A New Cr3+ Electrochemical Sensor Based on ATNA/Nafion/Glassy Carbon Electrode
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
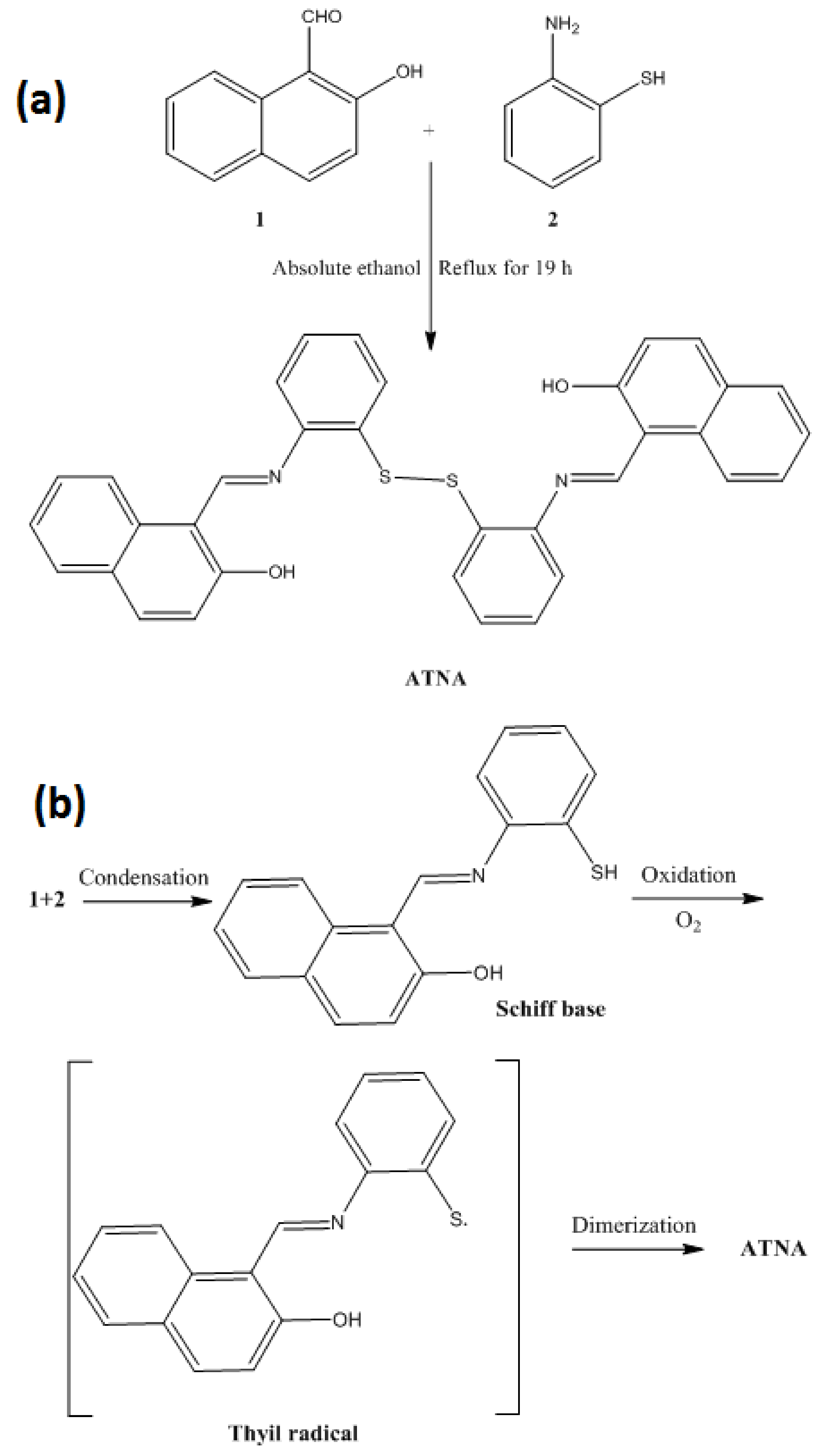
2.2. Synthesis of 1,1′-(-((disulfanediylbis(2,1-phenylene))bis(azaneylylidene))bis(methaneylylidene))bis(naphthalen-2-ol) (ATNA)
2.3. X-ray Crystallography of ATNA
2.4. Fabrication of ATNA/Nafion/GCE as a Selective Cr3+ Electrochemical Sensor
3. Results and Discussion
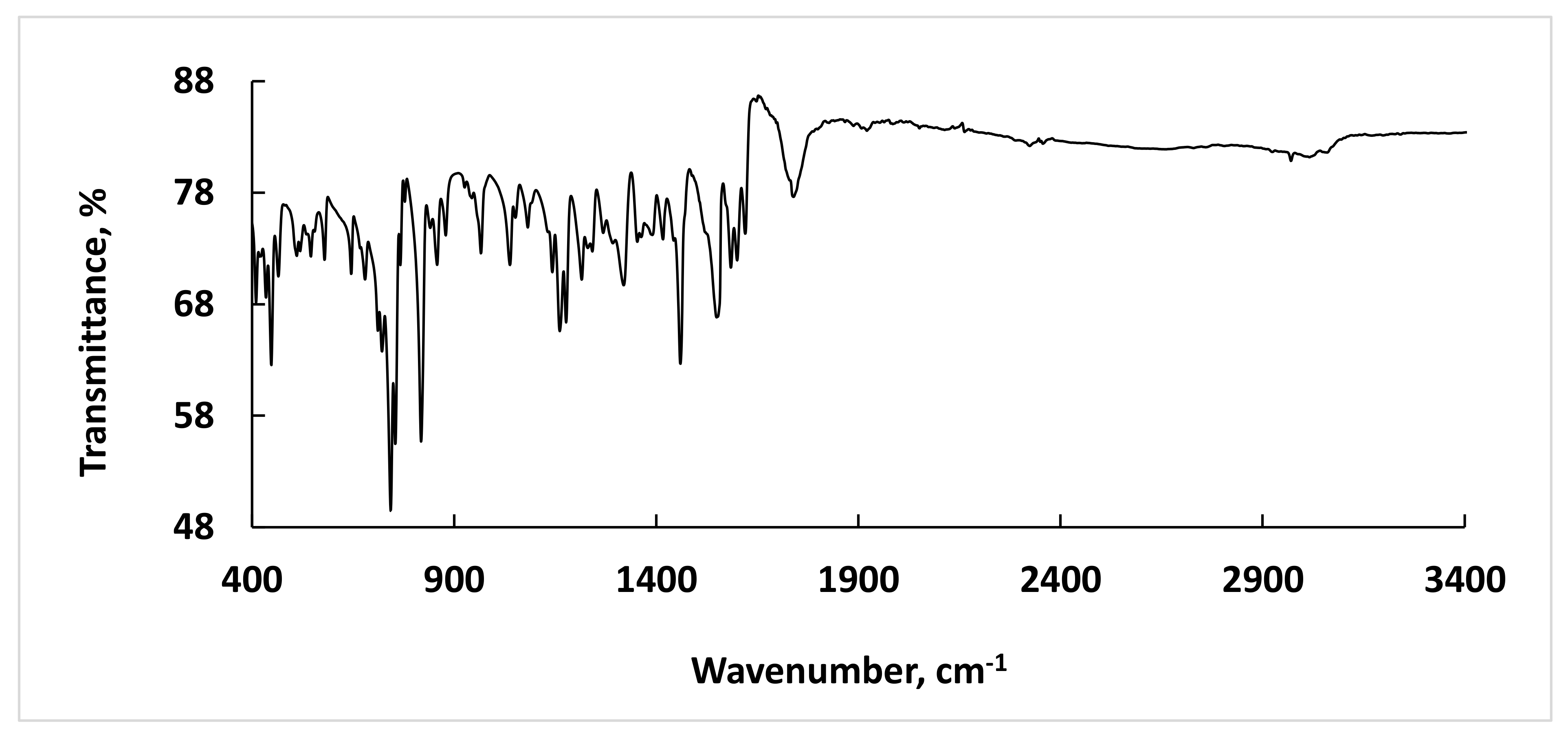
3.1. Synthesis
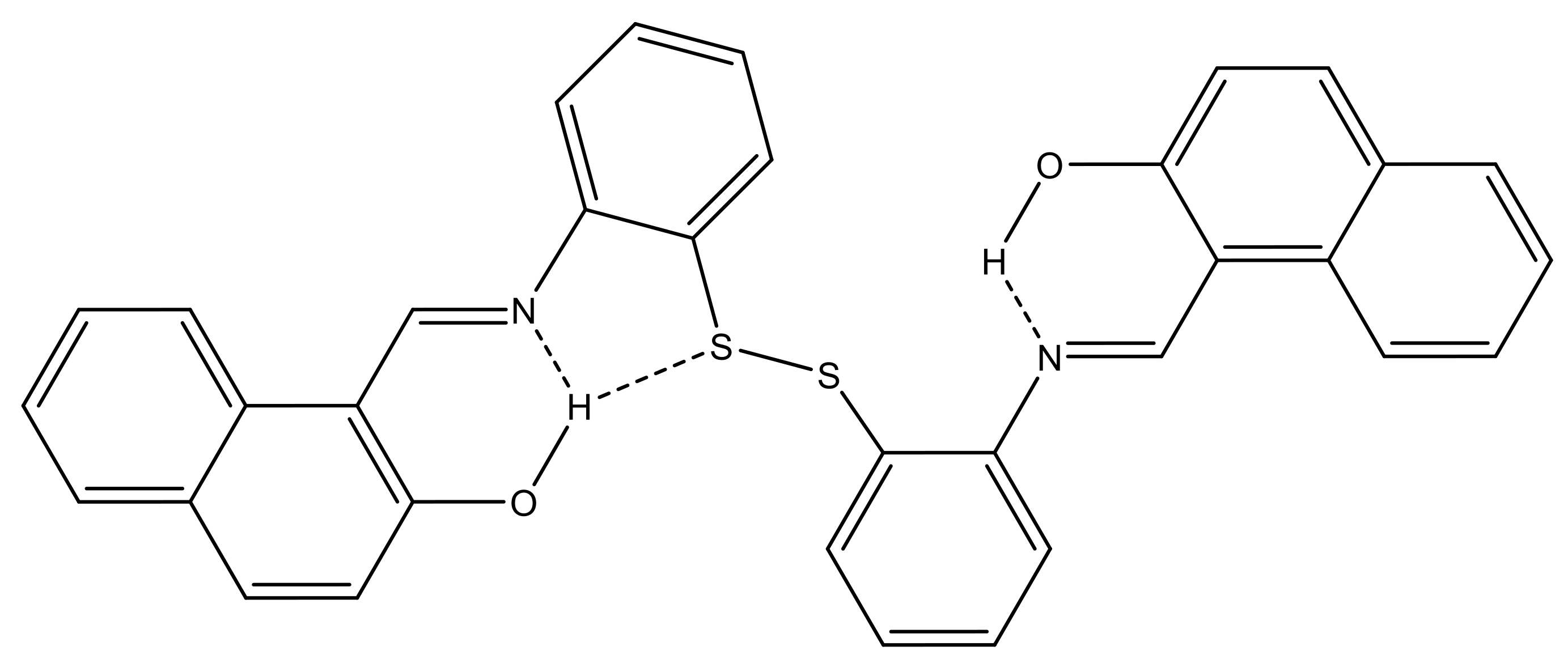
3.2. Crystal Description
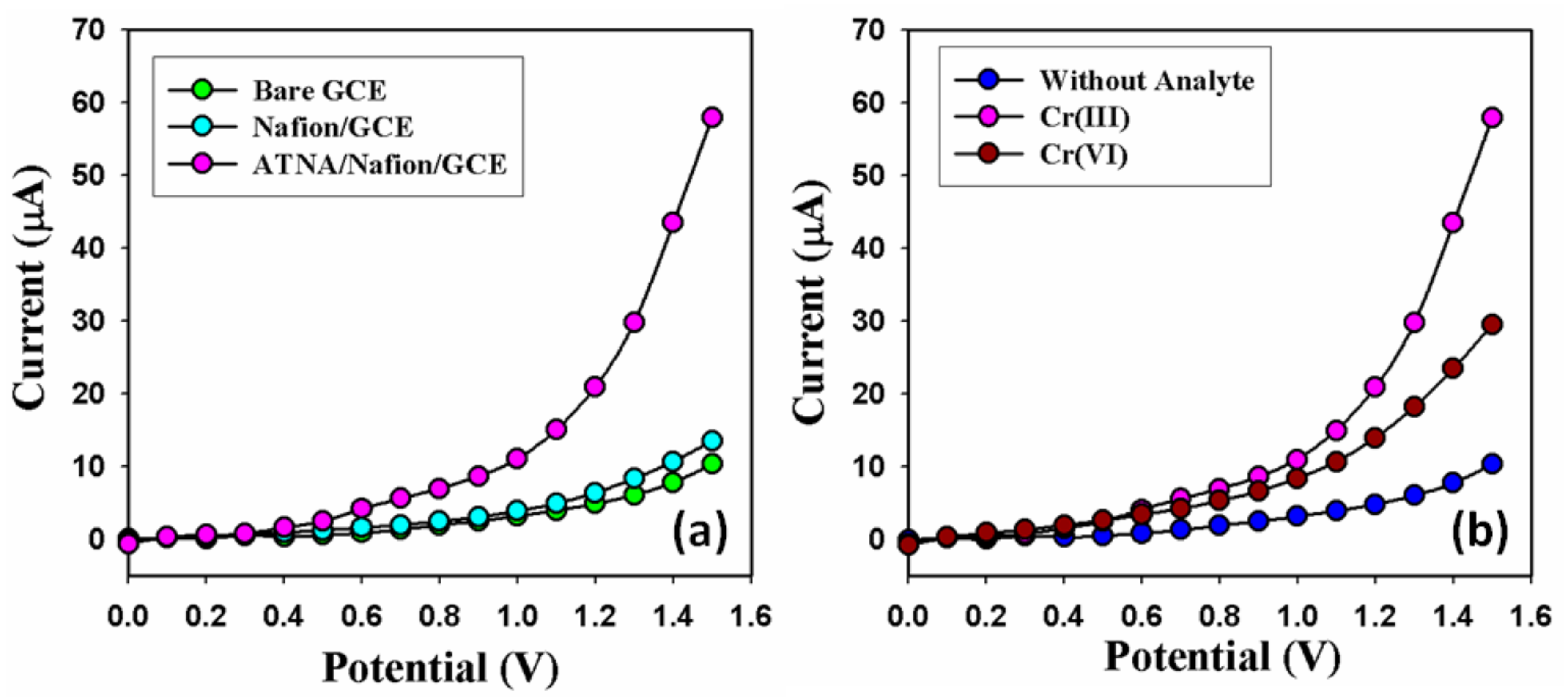
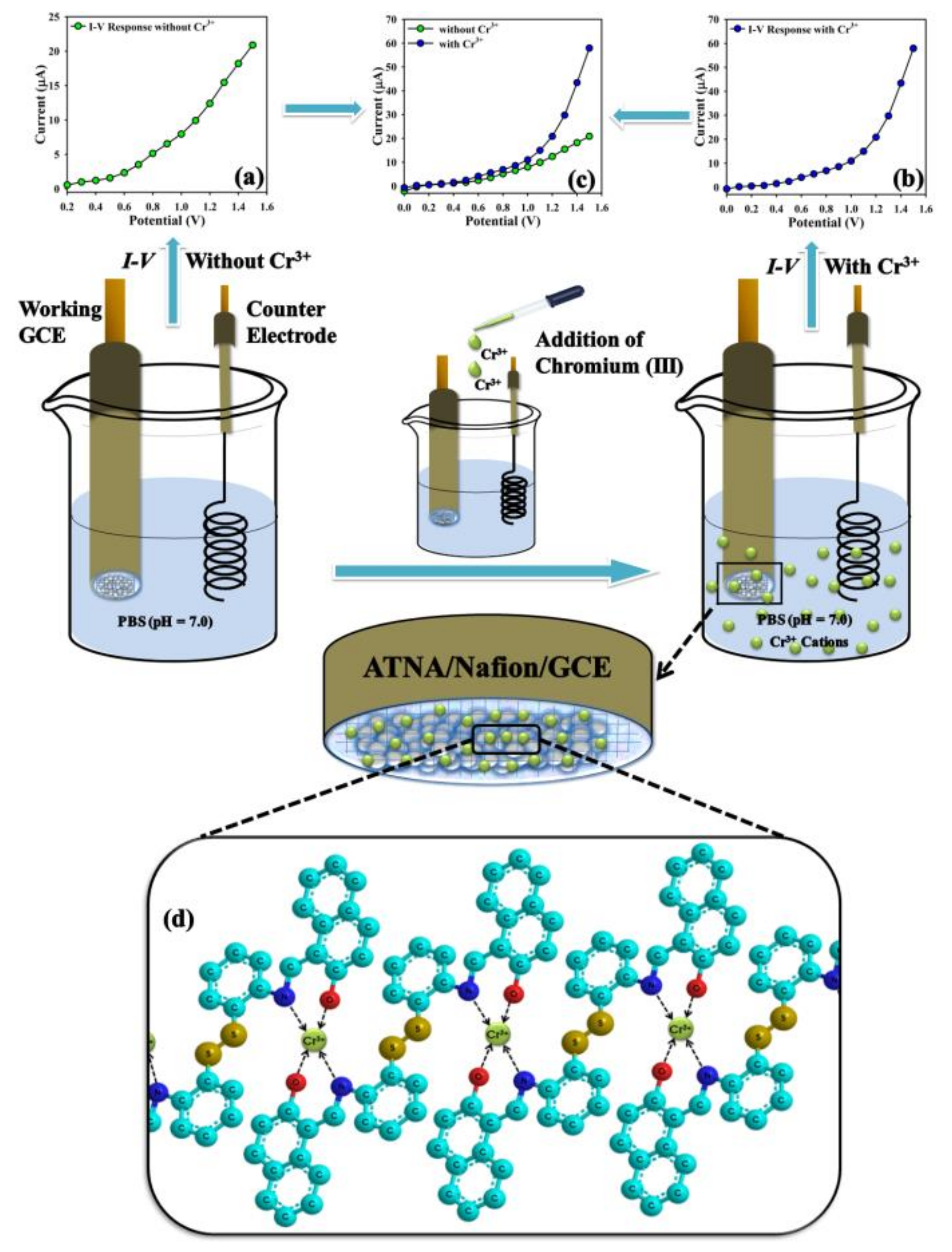
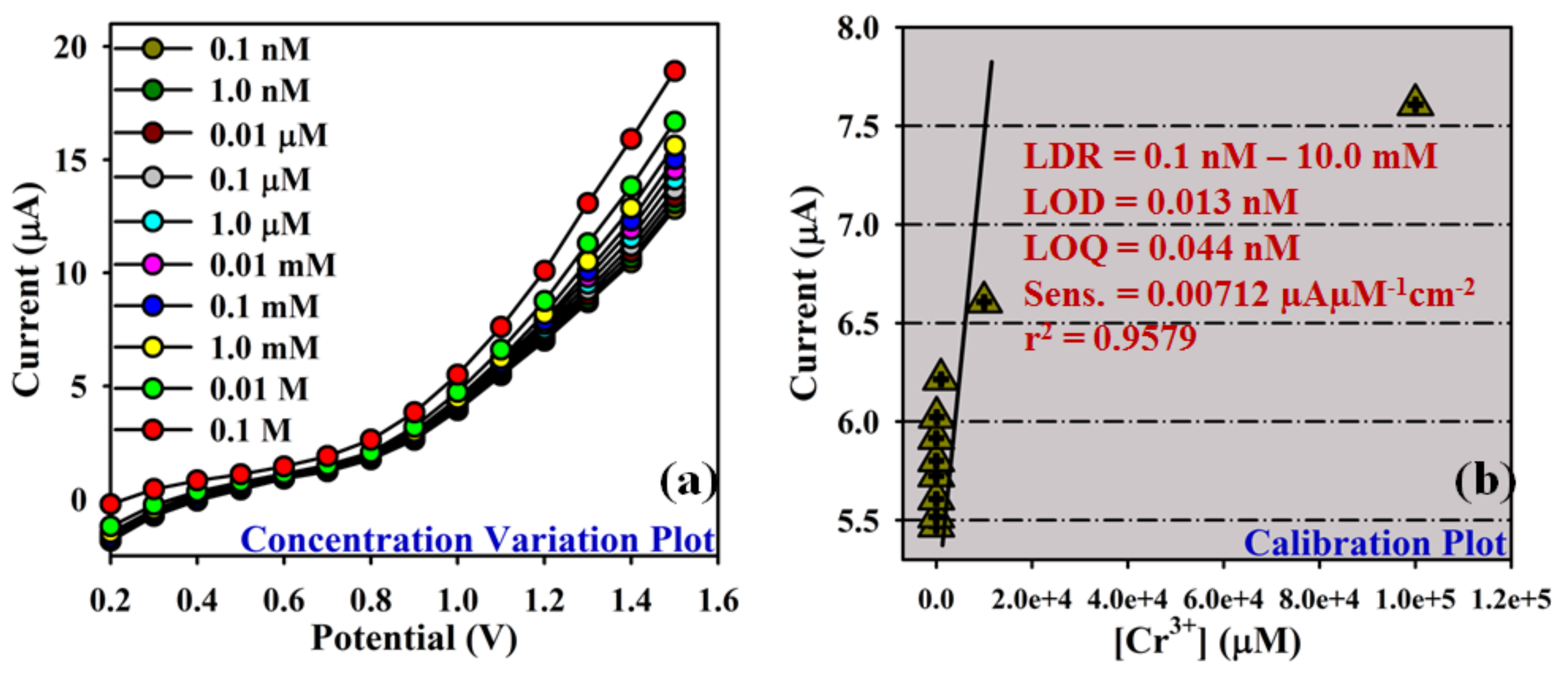
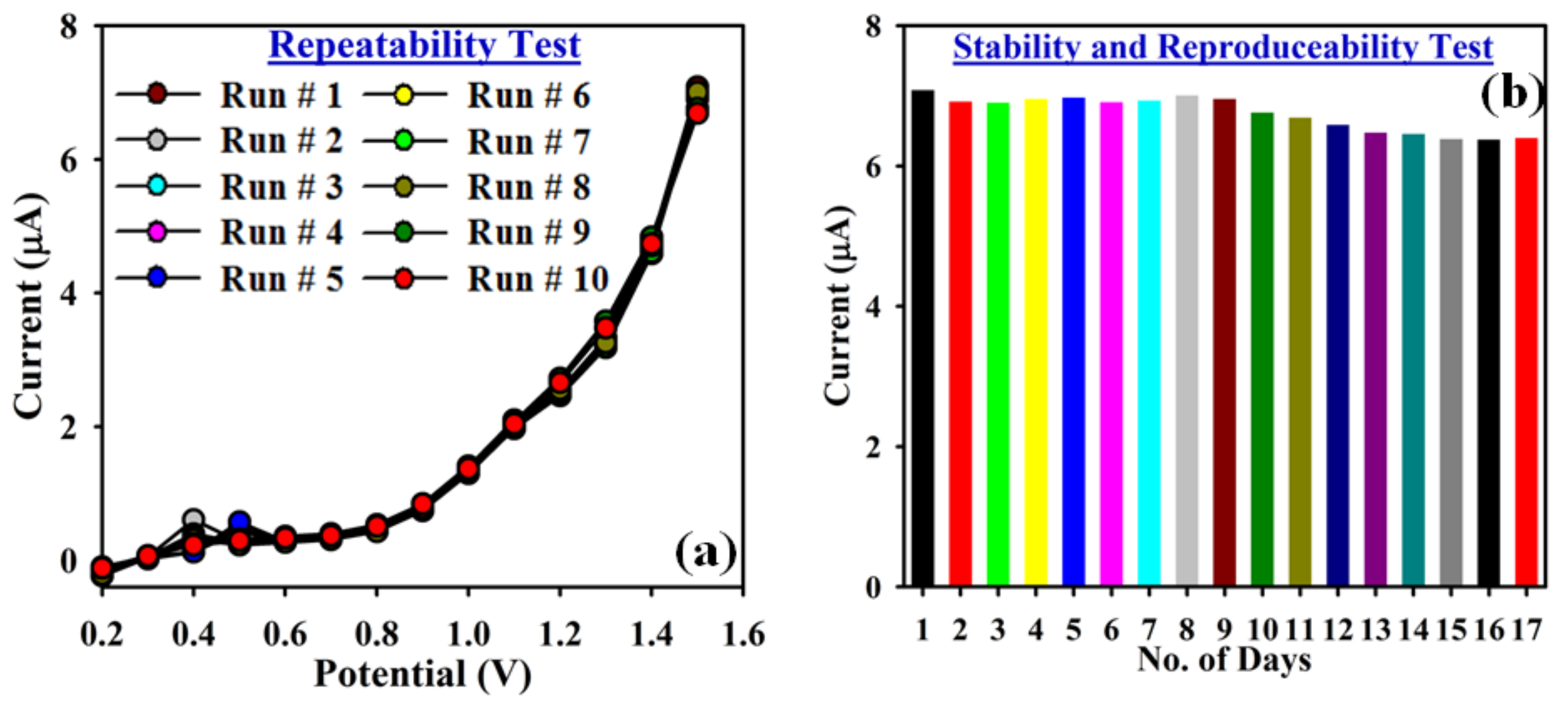
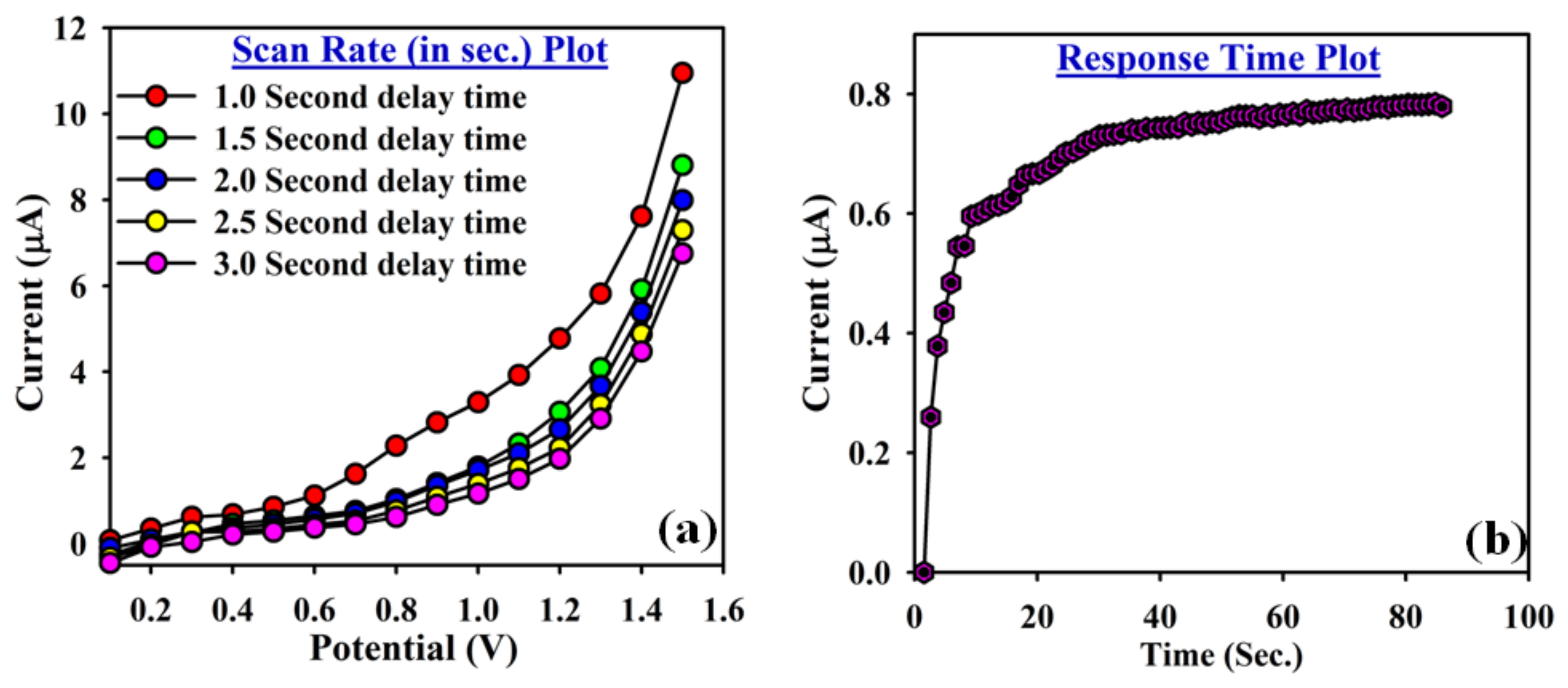
3.3. Application: Detection of Cr3+ Cation with ATNA Modified GCE
3.4. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Word Health Organization. Organization, Guidelines for Drinking Water Quality; Word Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Rassaei, L.; Marken, F.; Sillanpää, M.; Amiri, M.; Cirtiu, C.M.; Sillanpää, M. Nanoparticles in electrochemical sensors for environmental monitoring. Trends Anal. Chem. 2011, 30, 1704–1715. [Google Scholar] [CrossRef]
- Arvand, M.; Lashkari, Z. Sensitive and selective detection of trace copper in standard alloys, food and biological samples using a bulk optode based on N,N′-(4,4′-ethylene biphenyl) bis(3-methoxy salicylidine imine) as neutral carrier. Spectrochim. Acta Part A 2013, 107, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Kara, D.; Fisher, A. Modified silica gels and their use for the preconcentration of trace elements. Sep. Purif. Rev. 2012, 41, 267–317. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Mohamed, S.A.; Asiri, A.M.; Ahmed, N.S. Synthesis of hemicyanine-based chitosan ligands in dye-affinity chromatography for the purification of chewing stick peroxidase. Int. J. Biol. Macromol. 2020, 148, 401–414. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; Alhaddad, O.; El-Shishtawy, R.M.; Raffah, B.M. New two rings Schiff base liquid crystals; ball mill synthesis, mesomorphic, Hammett and DFT studies. J. Mol. Liq. 2020, 299, 112161. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef]
- Hussein, M.A.; El-Shishtawy, R.M.; Obaid, A.Y.; Salam, M.A. Influence of single-walled carbon nanotubes on the performance of Poly(Azomethine-Ether) composite materials. Polym. Technol. Eng. 2017, 57, 1150–1163. [Google Scholar] [CrossRef]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Zayed, M.E.; El-Shishtawy, R.M. Synthesis and antimicrobial activity of Aluminium(III), Nickel(II) and Zinc(II) schiff base complexes derived from o-phenylenediamine and salicylaldehyde. Asian J. Chem. 2013, 25, 2719–2721. [Google Scholar] [CrossRef]
- Ando, R.; Ono, H.; Yagyu, T.; Maeda, M. Spectroscopic characterization of mononuclear, binuclear, and insoluble polynuclear oxovanadium(IV)–Schiff base complexes and their oxidation catalysis. Inorg. Chim. Acta 2004, 357, 817–823. [Google Scholar] [CrossRef]
- Saravanamoorthy, S.; Sivan, V. Physiochemical interactions of chiral Schiff bases on high carbon steel surface: Corrosion inhibition in acidic media. Prog. Org. Coat. 2013, 76, 1527–1535. [Google Scholar] [CrossRef]
- Aqlan, F.M.; Alam, M.; Asiri, A.M.; Zayed, M.E.; Al-Eryani, D.A.; Al-Zahrani, F.A.; El-Shishtawy, R.M.; Uddin, J.; Rahman, M.M. Fabrication of selective and sensitive Pb2+ detection by 2,2′-(-(1,2-phenylenebis(azaneylylidene))bis(methaneylylidene))diphenol by electrochemical approach for environmental remediation. J. Mol. Liq. 2019, 281, 401–406. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Al-Ghamdi, H.A.; Alam, M.M.; Al-Amshany, Z.M.; Asiri, A.M.; Rahman, M.M. Development of Cd2+ sensor based on BZNA/Nafion/Glassy carbon electrode by electrochemical approach. Chem. Eng. J. 2018, 352, 225–231. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sheikh, T.A.; El-Shishtawy, R.M.; Arshad, M.N.; Al-Zahrani, F.A.M.; Asiri, A.M. Fabrication of Sb3+ sensor based on 1,1′-(-(naphthalene-2,3-diylbis(azanylylidene)) bis(methanylylidene))bis(naphthalen-2-ol)/nafion/glassy carbon electrode assembly by electrochemical approach. RSC Adv. 2018, 8, 19754–19764. [Google Scholar] [CrossRef]
- Ramadan, R.; Elantabli, F.M.; El-Medani, S.M. Conversion of thiol to homodisulfide-Schiff base derivative: Synthesis, molecular structure, crystal structure and DFT studies. J. Mol. Struct. 2019, 1196, 547–554. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, V.; Gupta, B. Chromium(III) selective membrane sensors based on Schiff bases as chelating ionophores. Anal. Chim. Acta 2007, 585, 171–178. [Google Scholar] [CrossRef]
- Vincent, J.B. Quest for the molecular mechanism of chromium action and its relationship to diabetes. Nutr. Rev. 2000, 58, 67–72. [Google Scholar] [CrossRef]
- Latva, S.; Jokiniemi, J.; Peräniemi, S.; Ahlgrén, M. Separation of picogram quantities of Cr(III) and Cr(VI) species in aqueous solutions and determination by graphite furnace atomic absorption spectrometry. J. Anal. At. Spectrom. 2003, 18, 84–86. [Google Scholar] [CrossRef]
- McRae, R.; Bagchi, P.; Sumalekshmy, S.; Fahrni, C.J. In situ imaging of metals in cells and tissues. Chem. Rev. 2009, 109, 4780–4827. [Google Scholar] [CrossRef]
- O’Brien, T.; Mandel, H.G.; Pritchard, D.E.; Patierno, S.R. Critical role of Chromium (Cr)−DNA interactions in the formation of Cr-induced polymerase arresting lesions. Biochemistry 2002, 41, 12529–12537. [Google Scholar] [CrossRef]
- Zhitkovich, A.; Quievryn, G.; Messer, J.; Motylevich, Z. Reductive activation with cysteine represents a chromium(III)-dependent pathway in the induction of genotoxicity by carcinogenic chromium(VI). Environ. Health Perspect. 2002, 110, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B. Synthesis and characterization of a new resin functionalized with 2-naphthol-3,6-disulfonic acid and its application for the speciation of chromium in natural water. Talanta 2002, 56, 145–152. [Google Scholar] [CrossRef]
- Beinrohr, E.; Manova, A.; Dzurov, J. Preconcentration of Cr(III) and total Cr in waters for flame AAS in a flow-through electrochemical/sorption cell. Anal. Bioanal. Chem. 1996, 355, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Posta, J.; Berndt, H.; Luo, S.K.; Schaldach, G. High-performance flow flame atomic absorption spectrometry for automated on-line separation and determination of chromium (III)/chromium (VI) and preconcentration of chromium (VI). Anal. Chem. 1993, 65, 2590–2595. [Google Scholar] [CrossRef]
- Cox, A.G.; Cook, I.G.; McLeod, C. Rapid sequential determination of chromium(III)-chromium(VI) by flow injection analysis-inductively coupled plasma atomic-emission spectrometry. Analyst 1985, 110, 331. [Google Scholar] [CrossRef]
- Wu, P.; Chen, H.; Cheng, G.; Hou, X. Exploring surface chemistry of nano-TiO2 for automated speciation analysis of Cr(iii) and Cr(vi) in drinking water using flow injection and ET-AAS detection. J. Anal. At. Spectrom. 2009, 24, 1098. [Google Scholar] [CrossRef]
- Cathum, S.; Brown, C.; Wong, W. Determination of Cr3+, CrO42−, and Cr2O72− in environmental matrixes by high-performance liquid chromatography with diode-array detection (HPLC–DAD). Anal. Bioanal. Chem. 2002, 373, 103–110. [Google Scholar] [CrossRef]
- Krull, I.S.; Bushee, D.; Savage, R.N.; Schleicher, R.G.; Smith, S.B., Jr. Speciation of Cr (III) and Cr (VI) via reversed phase HPLC with inductively coupled plasma emission spectroscopic detection (HPLC-ICP) 24. Anal. Lett. 1982, 15, 267–281. [Google Scholar] [CrossRef]
- Bond, A.M.; Wallace, G.G. Simultaneous determination of copper, nickel, cobalt, chromium(VI), and chromium(III) by liquid chromatography with electrochemical detection. Anal. Chem. 1982, 54, 1706–1712. [Google Scholar] [CrossRef]
- Wang, H.-J.; Du, X.-M.; Wang, M.; Wang, T.-C.; Ou-Yang, H.; Wang, B.; Zhu, M.-T.; Wang, Y.; Jia, G.; Feng, W.; et al. Using ion-pair reversed-phase HPLC ICP-MS to simultaneously determine Cr(III) and Cr(VI) in urine of chromate workers. Talanta 2010, 81, 1856–1860. [Google Scholar] [CrossRef]
- Jen, J.-F.; Ou-Yang, G.-L.; Chen, C.-S.; Yang, S.-M. Simultaneous determination of chromium(III) and chromium (VI) with reversed-phase ion-pair high-performance liquid chromatography. Analyst 1993, 118, 1281. [Google Scholar] [CrossRef]
- Bittner, M.; Broekaert, J. Speciation of chromium by solid-phase extraction coupled to reversed-phase liquid chromatography with UV detection. Anal. Chim. Acta 1998, 364, 31–40. [Google Scholar] [CrossRef]
- Gürleyük, H.; Wallschläger, D. Determination of chromium(iii) and chromium(vi) using suppressed ion chromatography inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2001, 16, 926–930. [Google Scholar] [CrossRef]
- Michalski, R. Trace level determination of Cr (III)/Cr (VI) in water samples using ion chromatography with UV detection. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2849–2862. [Google Scholar] [CrossRef]
- Hettipathirana, T.D. Speciation of sub-parts per billion levels of Cr(III) in waters by solid-phase adsorption followed by thin-layer X-ray fluorescence spectrometry. X-Ray Spectrom. 2001, 30, 330–337. [Google Scholar] [CrossRef]
- Elavarasi, M.; Alex, S.A.; Chandrasekaran, N.; Mukherjee, A. Simple fluorescence-based detection of Cr(iii) and Cr(vi) using unmodified gold nanoparticles. Anal. Methods 2014, 6, 9554–9560. [Google Scholar] [CrossRef]
- Wang, N.; Shiraishi, Y.; Hirai, T. A distyryl BODIPY derivative as a fluorescent probe for selective detection of chromium(III). Tetrahedron Lett. 2010, 51, 2545–2549. [Google Scholar] [CrossRef]
- Dong, C.; Wu, G.; Wang, Z.; Ren, W.; Zhang, Y.; Shen, Z.; Li, T.; Wu, A. Selective colorimetric detection of Cr(iii) and Cr(vi) using gallic acid capped gold nanoparticles. Dalton Trans. 2016, 45, 8347–8354. [Google Scholar] [CrossRef]
- Timerbaev, A.R.; Semenova, O.; Buchberger, W.; Bonn, G.K. Speciation studies by capillary electrophoresis- Simultaneous determination of chromium(III) and chromium(VI). Anal. Bioanal. Chem. 1996, 354, 414–419. [Google Scholar] [CrossRef]
- Himeno, S.; Nakashima, Y.; Sano, K.-I. Simultaneous determination of Chromium(VI) and Chromium(III) by capillary electrophoresis. Anal. Sci. 1998, 14, 369–373. [Google Scholar] [CrossRef]
- Seitz, W.R.; Suydam, W.W.; Hercules, D.M. Determination of trace amounts of chromium(III) using chemiluminescence analysis. Anal. Chem. 1972, 44, 957–963. [Google Scholar] [CrossRef]
- Stefan, R.-I.; Bairu, S.G.; Van Staden, J.F. Diamond paste-based electrodes for determination of Cr(III) in pharmaceutical compounds. Anal. Bioanal. Chem. 2003, 376, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Vukomanovic, D.V.; VanLoon, G.W.; Nakatsu, K.; Zoutman, D.E. Determination of Chromium (VI) and (III) by adsorptive stripping voltammetry with pyrocatechol violet. Microchem. J. 1997, 57, 86–95. [Google Scholar] [CrossRef]
- Jin, W.; Wu, G.; Chen, A. Sensitive and selective electrochemical detection of chromium(vi) based on gold nanoparticle-decorated titania nanotube arrays. Analyst 2014, 139, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, A.R.; Vazquez, M.D.; Tascon, M.L.; Batanero, P.S. Determination of chromium(VI) and chromium(III) by using a diphenylcarbazide-modified carbon paste electrode. Electroanalysis 1993, 5, 155–163. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. Electroanalytical sensing of chromium(iii) and (vi) utilising gold screen printed macro electrodes. Analyst 2012, 137, 896. [Google Scholar] [CrossRef]
- Renedo, O.D.; Lomillo, M.A.A.; Martinez, M.J.A. Optimisation procedure for the inhibitive determination of chromium (III) using an amperometrictyrosinase biosensor. Anal. Chim. Acta 2004, 521, 215–221. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies: Yarnton, UK, 2012. [Google Scholar]
- Sheldrick, G.M. A short history ofSHELX. Acta Crystallogr. A 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON-a Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2005. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Denes, F.; Pichowicz, M.; Povie, G.; Renaud, P. Thiyl radicals in organic synthesis. Chem. Rev. 2014, 114, 2587–2693. [Google Scholar] [CrossRef] [PubMed]
- Moosavi-Tekyeh, Z.; Dastani, N. Intramolecular hydrogen bonding in N-salicylideneaniline: FT-IR spectrum and quantum chemical calculations. J. Mol. Struct. 2015, 1102, 314–322. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2006; pp. 10815–11083. [Google Scholar]
- Raj, P.; Singh, A.; Singh, N.; Singh, N. Syntheses and photophysical properties of schiff base Ni(II) complexes: Application for sustainable antibacterial activity and cytotoxicity. Chem. Eng. 2017, 5, 6070–6080. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Sheikh, T.A.; Arshad, M.N.; Asiri, A.M.; Rahman, M.M.; Shaikh, T.A. Development of a selective and sensitive Ga3+ sensor for environmental safety: A comparative study of cyclohexyl and aromatic bis-sulphonamide fabricated glassy carbon electrodes. New J. Chem. 2018, 42, 13589–13601. [Google Scholar] [CrossRef]
- Sheikh, T.A.; Arshad, M.N.; Rahman, M.M.; Asiri, A.M.; Marwani, H.M.; Awual, R.; Bawazir, W.A. Trace electrochemical detection of Ni2+ ions with bidentate N, N′-(ethane-1, 2-diyl) bis (3, 4-dimethoxy benzenesulfonamide)[EDBDMBS] as a chelating agent. Inorg. Chim. Acta 2017, 464, 157–166. [Google Scholar] [CrossRef]
- Hussain, M.M.; Asiri, A.M.; Arshad, M.N.; Rahman, M.M. Synthesis, characterization, and crystal structure of (E)-Nʹ-(4-Bromobenzylidene)-benzenesulfonohydrazide and its application as a sensor of chromium ion detection from environmental samples. J. Mol. Struct. 2020, 1207, 127810. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.P.; Rahman, M.M.; Asiri, A.M.; Alamry, K. Preparation of polyaniline grafted graphene oxide–WO3 nanocomposite and its application as a chromium(iii) chemi-sensor. RSC Adv. 2015, 5, 105169–105178. [Google Scholar] [CrossRef]
- Asiri, A.M.; BahadarKhanab, S.; Alamry, K.; Marwani, H.; Rahman, M.M. Growth of Mn3O4 on cellulose matrix: Nanohybrid as a solid phase adsorbent for trivalent chromium. Appl. Surf. Sci. 2013, 270, 539–544. [Google Scholar] [CrossRef]
- Rahman, M.M.; Balkhoyor, H.B.; Asiri, A.M. Phenolic sensor development based on chromium oxide-decorated carbon nanotubes for environmental safety. J. Environ. Manag. 2017, 188, 228–237. [Google Scholar] [CrossRef]

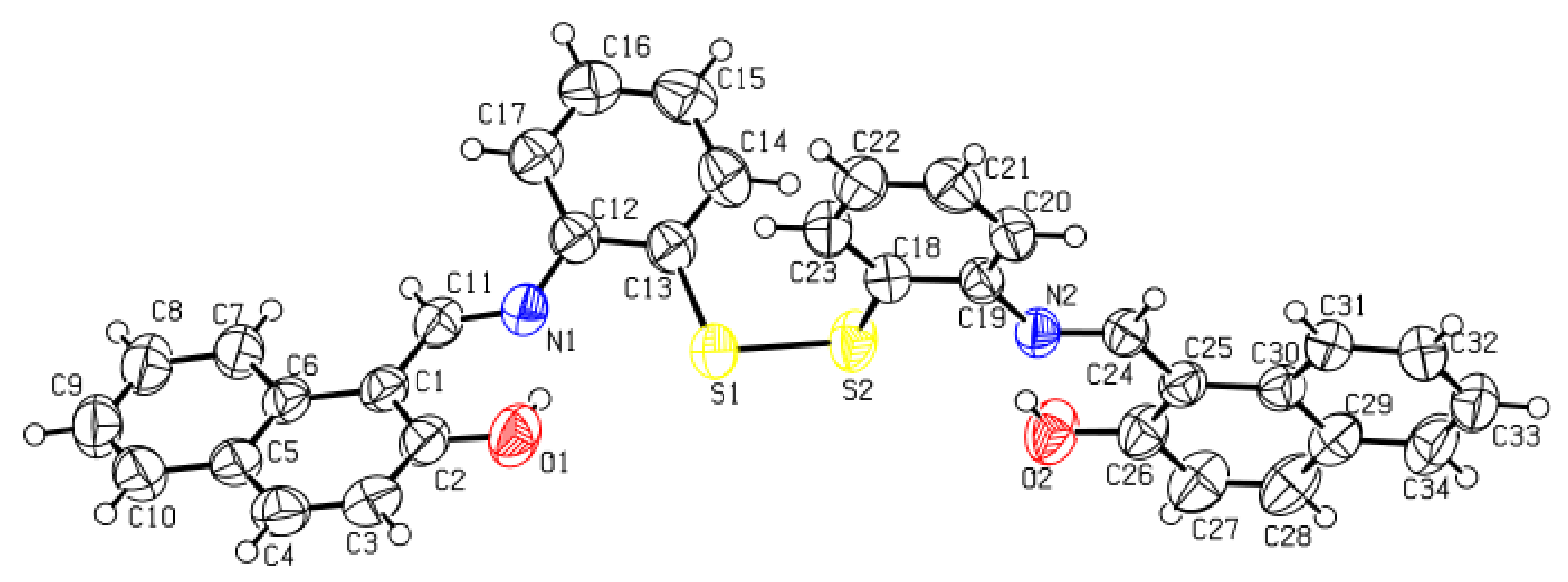
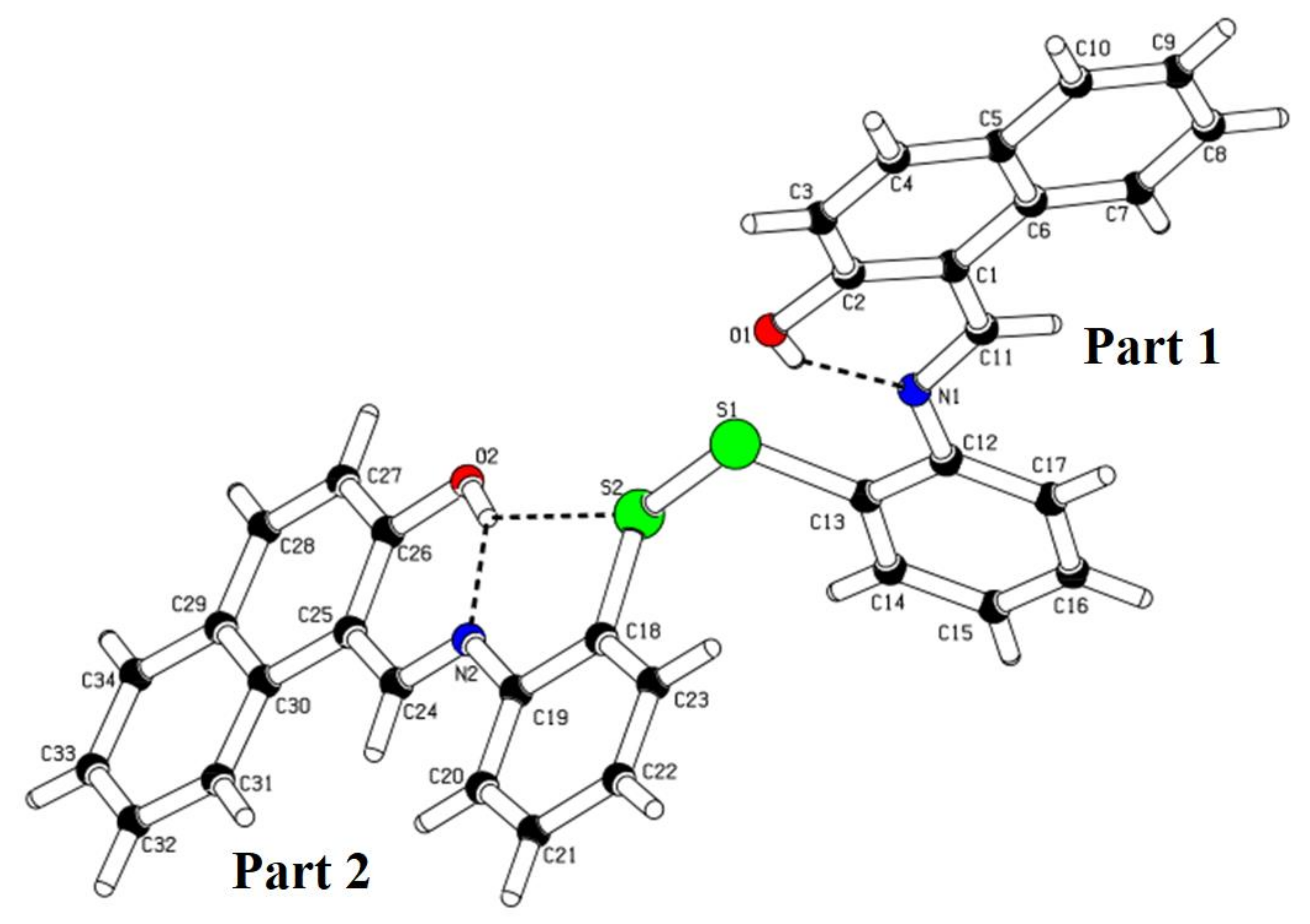

| CCDC Code (Cambridge Crystallographic Data Centre) | 1953254 |
|---|---|
| Empirical formula | C34H24N2O2S2 |
| Formula weight | 556.67 |
| Temperature/K | 296(2) |
| Crystal system | triclinic |
| Space group | P-1 |
| a/Å | 8.6843(5) |
| b/Å | 11.8282(6) |
| c/Å | 13.5159(9) |
| α/° | 78.828(5) |
| β/° | 82.291(5) |
| γ/° | 86.223(4) |
| Volume/Å3 | 1348.59(14) |
| Z | 2 |
| ρcalcg/cm3 | 1.371 |
| μ/mm−1 | 0.233 |
| F(000) | 580.0 |
| Crystal size/mm3 | 0.22 × 0.17 × 0.12 |
| Radiation | MoKα (λ = 0.71073) |
| 2θ range for data collection/° | 5.942 to 58.364 |
| Index ranges | −11 ≤ h ≤ 7, −15 ≤ k ≤ 15, −17 ≤ l ≤ 18 |
| Reflections collected | 12,053 |
| Independent reflections | 6334 (Rint = 0.0338, Rsigma = 0.0553) |
| Data/restraints/parameters | 6334/0/361 |
| Goodness-of-fit on F2 | 1.031 |
| Final R indexes indexes (I>=2σ (I)) | R1 = 0.0538, wR2 = 0.1211 |
| Final R indexes (all data) | R1 = 0.1039, wR2 = 0.1544 |
| Largest diff. peak/hole / e Å−3 | 0.23/−0.27 |
| D | H | A | d(D-H)/Å | d(H-A)/Å | d(D-A)/Å | D-H-A/° |
|---|---|---|---|---|---|---|
| O1 | H1O | N1 | 0.82 | 1.85 | 2.575(3) | 146.8 |
| O2 | H2O | N2 | 0.82 | 1.85 | 2.574(3) | 147.4 |
| O2 | H2O | S2 | 0.82 | 2.74 | 3.410(2) | 139.7 |
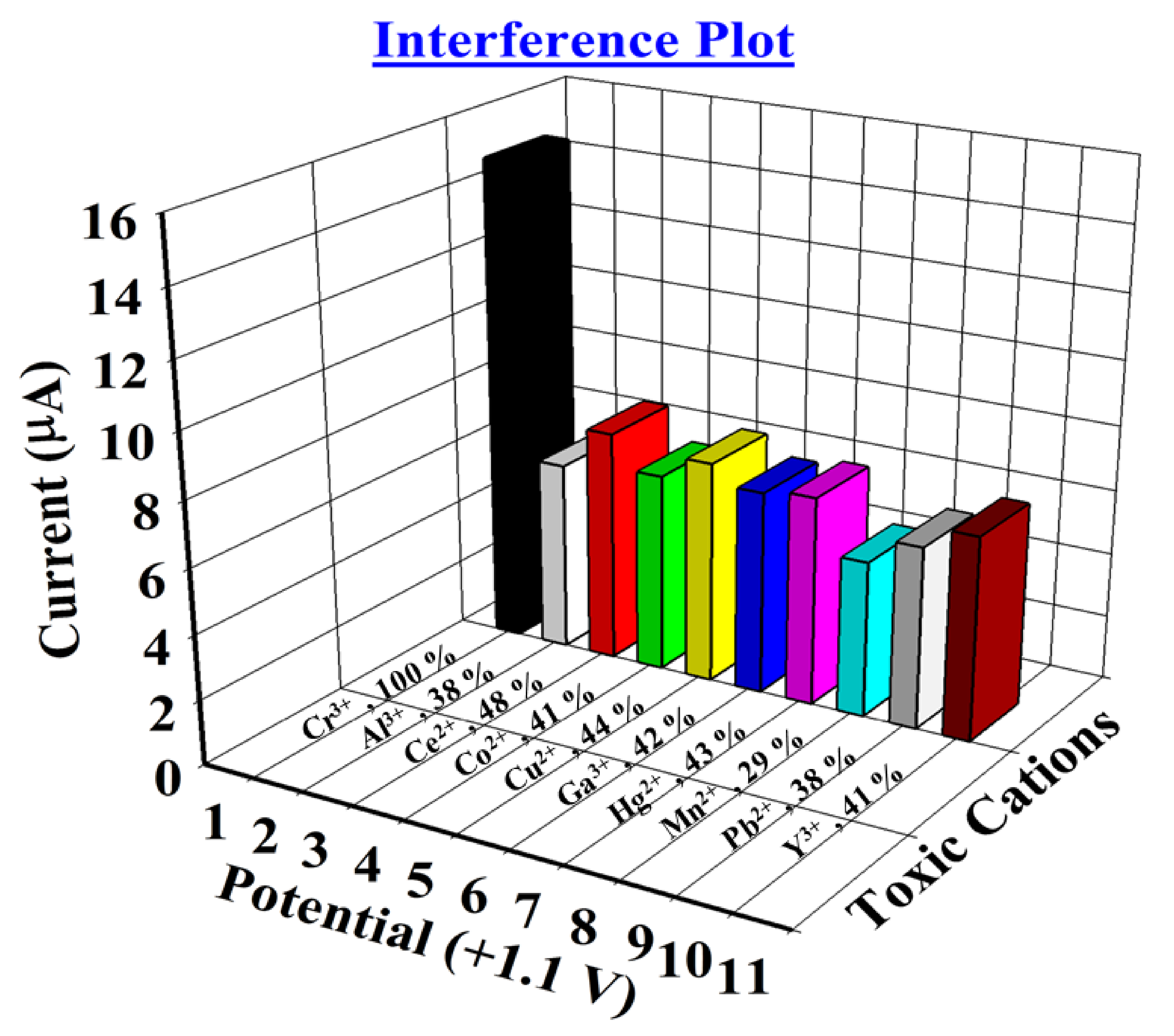
| Metal Ions | Observed Current (µA) | Interference Effect (%) | *SD (n= 3) | #RSD (%) (n = 3) | |||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | Average | ||||
| Cr3+ | 14.9618 | 14.8241 | 15.1423 | 14.9760 | 100 | 0.1595 | 1.07 |
| Al3+ | 5.2456 | 6.1323 | 5.8625 | 5.7468 | 38 | 0.4545 | 7.91 |
| Ce2+ | 7.1919 | 7.2031 | 7.1879 | 7.1943 | 48 | 0.0078 | 0.11 |
| Co2+ | 6.1792 | 6.2955 | 6.0095 | 6.1614 | 41 | 0.1438 | 2.33 |
| Cu2+ | 6.5441 | 6.6192 | 6.8994 | 6.6875 | 44 | 0.1872 | 2.80 |
| Ga3+ | 6.3739 | 6.2084 | 6.3215 | 6.3012 | 42 | 0.0845 | 1.34 |
| Hg2+ | 6.5512 | 6.4989 | 6.3003 | 6.4501 | 43 | 0.1323 | 2.05 |
| Mn2+ | 4.0958 | 4.3853 | 4.8554 | 4.4455 | 29 | 0.3833 | 8.62 |
| Pb2+ | 5.9285 | 5.7969 | 5.6714 | 5.7989 | 38 | 0.1285 | 2.22 |
| Y3+ | 5.8275 | 6.2791 | 6.3461 | 6.1509 | 41 | 0.2820 | 4.59 |
| Methods | Material | Sensitivity | *LDR | #LOD | @LOQ | Ref. |
|---|---|---|---|---|---|---|
| Amperometric | Tyrosinase biosensor | – | 2.0 × 10−4 M | 500.0 nM | – | [48] |
| Capillary electrophoresis | 1,2-Cyclohexanediaminetetraacetic acid (CDTA) | – | 0.0 M–0.0019 M | 961.6 nM | – | [40] |
| Capillary electrophoresis | Hexamolybdochromate | – | 5 × 10−6–1 × 10−5 M | 2000 nM | – | [41] |
| Chemiluminescence | Ethylene diamine tetra acetate (EDTA) | – | 0.0–1 × 10−6 M | 0.5 nM | – | [42] |
| Cyclic voltammetry&erometry | Gold nanoparticle-decorated titania nanotube arrays | 6.91 µAµM−1 | 0.10 µM–105 µM | 0.03 µM | – | [45] |
| Electrothermal atomic absorption spectrometric | Nano TiO2 | – | 1 × 10−3–0.5 M | 0.11 nM | – | [27] |
| Thin-layer X-ray fluorescence spectrometry | Solid-phase hydrous ferric hydroxide (HFO) | – | 0.0–1.0 µM | 16.9 nM | – | [36] |
| High-performance liquid chromatography with diode-array detection | Ammonium pyrrolidinedithiocarbamate (APDC) | – | 190 nM–0.76 mM | 76,900–134,000 nM | – | [28] |
| Inductively Coupled PlasmaAtomic-emission Spectrometry | Micro-column of activated alumina | – | 0.0–1.9 × 10−5 M | 26.0 nM | – | [26] |
| Electrochemical I-V method | ATNA/Nafion/GCE | 0.0071202 µAµM−1cm−2 | 1.0 nM–0.01 M | 0.013 nM | 0.04 nM | This work |
| Real Samples | Amount of 3-CP Added | No. of Readings | Measured Response in (µA) | % Recovery | Mean (% Recovery) | SD | RSD | SEM |
|---|---|---|---|---|---|---|---|---|
| Cr3+ | 0.1 µM, 25 µL | - | 13.6808 | 100 | - | - | - | - |
| Plastic baby feeding bottle | 0.1 µM, 25 µL | R1 | 13.2376 | 96.7 | 91.2 | 4.765 | 5.22 | 2.75 |
| R2 | 12.2129 | 89.3 | ||||||
| R3 | 12.0183 | 87.8 | ||||||
| Industrial effluent | 0.1 µM, 25.0 µL | R1 | 14.9859 | 109.5 | 106.7 | 3.143 | 2.95 | 1.81 |
| R2 | 14.6821 | 107.3 | ||||||
| R3 | 14.1258 | 103.3 | ||||||
| Sea water | 0.1 µM, 25.0 µL | R1 | 12.1036 | 88.5 | 88.1 | 1.833 | 2.08 | 1.05 |
| R2 | 11.7748 | 86.1 | ||||||
| R3 | 12.2757 | 89.7 | ||||||
| Tap water | 0.1 µM, 25.0 µL | R1 | 13.2457 | 96.8 | 97.9 | 1.850 | 1.89 | 1.06 |
| R2 | 13.6980 | 100.1 | ||||||
| R3 | 13.2682 | 97.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shishtawy, R.M.; Rahman, M.M.; Sheikh, T.A.; Nadeem Arshad, M.; Al-Zahrani, F.A.M.; Asiri, A.M. A New Cr3+ Electrochemical Sensor Based on ATNA/Nafion/Glassy Carbon Electrode. Materials 2020, 13, 2695. https://doi.org/10.3390/ma13122695
El-Shishtawy RM, Rahman MM, Sheikh TA, Nadeem Arshad M, Al-Zahrani FAM, Asiri AM. A New Cr3+ Electrochemical Sensor Based on ATNA/Nafion/Glassy Carbon Electrode. Materials. 2020; 13(12):2695. https://doi.org/10.3390/ma13122695
Chicago/Turabian StyleEl-Shishtawy, Reda M., Mohammed M. Rahman, Tahir Ali Sheikh, Muhammad Nadeem Arshad, Fatimah A. M. Al-Zahrani, and Abdullah M. Asiri. 2020. "A New Cr3+ Electrochemical Sensor Based on ATNA/Nafion/Glassy Carbon Electrode" Materials 13, no. 12: 2695. https://doi.org/10.3390/ma13122695
APA StyleEl-Shishtawy, R. M., Rahman, M. M., Sheikh, T. A., Nadeem Arshad, M., Al-Zahrani, F. A. M., & Asiri, A. M. (2020). A New Cr3+ Electrochemical Sensor Based on ATNA/Nafion/Glassy Carbon Electrode. Materials, 13(12), 2695. https://doi.org/10.3390/ma13122695










