Nanophotosensitizers for Folate Receptor-Targeted and Redox-Sensitive Delivery of Chlorin E6 against Cancer Cells
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Synthesis of FA–PEG3500-ss-Ce6tri Copolymer
2.3. Characterization of Copolymer
2.4. Preparation of FA–PEG3500-ss-Ce6tri Nanophotosensitizers
2.5. Nanophotosensitizer Characterization
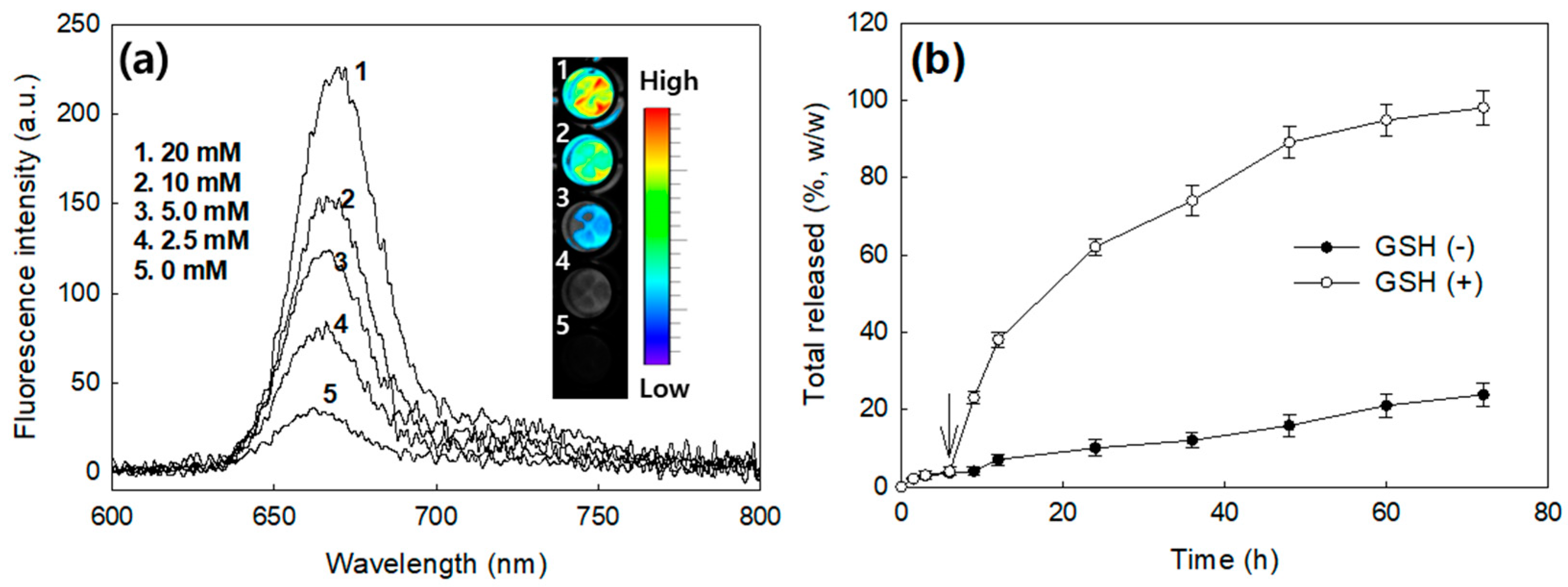
2.6. Ce6 Release Study
2.7. Cell Culture
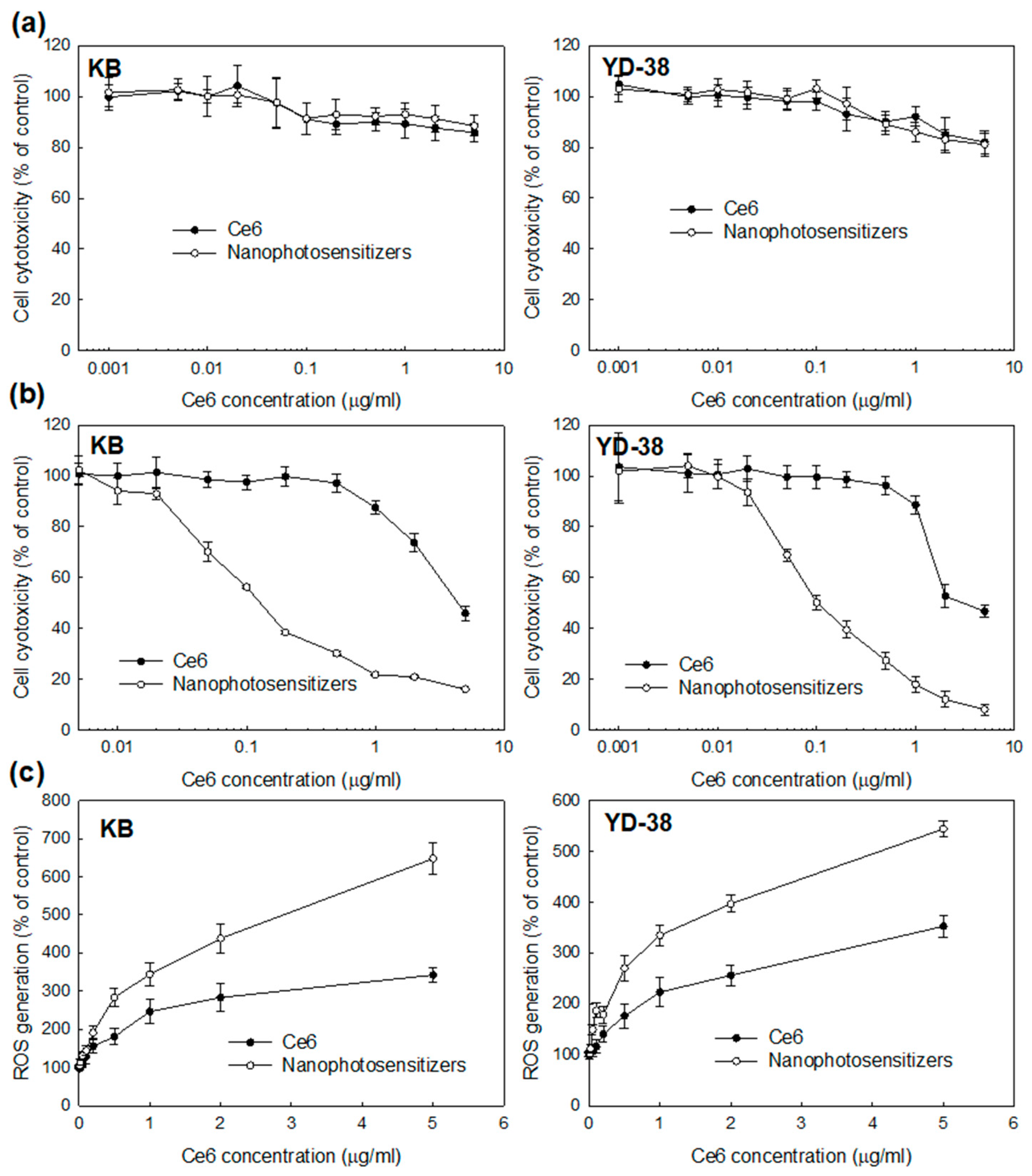
2.8. PDT Effect In Vitro
2.9. Relative Ce6 Uptake Comparison
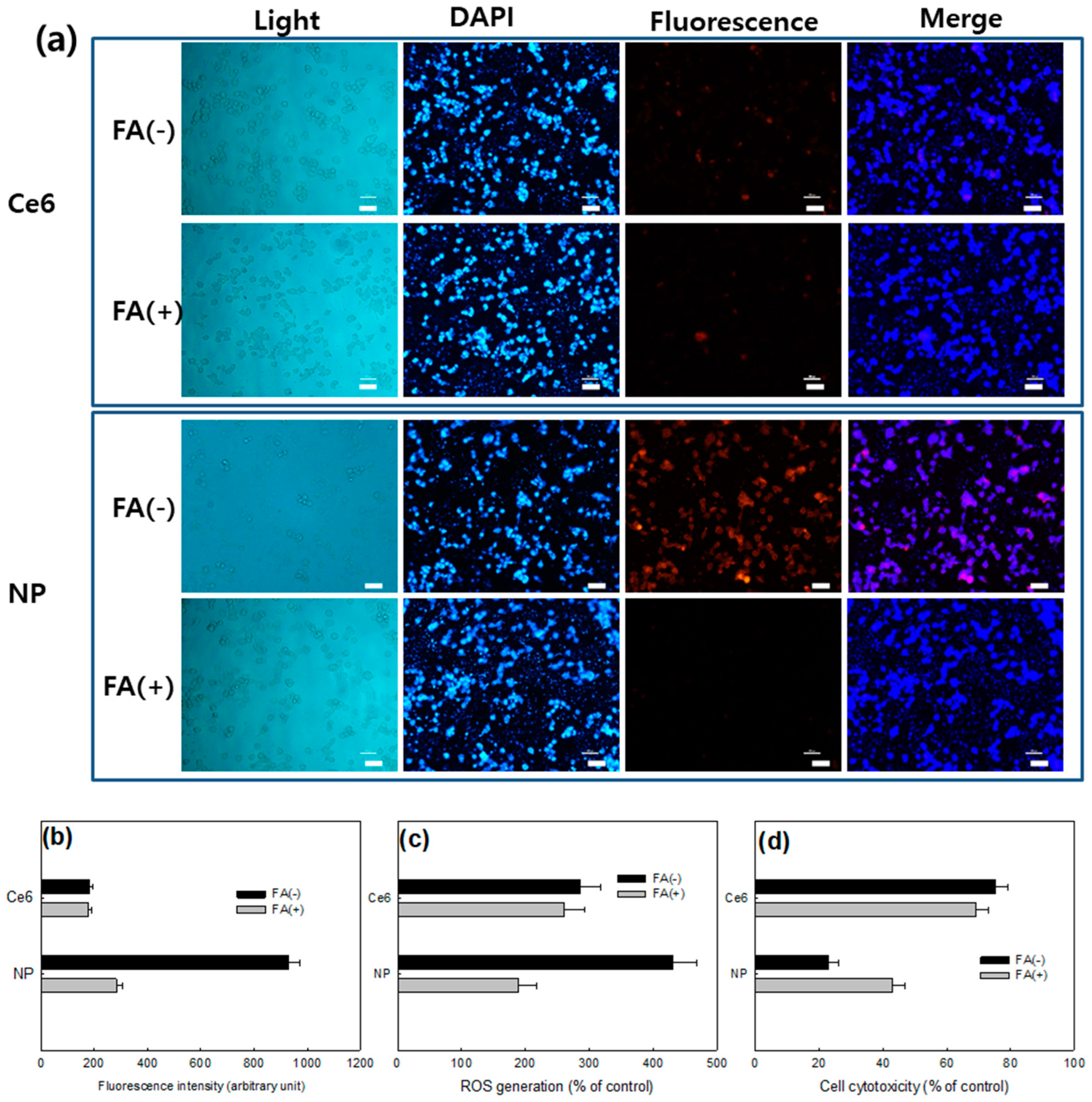
2.10. Fluorescence Microscopy for Observation of Cells
2.11. Folate Receptor-Targeting of KB Cells
2.12. ROS Production
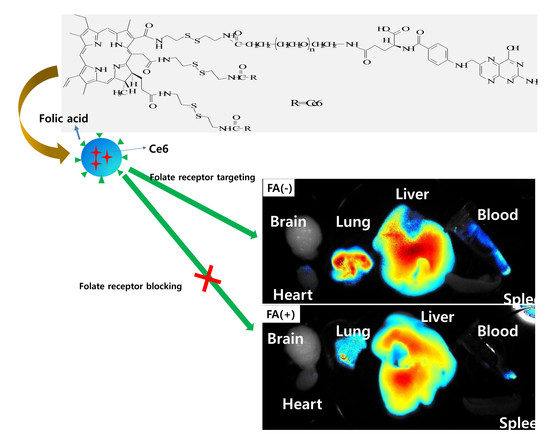
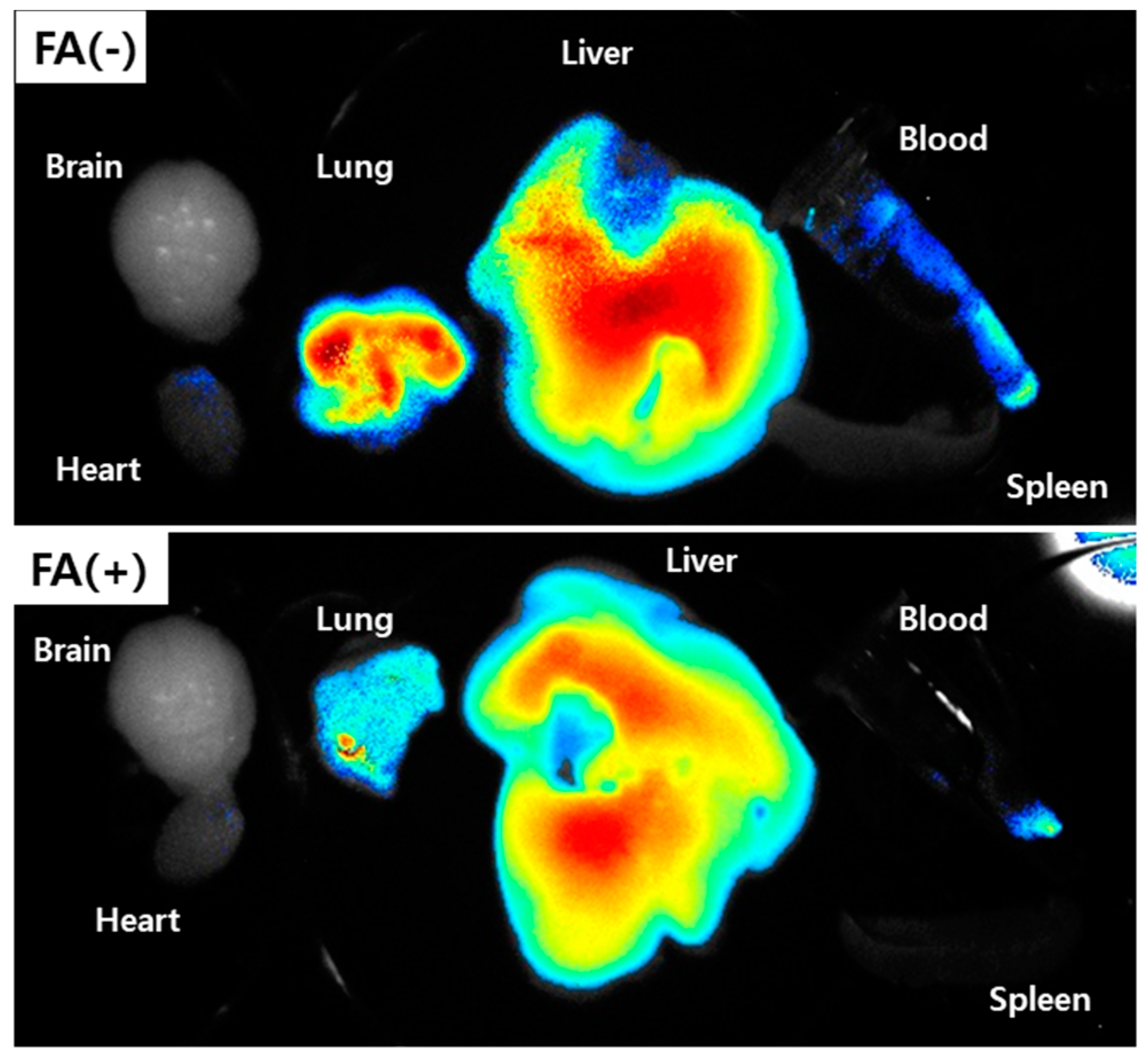
2.13. In Vivo Fluorescence Imaging Study Using Pulmonary Metastasis Model of KB Cells
2.14. Statistical Analysis
3. Results
3.1. Synthesis of FA–PEG-ss-Ce6tri Copolymer
3.2. Characterization of FA–PEG3500-ss-Ce6tri Copolymer Nanophotosensitizers
3.3. Biologic PDT Effect of Nanophotosensitizers against Cancer Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Pisani, P.; Parkin, D.M.; Bray, F.; Ferlay, J. Estimates of the worldwide mortality from 25 cancers in 1990. Int. J. Cancer 1999, 83, 18–29. [Google Scholar] [CrossRef]
- Jafari, A.; Najafi, S.; Moradi, F.; Kharazifard, M.; Khami, M. Delay in the diagnosis and treatment of oral cancer. J. Dent. 2013, 14, 146–150. [Google Scholar]
- Gigliotti, J.; Madathil, S.; Makhoul, N. Delays in oral cavity cancer. Int. J. Oral. Maxillofac. Surg. 2019, 48, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Neto, J.N.; de-Menezes, J.D.; Moura, L.B.; Massucato, E.M.; de-Andrade, C.R. Protocols for management of oral complications of chemotherapy and/or radiotherapy for oral cancer: Systematic review and meta-analysis current. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e15–e23. [Google Scholar] [CrossRef][Green Version]
- Furness, S.; Glenny, A.M.; Worthington, H.V.; Pavitt, S.; Oliver, R.; Clarkson, J.E.; Macluskey, M.; Chan, K.K.; Conway, D.I. CSROC Expert Panel. Interventions for the treatment of oral cavity and oropharyngeal cancer: Chemotherapy. Cochrane Database Syst. Rev. 2010, 9, CD006386. [Google Scholar]
- Marta, G.N.; Riera, R.; Bossi, P.; Zhong, L.P.; Licitra, L.; Macedo, C.R.; de Castro Junior, G.; Carvalho, A.L.; William, W.N., Jr.; Kowalski, L.P. Induction chemotherapy prior to surgery with or without postoperative radiotherapy for oral cavity cancer patients: Systematic review and meta-analysis. Eur. J. Cancer 2015, 51, 2596–2603. [Google Scholar] [CrossRef]
- Mohan, S.P.; Bhaskaran, M.K.; George, A.L.; Thirutheri, A.; Somasundaran, M.; Pavithran, A. Immunotherapy in Oral Cancer. J. Pharm. Bioallied Sci. 2019, 11, S107–S111. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Furukawa, K.; Sato, M.; Okunaka, T.; Kusunoki, Y.; Kawahara, M.; Fukuoka, M.; Miyazawa, T.; Yana, T.; Matsui, K.; et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 103–111. [Google Scholar] [CrossRef]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef]
- Lucena, S.R.; Salazar, N.; Gracia-Cazaña, T.; Zamarrón, A.; González, S.; Juarranz, Á.; Gilaberte, Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. Int. J. Mol. Sci. 2015, 16, 25912–25933. [Google Scholar] [CrossRef] [PubMed]
- Wakui, M.; Yokoyama, Y.; Wang, H.; Shigeto, T.; Futagami, M.; Mizunuma, H. Efficacy of a methyl ester of 5-aminolevulinic acid in photodynamic therapy for ovarian cancers. J. Cancer Res. Clin. Oncol. 2010, 136, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Berndt-Paetz, M.; Weimann, A.; Sieger, N.; Schastak, S.; Riyad, Y.M.; Griebel, J.; Arthanareeswaran, V.K.A.; Stolzenburg, J.U.; Neuhaus, J. Tetrahydroporphyrin-tetratosylat (THPTS): A near-infrared photosensitizer for targeted and efficient photodynamic therapy (PDT) of human bladder carcinoma. An in vitro study. Photodiagnosis Photodyn. Ther. 2017, 18, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Mallidi, S.; Liu, J.; Chiang, C.T.; Mai, Z.; Goldschmidt, R.; Ebrahim-Zadeh, N.; Rizvi, I.; Hasan, T. Photodynamic therapy synergizes with irinotecan to overcome compensatory mechanisms and improve treatment outcomes in pancreatic cancer. Cancer Res. 2016, 76, 1066–1077. [Google Scholar] [CrossRef]
- Nauta, J.M.; van Leengoed, H.L.; Star, W.M.; Roodenburg, J.L.; Witjes, M.J.; Vermey, A. Photodynamic therapy of oral cancer. A review of basic mechanisms and clinical applications. Eur. J. Oral Sci. 1996, 104, 69–81. [Google Scholar] [CrossRef]
- Saini, R.; Lee, N.V.; Liu, K.Y.; Poh, C.F. Prospects in the application of photodynamic therapy in oral cancer and premalignant lesions. Cancers 2016, 8, 83. [Google Scholar] [CrossRef]
- Nauta, J.M.; van Leengoed, H.L.; Witjes, M.J.; Nikkels, P.G.; Star, W.M.; Vermey, A.; Roodenburg, J.L. Photofrin-mediated photodynamic therapy of chemically-induced premalignant lesions and squamous cell carcinoma of the palatal mucosa in rats. Int. J. Oral Maxillofac. Surg. 1997, 26, 223–231. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Kwon, S.M.; Kim, Y.C.; Ahn, S.G.; Yoon, J.H. Pheophorbide a-mediated photodynamic therapy induces apoptotic cell death in murine oral squamous cell carcinoma in vitro and in vivo. Oncol. Rep. 2012, 27, 1772–1778. [Google Scholar]
- Low, K.P.; Bhuvaneswari, R.; Thong, P.S.; Bunte, R.M.; Soo, K.C. Novel delivery of Chlorin e6 using anti-EGFR antibody tagged virosomes for fluorescence diagnosis of oral cancer in a hamster cheek pouch model. Eur. J. Pharm. Sci. 2016, 83, 143–154. [Google Scholar] [CrossRef]
- Rosin, F.C.P.; Teixeira, M.G.; Pelissari, C.; Corrêa, L. Resistance of oral cancer cells to 5-ALA-mediated photodynamic therapy. J. Cell Biochem. 2018, 119, 3554–3562. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Muto, M.; Yoshimura, K.; Niimi, M.; Ezoe, Y.; Yoda, Y.; Yamamoto, Y.; Nishisaki, H.; Higashino, K.; Iishi, H. Phase I study of photodynamic therapy using talaporfin sodium and diode laser for local failure after chemoradiotherapy for esophageal cancer. Radiat. Oncol. 2012, 7, 113. [Google Scholar] [CrossRef]
- Sun, X.; Sun, J.; Lv, J.; Dong, B.; Liu, M.; Liu, J.; Sun, L.; Zhang, G.; Zhang, L.; Huang, G.; et al. Ce6-C6-TPZ co-loaded albumin nanoparticles for synergistic combined PDT-chemotherapy of cancer. J. Mater. Chem. B 2019, 7, 5797–5807. [Google Scholar] [CrossRef]
- Wang, B.Y.; Liao, M.L.; Hong, G.C.; Chang, W.W.; Chu, C.C. Near-Infrared-Triggered Photodynamic Therapy toward Breast Cancer Cells Using Dendrimer-Functionalized Upconversion Nanoparticles. Nanomaterials 2017, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeong, Y.I. Hybrid nanoparticles based on chlorin e6-conjugated hyaluronic acid/poly(l-histidine) copolymer for theranostic application to tumors. J. Mater. Chem B 2018, 6, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, D.M.; Lim, S.H.; Shim, Y.H.; Kwon, H.; Kim, D.H.; Lee, C.M.; Kim, B.H.; Jeong, Y.I. Hyaluronic Acid-Conjugated with Hyperbranched Chlorin e6 Using Disulfide Linkage and Its Nanophotosensitizer for Enhanced Photodynamic Therapy of Cancer Cells. Materials 2019, 12, 3080. [Google Scholar] [CrossRef]
- Ross, J.F.; Chaudhuri, P.K.; Ratnam, M. Differential Regulation of Folate Receptor Isoforms in Normal and Malignant Tissues in Vivo and in Established Cell Lines. Physiologic and Clinical Implications. Cancer 1994, 73, 2432–2443. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Lei, Y.; Yang, N.; Cabrera, R.M.; Finnell, R.H.; Ren, A. Gene variants in the folate pathway are associated with increased levels of folate receptor autoantibodies. Birth. Defects Res. 2018, 110, 973–981. [Google Scholar] [CrossRef]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R., Jr.; Kamen, B.A. Distribution of the Folate Receptor GP38 in Normal and Malignant Cell Lines and Tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar]
- Lee, S.J.; Shim, Y.H.; Oh, J.S.; Jeong, Y.I.; Park, I.K.; Lee, H.C. Folic-acid-conjugated pullulan/poly(DL-lactide-co-glycolide) graft copolymer nanoparticles for folate-receptor-mediated drug delivery. Nanoscale Res. Lett. 2015, 10, 43. [Google Scholar] [CrossRef]
- Stallivieri, A.; Colombeau, L.; Jetpisbayeva, G.; Moussaron, A.; Myrzakhmetov, B.; Arnoux, P.; Acherar, S.; Vanderesse, R.; Frochot, C. Folic acid conjugates with photosensitizers for cancer targeting in photodynamic therapy: Synthesis and photophysical properties. Bioorg. Med. Chem. 2017, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, P.; Lin, H.; Jiang, Z.; Guo, L.; Li, B. A novel chlorin–PEG–folate conjugate with higher water solubility, lower cytotoxicity, better tumor targeting and photodynamic activity. J. Photochem. Photobiol. B 2013, 127, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Alkan, D.; Guven, B.; Turer, C.C.; Balli, U.; Can, M. Folate-receptor 1 level in periodontal disease: A pilot study. BMC Oral. Health 2019, 19, 218. [Google Scholar] [CrossRef]
- Kenney, E.B.; Ash, M.M., Jr. Oxidation Reduction Potential of Developing Plaque, Periodontal Pockets and Gingival Sulci. J. Periodontol. 1969, 40, 630–633. [Google Scholar] [CrossRef]
- Wong, D.Y.; Hsiao, Y.L.; Poon, C.K.; Kwan, P.C.; Chao, S.Y.; Chou, S.T.; Yang, C.S. Glutathione concentration in oral cancer tissues. Cancer Lett. 1994, 81, 111–116. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Latorre, A.; Somoza, Á. Glutathione-triggered drug release from nanostructures. Curr. Top. Med. Chem. 2014, 14, 2662–2671. [Google Scholar] [CrossRef]
- Mallia, R.J.; Subhash, N.; Sebastian, P.; Kumar, R.; Thomas, S.S.; Mathews, A.; Madhavan, J. In vivo temporal evolution of ALA-induced normalized fluorescence at different anatomical locations of oral cavity: Application to improve cancer diagnostic contrast and potential. Photodiagnosis Photodyn. Ther. 2010, 7, 162–175. [Google Scholar] [CrossRef]
- Maloth, K.N.; Velpula, N.; Kodangal, S.; Sangmesh, M.; Vellamchetla, K.; Ugrappa, S.; Meka, N. Photodynamic Therapy—A Non-invasive Treatment Modality for Precancerous Lesions. J. Lasers Med. Sci. 2016, 7, 30–36. [Google Scholar] [CrossRef]
- Park, H.; Na, K. Conjugation of the Photosensitizer Chlorin e6 to Pluronic F127 for Enhanced Cellular Internalization for Photodynamic Therapy. Biomaterials 2013, 34, 6992–7000. [Google Scholar] [CrossRef]



| Ce6 Contents (%, w/w) a | Particle Size (nm) b | ||
|---|---|---|---|
| Theoretical | Experimental | ||
| FA–PEG3500 conjugates | - | - | - |
| FA–PEG3500-ss-Ce6 | 12.2 | 11.8 | 96.2 ± 7.3 |
| FA–PEG-ss-Ce6tri | 29.5 | 28.3 | 189.1 ± 10.8 |
| IC50 (μg/mL) a | ||
|---|---|---|
| KB Cells | YD-38 Cells | |
| Ce6 | 4.52 | 3.13 |
| Nanophotosensitizers | 0.13 | 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kook, M.-S.; Lee, C.-M.; Jeong, Y.-I.; Kim, B.-H. Nanophotosensitizers for Folate Receptor-Targeted and Redox-Sensitive Delivery of Chlorin E6 against Cancer Cells. Materials 2020, 13, 2810. https://doi.org/10.3390/ma13122810
Kook M-S, Lee C-M, Jeong Y-I, Kim B-H. Nanophotosensitizers for Folate Receptor-Targeted and Redox-Sensitive Delivery of Chlorin E6 against Cancer Cells. Materials. 2020; 13(12):2810. https://doi.org/10.3390/ma13122810
Chicago/Turabian StyleKook, Min-Suk, Chang-Min Lee, Young-Il Jeong, and Byung-Hoon Kim. 2020. "Nanophotosensitizers for Folate Receptor-Targeted and Redox-Sensitive Delivery of Chlorin E6 against Cancer Cells" Materials 13, no. 12: 2810. https://doi.org/10.3390/ma13122810
APA StyleKook, M.-S., Lee, C.-M., Jeong, Y.-I., & Kim, B.-H. (2020). Nanophotosensitizers for Folate Receptor-Targeted and Redox-Sensitive Delivery of Chlorin E6 against Cancer Cells. Materials, 13(12), 2810. https://doi.org/10.3390/ma13122810