Synthesis and Effects of Two Novel Rare-Earth Energetic Complexes on Thermal Decomposition of Cyclotetramethylene Tetranitramine (HMX)
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of [La(tza)(NO3)2(H2O)4]n (1) and [Ce(tza)(NO3)2(H2O)4]n (2)
2.2. X-ray Single-Crystal Diffraction (XRD)
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. X-ray Powder Diffraction (PXRD)
2.5. TG-DSC Thermal Analysis
2.6. Effect of the Energetic Complexes on Thermal Degradation of HMX
2.7. Compatibility Test between HMX and Complex 1
3. Results and Discussion
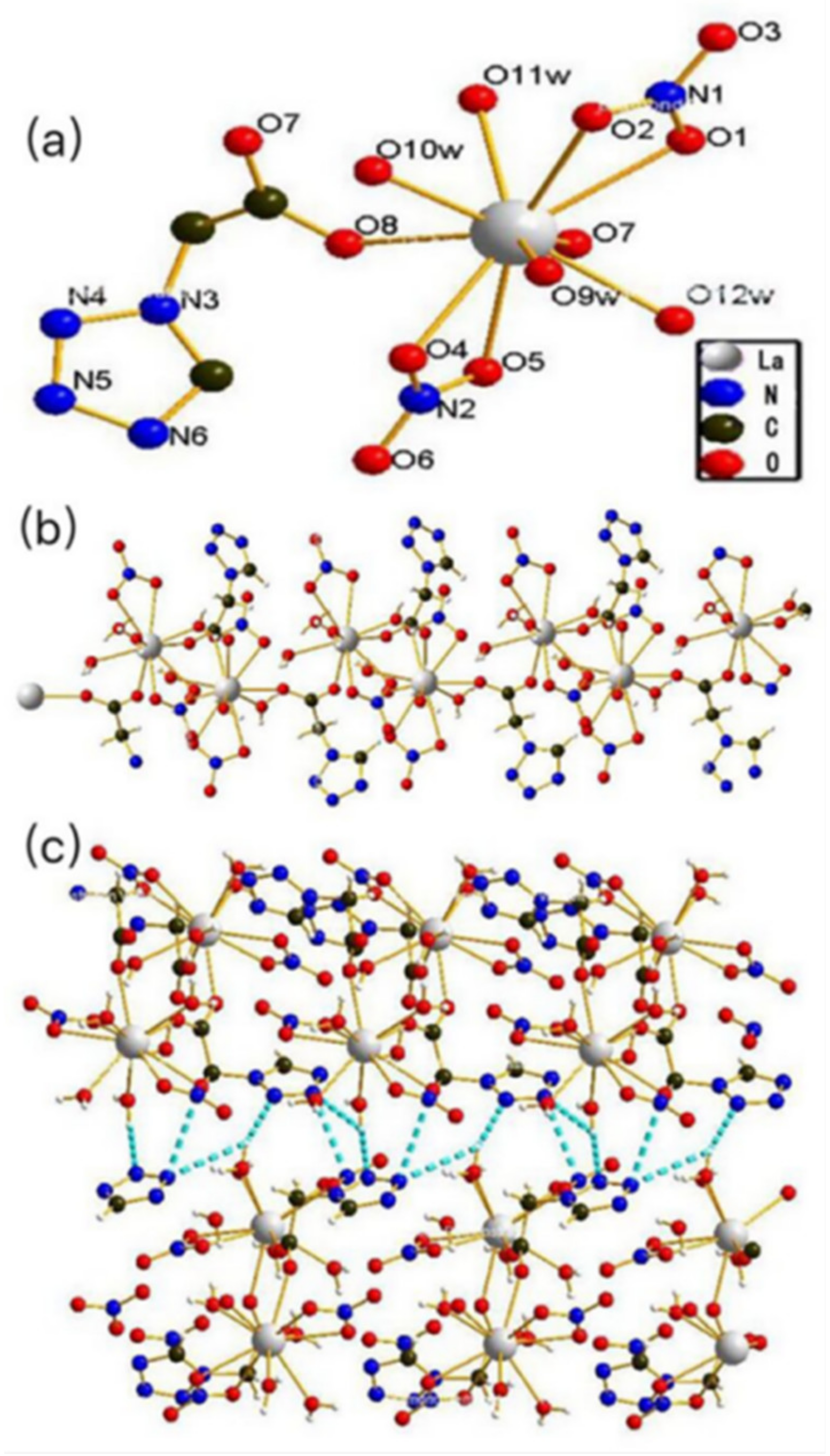
3.1. Structure Descriptions
3.1.1. Structures of [La(tza)(NO3)2(H2O)4]n (1)
3.1.2. Structures of [Ce(tza)(NO3)2(H2O)4]n (2)
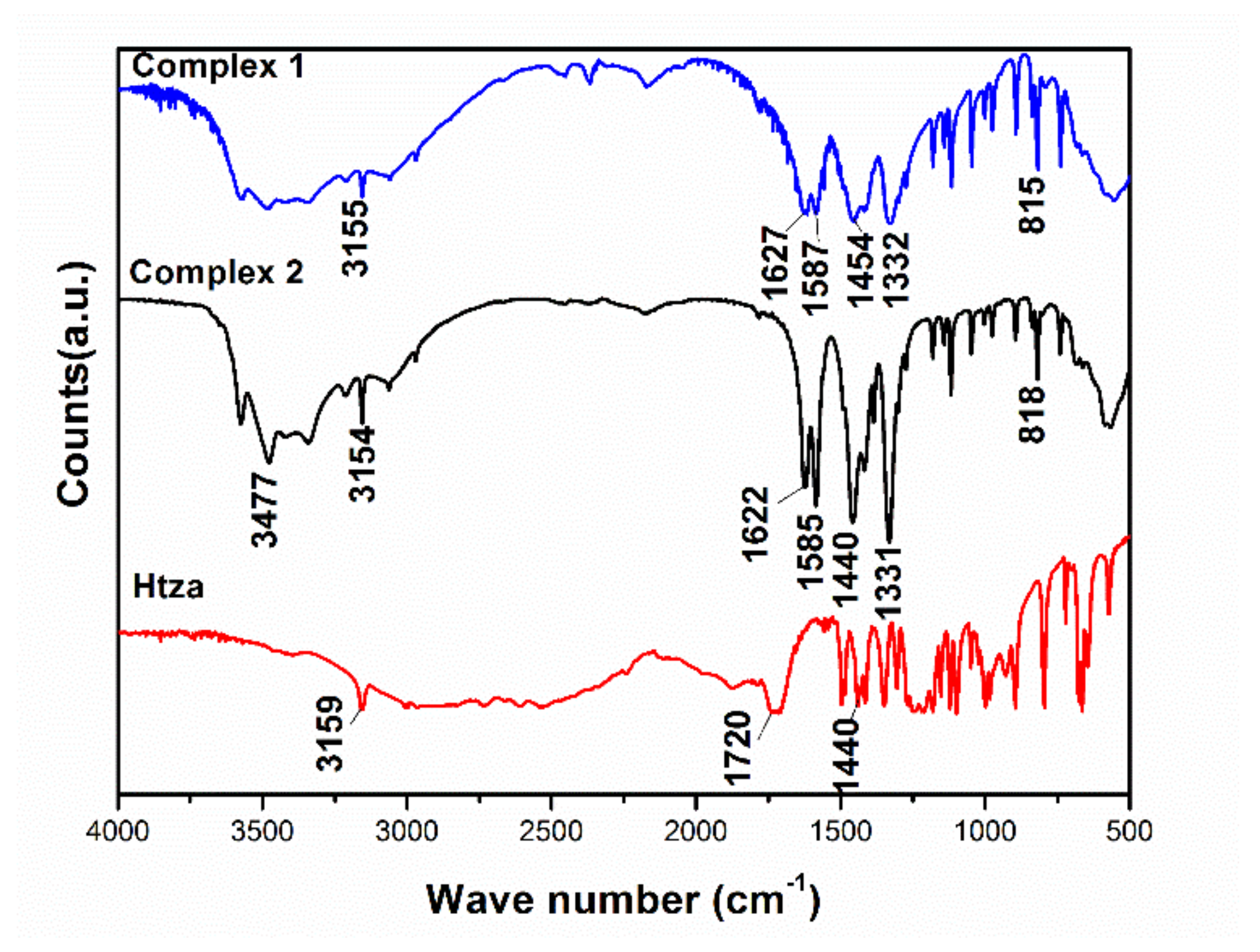
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
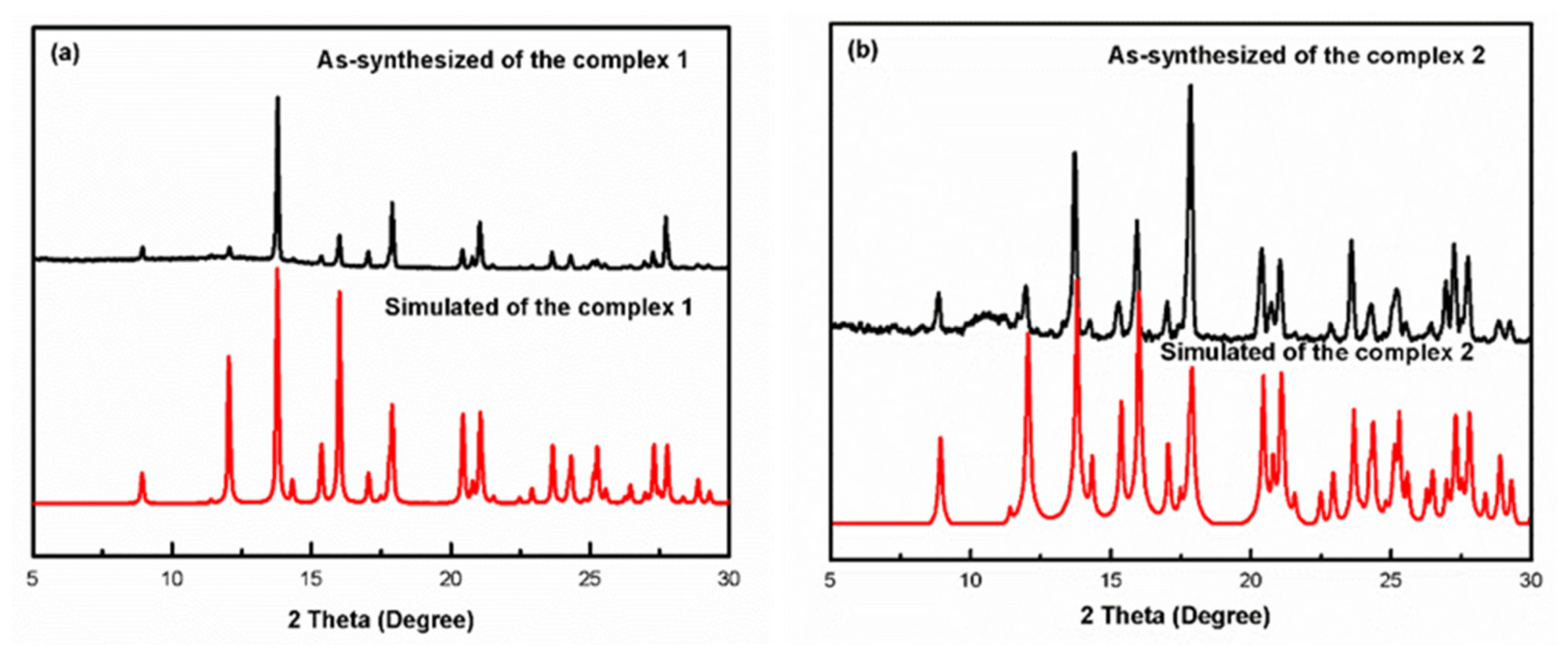
3.3. PXRD Diffraction Analysis
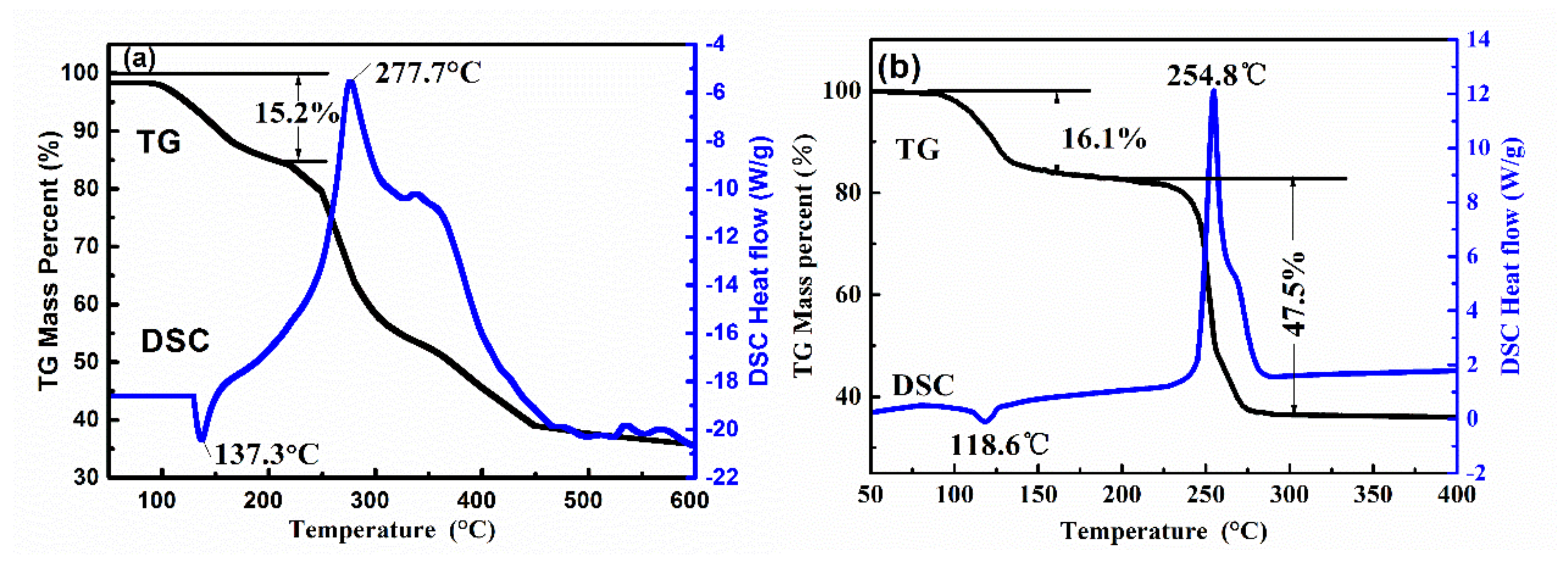
3.4. TG-DSC Thermal Analysis of Energetic Complexes 1 and 2
3.5. Effect of Energetic Complexes 1 and 2 on Thermal Degradation of HMX
3.6. Compatibility between HMX and Complex 1
3.6.1. DSC Analysis
3.6.2. FTIR Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, W.E.; Chen, C.; Fu, X.; Ding, C.; Wang, G. The correlation between chemical stability and binder network structure in NEPE propellant. Propell. Explos. Pyrot. 2017, 42, 541–546. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Bai, L.F.; Huang, S.L.; Yan, G.Y.; Xie, L. Acceleration of δ- to β-HMX-D8 phase retransformation with D2O and intergranular strain evolution in a HMX-based polymer-bonded explosive. J. Phys. Chem. C 2019, 123, 6958–6964. [Google Scholar] [CrossRef]
- Wu, X.W.; Liu, Z.C.; Zhu, W.H. Conformational changes and decomposition mechanisms of HMX-based cocrystal explosives at high temperatures. J. Phys. Chem. C 2020, 124, 25–36. [Google Scholar] [CrossRef]
- Sun, Y.L.; Ren, H.; Jiao, Q.J. Comparison of thermal behaviors and decomposition kinetics of NEPE propellant before and after storage. J. Therm. Anal. Calorim. 2018, 131, 101–111. [Google Scholar] [CrossRef]
- Yim, Y.J.; Jang, M.W.; Park, E.Y.; Lee, J.S.; Han, H.; Lee, W.B.; Song, S.H.; Kim, M.T.; Yoo, J.C.; Yoon, M.W. Infrared irradiance reduction in minimum smoke propellants by addition of potassium salt. Propell. Explos. Pyrot. 2015, 40, 74–80. [Google Scholar] [CrossRef]
- Gao, D.X.; Huang, J.; Lin, X.H.; Yang, D.L.; Wang, Y.J.; Zheng, H.Y. Phase transitions and chemical reactions of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine under high pressure and high temperature. RSC Adv. 2019, 9, 5825–5833. [Google Scholar] [CrossRef]
- Tarver, C.M. High Energy Materials, Propellants, Explosives and Pyrotechnics, Jai Prakash Agrawal. Propell. Explos. Pyrot. 2010, 35. [Google Scholar] [CrossRef]
- Wei, T.T.; Zhang, Y.; Xu, K.Z.; Ren, Z.Y.; Gao, H.X.; Zhao, F.Q. Catalytic action of nano Bi2WO6 on thermal decompositions of AP, RDX, HMX and combustion of NG/NC propellant. RSC Adv. 2015, 5, 70323–70328. [Google Scholar] [CrossRef]
- Gao, Y.; Ao, W.; Chen, S.W.; Kong, J.; Wang, Y.; Zhang, Q.H.; Yan, Q.L. Effects of nanosized metals and metal oxides on the thermal behaviors of insensitive high energetic compound ICM-102. J. Phys. Chem. C 2019, 123, 31108–31118. [Google Scholar] [CrossRef]
- Shao, E.S.; Li, D.D.; Li, J.Z.; Zhang, G.F.; Zhang, W.Q.; Gao, Z.W. Mono- and dinuclear ferrocenyl lonic complexes with polycyano anions. Characterization, migration, and catalytic effects on thermal decomposition of energetic complexes. Z. Anorg. Allg. Chem. 2016, 642, 871–881. [Google Scholar] [CrossRef]
- Yang, Q.; Song, X.X.; Zhao, G.W.; Yang, G.L.; Yang, L.L.; Wei, Q.; Xie, G.; Chen, S.P.; Gao, S.L. A 3D cuii-based energetic MOF: Synthesis, structure, and energetic performance. Eur. J. Inorg. Chem. 2016, 2016, 5052–5056. [Google Scholar] [CrossRef]
- Ma, X.H.; Cai, C.; Sun, W.J.; Song, W.M. Enhancing energetic performance of multinuclear Ag(I)-cluster MOF-based high-energy-density materials by thermal dehydration. ACS Appl. Mater. Interfaces 2019, 11, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Wei, Z.X.; Kang, L. Progress on combustion catalysts of solid propellant. Chin. J. Energ. Mater. 2015, 23, 89–98. [Google Scholar] [CrossRef]
- Yan, Q.L.; Zhao, F.Q.; Kuo, K.K.; Zhang, X.H.; Zeman, S.; DeLuca, L.T. Catalytic effects of nano additives on decomposition and combustion of RDX-, HMX-, and AP-based energetic compositions. Prog. Energy Combust. Sci. 2016, 57, 75–136. [Google Scholar] [CrossRef]
- Bu, R.P.; Li, H.Z.; Zhang, C.Y. Polymorphic transition in traditional energetic materials: Influencing factors and effects on structure, property, and performance. Cryst. Growth Des. 2020, 20, 3561–3576. [Google Scholar] [CrossRef]
- Jeon, W.C.; Lee, J.H.; Kim, J.C.; Jung, S.H.; Cho, S.G.; Kwak, S.K. Controllable explosion of nanobomb by modifying nanocontainer and external shocks. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Chen, D.; Huang, S.L.; Zhang, Q.; Yu, Q.; Zhou, X.Q.; Li, H.Z.; Li, J.S. Two nitrogen-rich Ni(II) coordination complexes based on 5, 5′-azotetrazole: Synthesis, characterization and effect on thermal decomposition for RDX, HMX and AP. RSC Adv. 2015, 5, 32872–32879. [Google Scholar] [CrossRef]
- Wei, J.P.; Zhang, D.; Yang, Q.; Chen, S.P.; Gao, S.L. 0D Cu (II) and 1D mixed-valence Cu (I)/Cu (II) coordination complexes based on mixed ligands: Syntheses, structures and catalytic thermal decomposition for HMX. Inorg. Chem. Commun. 2013, 30, 13–16. [Google Scholar] [CrossRef]
- Zhao, C.D.; Chi, Y.; Yu, Q.; Wang, X.F.; Fan, G.J.; Yu, K. Comprehensive study of the interaction and mechanism between bistetrazole ionic salt and ammonium nitrate explosive in thermal decomposition. J. Phys. Chem. C 2019, 123, 27286–27294. [Google Scholar] [CrossRef]
- Yang, Q.; Ge, J.; Gong, Q.B.; Song, X.X.; Zhao, J.W.; Wei, Q.; Xie, G.; Chen, S.P.; Gao, S.L. Two energetic complexes incorporating 3, 5-dinitrobenzoic acid and azole ligands: Microwave-assisted synthesis, favorable detonation properties, insensitivity and effects on the thermal decomposition of RDX. New J. Chem. 2016, 40, 7779–7786. [Google Scholar] [CrossRef]
- Han, J.; Li, T.; Li, B. A new nickel (II) coordination complex constructed by pyridyl-triazole and oxybis (benzoic acid): Synthesis, crystal structure and the effect on the thermal decomposition of ammonium perchlorate. Chin. J. Struct. Chem. 2015, 34, 253–259. [Google Scholar] [CrossRef]
- Gao, W.J.; Liu, X.Y.; Su, Z.Y.; Zhang, S.; Yang, Q.; Wei, Q.; Chen, S.P.; Xie, G.; Yang, X.W.; Gao, S.L. High-energy-density materials with remarkable thermostability and insensitivity: Syntheses, structures and physicochemical properties of Pb (II) complexes with 3-(tetrazol-5-yl) triazol. J. Marer. Chem. A 2014, 2, 11958–11965. [Google Scholar] [CrossRef]
- Tao, G.H.; Parrish, D.A.; Shreeve, J.M. Nitrogen-ich 5-(1-methylhydraziny) tetrazole and its copper and silver complexes. Inorg. Chem. 2012, 51, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Qu, X.N.; Zhang, S.; Ke, H.S.; Yang, Q.; Shi, Q.; Wei, Q.; Xie, G.; Chen, S.P. High-performance energetic characteristics and magnetic properties of a threedimensional cobalt(II) metal-organic framework assembled with azido and triazole. Inorg. Chem. 2015, 54, 11520–11525. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.N.; Zhang, S.; Wang, B.Z.; Yang, Q.; Han, J.; Wei, Q.; Xie, G.; Chen, S.P. An Ag(I) energetic metal-organic framework assembled with the energetic combination of furazan and tetrazole synthesis structure and energetic performance. Dalton Trans. 2016, 45, 6968–6973. [Google Scholar] [CrossRef] [PubMed]
- Szimhardt, N.; Wurzenberger, M.H.H.; Zeisel, L.; Gruhne, M.S.; Lommel, M.; Stierstorfer, J. Maximization of the energy capability level in transition metal complexes through application of 1-amino-and 2-amino-5 H-tetrazole ligands. J. Mater. Chem. A 2018, 6, 16257–16272. [Google Scholar] [CrossRef]
- Zhang, X.Q. Studies on the Synthesis, Crystal Structures, Spectroscopic Characterization and Magnetic Properties of Complexes of Tetrazole-1-Acetic Acid; Guangxi Normal University: Guilin, China, 2006. [Google Scholar]
- Li, Q.Y.; Yang, G.W.; Tang, X.Y.; Ma, Y.S.; Yao, W.; Zhou, Y.F.; Chen, J.; Zhou, H. Constructions of a set of new lanthanide-based coordination polymers with hatza ligands (hatza = 5-aminotetrazole − 1-acetic acid). Cryst. Growth Des. 2010, 10, 165–170. [Google Scholar] [CrossRef]
- Qu, Y.Y.; Wei, Z.X.; Kang, L.; Xie, F.; Zhang, H.D.; Yue, P. Synergistic catalytic effect of a series of energetic coordination complexes based on tetrazole-1-acetic acid on thermal decomposition of HMX. Z. Anog. Allg. Chem. 2017, 643, 742–748. [Google Scholar] [CrossRef]
- Xie, Y.S.; Tan, J.Y.; Hu, M.L. Application of nanotechnology in post-treatment of automobile tail gas nitrogen oxides. Nanosci. Nanotech. 2017, 1, 26–29. [Google Scholar] [CrossRef]
- Papavasiliou, A.; Tsiourvas, D.; Deze, E.G. Hyperbranched polyethyleneimine towards the development of homogeneous and highly porous CuO–CeO2–SiO2 catalytic materials. Chem. Eng. J. 2016, 300, 343–357. [Google Scholar] [CrossRef]
- Wei, Z.X.; Wang, Y.; Zhang, X.J.; Hu, C.W. Combustion synthesis and effect of LaMnO3 and LaOCl powder mixture on HMX thermal decomposition. Thermochim. Acta 2010, 499, 111–116. [Google Scholar] [CrossRef]
- Mazzeu, M.A.C.; Mattos, E.D.C.; Iha, K. Studies on compatibility of energetic materials by thermal methods. JATM 2010, 2, 53–58. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, R.; Wang, Q.; Wang, E.Y.; Zhao, L.M.; Wang, P.; Chen, F.X.; Huang, J.L. Effects of novel energetic material NOG2Tz on thermal decomposition behavior of HMX. Chin. J. Chem. Eng. 2014, 22, 22–25. [Google Scholar] [CrossRef]
- Guo, W.X.; Han, Z.W.; Lin, Q.H.; Wang, B. Pre-formulation compatibility studies of 5-amino-1H-tetrazole nitrate with several typical materials by thermal and non-thermal techniques. Cent. Eur. J. Energ. Mater. 2018, 15, 100–114. [Google Scholar] [CrossRef]
- De Lima, I.P.B.; Lima, N.G.P.B.; Barros, D.M.C.; Oliveira, T.S.; Mendonc, C.M.S. Compatibility study between hydroquinone and the excipients used in semi-solid pharmaceutical forms by thermal and non-thermal techniques. J. Therm. Anal. Calorim. 2015, 120, 719–732. [Google Scholar] [CrossRef]
- Cheng, G.Z.; Zhu, C.F.; Zhang, M.T.; Li, C.; Yuan, G. Infrared absorption spectrum of evaporated water molecules in slit. Chin. J. Lumin. 2020, 41, 110–116. [Google Scholar] [CrossRef]
- Wang, J.S.; Xu, M.C.; Li, C.; Yuan, G. Influence of infrared adsorption properties of sodium nitrate with silver/diamond powder (Ag/DP) composites. Spectrosc. Spect. Anal. 2017, 37, 2737–2742. [Google Scholar] [CrossRef]
- Nie, F.D.; Zhang, J.; Guo, Q.X.; Qiao, Z.Q.; Zeng, G.Y. Sol–gel synthesis of nanocomposite crystalline HMX/AP coated by resorcinol–formaldehyde. J. Phys. Chem. Solids 2010, 71, 109–113. [Google Scholar] [CrossRef]
- Ordzhonikidze, O.; Pivkina, A.; Frolov, Y.; Muravyev, N.; Monogarov, K. Comparative study of HMX and CL-20. J. Therm. Anal. Calorim. 2011, 105, 529–534. [Google Scholar] [CrossRef]
- Yong, G.P.; Zhang, Y.M.; Zhang, B. Hydrothermal syntheses, crystal structures and properties of novel quinone biradical and mixed-valence copper coordination polymer with semiquinone radical ligand generated in situ. Cryst. Eng. 2012, 14, 8620–8625. [Google Scholar] [CrossRef]
- Liu, Z.R.; Liu, Y.; Fan, X.P.; Zhao, F.Q. Thermal decomposition of RDX and HMX explosives part III: Mechanism of thermal decomposition. Chin. J. Explos. Propel. 2006, 29, 14–18. [Google Scholar] [CrossRef]
- Tarver, C.M.; Chidester, S.K.; Nichols, A.L. Critical conditions for impact-and shock-induced hot spots in solid explosives. J. Phys. Chem. 1996, 100, 5794–5799. [Google Scholar] [CrossRef]
- Ye, C.C.; An, Q.; Zhang, W.Q.; Goddard, W.A., III. Initial decomposition of HMX energetic material from quantum molecular dynamics and the molecular structure transition of β-HMX to δ-HMX. J. Phys. Chem. C 2019, 123, 9231–9236. [Google Scholar] [CrossRef]
- Zhang, H.D.; Wei, Z.X.; Cao, X.F. Synthesis of a series of coordination compounds based on tetrazole-1-acetic acid and effect on HMX thermal decomposition. J. Shanxi Univ. (Nat. Sci. Ed.) 2020. [Google Scholar] [CrossRef]
- Ai, L.H.; Zhang, C.H.; Li, L.L.; Jiang, J. Iron terephthalate metal–organic framework: Revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation. Appl. Catal. B 2014, 148, 191–200. [Google Scholar] [CrossRef]
- Jin, B.; Shen, J.; Peng, R.F.; Shu, Y.J.; Tan, B.S.; Chu, S.J.; Dong, H.S. Synthesis, characterization, thermal stability and sensitivity properties of the new energetic polymer through the azidoacetylation of poly (vinyl alcohol). Polym. Degrad. Stab. 2012, 97, 473–480. [Google Scholar] [CrossRef]
- Li, J.Z.; Fan, X.Z.; Feng, X.P.; Zhang, F.Q.; Han, R.Z. Compatibility study of 1,3,3-trinitroazetidinewith some energetic components and inert materials. J. Therm. Anal. Calorim. 2006, 85, 779–784. [Google Scholar] [CrossRef]
- Liao, N. Preparation and characterization of nanocomposite energetic material based on HMX and AP. Chin. J. Energ. Mater 2015, 23, 709–711. [Google Scholar]

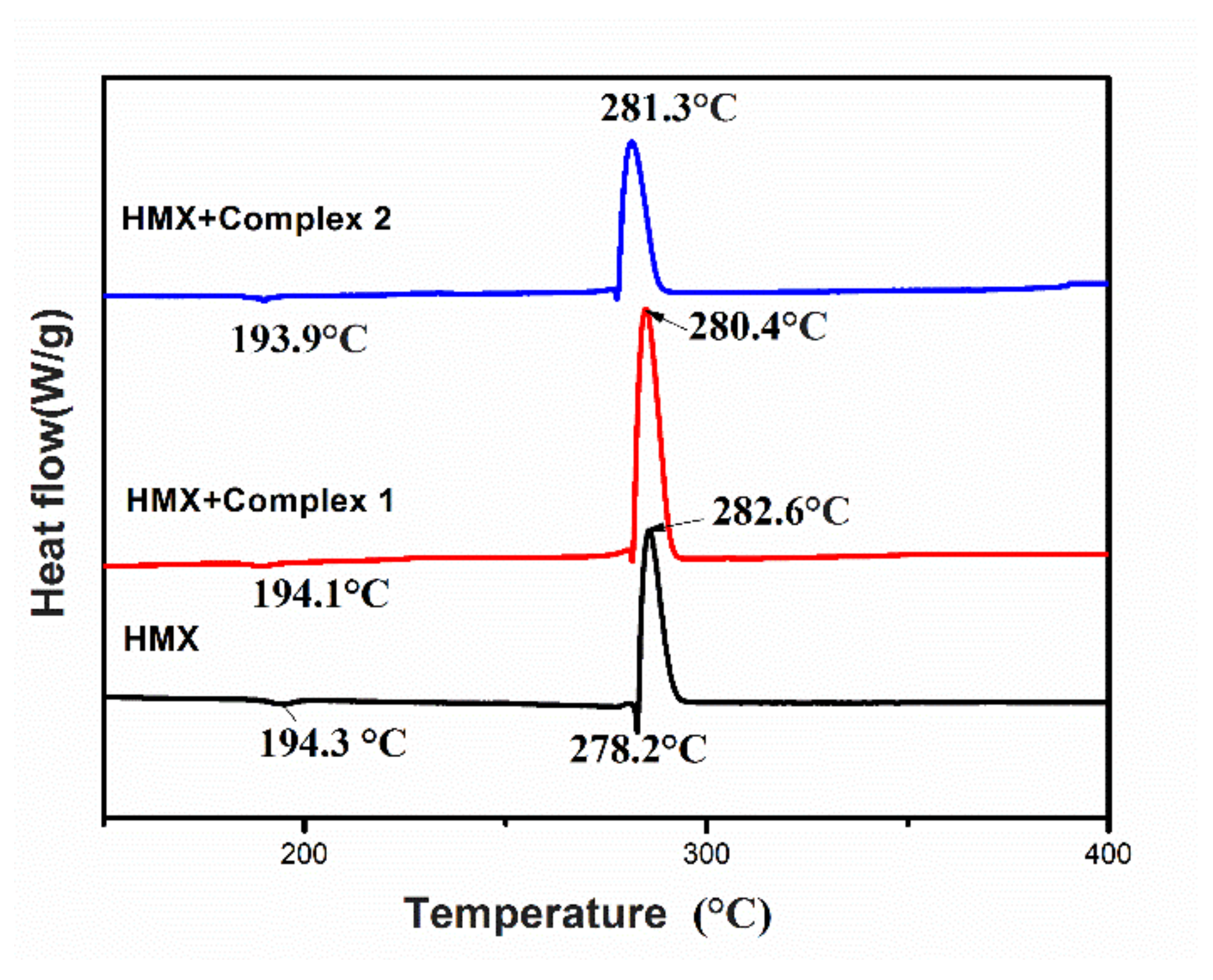
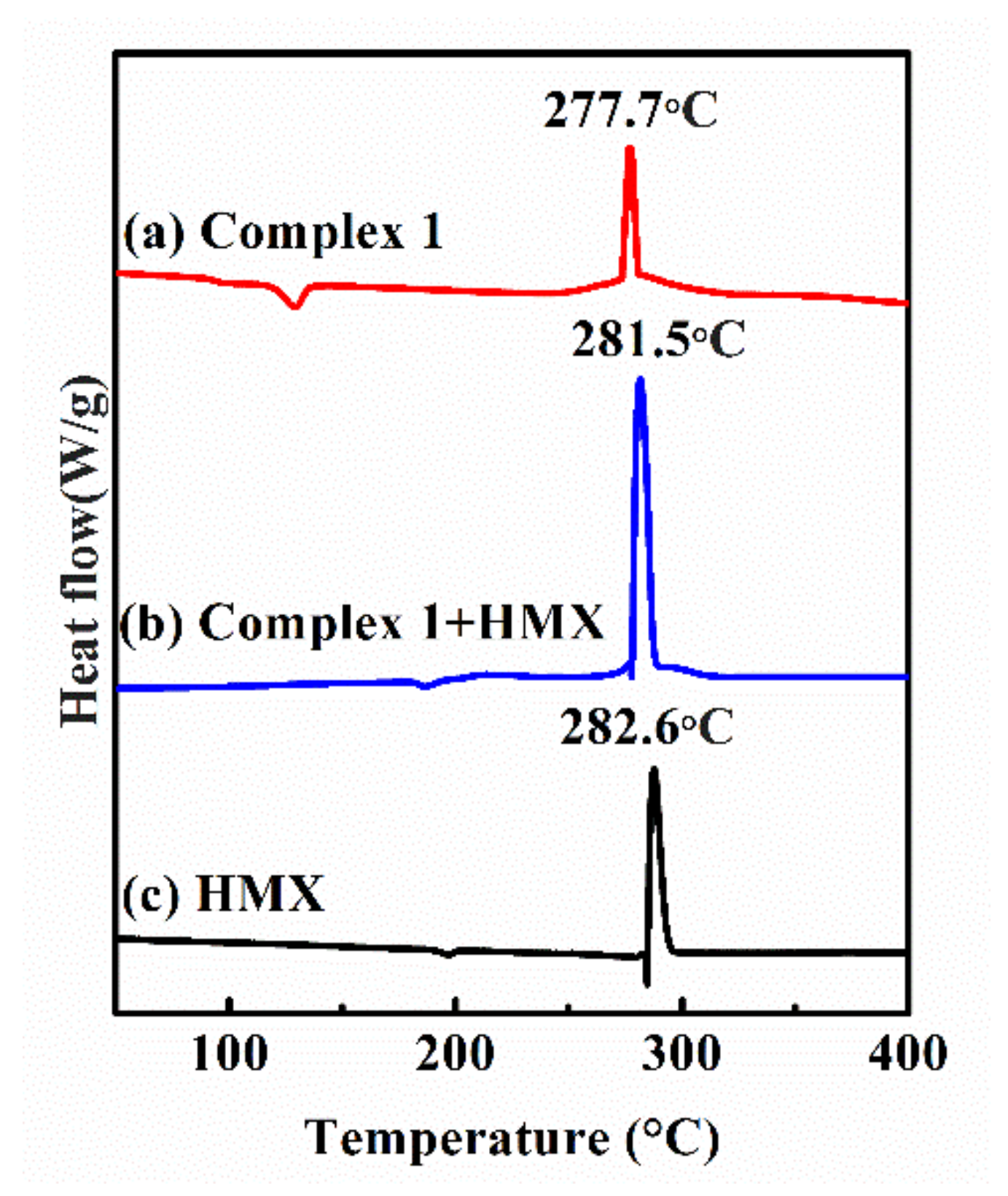
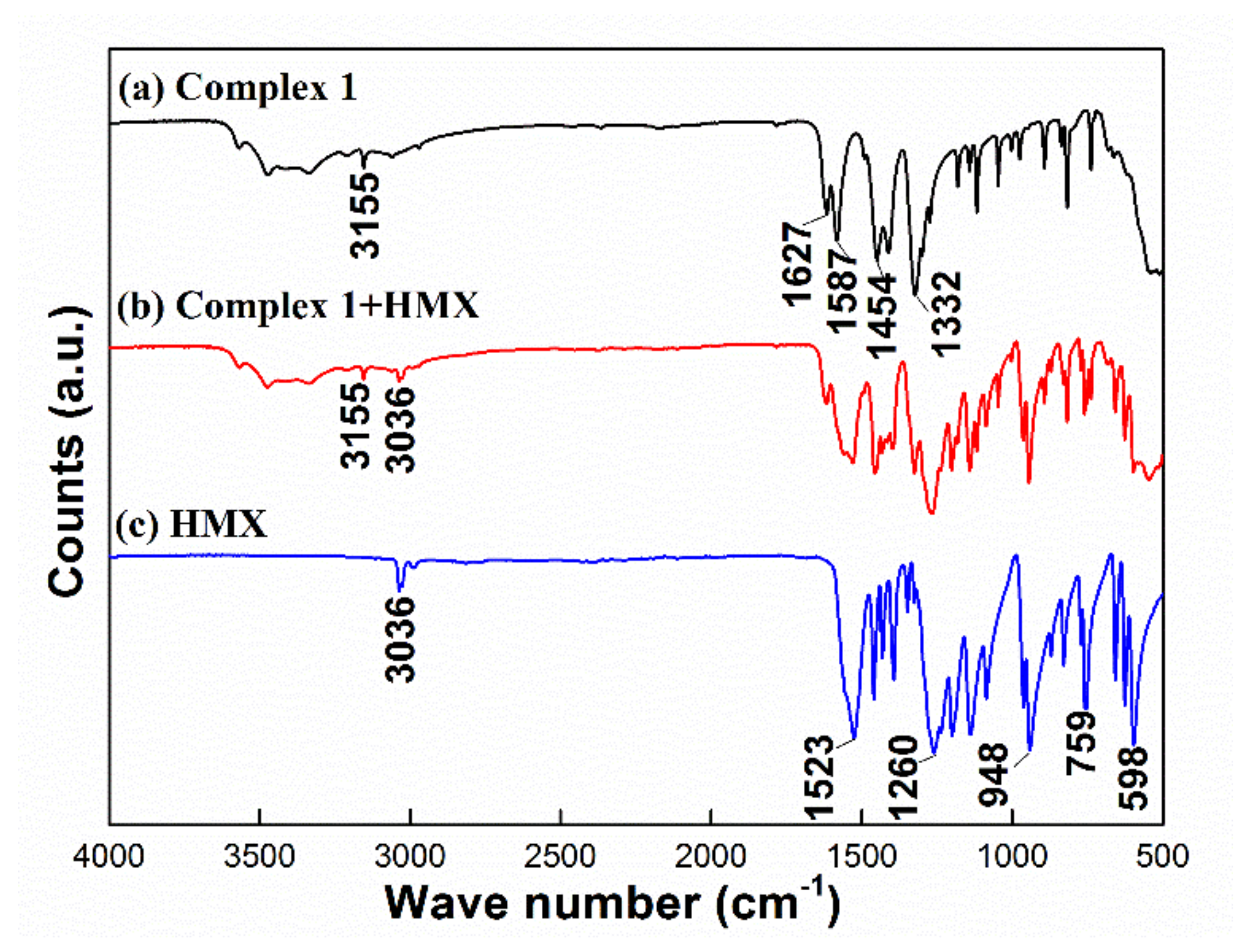
| Sample | Texo (°C) | H1 (J·g−1) | ΔH 2 (J·g−1) | ΔT 3 (°C) |
|---|---|---|---|---|
| HMX | 282.6 | 1198.3 | - | - |
| HMX + complex 1 | 280.4 | 1345.7 | 129.9 | 2.2 |
| HMX + complex 2 | 281.3 | 1226.4 | 41.5 | 1.3 |
| HMX + complex 3 | 280.9 | 1286.3 | 109.8 | 1.7 |
| HMX + complex 4 | 281.0 | 1213.2 | 37.7 | 1.6 |
| HMX + complex 5 | 281.2 | 1234.3 | 53.1 | 1.4 |
| Standard (ΔTp/°C) | Rating | ||
|---|---|---|---|
| ≦2 | A | Compatible or good compatibility | Safe for use in any explosive design. |
| 3–5 | B | Slightly sensitized or fair compatibility | Safe for use in testing, when the device is used in a very short period of time; Not to be used as a binder material, or when long-term storage is desired. |
| 6–15 | C | Sensitized or poor compatibility | Not recommended for use with explosive items. |
| >15 | D | Hazardous or bad compatibility | Hazardous. Do not use under any conditions. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Wei, Z.; Song, J.; Zhang, H.; Qu, Y.; Xie, F. Synthesis and Effects of Two Novel Rare-Earth Energetic Complexes on Thermal Decomposition of Cyclotetramethylene Tetranitramine (HMX). Materials 2020, 13, 2811. https://doi.org/10.3390/ma13122811
Cao X, Wei Z, Song J, Zhang H, Qu Y, Xie F. Synthesis and Effects of Two Novel Rare-Earth Energetic Complexes on Thermal Decomposition of Cyclotetramethylene Tetranitramine (HMX). Materials. 2020; 13(12):2811. https://doi.org/10.3390/ma13122811
Chicago/Turabian StyleCao, Xuefang, Zhixian Wei, Jiangfeng Song, Hedan Zhang, Yuanyuan Qu, and Fei Xie. 2020. "Synthesis and Effects of Two Novel Rare-Earth Energetic Complexes on Thermal Decomposition of Cyclotetramethylene Tetranitramine (HMX)" Materials 13, no. 12: 2811. https://doi.org/10.3390/ma13122811
APA StyleCao, X., Wei, Z., Song, J., Zhang, H., Qu, Y., & Xie, F. (2020). Synthesis and Effects of Two Novel Rare-Earth Energetic Complexes on Thermal Decomposition of Cyclotetramethylene Tetranitramine (HMX). Materials, 13(12), 2811. https://doi.org/10.3390/ma13122811





