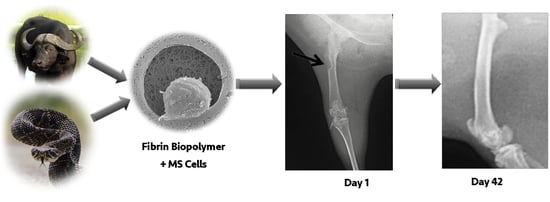
Highly Effective Fibrin Biopolymer Scaffold for Stem Cells Upgrading Bone Regeneration
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Ethical Approval
2.2. Fibrin Biopolymer (FBP)
2.3. Cell Isolation and Culture
2.4. Osteogenic Differentiation of MSCs
2.5. Animals and Surgical Protocols
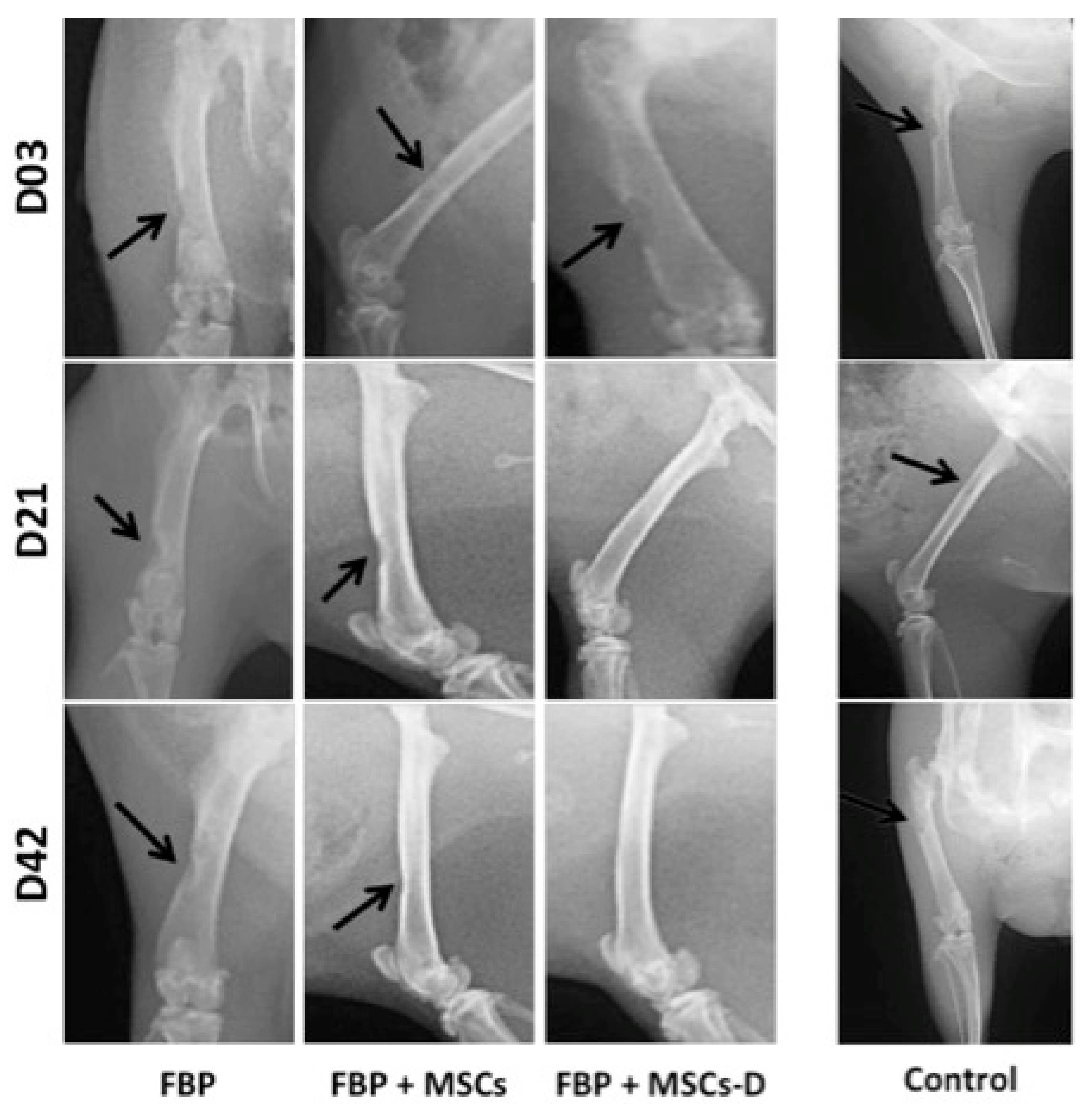
2.6. Radiographic Evaluation
2.7. Histological Analysis
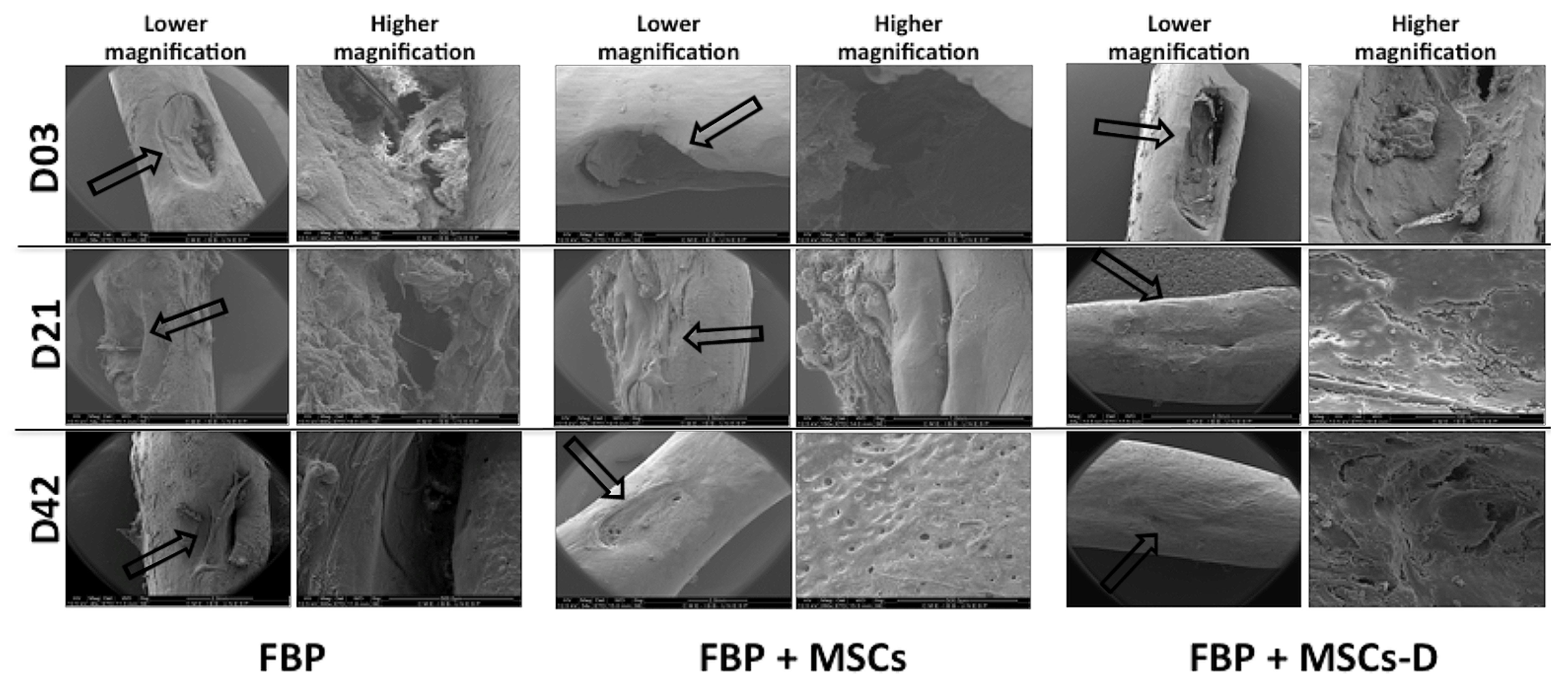
2.8. Scanning Electron Microscopy (SEM)
3. Results
3.1. MSCs Expansion and Characterization
3.2. MSCs Osteogenic Differentiation
3.3. Radiographic Evaluation
3.4. Scanning Electron Microscopy (SEM)
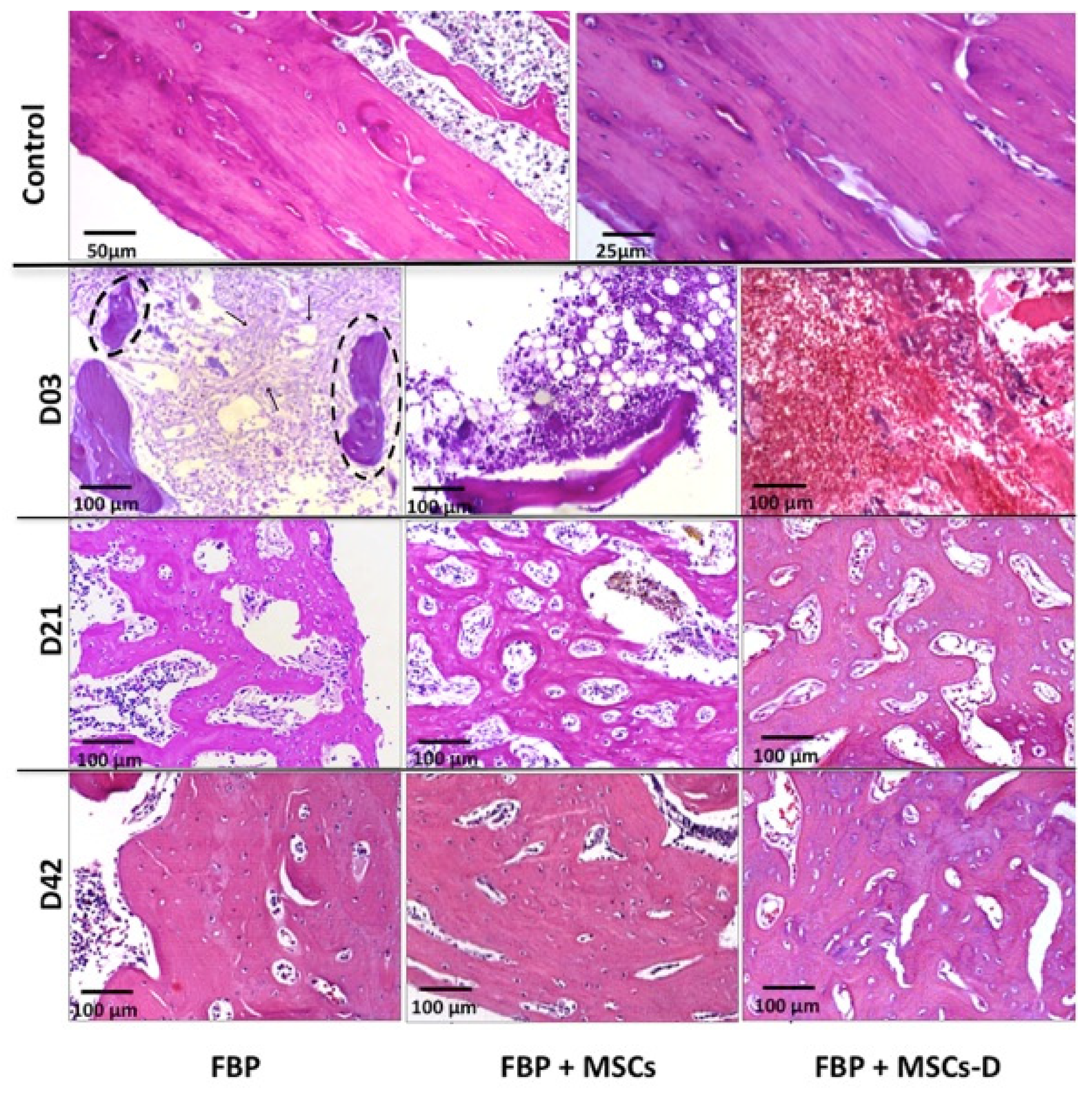
3.5. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weir, M.D.; Xu, H.H.K. Human bone marrow stem cell-encapsulating calcium Phosphate scaffolds for bone repair. Acta Biomater. 2010, 6, 4118–4126. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E. Biopolymers for Hard and Soft Engineered Tissues: Application in Odontoiatric and Plastic Surgery Field. Polymers 2011, 3, 509–526. [Google Scholar] [CrossRef]
- Stock, U.A.; Vacanti, J.P. Tissue engineering: Current state and prospects. Ann. Rev. Med. 2001, 52, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Griffith, M.; Hincke, M. Characterization and inhibition of fibrin hydrogel degrading enzymes during development of tissue engineering scaffolds. Tissue Eng. 2007, 13, 1469–1477. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Dare, E.V.; Griffith, M.; Poitras, P.; Kaupp, J.A.; Waldman, S.D.; Carlsson, D.J. Genipin cross-linked fibrin hydrogels for in vitro human articular cartilage tissue-engineered regeneration. Cells Tissues Organs. 2009, 190, 313–325. [Google Scholar] [CrossRef]
- Clines, G.A. Prospects for osteoprogenitor stem cells in fracture repair and osteoporosis. Curr. Opin. Organ. Transplant. 2010, 15, 73–78. [Google Scholar] [CrossRef]
- Vilquin, J.T.; Rosset, P. Mesenchymal stem cells in bone and cartilage repair: Current status. Regen. Med. 2006, 1, 589–604. [Google Scholar] [CrossRef]
- Panetta, N.J.; Gupta, D.M.; Quarto, N.; Longaker, M.T. Mesenchymal cells for skeletal tissue engineering. Panminerva Med. 2009, 51, 25–41. [Google Scholar]
- Buchaim, D.V.; Cassaro, C.V.; Shindo, J.V.T.C.; Coletta, B.B.D.; Pomini, K.T.; Rosso, M.P.O.; Campos, L.M.G.; Ferreira, R.S., Jr.; Barraviera, B.; Buchaim, R.L. Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties: A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190038. [Google Scholar] [CrossRef]
- Cassaro, C.V.; Justulin, L.A., Jr.; de Lima, P.R.; Golim, M.A.; Biscola, N.P.; de Castro, M.V.; de Oliveira, A.L.R.; Doiche, D.P.; Pereira, E.J.; Ferreira, R.S., Jr.; et al. Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190027. [Google Scholar] [CrossRef] [PubMed]
- Boo, J.S.; Yamada, Y.; Okazaki, Y.; Hibino, Y.; Okada, K.; Hata, K. Tissue-engineered bone using mesenchymal stem cells and a biodegradable scaffold. J. Craniofac. Surg. 2002, 13, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Song, J.H.Z.; Huang, A.P.K.; Zhipeng, G. The scaffold microenvironment for stem cell based bone tissue engineering. Biomater. Sci. 2017, 5, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, S.; Okamoto, Y.; Uchiyama, S.; Nakatsuma, A.; Hashimoto, K.; Ohnishi, S.T. Royal jelly prevents osteoporosis in rats: Beneficial effects in ovariectomy model and in bone tissue culture model. Evid. Based Complement. Alternat. Med. 2006, 3, 339–348. [Google Scholar] [CrossRef]
- Gasparotto, V.P.O.; Landim-Alvarenga, F.C.; Oliveira, A.L.R.; Simões, G.F.; Lima-Neto, J.F.; Barraviera, B.; Ferreira, R.S., Jr. A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C- Mater. Biol. 2017, 1, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Bruder, S.P.; Jaiswal, N.; Ricalton, N.S.; Mosca, J.D.; Kraus, K.H.; Kadiyala, S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin. Orthop. Relat. Res. 1998, 355, 247–356. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Giulivi, A.; Griffith, M.; Hincke, M. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage substitute. Tissue Eng. Part A 2001, 17, 323–335. [Google Scholar] [CrossRef]
- Alving, B.M.; Weinstein, M.J.; Finlayson, J.S.; Menitove, J.E.; Fratantoni, J.C. Fibrin sealant: Summary of a conference on characteristics and clinical uses. Transfusion 1995, 35, 783–790. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Fibrin Sealant: The only approved hemostat, sealant, and adhesive- A laboratory and clinical perspective. ISRN Surg. 2014, 203943, 1–28. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.C.; Ferreira, R.S., Jr.; Barraviera, S.R.C.S.; Stolf, H.O.; Thomazini-Santos, I.A.; Mendes-Giannini, M.J.; Barraviera, B. A new fibrin sealant from Crotalus durissus terrificus venom: Applications in medicine. J. Toxicol. Environ. Health 2009, 12, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Thomazini-Santos, I.A.; Barraviera, S.R.C.S.; Mendes-Giannini, M.J.; Barraviera, B. Surgical adhesives. J. Venom Anim. Toxins 2001, 7, 1–10. [Google Scholar] [CrossRef]
- Hino, M.; Ishiko, O.; Honda, K.I.; Yamane, T.; Ohta, K.; Takubo, T. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br. J. Haematol. 2000, 108, 194–205. [Google Scholar] [CrossRef]
- Kawamura, M.; Sawafuji, M.; Watanabe, M.; Horinouchi, H.; Kobayashi, K. Frequency of transmission of human parvovirus B19 infection by fibrin sealant used during thoracic surgery. Ann. Thorac. Surg. 2002, 73, 1098–1100. [Google Scholar] [CrossRef]
- Dhillon, S. Fibrin sealant (evicel® [quixil®/crosseal™]): A review of its use as supportive treatment for haemostasis in surgery. Drugs 2011, 71, 1893–1915. [Google Scholar] [CrossRef]
- Barros, L.C.; Soares, A.M.; Costa, F.L.; Rodrigues, V.M.; Fuly, A.L.; Giglio, J.R.; Barraviera, B.; Ferreira, R.S., Jr. Biochemical and biological evaluation of gyroxin isolated from Crotalus durissus terrificus venom. J. Venom Anim. Toxins Incl. Trop. Dis. 2011, 17, 23–33. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Andreo, J.C.; Barraviera, B.; Ferreira Junior, R.S.; Buchaim, D.V.; Rosa Junior, G.M.; de Oliveira, A.L.; Rodrigues, A.C. Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom. Injury 2015, 46, 655–660. [Google Scholar] [CrossRef]
- Cunha, M.R.D.; Menezes, F.A.; Santos, G.R.; Pinto, C.A.L.; Barraviera, B.; Martins, V.C.A.; Plepis, A.M.G.; Ferreira, R.S., Jr. Hydroxyapatite and a new fibrin sealant derived from snake venom as scaffold to treatment of cranial defects in rats. Mater. Res. 2015, 18, 196–203. [Google Scholar] [CrossRef]
- Machado, E.G.; Issa, J.P.; Figueiredo, F.A.; Santos, G.R.; Galdeano, E.A.; Alves, M.C.; Chacon, E.L.; Ferreira Junior, R.S.; Barraviera, B.; Cunha, M.R. A new heterologous fibrin sealant as scaffold to recombinant human bone morphogenetic protein-2 (rhBMP-2) and natural latex proteins for the repair of tibial bone defects. Acta Histochem. 2015, 117, 288–296. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Rodrigues, A.C.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.; Junior, G.M.R.; Souza, B.C.R.; Roque, D.D.; Dias, D.V.; Dare, L.R.; et al. The new heterologous fibrin sealant in combination with low-level laser therapy (LLLT) in the repair of the buccal branch of the facial nerve. Lasers Med. Sci. 2016, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.S., Jr.; Barros, L.C.; Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; Pontes, L.G.; Santos, L.D.; Barraviera, B. Heterologous fibrin sealant derived from snake venom: From bench to bedside—An overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 21. [Google Scholar] [CrossRef] [PubMed]
- Biscola, N.P.; Cartarozzi, L.P.; Ulian-Benitez, S.; Barbizan, R.; Castro, M.V.; Spejo, A.B.; Ferreira, R.R., Jr.; Barraviera, B.; Oliveira, A.L.R. Multiple uses of fibrin sealant for nervous system treatment following injury and disease. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Cartarozzi, L.P.; Spejo, A.B.; Ferreira, R.S.; Barraviera, B.; Duek, E.; Carvalho, J.L.; Góes, A.M.; Oliveira, A.L.R. Mesenchymal stem cells engrafted in a fibrin scaffold stimulate Schwann cell reactivity and axonal regeneration following sciatic nerve tubulization. Brain Res. Bull. 2015, 112, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; Ferreira, R.S., Jr.; Carneiro, M.T.R.; Medolago, N.B.; Barraviera, B. A new fibrin sealant derived from snake venom candidate to treat chronic venous ulcers. J. Am. Acad. Dermatol. 2015, 72, AB271. [Google Scholar]
- Orsi, P.R.; Landim-Alvarenga, F.C.; Justulin, L.A.; Kaneno, R.; Golim, M.A.; Dos Santos, D.C.; Creste, C.F.Z.; Oba, E.; Maia, L.; Barraviera, B.; et al. A unique heterologous fibrin sealant (HFS) as a candidate biological scaffold for mesenchymal stem cells in osteoporotic rats. Stem Cell Res. Ther. 2017, 8, 205. [Google Scholar] [CrossRef]
- Ben-Ari, A.; Rivkin, R.; Frishman, M.; Gaberman, E.; Levdansky, L.; Gorodetsky, R. Isolation and implantation of bone marrow-derived mesenchymal stem cells with fibrin micro beads to repair a critical-size bone defect in mice. Tissue Eng. A 2009, 15, 2537–2546. [Google Scholar] [CrossRef]
- Langenbach, F.; Naujoks, C.; Laser, A.; Kelz, M.; Kersten-Thiele, P.; Berr, K.; Depprich, R.; Kübler, N.; Kögler, G.; Handschel, J. Improvement of the cell-loading efficiency of biomaterials by inoculation with stem cell-based microspheres, in osteogenesis. J. Biomater. 2012, 26, 549–564. [Google Scholar] [CrossRef]
- Khodakaram-Tafti, A.; Mehrabani, D.; Shaterzadeh-Yazdi, H. An overview on autologous fibrin glue in bone tissue engineering of maxillofacial surgery. Dent. Res. J. 2017, 14, 79–86. [Google Scholar]
- Roura, S.; Gálvez-Montón, C.; Bayes-Genis, A. Fibrin, the preferred scaffold for cell transplantation after myocardial infarction? An old molecule with a new life. J. Tissue Eng. Regen. Med. 2016, 11, 2304–2313. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W.I.; Park, W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial. J. Pediatr. 2014, 164, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, S.H.; Youn, Y.J.; Ahn, M.S.; Kim, J.Y.; Yoo, B.S.; Yoon, J.; Kwon, W.; Hong, I.S.; Lee, K.; et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J. Korean Med. Sci. 2014, 29, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A.; Lu, H.H.; Ko, F.K.; Freeman, J.W.; Laurencin, C.T. Fiber based tissue engineering scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials 2005, 26, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Partially nanofibrous architecture of 3D tissue engineering scaffolds. Biomaterials 2009, 30, 6426–6434. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M.; Hoque, M.E.; Prasad, R.G.; Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. J. Biomed. Mater. Res. A 2014, 103, 2460–2481. [Google Scholar] [CrossRef]
- Tour, G.; Wendel, M.; Tcacencu, I. Cell-derived matrix enhances osteogenic properties of hydroxyapatite. Tissue Eng. Part A 2010, 17, 127–137. [Google Scholar] [CrossRef]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Xu, C.; Su, P.; Wang, Y.; Chen, X.; Meng, Y.; Liu, C.; Yu, X.; Yang, X.; Yu, W.; Zhang, X.; et al. A novel biomimetic composite scaffold hybridized with mesenchymal stem cells in repair of rat bone defects models. J. Biomed. Mater. Res. 2010, 95, 465–503. [Google Scholar]
- Xiao, Q.; Wang, S.K.; Tian, H.; Xin, L.; Zou, Z.G.; Hu, Y.L.; Chang, C.M.; Wang, X.Y.; Yin, Q.S.; Zhang, X.H.; et al. TNF-α increases bone marrow mesenchymal stem cell migration to ischemic tissues. Cell Biochem. Biophys. 2012, 62, 409–414. [Google Scholar] [CrossRef]
- Dayan, D.; Hiss, Y.; Hirshberg, A.; Bubis, J.J.; Wolman, M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry 1989, 93, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, C.; Ng, R.; Tan, S.; Kim, S.; Wu, B.; Lee, M. Photopolymerizable chitosan-collagen hydrogels for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 64–74. [Google Scholar] [CrossRef]
- Laurencin, C.; Khan, Y.; EI-Amin, S.F. Bone grafts substitutes. Expert Rev. Med. Devices 2006, 3, 49–57. [Google Scholar] [CrossRef]
- Kong, L.; Zheng, L.Z.; Qin, L.; Ho, K.K.W. Role of mesenchymal stem cells in osteoarthritis treatment. J. Orthop. Transl. 2017, 9, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D.; Prabhu, R. Fibrin sealant tissue adhesive–review and update. J. Long Term Eff. Med. Implants 2005, 15, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Spejo, A.B.; Chiarotto, G.B.; Ferreira, A.D.F.; Gomes, D.A.; Ferreira, R.S.; Barraviera, B.; Oliveira, A.L.R. Neuroprotection and immunomodulation following intraspinalaxotomy of motoneurons by treatment with adult mesenchymal stem cells. J. Neuroinflammation 2018, 15, 230. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F. Bone developmented and its relation to factory repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cells Mater. 2008, 15, 56–73. [Google Scholar]
- Boxall, S.A.; Jones, E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012, 97587, 1–12. [Google Scholar]
- Casteilla, L.; Benard, V.P.; Laharrague, P.; Cousin, B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J. Stem Cells 2011, 3, 25–33. [Google Scholar] [CrossRef]
- Mankani, M.H.; Kuznetsov, S.A.; Avila, N.A.; Kingman, A.; Robey, P.G. Bone formation in transplants of human bone marrow stromal cells and hydroxyapatite-tricalcium phosphate: Prediction with quantitative CT in mice. Radiology 2004, 230, 369–376. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Zwingenberger, S.; Gibon, E.; Goodman, S.B.; Li, G. Mesenchymal stem cells homing to improve bone healing. J. Orthop. Transl. 2017, 9, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Onimowo, J.O.; Khan, W.S. Bone marrow derived stem cells in trauma and orthopaedics: A review of the current trend. Curr. Stem Cell Res. Ther. 2014, 10, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, W.; Yang, T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol. Res. 2015, 48, 62. [Google Scholar] [CrossRef]
- Ito, H. Chemokines in mesenchymal stem cell therapy for bone repair: A novel concept of recruiting mesenchymal stem cells and the possible cell sources. Mod. Rheumatol. 2011, 21, 113–121. [Google Scholar] [CrossRef]
- Nakamizo, A.; Marini, F.; Amano, T.; Khan, A.; Studeny, M.; Gumin, J.; Chen, J.; Hentschel, S.; Vecil, G.; Dembinski, J.; et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005, 65, 3307–3318. [Google Scholar] [CrossRef]
- Dwyer, R.M.; Khan, S.; Barry, F.P.; O’Brien, T.; Kerin, M.J. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res. Ther. 2010, 1, 25. [Google Scholar] [CrossRef]
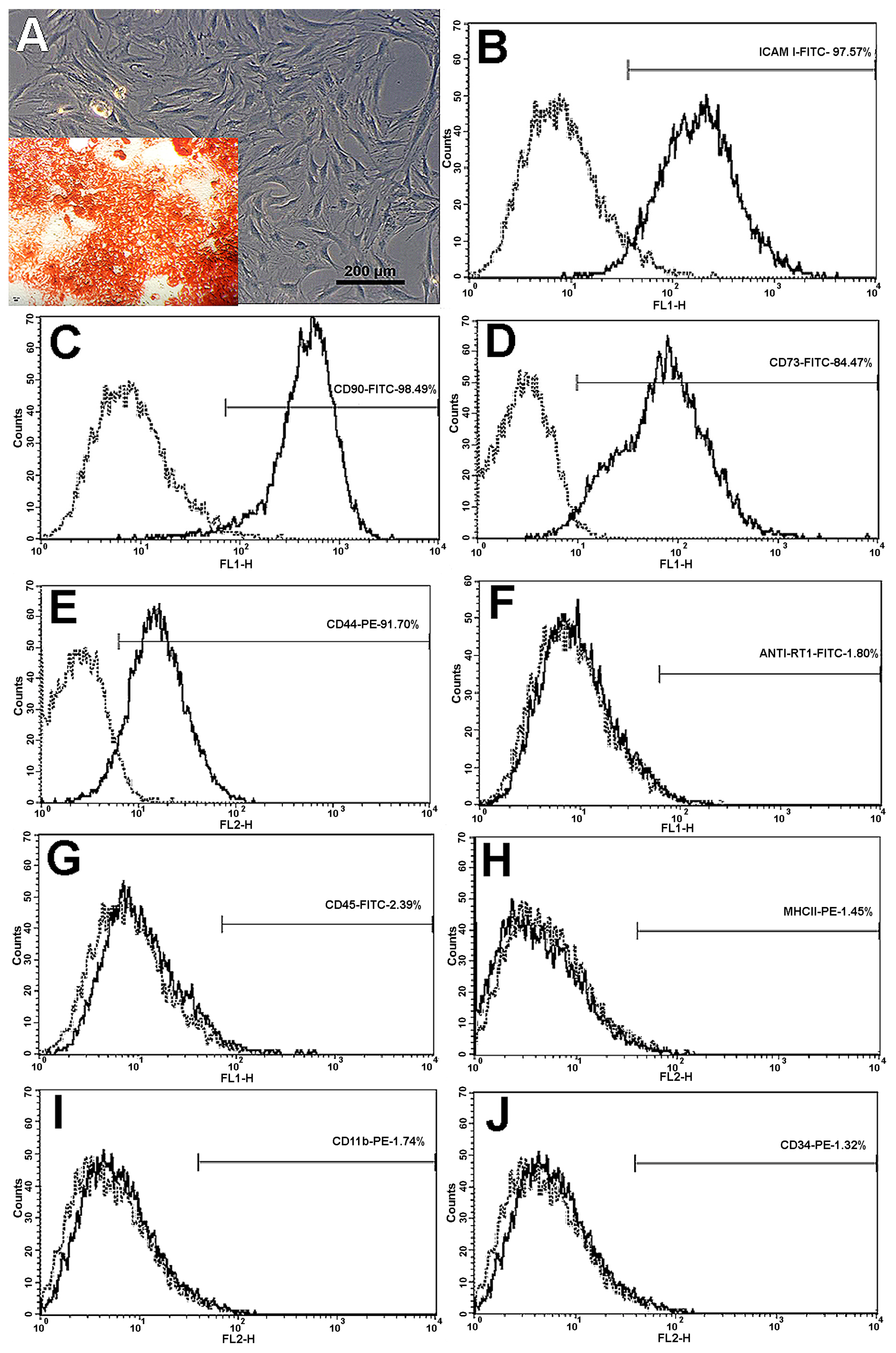

| Negative Markers | |
| RT1 | anti-RT1-Aw2-FITC, clone MRC OX-18; Abcam, Cambridge, MA, USA |
| CD34 | anti-CD34-PE, clone ICO-115; Abcam, Cambridge, MA, USA |
| CD11b | anti-CD11b-PE, clone ED8; Abcam, Cambridge, MA, USA |
| CD45 | anti-CD45-FITC, clone MRC OX-1; Abcam, Cambridge, MA, USA |
| MHCII | anti-rat MHC CLASS II RT1D-PE, clone MRC OX-17; Abcam, Cambridge, MA, USA |
| Positive Markers | |
| CD73 | purified mouse anti-rat CD73; clone 5F/B9, BD Pharmingen, San Diego, CA, USA |
| CD90 | anti-CD90/Thy1-FITC, clone FITC.MRC OX-7; Abcam, Cambridge, MA, USA |
| CD44 | anti-CD44-PE, clone OX-50; Abcam, Cambridge, MA, USA |
| ICAM-I | anti-ICAM-I-FITC, clone 1A29; Abcam, Cambridge, MA, USA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Creste, C.F.Z.; Orsi, P.R.; Landim-Alvarenga, F.C.; Justulin, L.A.; Golim, M.d.A.; Barraviera, B.; Ferreira, R.S., Jr. Highly Effective Fibrin Biopolymer Scaffold for Stem Cells Upgrading Bone Regeneration. Materials 2020, 13, 2747. https://doi.org/10.3390/ma13122747
Creste CFZ, Orsi PR, Landim-Alvarenga FC, Justulin LA, Golim MdA, Barraviera B, Ferreira RS Jr. Highly Effective Fibrin Biopolymer Scaffold for Stem Cells Upgrading Bone Regeneration. Materials. 2020; 13(12):2747. https://doi.org/10.3390/ma13122747
Chicago/Turabian StyleCreste, Camila Fernanda Zorzella, Patrícia Rodrigues Orsi, Fernanda Cruz Landim-Alvarenga, Luis Antônio Justulin, Marjorie de Assis Golim, Benedito Barraviera, and Rui Seabra Ferreira, Jr. 2020. "Highly Effective Fibrin Biopolymer Scaffold for Stem Cells Upgrading Bone Regeneration" Materials 13, no. 12: 2747. https://doi.org/10.3390/ma13122747
APA StyleCreste, C. F. Z., Orsi, P. R., Landim-Alvarenga, F. C., Justulin, L. A., Golim, M. d. A., Barraviera, B., & Ferreira, R. S., Jr. (2020). Highly Effective Fibrin Biopolymer Scaffold for Stem Cells Upgrading Bone Regeneration. Materials, 13(12), 2747. https://doi.org/10.3390/ma13122747