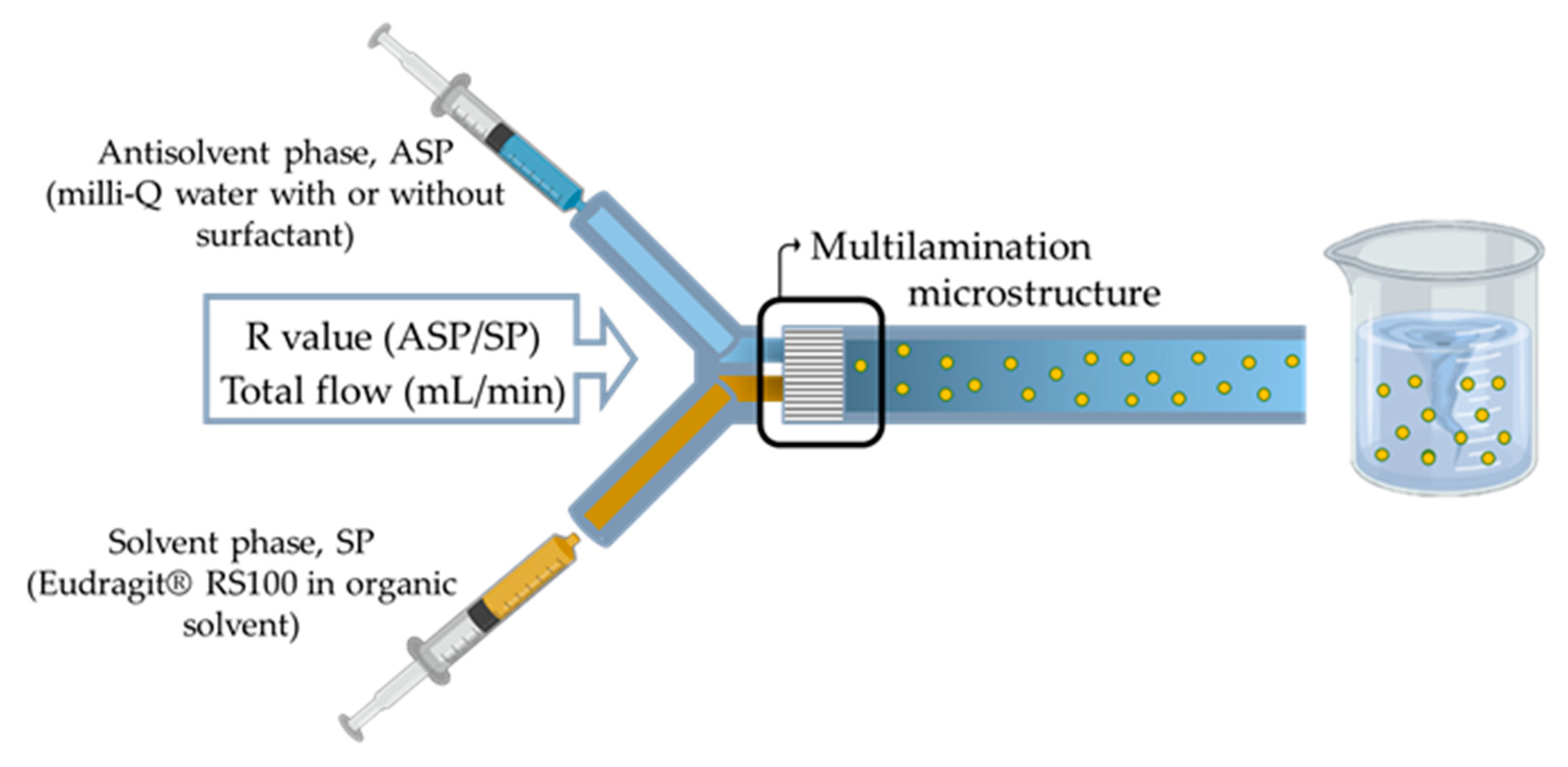
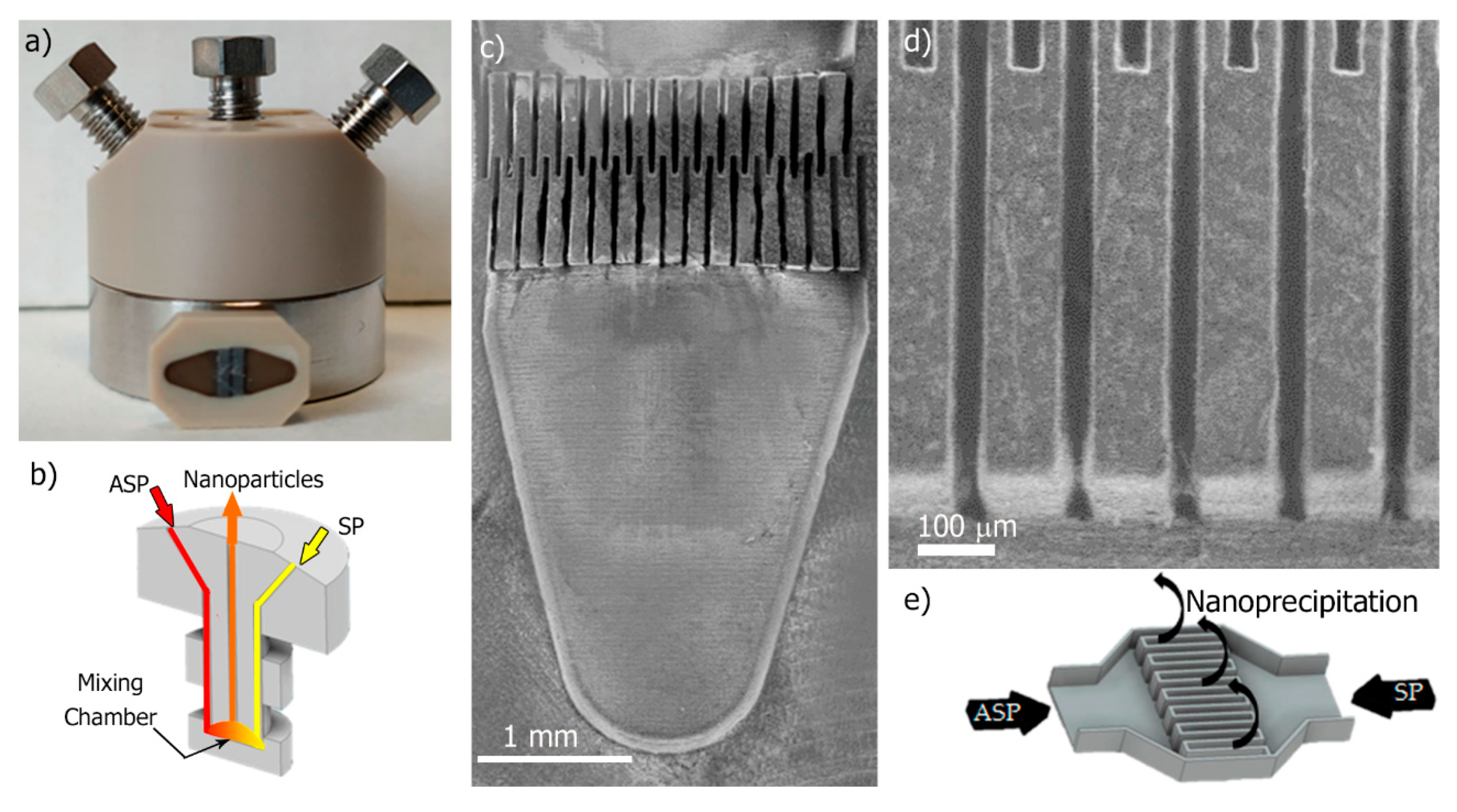
2.4. Synthesis of Eudragit® RS100 Nanoparticles by Micromixer-Assisted Nanoprecipitation
2.5. Rifampicin Encapsulation in Eudragit® RS100 Nanoparticles
2.6. Nanoparticle Characterization
2.6.1. Determination of the Entrapped Rifampicin in Eudragit® RS100 Nanoparticles
2.6.2. In vitro Release Study of Rifampicin from Nanoparticles
2.6.3. Size Distribution and Zeta-Potential Measurements
2.6.4. Transmission Electron Microscopy (TEM) Characterization
2.6.5. Scanning Electron Microscopy Characterization
2.6.6. Statistical Analysis
3. Results and Discussion
3.1. Selection of Class 3 Solvents
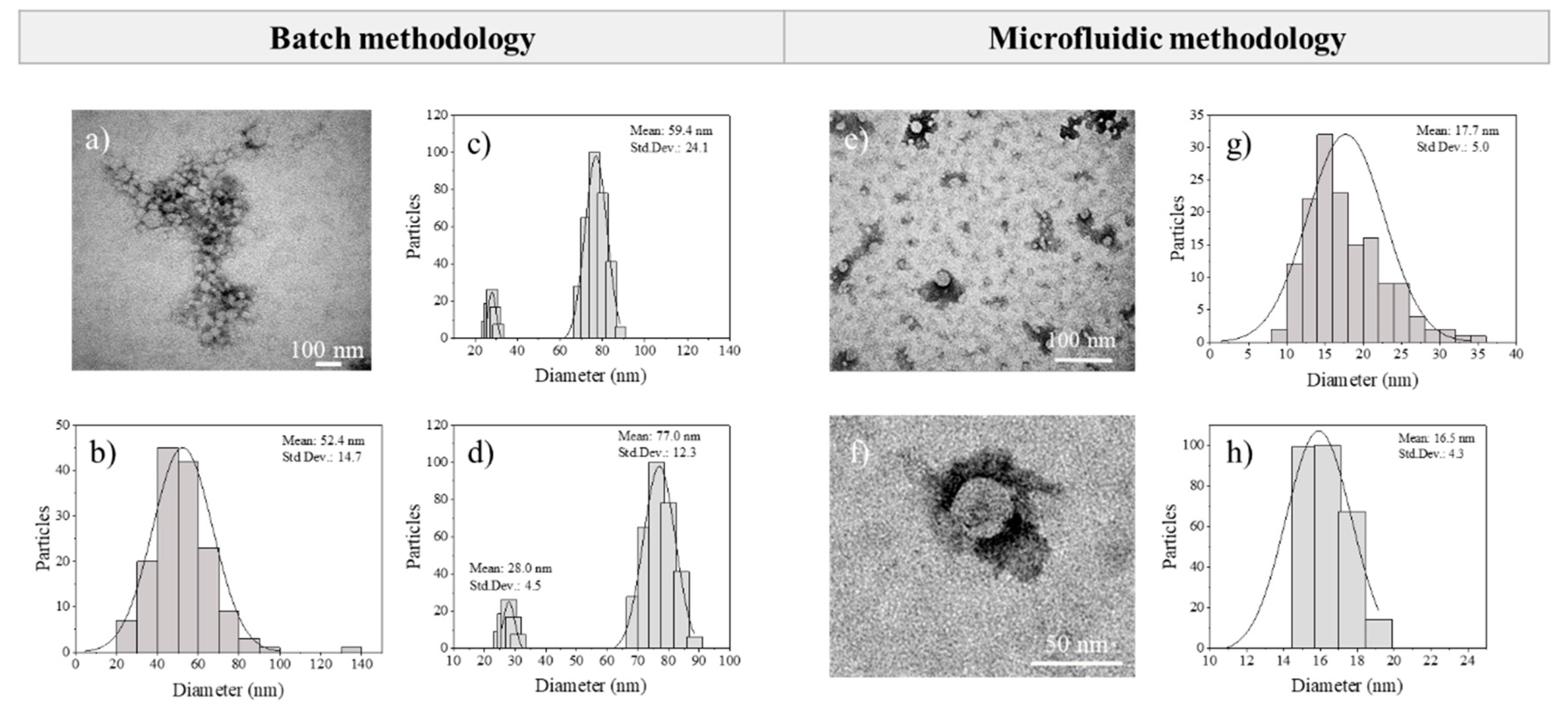
3.2. Analysis of the Results Obtained by the Conventional Batch Method
3.3. Eudragit® RS100 NP Synthesis Based on the Micromixer-Assisted Nanoprecipitation: Study of Parameter Effects
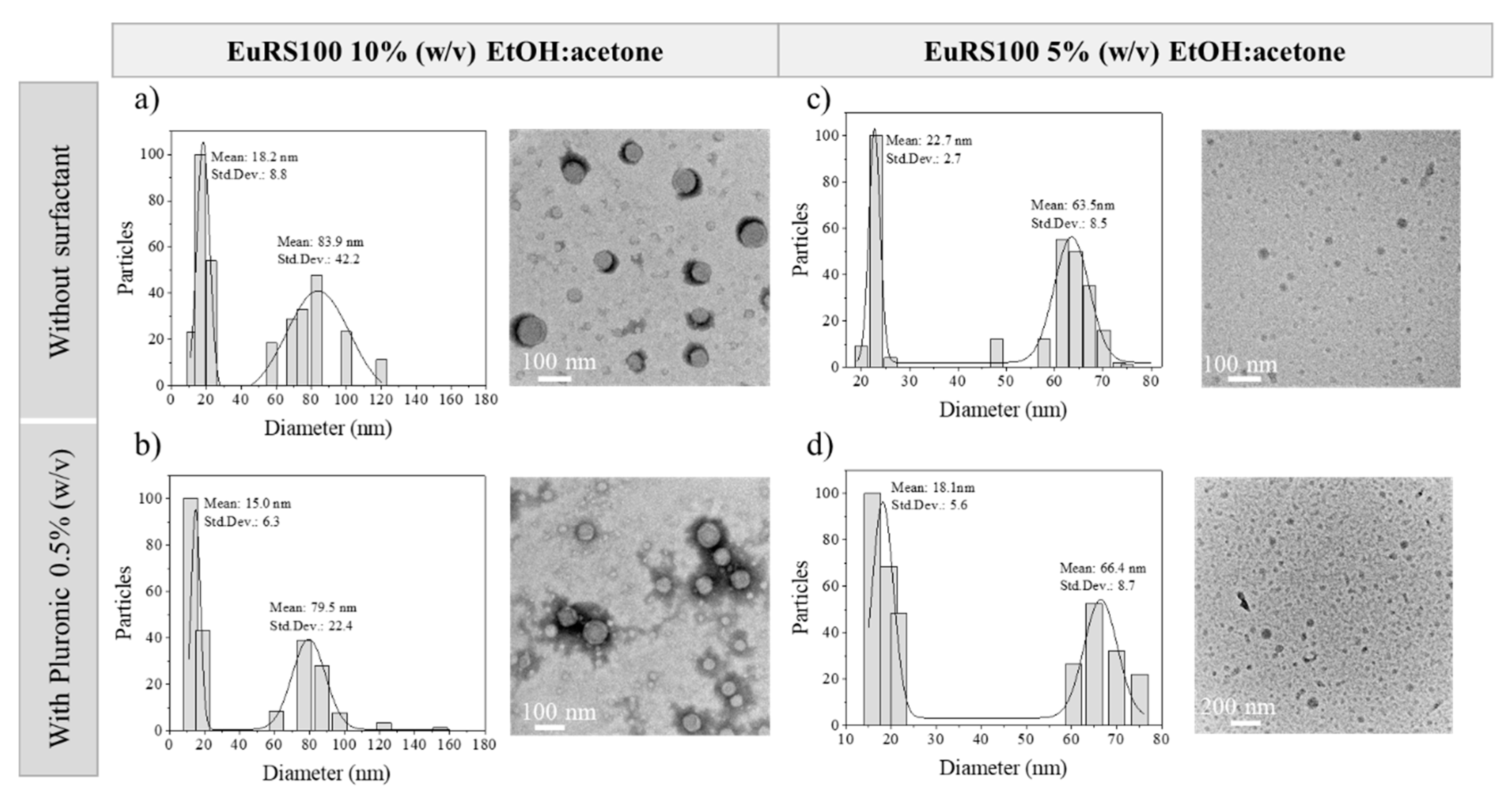
3.3.1. Influence of Polymer Concentration
3.3.2. Influence of the Type of Solvent
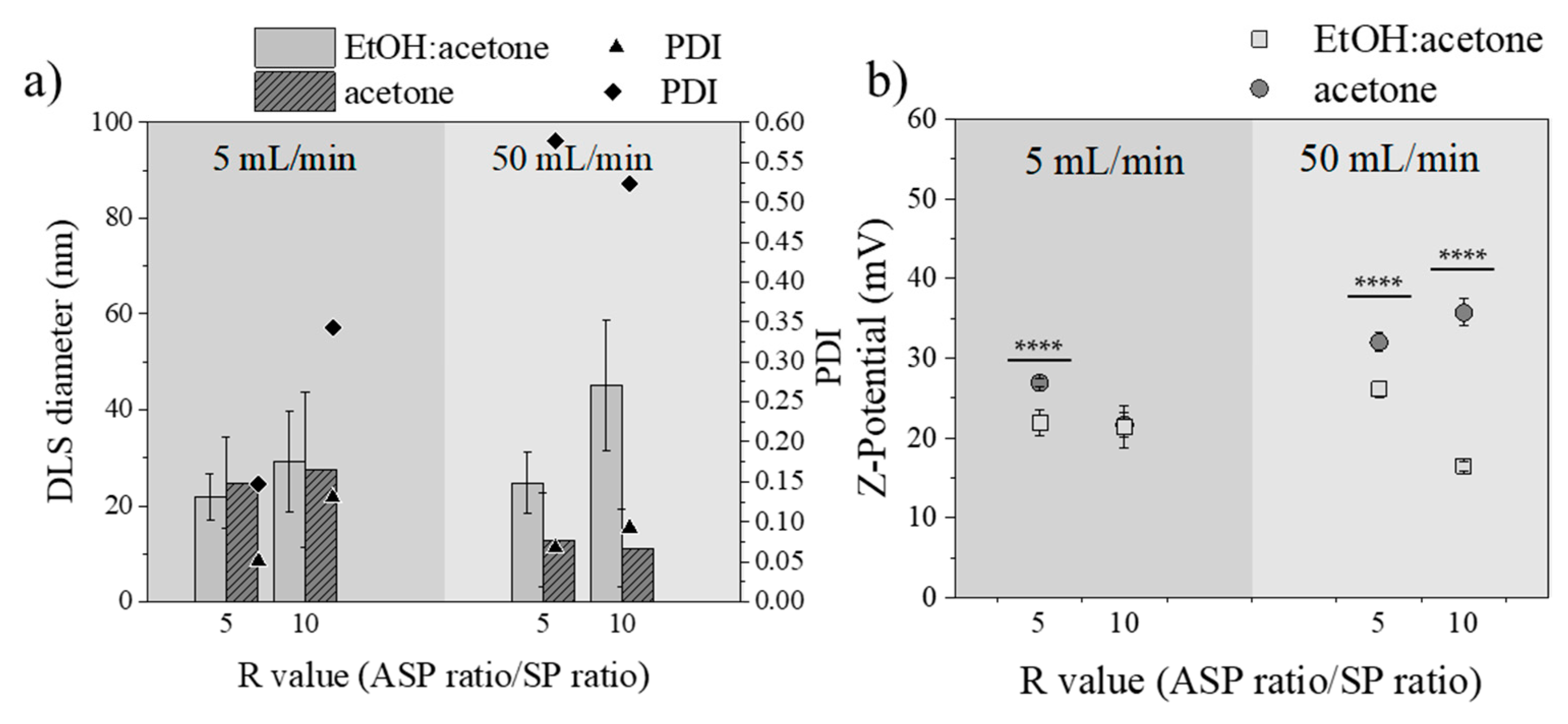
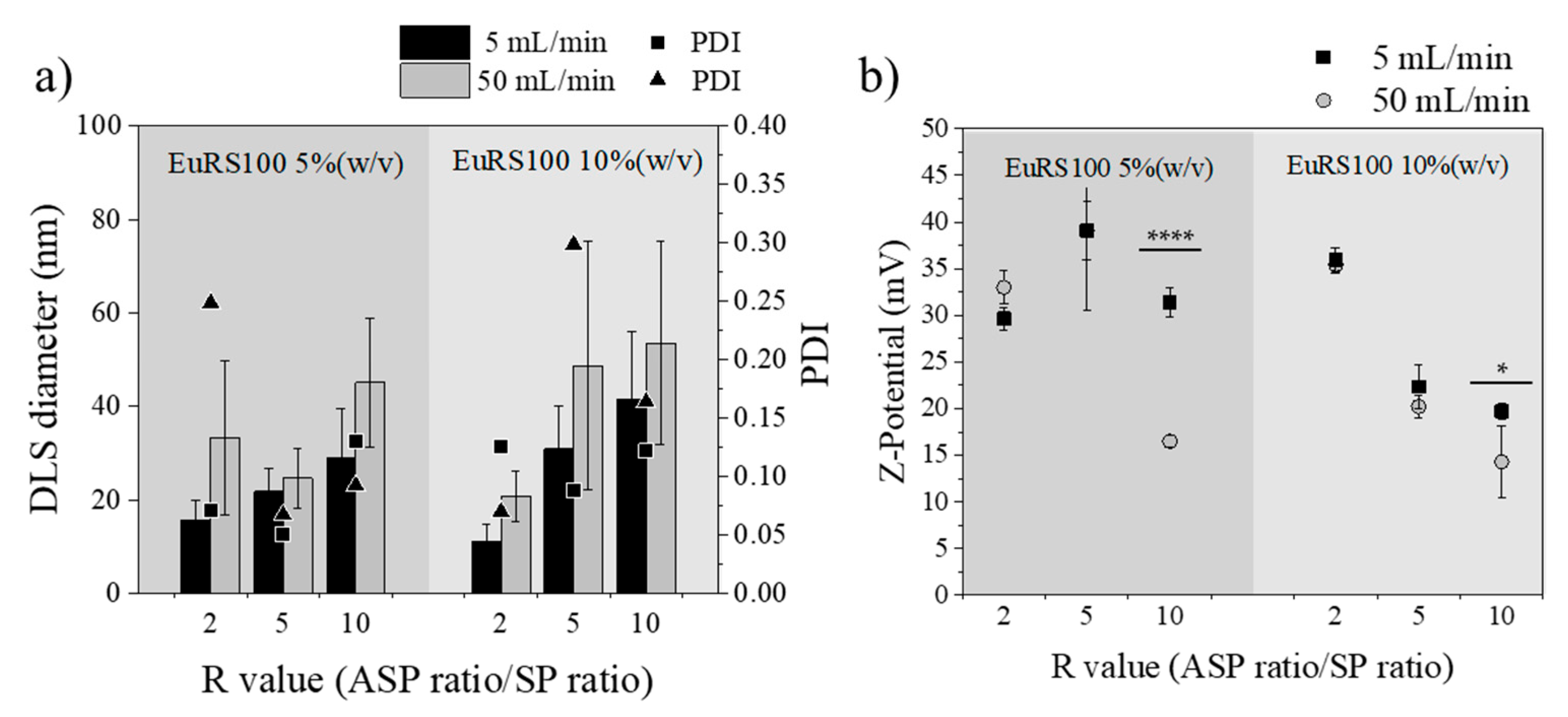
3.3.3. Influence of Total Flow Rate
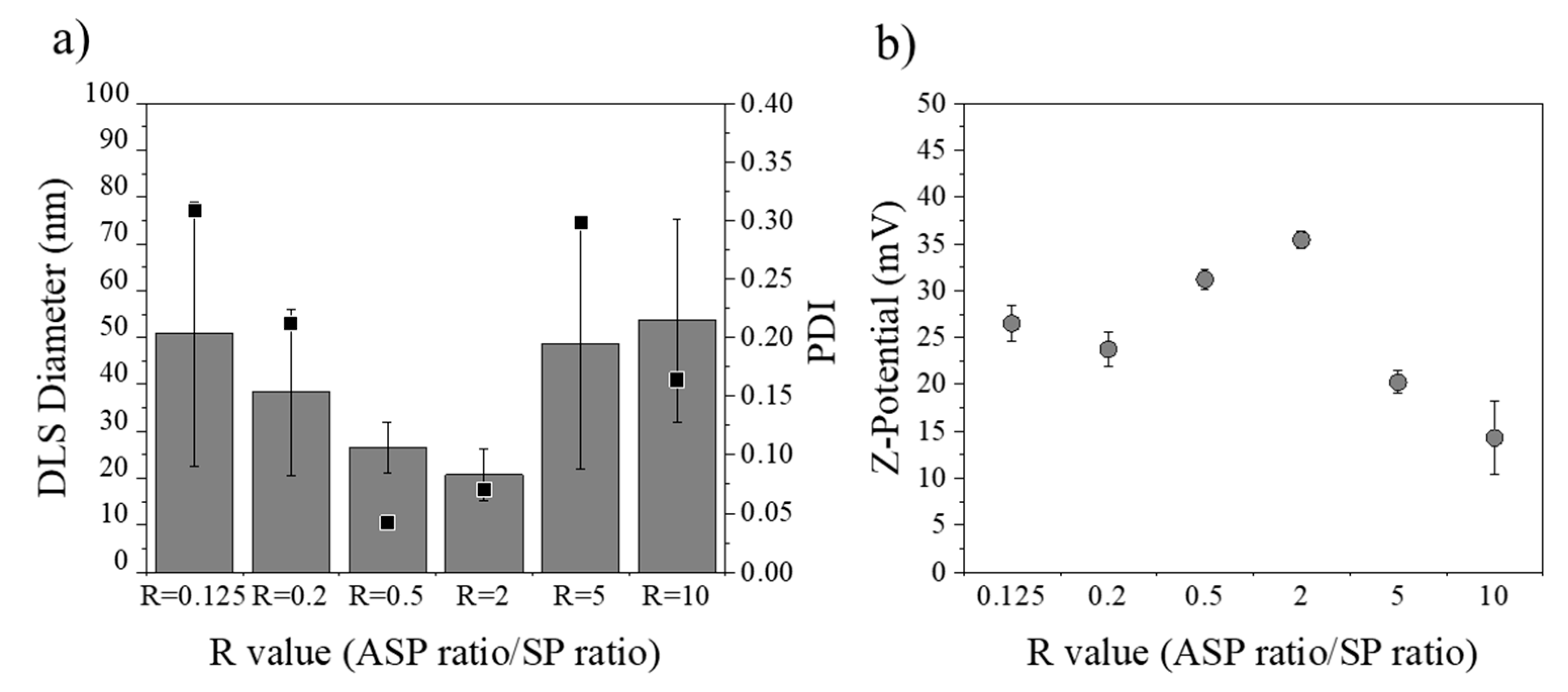
3.3.4. Influence of R Ratio Value
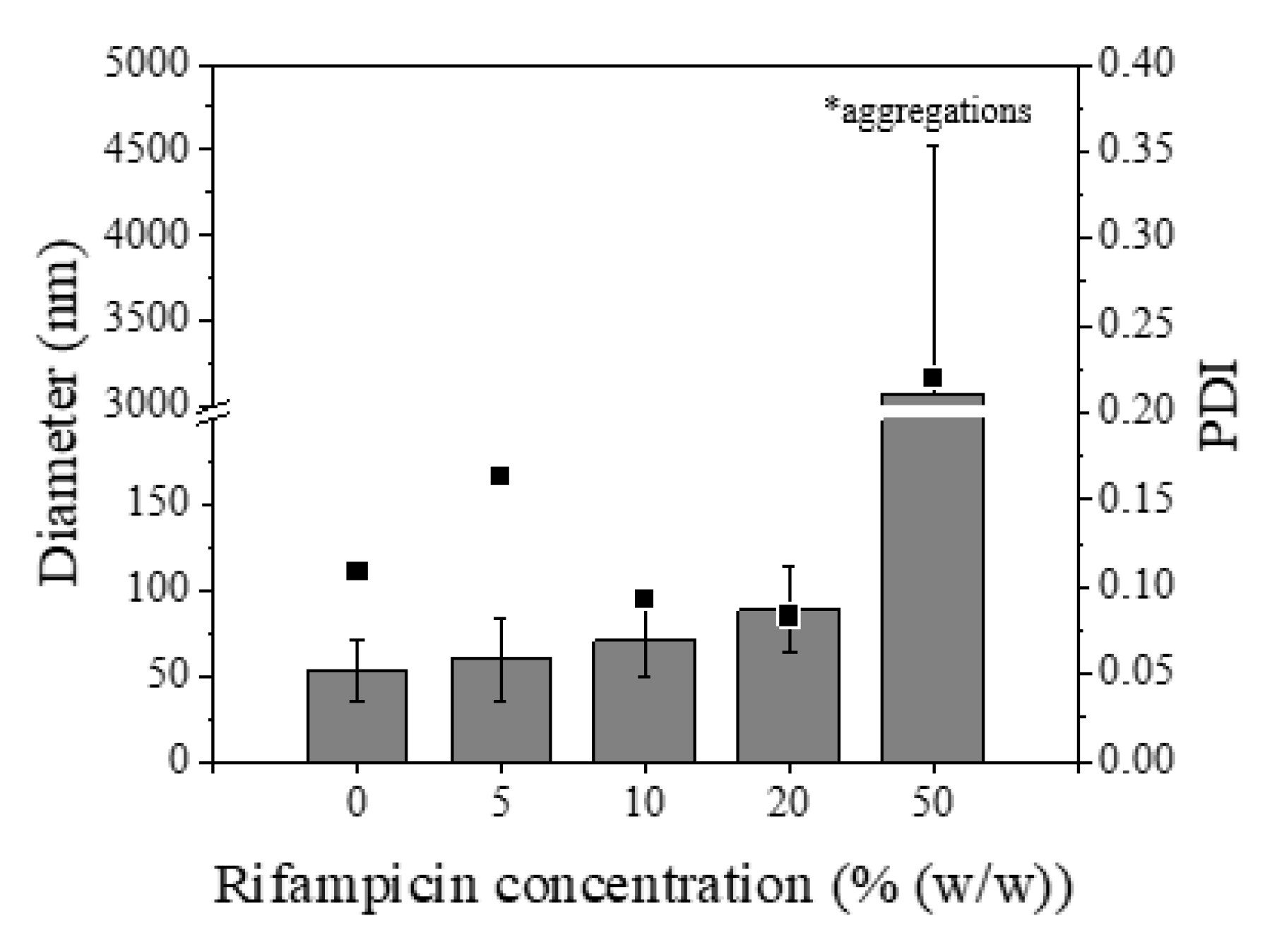
3.4. Study of Rifampicin Loading and Entrapment Efficiency by Batch and Micromixing-Assisted Approaches
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anton, N.; Benoit, J.P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates-A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Calderó, G.; García-Celma, M.J.; Solans, C. Formation of polymeric nano-emulsions by a low-energy method and their use for nanoparticle preparation. J. Colloid Interface Sci. 2011, 353, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Albisa, A.; Espanol, L.; Prieto, M.; Sebastian, V. Polymeric Nanomaterials as Nanomembrane Entities for Biomolecule and Drug Delivery. Curr. Pharm. Des. 2017, 23, 263–280. [Google Scholar] [CrossRef]
- Kuchler-Bopp, S.; Larrea, A.; Petry, L.; Idoux-Gillet, Y.; Sebastian, V.; Ferrandon, A.; Schwinté, P.; Arruebo, M.; Benkirane-Jessel, N. Promoting bioengineered tooth innervation using nanostructured and hybrid scaffolds. Acta Biomater. 2017, 50, 493–501. [Google Scholar] [CrossRef]
- Luque-Michel, E.; Larrea, A.; Lahuerta, C.; Sebastian, V.; Imbuluzqueta, E.; Arruebo, M.; Blanco-Prieto, M.J.; Santamaría, J. A simple approach to obtain hybrid Au-loaded polymeric nanoparticles with a tunable metal load. Nanoscale 2016, 8, 6495–6506. [Google Scholar] [CrossRef] [PubMed]
- Luque-Michel, E.; Sebastian, V.; Larrea, A.; Marquina, C.; Blanco-Prieto, M.J. Co-encapsulation of superparamagnetic nanoparticles and doxorubicin in PLGA nanocarriers: Development, characterization and in vitro antitumor efficacy in glioma cells. Eur. J. Pharm. Biopharm. 2019, 145, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Harguindey, A.; Domaille, D.W.; Fairbanks, B.D.; Wagner, J.; Bowman, C.N.; Cha, J.N. Synthesis and Assembly of Click-Nucleic-Acid-Containing PEG–PLGA Nanoparticles for DNA Delivery. Adv. Mater. 2017, 29, 10–15. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Barbé, C.; Bartlett, J.; Kong, L.; Finnie, K.; Lin, H.Q.; Larkin, M.; Calleja, S.; Bush, A.; Calleja, G. Silica particles: A novel drug-delivery system. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; What is the appropriate target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Quan, J.S.; Jiang, H.L.; Kim, E.M.; Jeong, H.J.; Choi, Y.J.; Guo, D.D.; Yoo, M.K.; Lee, H.G.; Cho, C.S. pH-sensitive and mucoadhesive thiolated Eudragit-coated chitosan microspheres. Int. J. Pharm. 2008, 359, 205–210. [Google Scholar] [CrossRef]
- Chaves, P.D.S.; Frank, L.A.; Frank, A.G.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Mucoadhesive Properties of Eudragit®RS100, Eudragit®S100, and Poly(ε-caprolactone) Nanocapsules: Influence of the Vehicle and the Mucosal Surface. AAPS PharmSciTech 2018, 19, 1637–1646. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
- Animesh, K.; Afrasim, M.; Bommareddy, R.R.; Ayaz, A.; Shruthi, R.; Shivakumar, H.G. Applicability and approaches of (Meth) acrylate copolymers (Eudragits) in novel drug delivery systems. Curr. Drug Ther. 2012, 7, 219–234. [Google Scholar] [CrossRef]
- Dillen, K.; Vandervoort, J.; Van den Mooter, G.; Ludwig, A. Evaluation of ciprofloxacin-loaded Eudragit® RS100 or RL100/PLGA nanoparticles. Int. J. Pharm. 2006, 314, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, C.; Priya, R.; Swain, S.; Kumar, G.; Charan, K.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Future J. Pharm. Sci. 2017, 3. [Google Scholar] [CrossRef]
- Trapani, A.; Laquintana, V.; Denora, N.; Lopedota, A.; Cutrignelli, A.; Franco, M.; Trapani, G.; Liso, G. Eudragit RS 100 microparticles containing 2-hydroxypropyl-β-cyclodextrin and glutathione: Physicochemical characterization, drug release and transport studies. Eur. J. Pharm. Sci. 2007, 30, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Dos Chaves, P.S.; Ourique, A.F.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Carvedilol-loaded nanocapsules: Mucoadhesive properties and permeability across the sublingual mucosa. Eur. J. Pharm. Biopharm. 2017, 114, 88–95. [Google Scholar] [CrossRef]
- Gracia, R.; Yus, C.; Abian, O.; Mendoza, G.; Irusta, S.; Sebastian, V.; Andreu, V.; Arruebo, M. Enzyme structure and function protection from gastrointestinal degradation using enteric coatings. Int. J. Biol. Macromol. 2018, 119, 413–422. [Google Scholar] [CrossRef]
- Contri, R.V.; Fiel, L.A.; Alnasif, N.; Pohlmann, A.R.; Guterres, S.S.; Schäfer-Korting, M. Skin penetration and dermal tolerability of acrylic nanocapsules: Influence of the surface charge and a chitosan gel used as vehicle. Int. J. Pharm. 2016, 507, 12–20. [Google Scholar] [CrossRef]
- Pignatello, R.; Bucolo, C.; Ferrara, P.; Maltese, A.; Puleo, A.; Puglisi, G. Eudragit RS100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur. J. Pharm. Sci. 2002, 16, 53–61. [Google Scholar] [CrossRef]
- Frank, L.A.; Sandri, G.; D’Autilia, F.; Contri, R.V.; Bonferoni, M.C.; Caramella, C.; Frank, A.G.; Pohlmann, A.R.; Guterres, S.S. Chitosan gel containing polymeric nanocapsules: A new formulation for vaginal drug delivery. Int. J. Nanomed. 2014, 9, 3151–3161. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Barar, J.; Valizadeh, H.; Shadrou, S.; Nokhodchi, A. Formulation, characterization and in vitro evaluation of theophylline-loaded Eudragit RS 100 microspheres prepared by an emulsion-solvent diffusion/evaporation technique. Pharm. Dev. Technol. 2011, 16, 637–644. [Google Scholar] [CrossRef]
- Yus, C.; Gracia, R.; Larrea, A.; Andreu, V.; Irusta, S.; Sebastian, V.; Mendoza, G.; Arruebo, M. Targeted release of probiotics from enteric microparticulated formulations. Polymers 2019, 11, 1668. [Google Scholar] [CrossRef]
- Zweers, M.L.T.; Grijpma, D.W.; Engbers, G.H.M.; Feijen, J. The Preparation of Monodisperse Biodegradable Polyester Nanoparticles with a Controlled Size. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 66, 559–566. [Google Scholar] [CrossRef]
- Perevyazko, I.Y.; Vollrath, A.; Pietsch, C.; Schubert, S.; Pavlov, G.M.; Schubert, U.S. Nanoprecipitation of poly(methyl methacrylate)-based nanoparticles: Effect of the molar mass and polymer behavior. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2906–2913. [Google Scholar] [CrossRef]
- Okuyama, K.; Abdullah, M.; Lenggoro, I.W.; Iskandar, F. Preparation of functional nanostructured particles by spray drying. Adv. Powder Technol. 2006, 17, 587–611. [Google Scholar] [CrossRef]
- Desgouilles, S.; Vauthier, C.; Bazile, D.; Vacus, J.; Grossiord, J.L.; Veillard, M.; Couvreur, P. The Design of Nanoparticles Obtained by Solvent Evaporation: A Comprehensive Study. Langmuir 2003, 19, 9504–9510. [Google Scholar] [CrossRef]
- Katara, R.; Sachdeva, S.; Majumdar, D.K. Design, Characterization, and Evaluation of Aceclofenac-loaded Eudragit RS 100 Nanoparticulate System for Ocular Delivery; Taylor & Francis: London, UK, 2019; Volume 24, ISBN 9196945257. [Google Scholar]
- Deshmukh, R.; Mujumdar, A.; Naik, J. Production of aceclofenac-loaded sustained release micro/nanoparticles using pressure homogenization and spray drying. Dry. Technol. 2018, 36, 459–467. [Google Scholar] [CrossRef]
- Adibkia, K.; Javadzadeh, Y.; Dastmalchi, S.; Mohammadi, G.; Niri, F.K.; Alaei-Beirami, M. Naproxen-eudragit® RS100 nanoparticles: Preparation and physicochemical characterization. Colloids Surf. B Biointerfaces 2011, 83, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, 1–4. [Google Scholar] [CrossRef]
- Chu, D.B.K.; Owen, J.S.; Peters, B. Nucleation and Growth Kinetics from LaMer Burst Data. J. Phys. Chem. A 2017, 121, 7511–7517. [Google Scholar] [CrossRef]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Albisa, A.; Piacentini, E.; Arruebo, M.; Sebastian, V.; Giorno, L. Sustainable Production of Drug-Loaded Particles by Membrane Emulsification. ACS Sustain. Chem. Eng. 2018, 6, 6663–6674. [Google Scholar] [CrossRef]
- Ba, J.; Orciuch, W. Some hydrodynamic aspects of precipitation. Powder Technol. 2001, 121, 9–19. [Google Scholar] [CrossRef]
- Bally, F.; Garg, D.K.; Serra, C.A.; Hoarau, Y.; Anton, N.; Brochon, C.; Parida, D.; Vandamme, T.; Hadziioannou, G. Improved size-tunable preparation of polymeric nanoparticles by microfluidic nanoprecipitation. Polymer 2012, 53, 5045–5051. [Google Scholar] [CrossRef]
- Tseng, C.H.T.; Paul, B.K.; Chang, C.H.; Engelhard, M.H. Continuous precipitation of ceria nanoparticles from a continuous flow micromixer. Int. J. Adv. Manuf. Technol. 2013, 64, 579–586. [Google Scholar] [CrossRef]
- Ding, S.; Anton, N.; Vandamme, T.F.; Serra, C.A. Microfluidic nanoprecipitation systems for preparing pure drug or polymeric drug loaded nanoparticles: An overview. Expert Opin. Drug Deliv. 2016, 13, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- De Solorzano, I.O.; Uson, L.; Larrea, A.; Miana, M.; Sebastian, V.; Arruebo, M. Continuous synthesis of drug-loaded nanoparticles using microchannel emulsification and numerical modeling: Effect of passive mixing. Int. J. Nanomed. 2016, 11, 3397–3416. [Google Scholar] [CrossRef]
- Nguyen, N.-T. Micromixers. Fundamentals, Design and Fabrication. In Micro and Nano Technologies; William Andrew Publishing: Norwich, NY, USA, 2008; ISBN 978-0-8155-1543-2. [Google Scholar]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Farokhzad, O.C.; Karnik, R.; Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 2012, 7, 623–629. [Google Scholar] [CrossRef]
- Larrea, A.; Clemente, A.; Luque-Michel, E.; Sebastian, V. Efficient production of hybrid bio-nanomaterials by continuous microchannel emulsification: Dye-doped SiO2 and Au-PLGA nanoparticles. Chem. Eng. J. 2017, 316, 663–672. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics; CRC Press: New York, NY, USA, 1998. [Google Scholar]
- Beck-Broichsitter, M.; Rytting, E.; Lebhardt, T.; Wang, X.; Kissel, T. Preparation of nanoparticles by solvent displacement for drug delivery: A shift in the “ouzo region” upon drug loading. Eur. J. Pharm. Sci. 2010, 41, 244–253. [Google Scholar] [CrossRef]
- Saad, W.S.; Prud’Homme, R.K. Principles of nanoparticle formation by flash nanoprecipitation. Nano Today 2016, 11, 212–227. [Google Scholar] [CrossRef]
- Vossen, L.I.; Wedepohl, S.; Calderón, M. A facile, one-pot, surfactant-free nanoprecipitation method for the preparation of nanogels from polyglycerol-drug conjugates that can be freely assembled for combination therapy applications. Polymers 2018, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Dobhal, A.; Kulkarni, A.; Dandekar, P.; Jain, R. A microreactor-based continuous process for controlled synthesis of poly-methyl-methacrylate-methacrylic acid (PMMA) nanoparticles. J. Mater. Chem. B 2017, 5, 3404–3417. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Han, H.Y.; Alakhov, V.Y.; Miller, D.W.; Kabanov, A.V. Effects of pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm. Res. 1998, 15, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Ganachaud, F.; Katz, J.L. Nanoparticles and nanocapsules created using the ouzo effect: Spontaneous emulsification as an alternative to ultrasonic and high-shear devices. ChemPhysChem 2005, 6, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.K.; Prud’homme, R.K. Mechanism for rapid self-assembly of block copolymer nanoparticles. Phys. Rev. Lett. 2003, 91, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-loaded PLGA nanoparticles: Systematic study of particle size and drug content. Int. J. Pharm. 2007, 336, 367–375. [Google Scholar] [CrossRef]
- Stainmesse, S.; Orecchioni, A.M.; Nakache, E.; Puisieux, F.; Fessi, H. Formation and stabilization of a biodegradable polymeric colloidal suspension of nanoparticles. Colloid Polym. Sci. 1995, 273, 505–511. [Google Scholar] [CrossRef]
- Doane, T.L.; Chuang, C.H.; Hill, R.J.; Burda, C. Nanoparticle ζ -potentials. Acc. Chem. Res. 2012, 45, 317–326. [Google Scholar] [CrossRef]
- Skoglund, S.; Hedberg, J.; Yunda, E.; Godymchuk, A.; Blomberg, E.; Odnevall Wallinder, I. Difficulties and flaws in performing accurate determinations of zeta potentials of metal nanoparticles in complex solutions—Four case studies. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef]
- Holmberg, J.P.; Ahlberg, E.; Bergenholtz, J.; Hassellöv, M.; Abbas, Z. Surface charge and interfacial potential of titanium dioxide nanoparticles: Experimental and theoretical investigations. J. Colloid Interface Sci. 2013, 407, 168–176. [Google Scholar] [CrossRef]
- Reisch, A.; Runser, A.; Arntz, Y.; Mély, Y.; Klymchenko, A.S. Charge-controlled nanoprecipitation as a modular approach to ultrasmall polymer nanocarriers: Making bright and stable nanoparticles. ACS Nano 2015, 9, 5104–5116. [Google Scholar] [CrossRef] [PubMed]
- Legrand, P.; Lesieur, S.; Bochot, A.; Gref, R.; Raatjes, W.; Barratt, G.; Vauthier, C. Influence of polymer behaviour in organic solution on the production of polylactide nanoparticles by nanoprecipitation. Int. J. Pharm. 2007, 344, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Pinho, B.; Torrente-Murciano, L. Continuous manufacturing of silver nanoparticles between 5 and 80 nm with rapid online optical size and shape evaluation. React. Chem. Eng. 2020, 5, 342–355. [Google Scholar] [CrossRef]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Aubry, J.; Ganachaud, F.; Addad, J.P.C.; Cabane, B. Nanoprecipitation of polymethylmethacrylate by solvent shifting: 1. Boundaries. Langmuir 2009, 25, 1970–1979. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S.K.; Kumar, S.; Bhanjana, G.; Dilbaghi, N. Coencapsulation of Hydrophobic and Hydrophilic Antituberculosis Drugs in Synergistic Brij 96 Microemulsions: A Biophysical Characterization. J. Pharm. Sci. 2015, 104, 2203–2212. [Google Scholar] [CrossRef]
- Tao, J.; Chow, S.F.; Zheng, Y. Application of flash nanoprecipitation to fabricate poorly water-soluble drug nanoparticles. Acta Pharm. Sin. B 2019, 9, 4–18. [Google Scholar] [CrossRef]




| Eudragit® RS100 Concentrations | Solvents | R Value | Surfactant |
|---|---|---|---|
| 5% (w/v) | Ethanol | 5 | Pluronic 0.5% (w/v) |
| Acetone | |||
| 10% (w/v) | EtOH:acetone (1:1) | No surfactant |
| Eudragit® RS100 Concentrations | Solvents | Total Flow | R Value |
|---|---|---|---|
| 5% (w/v) | Acetone | 5 mL/min | 2 |
| 5 | |||
| 10% (w/v) | EtOH:acetone (1:1) | 50 mL/min | 0.5 |
| 0.2 | |||
| 0.125 |
| Eudragit® RS100 | |||
|---|---|---|---|
| 0% (w/v) | 5% (w/v) | 10% (w/v) | |
| Ethanol | 1.07 a | - | - |
| Acetone | 0.31 a | 1.57 ± 0.04 | 3.61 ± 0.01 |
| EtOH:acetone (1:1) | 0.69 | 4.56 ± 0.16 | 6.08 ± 0.58 |
| Synthesis | Mean Diameter ± Std.Dev. (nm) | PDI | Z-potential (mV) |
|---|---|---|---|
| EuRS100 10% (w/v) EtOH:acetone | 62.2 ± 25.6 | 0.17 | 21.3 ± 6.9 |
| EuRS100 10% (w/v) EtOH:acetone, Pluronic 0.5% (w/v) | 65.4 ± 25.4 | 0.15 | 22.0 ± 7.6 |
| EuRS100 10% (w/v) acetone | 27.9 ± 11.4 | 0.17 | 19.6 ± 2.5 |
| EuRS100 5% (w/v) EtOH:acetone | 53.8 ± 17.9 | 0.11 | 13.2 ± 3.5 |
| EuRS100 5% (w/v) EtOH:acetone, Pluronic 0.5% (w/v) | 50.6 ± 18.9 | 0.14 | 14.32 ± 4.1 |
| EuRS100 5% (w/v) acetone | 20.6 ± 9.2 | 0.20 | 17.6 ± 0.6 |
| Methodology | Synthesis | Mean Diameter ± Std.Dev. (nm) | PDI |
|---|---|---|---|
| By batch (R = 5) | EuRS100 10% (w/v) EtOH:acetone | 62.2 ± 25.6 | 0.17 |
| EuRS100 5% (w/v) EtOH:acetone | 53.8 ± 17.9 | 0.11 | |
| By microfluidic (R = 5, Q = 5 mL/min) | EuRS100 10% (w/v) EtOH:acetone | 31.0 ± 9.2 | 0.09 |
| EuRS100 5% (w/v) EtOH:acetone | 21.8 ± 4.9 | 0.05 |
| Methodology | Synthesis | Mean Diameter ± Std. Dev. (nm) | PDI |
|---|---|---|---|
| By batch (R = 5) | EuRS100 5% (w/v) acetone | 28.6 ± 12.4 | 0.19 |
| EuRS100 5% (w/v) EtOH:acetone | 53.8 ± 17.9 | 0.11 | |
| By microfluidic (R = 5, Q = 5 mL/min) | EuRS100 5% (w/v) acetone | 24.8 ± 9.5 | 0.15 |
| EuRS100 5% (w/v) EtOH:acetone | 21.8 ± 4.9 | 0.05 |
| Methodology | Drug:polymer | Mean Size (nm) | DLS Mean Size (nm) | PDI | Z-potential (mV) | EE (%) | DL (%) |
|---|---|---|---|---|---|---|---|
| In batch | - | 55.3 ± 15.9 | 53.8 ± 17.9 | 0.11 | 13.2 ± 3.5 | - | - |
| 1:20 | 52.4 ± 14.7 | 59.4 ± 24.1 | 0.17 | 13.7 ± 7.6 | 30.1 ± 5.7 | 1.4 ± 0.3 | |
| By microfluidic | - | 18.4 ± 5.5 | 21.8 ± 4.9 | 0.05 | 31.9 ± 8.6 | - | - |
| 1:20 | 17.7 ± 5.0 | 16.5 ± 4.3 | 0.07 | 17.4 ± 2.2 | 42.3 ± 2.4 | 2.0 ± 0.1 |
| Conventional Batch Synthesis | Total Flow 5 mL/min Synthesis | ||||
|---|---|---|---|---|---|
| Time | Cumulative Amount Released (%) | Time | Cumulative Amount Released (%) | ||
| Hours | Mean | Std. Dev. | Hours | Mean | Std. Dev. |
| 0.5 | 19.0 | 4.7 | 0.5 | 15.9 | 4.6 |
| 1 | 36.3 | 4.4 | 1 | 28.4 | 4.6 |
| 2 | 52.4 | 5.5 | 2 | 37.6 | 6.5 |
| 4 | 70.2 | 10.7 | 4 | 53.8 | 8.4 |
| 8 | 87.5 | 3.9 | 8 | 77.4 | 9.0 |
| 24 | 89.4 | 3.8 | 24 | 89.7 | 6.6 |
| 48 | 90.9 | 4.8 | 48 | 90.3 | 1.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yus, C.; Arruebo, M.; Irusta, S.; Sebastián, V. Microflow Nanoprecipitation of Positively Charged Gastroresistant Polymer Nanoparticles of Eudragit® RS100: A Study of Fluid Dynamics and Chemical Parameters. Materials 2020, 13, 2925. https://doi.org/10.3390/ma13132925
Yus C, Arruebo M, Irusta S, Sebastián V. Microflow Nanoprecipitation of Positively Charged Gastroresistant Polymer Nanoparticles of Eudragit® RS100: A Study of Fluid Dynamics and Chemical Parameters. Materials. 2020; 13(13):2925. https://doi.org/10.3390/ma13132925
Chicago/Turabian StyleYus, Cristina, Manuel Arruebo, Silvia Irusta, and Victor Sebastián. 2020. "Microflow Nanoprecipitation of Positively Charged Gastroresistant Polymer Nanoparticles of Eudragit® RS100: A Study of Fluid Dynamics and Chemical Parameters" Materials 13, no. 13: 2925. https://doi.org/10.3390/ma13132925
APA StyleYus, C., Arruebo, M., Irusta, S., & Sebastián, V. (2020). Microflow Nanoprecipitation of Positively Charged Gastroresistant Polymer Nanoparticles of Eudragit® RS100: A Study of Fluid Dynamics and Chemical Parameters. Materials, 13(13), 2925. https://doi.org/10.3390/ma13132925








