Optimization of Washing Processes in Solvothermal Synthesis of Nickel-Based MOF-74
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
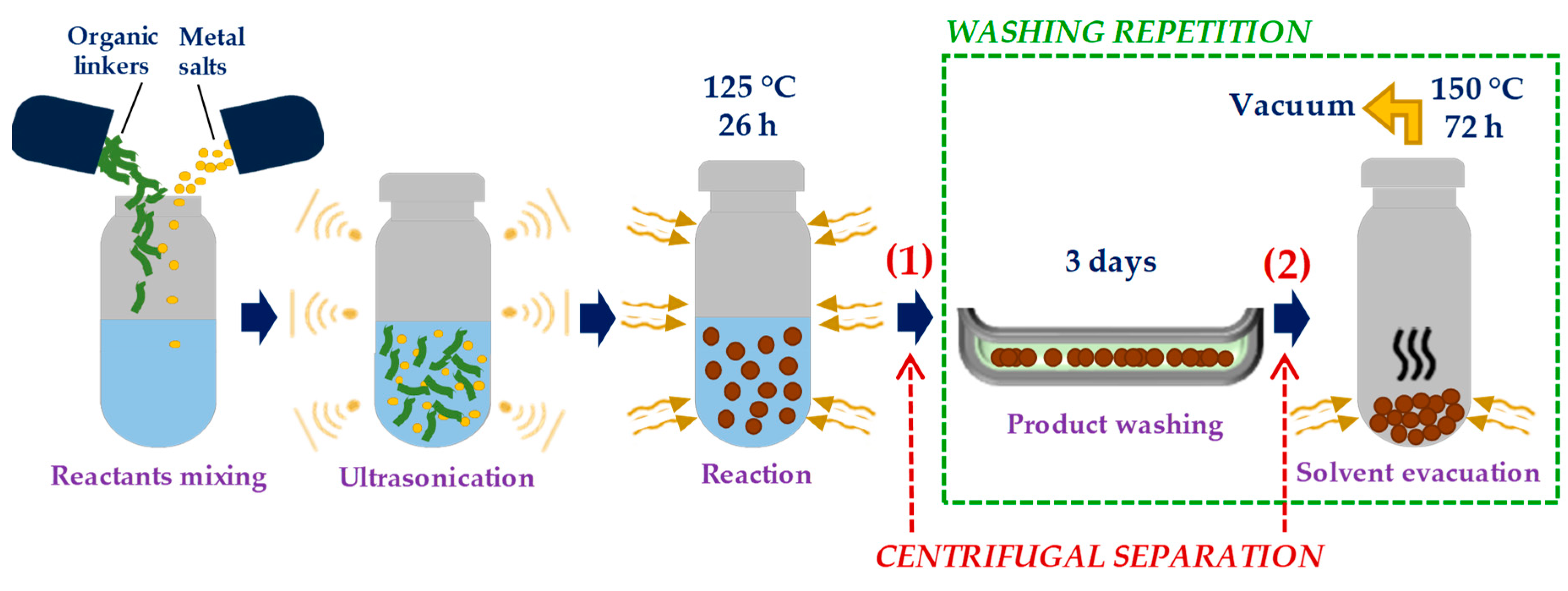
2.2. Synthesis of Ni-MOF-74
2.3. Optimization of Washing Processes
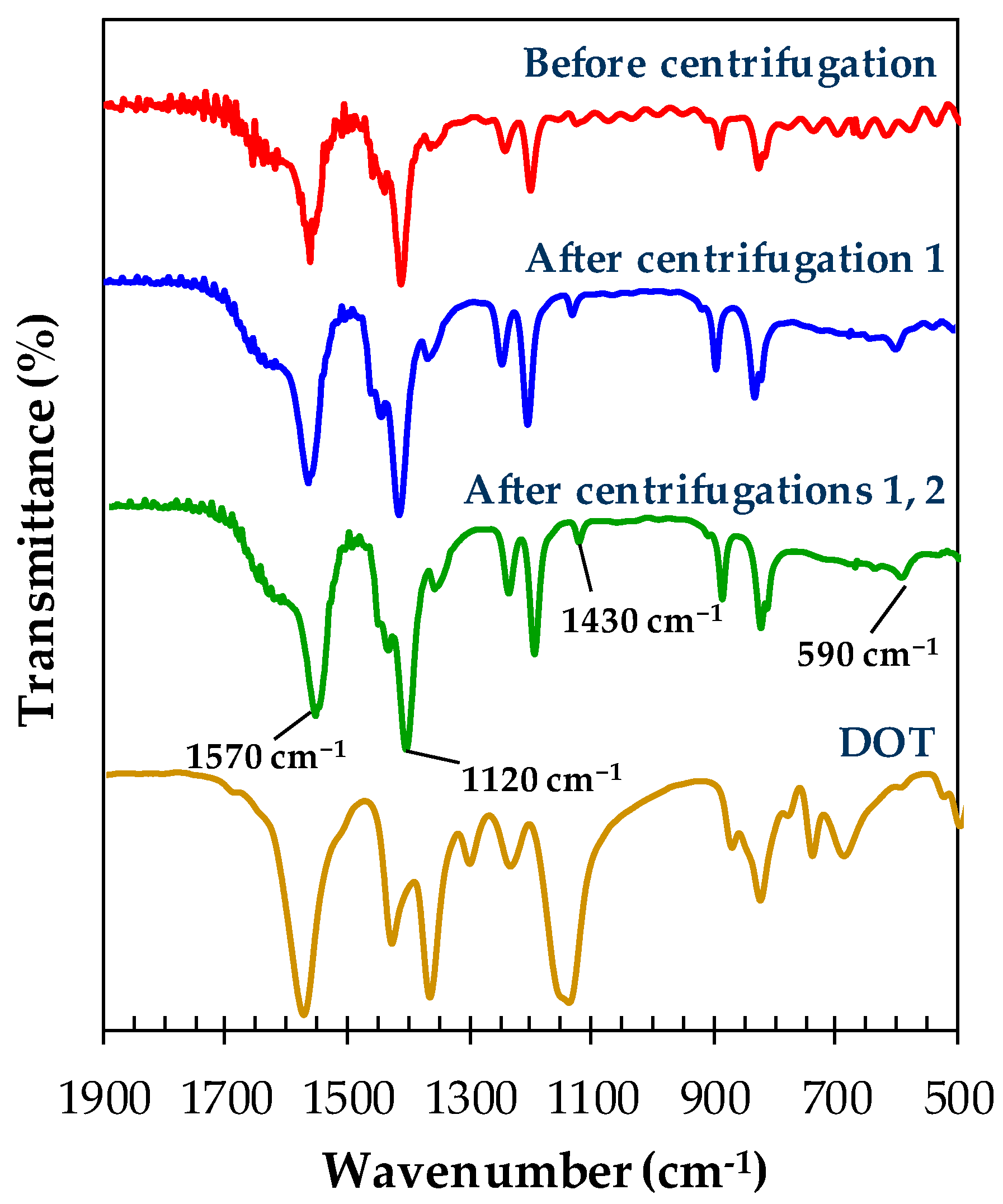
2.4. Characterization of Materials
2.5. Adsorption Isotherm Measurements
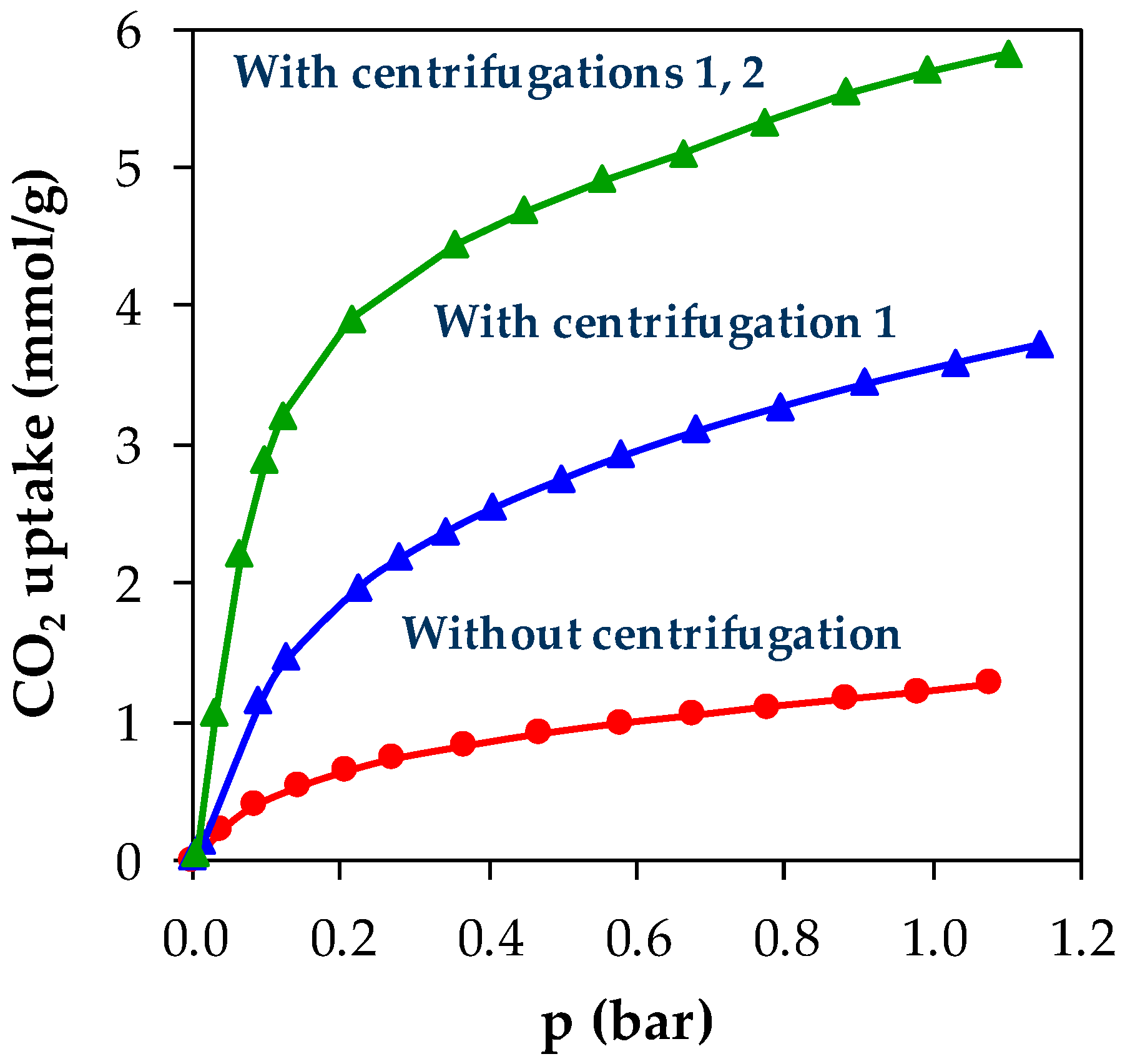
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 1999, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Wang, H.; Deibert, B.J.; Li, J. Chapter 4: Alkaline earth metal-based metal-organic frameworks: Synthesis, Properties and Applications. In The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications, 1st ed.; Kaskel, S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 73–99. [Google Scholar]
- Yusran, Y.; Xu, D.; Fang, Q.; Zhang, D.; Qiu, S. MOF-derived Co@NC nanocatalyst for catalytic reduction of 4-nitrophenol to 4-aminophenol. Micropor. Mesopor. Mater. 2017, 241, 346–354. [Google Scholar] [CrossRef]
- Pentyala, V.; Davydovskaya, P.; Pohle, R.; Urban, G.; Yurchenko, O. Mg-MOF74 and Co-MOF74 as sensing layers for CO2 detection. Procedia Eng. 2014, 87, 1071–1074. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal-organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. 2006, 118, 6120–6124. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Deep, A.; Kwon, E.E.; Brown, R.J.C.; Kim, K.-H. Performance comparison of MOF and other sorbent materials in removing key odorants emitted from pigpen slurry. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shioyama, H.; Jiang, H.; Zhang, X.; Xu, Q. Metal-organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor. Carbon 2010, 48, 456–463. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2011, 112, 1126–1162. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline metal-organic frameworks (MOFs): Synthesis, structure and function. Acta Crystallogr. Sect. B 2014, 70, 3–10. [Google Scholar] [CrossRef]
- Lopez, M.; Canepa, P.; Thonhauser, T. NMR study of small molecule adsorption in MOF-74-Mg. J. Chem. Phys. 2013, 138, 154704. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Palomino, G.T.; Areán, C.O.; Bordiga, S. Computational and experimental studies on the adsorption of CO, N2, and CO2 on Mg-MOF-74. J. Phys. Chem. C 2010, 114, 11185–11191. [Google Scholar] [CrossRef]
- Yang, D.-A.; Cho, H.-Y.; Kim, J.; Yang, S.-T.; Ahn, W.-S. CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ. Sci. 2012, 5, 6465–6473. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Yang, D.-A.; Kim, J.; Jeong, S.-Y.; Ahn, W.-S. CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating. Catal. Today 2012, 185, 35–40. [Google Scholar] [CrossRef]
- Wu, X.; Bao, Z.; Yuan, B.; Wang, J.; Sun, Y.; Luo, H.; Deng, S. Microwave synthesis and characterization of MOF-74 (M = Ni, Mg) for gas separation. Micropor. Mesopor. Mater. 2013, 180, 114–122. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lv, D.F.; Wu, J.L.; Xiao, J.; Xi, H.X.; Xia, Q.B.; Li, Z. A new MOF-505@GO composite with high selectivity for CO2/CH4 and CO2/N2 separation. Chem. Eng. J. 2017, 308, 1065–1072. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Yi, T.-F.; Jiang, L.-J.; Shu, J.; Yue, C.-B.; Zhu, R.-S.; Qiao, H.-B. Recent development and application of Li4Ti5O12 as anode material of lithium ion battery. J. Phys. Chem. Solids 2010, 71, 1236–1242. [Google Scholar] [CrossRef]
- Larabi, C. Surface Organometallic Chemistry on Metal Organic Frameworks (MOF): Synthesis, Characterization and Their Application in Catalysis. Ph.D. Thesis, Université Claude Bernard–Lyon I, Lyon, France, 13 January 2011. [Google Scholar]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef]
- Britt, D.; Furukawa, H.; Wang, B.; Glover, T.G.; Yaghi, O.M. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proc. Natl. Acad. Sci. USA 2009, 106, 20637–20640. [Google Scholar] [CrossRef]
- Dietzel, P.D.C.; Besikiotis, V.; Blom, R. Application of metal-organic frameworks with coordinatively unsaturated metal sites in storage and separation of methane and carbon dioxide. J. Mater. Chem. 2009, 19, 7362–7370. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, L.; Ren, Q.; Lu, X.; Deng, S. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. J. Colloid Interface Sci. 2011, 353, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Yang, J.; Li, L.; Li, J. Adsorption and separation of CO2 on Fe(II)-MOF-74: Effect of the open metal coordination site. J. Solid State Chem. 2014, 213, 224–228. [Google Scholar] [CrossRef]
- Chen, D.-L.; Shang, H.; Zhu, W.; Krishna, R. Transient breakthroughs of CO2/CH4 and C3H6/C3H8 mixtures in fixed beds packed with Ni-MOF-74. Chem. Eng. Sci. 2014, 117, 407–415. [Google Scholar] [CrossRef]
- Adhikari, A.K.; Lin, K.-S. Improving CO2 adsorption capacities and CO2/N2 separation efficiencies of MOF-74 (Ni, Co) by doping palladium-containing activated carbon. Chem. Eng. J. 2016, 284, 1348–1360. [Google Scholar] [CrossRef]
- Stuart, B.H. Chapter 4: Organic molecules. In Infrared spectroscopy: Fundamentals and applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 71–93. [Google Scholar]
- Griffiths, P.R. Beer’s Law. In Handbook of Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Maikala, R.V. Modified Beer’s law-historical perspectives and relevance in near-infrared monitoring of optical properties of human tissue. Int. J. Ind. Ergon. 2010, 40, 125–134. [Google Scholar] [CrossRef]
- Kamal, K.; Grekov, D.; Hamon, L.; Shariff, A.M.; Bustam, M.A.; Pré, P. Improving textural properties of magnesium-based metal-organic framework for gas adsorption by doping carbonaceous material. Micropor. Mesopor. Mater. 2020. under review. [Google Scholar]
- Haydar, M.A.L.; Abid, H.R.; Sunderland, B.; Wang, S. Metal organic frameworks as a drug delivery system for flurbiprofen. Drug Des. Dev. Ther. 2017, 11, 2685. [Google Scholar] [CrossRef]
- Dutta, R.; Rao, M.N.; Kumar, A. Investigation of ionic liquid interaction with ZnBDC-metal organic framework through Scanning eXAfS and inelastic neutron Scattering. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Sun, X.; Xia, Q.; Zhao, Z.; Li, Y.; Li, Z. Synthesis and adsorption performance of MIL-101(Cr)/graphite oxide composites with high capacities of n-hexane. Chem. Eng. J. 2014, 239, 226–232. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, X.; Xia, Q.; Peng, J.; Wang, H.; Li, Z. Preparation and adsorption performance of GrO@Cu-BTC for separation of CO2/CH4. Ind. Eng. Chem. Res. 2014, 53, 11176–11184. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Y.; Zhong, Q. CO2 capture on metal-organic framework and graphene oxide composite using a high-pressure static adsorption apparatus. J. Clean Energy Technol. 2014, 2, 34–37. [Google Scholar] [CrossRef]
- Yu, Z.; Deschamps, J.; Hamon, L.; Prabhakaran, P.K.; Pré, P. Hydrogen adsorption and kinetics in MIL-101 (Cr) and hybrid activated carbon-MIL-101 (Cr) materials. Int. J. Hydrog. Energy 2017, 42, 8021–8031. [Google Scholar] [CrossRef]
- Speakman, S.A. Fundamentals of Rietveld Refinement, II. Refinement of a Single Phase. MIT Cent. Mater. Sci. Eng. 2011. Available online: http://prism.mit.edu/xray/Rietveld_SinglePhase.pdf (accessed on 29 November 2011).
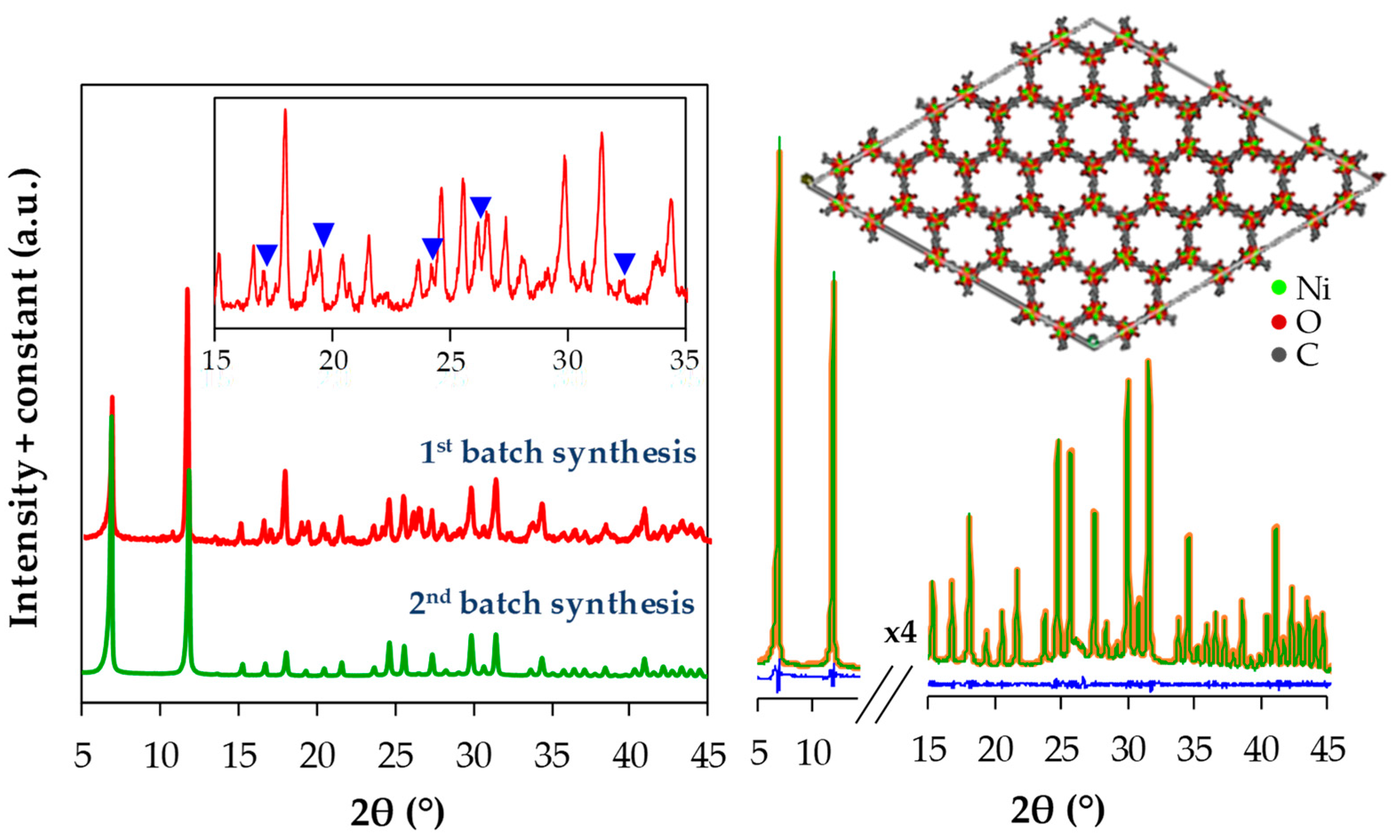
| Ni-MOF-74 Sample | Second-Batch Synthesis | Calculated from CCDC, FIJDOS 265095 |
|---|---|---|
| Crystal system | Trigonal (hexagonal axes) | |
| Angles | α = β = 90°, γ = 120° | |
| Space group | R-3 | |
| Lattice parameters | ||
| a = b (Å) | 26.009 (±3) | 26.026 |
| c (Å) | 6.777 (±1) | 6.759 |
| Unit cell volume (Å3) | 3970 | 3965 |
| Rexp | 2.39 | |
| Rp | 3.32 | |
| Rwp | 4.67 | |
| Goodness of fit | 1.95 | |
| Stage | Synthesis | CO2 Adsorption Uptake | Observation |
|---|---|---|---|
| 1 | First batch | Increases from 0.34 to 0.85 mmol/g after three successive adsorption cycles | Activation of some micropores by progressive outgassing. |
| 2 | First batch with washing repetitions | 1.22 mmol/g | Activation of some micropores by more times of washing. |
| 3 | Second batch with centrifugation (1) | 3.70 mmol/g | Good separation of reactive medium from MOF powder. |
| 4 | Second batch with centrifugations (1) and (2) | 5.80 mmol/g | Good separation of reactive medium and washing solvents from MOF powder. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, K.; Bustam, M.A.; Ismail, M.; Grekov, D.; Mohd Shariff, A.; Pré, P. Optimization of Washing Processes in Solvothermal Synthesis of Nickel-Based MOF-74. Materials 2020, 13, 2741. https://doi.org/10.3390/ma13122741
Kamal K, Bustam MA, Ismail M, Grekov D, Mohd Shariff A, Pré P. Optimization of Washing Processes in Solvothermal Synthesis of Nickel-Based MOF-74. Materials. 2020; 13(12):2741. https://doi.org/10.3390/ma13122741
Chicago/Turabian StyleKamal, Khaliesah, Mohamad Azmi Bustam, Marhaina Ismail, Denys Grekov, Azmi Mohd Shariff, and Pascaline Pré. 2020. "Optimization of Washing Processes in Solvothermal Synthesis of Nickel-Based MOF-74" Materials 13, no. 12: 2741. https://doi.org/10.3390/ma13122741
APA StyleKamal, K., Bustam, M. A., Ismail, M., Grekov, D., Mohd Shariff, A., & Pré, P. (2020). Optimization of Washing Processes in Solvothermal Synthesis of Nickel-Based MOF-74. Materials, 13(12), 2741. https://doi.org/10.3390/ma13122741






